Modulation of the Cellular Microenvironment by Mechanical Fluid Shear Stress and Hypoxia Alters the Differentiation Capacity of Skeletal Muscle-Derived Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Myogenic Differentiation
- τ = FSS (Pa)
- µ = Fluid viscosity (Pa s)
- θmax = Maximum rocking angle (rad)
- δ = Liquid depth/length-ratio (h0/(2R))
- h0 = Liquid depth (mm)
- R = Radius of the cultivation plate (mm)
- T = Rocking period, duration of one cycle (s)
2.3. Hypoxia Induction
2.4. Colony Formation Unit Assay
2.5. MTT Assay
2.6. Sulforhodamine B Assay
2.7. Immunocytochemistry
2.8. Phalloidin Staining
2.9. DAF-FM Staining
2.10. RT-qPCR
2.11. Statistical Analysis
3. Results
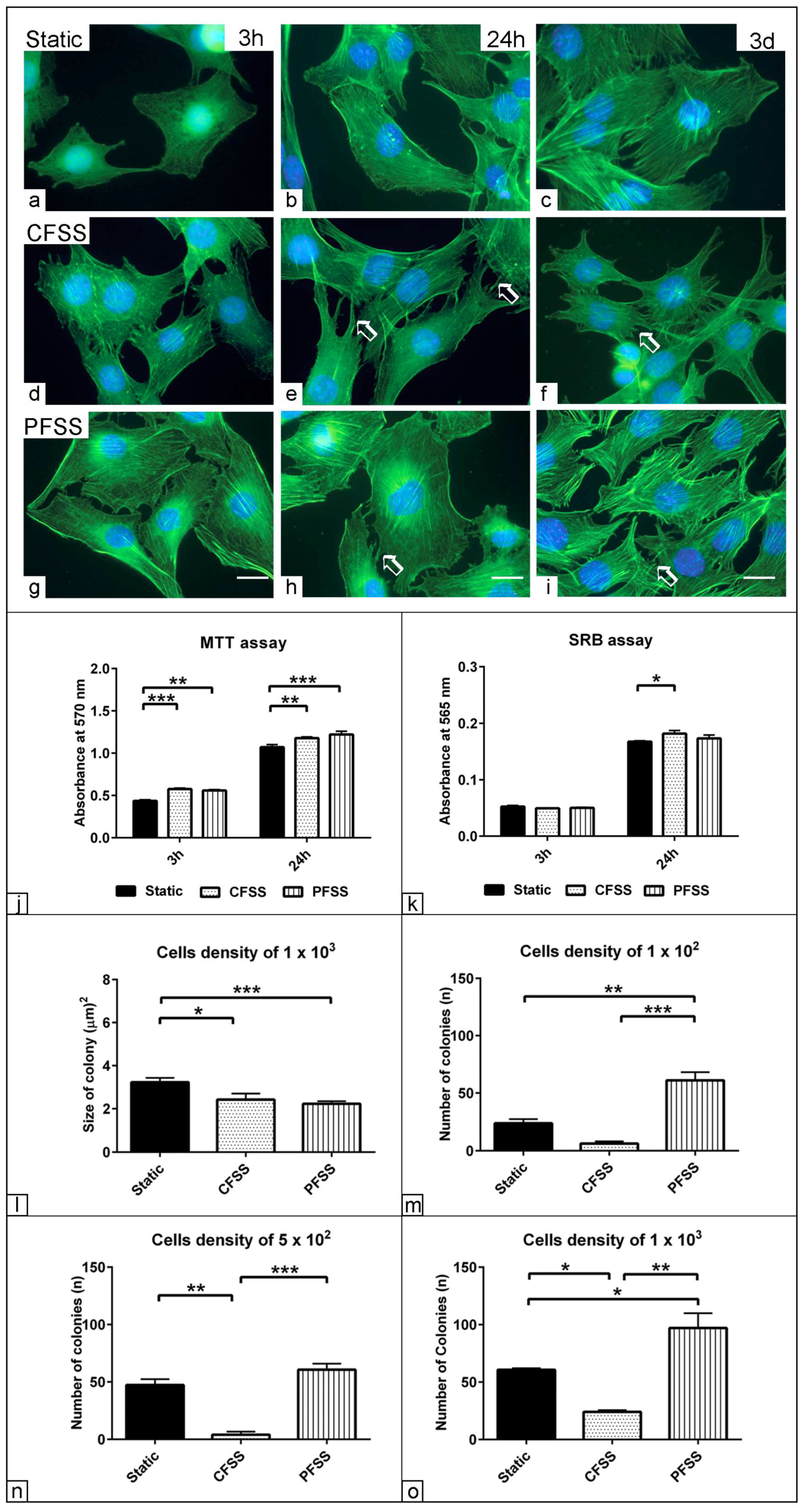
3.1. Effect of Optimized Mechanical Fluid Shear Stress on the Cell Viability
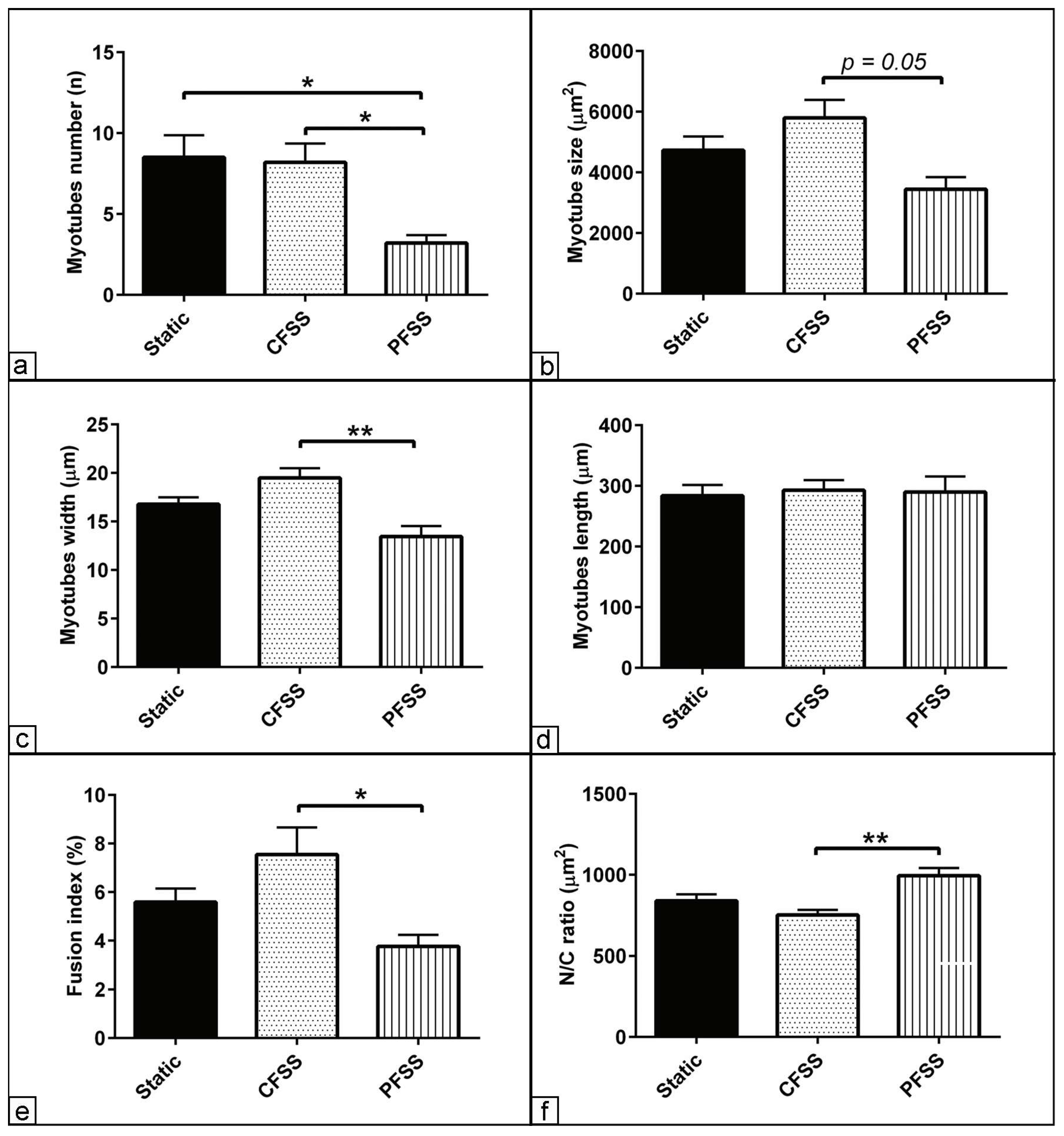
3.2. Effect of Optimized Mechanical Fluid Shear Stress on Myogenic Differentiation
3.3. Morphometric Assessment of Myogenic Differentiation under Mechanical Fluid Shear Stress
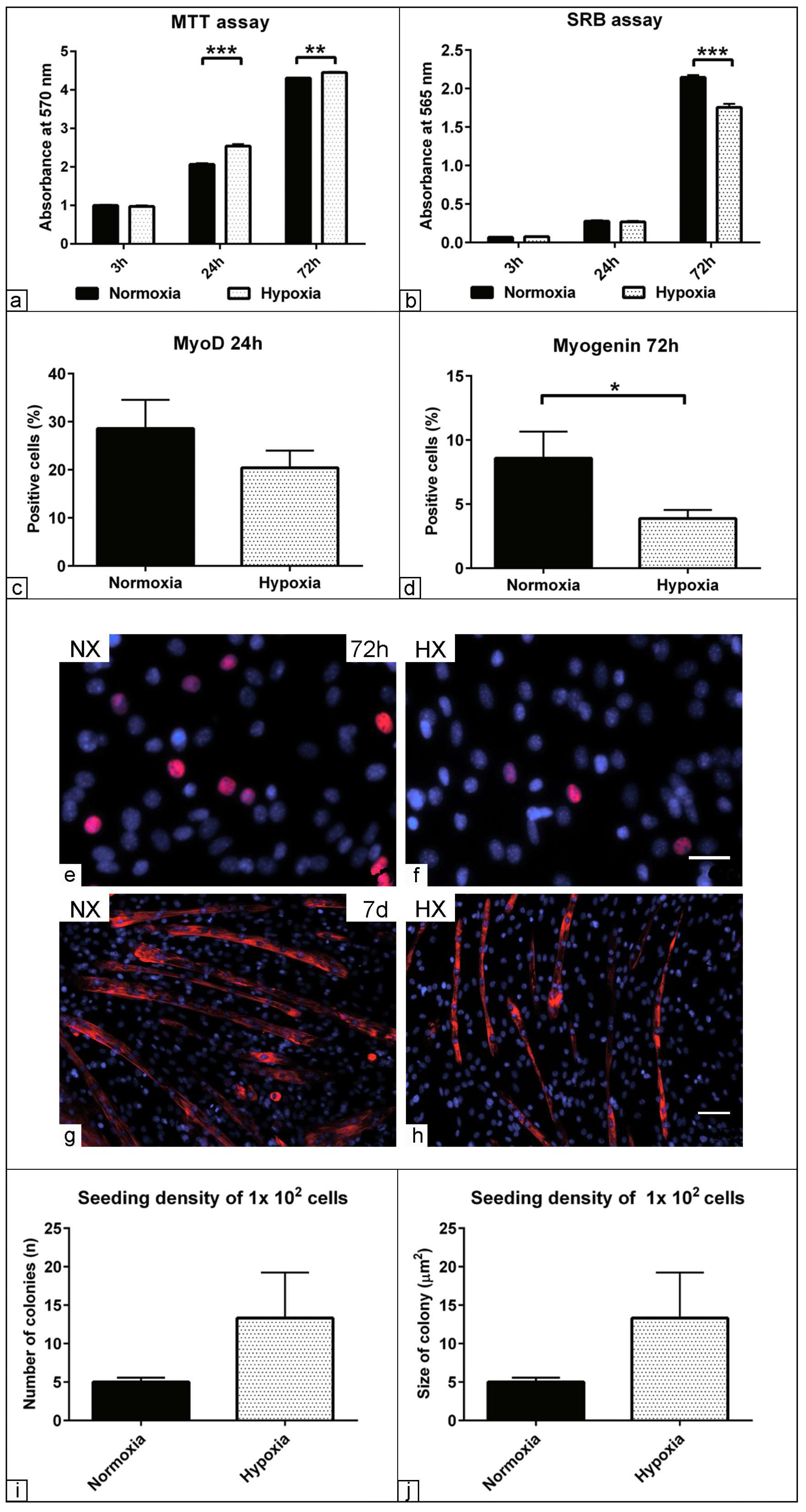
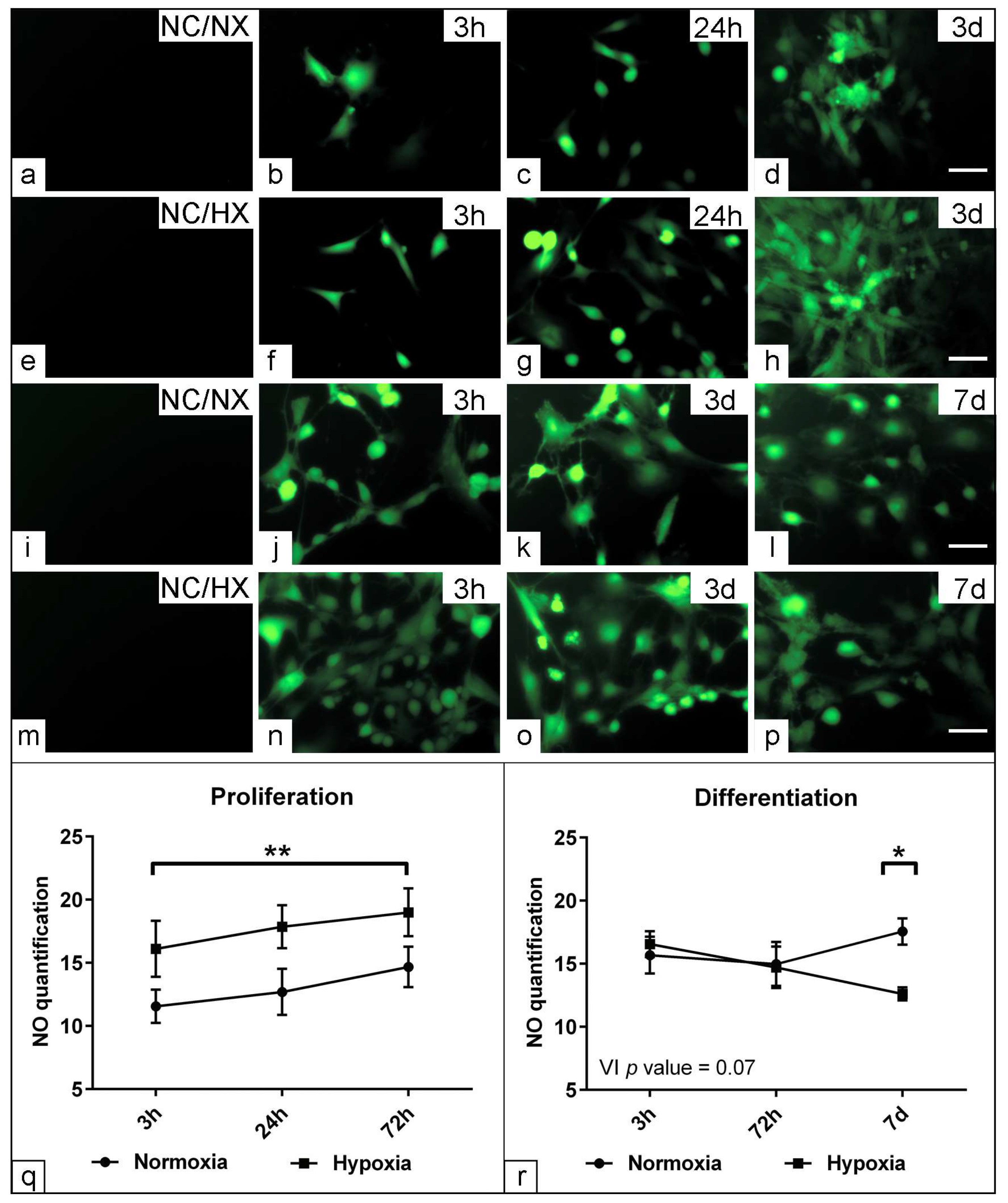
3.4. Effect of Low Oxygen Concentration on the Physiological Parameters of Muscle Precursors
3.5. The Effect of Hypoxia on Myogenic Relative Markers Expression
3.6. Morphometric Assessment of Myogenic Differentiation under Hypoxia
3.7. Assessment the Effect of Hypoxia on Nitric Oxide Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, A.-S.; Launay, T.; Della Gaspera, B.; Charbonnier, F.; Gallien, C.L.; Chanoine, C. Effects of eccentric treadmill running on mouse soleus: Degeneration/regeneration studied with Myf-5 and MyoD probes. Acta Physiol. Scand. 2003, 179, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Mann, C.J.; Vidal, B.; Ardite, E.; Perdiguero, E.; Muñoz-Cánoves, P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr. Top. Dev. Biol. 2011, 96, 167–201. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.N.; Tajbakhsh, S.; Mouly, V.; Cossu, G.; Buckingham, M.; Butler-Browne, G.S. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 1999, 112 Pt 17, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Zhou, Z.; Bornemann, A. MRF4 protein expression in regenerating rat muscle. J. Muscle Res. Cell Motil. 2001, 22, 311–316. [Google Scholar] [CrossRef]
- Boers, H.E.; Haroon, M.; Le Grand, F.; Bakker, A.D.; Klein-Nulend, J.; Jaspers, R.T. Mechanosensitivity of aged muscle stem cells. J. Orthop. Res. 2018, 36, 632–641. [Google Scholar] [CrossRef]
- Park, J.-H.; Ushida, T.; Akimoto, T. Control of cell differentiation by mechanical stress. JPFSM 2013, 2, 49–62. [Google Scholar] [CrossRef]
- Li, E.W.; McKee-Muir, O.C.; Gilbert, P.M. Cellular Biomechanics in Skeletal Muscle Regeneration. Curr. Top. Dev. Biol. 2018, 126, 125–176. [Google Scholar] [CrossRef]
- McCullen, S.D.; Haslauer, C.M.; Loboa, E.G. Musculoskeletal mechanobiology: Interpretation by external force and engineered substratum. J. Biomech. 2010, 43, 119–127. [Google Scholar] [CrossRef]
- Simmons, C.A.; Matlis, S.; Thornton, A.J.; Chen, S.; Wang, C.Y.; Mooney, D.J. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J. Biomech. 2003, 36, 1087–1096. [Google Scholar] [CrossRef]
- Sen, B.; Xie, Z.; Case, N.; Ma, M.; Rubin, C.; Rubin, J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 2008, 149, 6065–6075. [Google Scholar] [CrossRef] [PubMed]
- Vandenburgh, H.; Kaufman, S. In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 1979, 203, 265–268. [Google Scholar] [CrossRef]
- Kook, S.-H.; Son, Y.-O.; Lee, K.-Y.; Lee, H.-J.; Chung, W.-T.; Choi, K.-C.; Lee, J.-C. Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell Biol. Int. 2008, 32, 871–878. [Google Scholar] [CrossRef]
- Tatsumi, R. Mechano-biology of skeletal muscle hypertrophy and regeneration: Possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim. Sci. J. 2010, 81, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Grodzinsky, A.J.; Levenston, M.E.; Jin, M.; Frank, E.H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2000, 2, 691–713. [Google Scholar] [CrossRef] [PubMed]
- Rath, B.; Nam, J.; Knobloch, T.J.; Lannutti, J.J.; Agarwal, S. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J. Biomech. 2008, 41, 1095–1103. [Google Scholar] [CrossRef]
- Cezar, C.A.; Roche, E.T.; Vandenburgh, H.H.; Duda, G.N.; Walsh, C.J.; Mooney, D.J. Biologic-free mechanically induced muscle regeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 1534–1539. [Google Scholar] [CrossRef]
- Arora, S.; Srinivasan, A.; Leung, C.M.; Toh, Y.-C. Bio-mimicking Shear Stress Environments for Enhancing Mesenchymal Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2020, 15, 414–427. [Google Scholar] [CrossRef]
- Kim, H.W.; Lim, J.; Rhie, J.W.; Kim, D.S. Investigation of effective shear stress on endothelial differentiation of human adipose-derived stem cells with microfluidic screening device. Microelectron. Eng. 2017, 174, 24–27. [Google Scholar] [CrossRef]
- Elashry, M.I.; Gegnaw, S.T.; Klymiuk, M.C.; Wenisch, S.; Arnhold, S. Influence of mechanical fluid shear stress on the osteogenic differentiation protocols for Equine adipose tissue-derived mesenchymal stem cells. Acta Histochem. 2019, 121, 344–353. [Google Scholar] [CrossRef]
- Evertz, L.Q.; Greising, S.M.; Morrow, D.A.; Sieck, G.C.; Kaufman, K.R. Analysis of fluid movement in skeletal muscle using fluorescent microspheres. Muscle Nerve 2016, 54, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Juffer, P.; Bakker, A.D.; Klein-Nulend, J.; Jaspers, R.T. Mechanical loading by fluid shear stress of myotube glycocalyx stimulates growth factor expression and nitric oxide production. Cell Biochem. Biophys. 2014, 69, 411–419. [Google Scholar] [CrossRef]
- Naskar, S.; Kumaran, V.; Basu, B. On The Origin of Shear Stress Induced Myogenesis Using PMMA Based Lab-on-Chip. ACS Biomater. Sci. Eng. 2017, 3, 1154–1171. [Google Scholar] [CrossRef]
- Haroon, M.; Boers, H.E.; Bakker, A.D.; Bloks, N.G.C.; Hoogaars, W.M.H.; Giordani, L.; Musters, R.J.P.; Deldicque, L.; Koppo, K.; Le Grand, F.; et al. Reduced growth rate of aged muscle stem cells is associated with impaired mechanosensitivity. Aging 2022, 14, 28–53. [Google Scholar] [CrossRef]
- Beaudry, M.; Hidalgo, M.; Launay, T.; Bello, V.; Darribère, T. Regulation of myogenesis by environmental hypoxia. J. Cell Sci. 2016, 129, 2887–2896. [Google Scholar] [CrossRef]
- Chaillou, T.; Lanner, J.T. Regulation of myogenesis and skeletal muscle regeneration: Effects of oxygen levels on satellite cell activity. FASEB J. 2016, 30, 3929–3941. [Google Scholar] [CrossRef] [PubMed]
- Csete, M. Oxygen in the cultivation of stem cells. Ann. N. Y. Acad. Sci. 2005, 1049, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 336–361. [Google Scholar] [CrossRef]
- Jash, S.; Adhya, S. Effects of Transient Hypoxia versus Prolonged Hypoxia on Satellite Cell Proliferation and Differentiation In Vivo. Stem Cells Int. 2015, 2015, 961307. [Google Scholar] [CrossRef]
- Matsubara, Y.; Matsumoto, T.; Aoyagi, Y.; Tanaka, S.; Okadome, J.; Morisaki, K.; Shirabe, K.; Maehara, Y. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J. Vasc. Surg. 2015, 61, 945–950. [Google Scholar] [CrossRef]
- de Theije, C.; Costes, F.; Langen, R.C.; Pison, C.; Gosker, H.R. Hypoxia and muscle maintenance regulation: Implications for chronic respiratory disease. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 548–553. [Google Scholar] [CrossRef]
- Chaillou, T.; Koulmann, N.; Meunier, A.; Pugnière, P.; McCarthy, J.J.; Beaudry, M.; Bigard, X. Ambient hypoxia enhances the loss of muscle mass after extensive injury. Pflugers Arch. 2014, 466, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Favier, F.B.; Costes, F.; Defour, A.; Bonnefoy, R.; Lefai, E.; Baugé, S.; Peinnequin, A.; Benoit, H.; Freyssenet, D. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1659–R1666. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, T.; Koulmann, N.; Meunier, A.; Chapot, R.; Serrurier, B.; Beaudry, M.; Bigard, X. Effect of hypoxia exposure on the recovery of skeletal muscle phenotype during regeneration. Mol. Cell. Biochem. 2014, 390, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Csete, M.; Groves, A.K.; Melega, W.; Wold, B.; Anderson, D.J. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 2000, 20, 7370–7376. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Spangenburg, E.E.; Booth, F.W. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cell. Mol. Life Sci. 2001, 58, 1150–1158. [Google Scholar] [CrossRef]
- Csete, M.; Walikonis, J.; Slawny, N.; Wei, Y.; Korsnes, S.; Doyle, J.C.; Wold, B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell. Physiol. 2001, 189, 189–196. [Google Scholar] [CrossRef]
- Di Carlo, A.; de Mori, R.; Martelli, F.; Pompilio, G.; Capogrossi, M.C.; Germani, A. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J. Biol. Chem. 2004, 279, 16332–16338. [Google Scholar] [CrossRef]
- Elashry, M.I.; Kinde, M.; Klymiuk, M.C.; Eldaey, A.; Wenisch, S.; Arnhold, S. The effect of hypoxia on myogenic differentiation and multipotency of the skeletal muscle-derived stem cells in mice. Stem Cell Res. Ther. 2022, 13, 56. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Liu, Z.; Chen, L.; Liu, X.; Kuang, S. Hypoxia Inhibits Myogenic Differentiation through p53 Protein-dependent Induction of Bhlhe40 Protein. J. Biol. Chem. 2015, 290, 29707–29716. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Accili, D.; Duan, C. Hypoxia converts the myogenic action of insulin-like growth factors into mitogenic action by differentially regulating multiple signaling pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 5857–5862. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhou, Y.; Li, L.; Zhao, J.; Qin, W.; Jin, Z.; Liu, W. Increase in HDAC9 suppresses myoblast differentiation via epigenetic regulation of autophagy in hypoxia. Cell Death Dis. 2019, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.V.; Zheng, X.; Pereira, T.; Gradin, K.; Jin, S.; Lundkvist, J.; Ruas, J.L.; Poellinger, L.; Lendahl, U.; Bondesson, M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 2005, 9, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wen, Y.; Bi, P.; Lai, X.; Liu, X.S.; Liu, X.; Kuang, S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 2012, 139, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Itoigawa, Y.; Kishimoto, K.N.; Okuno, H.; Sano, H.; Kaneko, K.; Itoi, E. Hypoxia induces adipogenic differentitation of myoblastic cell lines. Biochem. Biophys. Res. Commun. 2010, 399, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.T.; Vajanto, I.; Ylä-Herttuala, S. Gene therapy for therapeutic angiogenesis in critically ischaemic lower limb—On the way to the clinic. Eur. J. Clin. Investig. 2001, 31, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey Man, H.S.; Tsui, A.K.Y.; Marsden, P.A. Nitric oxide and hypoxia signaling. Vitam. Horm. 2014, 96, 161–192. [Google Scholar] [CrossRef]
- Michel, T.; Feron, O. Nitric oxide synthases: Which, where, how, and why? J. Clin. Investig. 1997, 100, 2146–2152. [Google Scholar] [CrossRef]
- Blau, H.M.; Pavlath, G.K.; Hardeman, E.C.; Chiu, C.P.; Silberstein, L.; Webster, S.G.; Miller, S.C.; Webster, C. Plasticity of the differentiated state. Science 1985, 230, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, D.; You, L.; Wang, L. Quantifying fluid shear stress in a rocking culture dish. J. Biomech. 2010, 43, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio-Protocol 2016, 6, e1984. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wang, Q.; Pei, S.; Lu, X.L.; Wang, L.; Wu, Q. On the characterization of interstitial fluid flow in the skeletal muscle endomysium. J. Mech. Behav. Biomed. Mater. 2020, 102, 103504. [Google Scholar] [CrossRef] [PubMed]
- Ponik, S.M.; Triplett, J.W.; Pavalko, F.M. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J. Cell. Biochem. 2007, 100, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Franco-Obregón, A.; Casarosa, M.; Küster, S.K.; Wuertz-Kozak, K.; Dittrich, P.S. Transient receptor potential vanilloid 2-mediated shear-stress responses in C2C12 myoblasts are regulated by serum and extracellular matrix. FASEB J. 2015, 29, 4726–4737. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Klein-Nulend, J.; Bakker, A.D.; Jin, J.; Seddiqi, H.; Offringa, C.; de Wit, G.M.J.; Le Grand, F.; Giordani, L.; Liu, K.J.; et al. Myofiber stretch induces tensile and shear deformation of muscle stem cells in their native niche. Biophys. J. 2021, 120, 2665–2678. [Google Scholar] [CrossRef]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109 Pt 4, 713–726. [Google Scholar] [CrossRef]
- Verma, D.; Ye, N.; Meng, F.; Sachs, F.; Rahimzadeh, J.; Hua, S.Z. Interplay between cytoskeletal stresses and cell adaptation under chronic flow. PLoS ONE 2012, 7, e44167. [Google Scholar] [CrossRef]
- Xu, H.; Duan, J.; Ren, L.; Yang, P.; Yang, R.; Li, W.; Zhao, D.; Shang, P.; Jiang, J.X. Impact of flow shear stress on morphology of osteoblast-like IDG-SW3 cells. J. Bone Miner. Metab. 2018, 36, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Le Gac, S.; Verdonschot, N.; van den Berg, A.; Koopman, B.; Rouwkema, J. A medium throughput device to study the effects of combinations of surface strains and fluid-flow shear stresses on cells. Lab Chip 2015, 15, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, B.; Chen, J.; Tang, Z.; Zong, C.; Shen, D.; Zheng, Q.; Tong, X.; Gao, C.; Wang, J. Different effects of intermittent and continuous fluid shear stresses on osteogenic differentiation of human mesenchymal stem cells. Biomech. Model. Mechanobiol. 2012, 11, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Riddle, R.C.; Taylor, A.F.; Genetos, D.C.; Donahue, H.J. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am. J. Physiol. Cell Physiol. 2006, 290, C776–C784. [Google Scholar] [CrossRef] [PubMed]
- Pochampally, R. Colony forming unit assays for MSCs. Methods Mol. Biol. 2008, 449, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, X.; Xu, H.; Zhou, B.; Wen, W.; Qin, J. Regulation of cell migration and osteogenic differentiation in mesenchymal stem cells under extremely low fluidic shear stress. Biomicrofluidics 2014, 8, 052008. [Google Scholar] [CrossRef]
- Yuan, L.; Sakamoto, N.; Song, G.; Sato, M. Migration of human mesenchymal stem cells under low shear stress mediated by mitogen-activated protein kinase signaling. Stem Cells Dev. 2012, 21, 2520–2530. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Chang, T.-H.; Hsu, W.-T.; Zhou, J.; Lee, H.-H.; Hui-Chun Ho, J.; Chien, S.; Lee, O.K.-S. Oscillatory shear stress mediates directional reorganization of actin cytoskeleton and alters differentiation propensity of mesenchymal stem cells. Stem Cells 2015, 33, 429–442. [Google Scholar] [CrossRef]
- Pedraza-Alva, G.; Zingg, J.M.; Jost, J.P. AP-1 binds to a putative cAMP response element of the MyoD1 promoter and negatively modulates MyoD1 expression in dividing myoblasts. J. Biol. Chem. 1994, 269, 6978–6985. [Google Scholar] [CrossRef]
- Akimoto, T.; Ushida, T.; Miyaki, S.; Tateishi, T.; Fukubayashi, T. Mechanical stretch is a down-regulatory signal for differentiation of C2C12 myogenic cells. Mater. Sci. Eng. C 2001, 17, 75–78. [Google Scholar] [CrossRef]
- Koning, M.; Werker, P.M.N.; van Luyn, M.J.A.; Harmsen, M.C. Hypoxia promotes proliferation of human myogenic satellite cells: A potential benefactor in tissue engineering of skeletal muscle. Tissue Eng. Part A 2011, 17, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Lin, Q.; Giaccia, A.J. Adaptive myogenesis under hypoxia. Mol. Cell. Biol. 2005, 25, 3040–3055. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Resmini, G.; Ghiroldi, A.; Piccoli, M.; Bergante, S.; Tettamanti, G.; Anastasia, L. Activation of the hypoxia-inducible factor 1α promotes myogenesis through the noncanonical Wnt pathway, leading to hypertrophic myotubes. FASEB J. 2017, 31, 2146–2156. [Google Scholar] [CrossRef]
- Sellathurai, J.; Nielsen, J.; Hejbøl, E.K.; Jørgensen, L.H.; Dhawan, J.; Nielsen, M.F.B.; Schrøder, H.D. Low Oxygen Tension Enhances Expression of Myogenic Genes When Human Myoblasts Are Activated from G0 Arrest. PLoS ONE 2016, 11, e0158860. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Skuli, N.; Mesquita, R.C.; Kim, M.N.; Yodh, A.G.; Nguyen-McCarty, M.; Simon, M.C. O2 regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Mol. Cell. Biol. 2012, 32, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Lee, D.S.M.; Skuli, N.; Mesquita, R.C.; Kim, M.N.; Yodh, A.G.; Nguyen-McCarty, M.; Li, B.; Simon, M.C. HIF modulation of Wnt signaling regulates skeletal myogenesis in vivo. Development 2015, 142, 2405–2412. [Google Scholar] [CrossRef]
- Ono, Y.; Sensui, H.; Sakamoto, Y.; Nagatomi, R. Knockdown of hypoxia-inducible factor-1alpha by siRNA inhibits C2C12 myoblast differentiation. J. Cell. Biochem. 2006, 98, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, A.; Kotake, N.; Yamada, S. Spatial and temporal expression of hypoxia-inducible factor-1α during myogenesis in vivo and in vitro. Mol. Cell. Biochem. 2011, 347, 145–155. [Google Scholar] [CrossRef]
- Stroka, D.M.; Burkhardt, T.; Desbaillets, I.; Wenger, R.H.; Neil, D.A.H.; Bauer, C.; Gassmann, M.A.X.; Candinas, D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001, 15, 2445–2453. [Google Scholar] [CrossRef]
- Kobzik, L.; Reid, M.B.; Bredt, D.S.; Stamler, J.S. Nitric oxide in skeletal muscle. Nature 1994, 372, 546–548. [Google Scholar] [CrossRef]
- Javeshghani, D.; Sakkal, D.; Mori, M.; Hussain, S.N. Regulation of diaphragmatic nitric oxide synthase expression during hypobaric hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L520–L527. [Google Scholar] [CrossRef] [PubMed]
- Nagahisa, H.; Miyata, H. Influence of hypoxic stimulation on angiogenesis and satellite cells in mouse skeletal muscle. PLoS ONE 2018, 13, e0207040. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.E.; Toporsian, M.; Scott, J.A.; Teoh, H.; Govindaraju, V.; Quan, A.; Wener, A.D.; Wang, G.; Bevan, S.C.; Newton, D.C.; et al. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J. Clin. Investig. 2005, 115, 3128–3139. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.; Pearson, T.; Vasilaki, A. Manipulation of environmental oxygen modifies reactive oxygen and nitrogen species generation during myogenesis. Redox Biol. 2016, 8, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoity, A.; Padre, R.C.; Burzynski, K.E.; Stull, J.T.; Lau, K.S. Neuronal nitric oxide synthase localizes through multiple structural motifs to the sarcolemma in mouse myotubes. FEBS Lett. 2000, 482, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Montagna, C.; Rizza, S.; Cirotti, C.; Maiani, E.; Muscaritoli, M.; Musarò, A.; Carrí, M.T.; Ferraro, E.; Cecconi, F.; Filomeni, G. nNOS/GSNOR interaction contributes to skeletal muscle differentiation and homeostasis. Cell Death Dis. 2019, 10, 354. [Google Scholar] [CrossRef]
- de Palma, C.; Clementi, E. Nitric oxide in myogenesis and therapeutic muscle repair. Mol. Neurobiol. 2012, 46, 682–692. [Google Scholar] [CrossRef]
- Balon, T.W.; Nadler, J.L. Evidence that nitric oxide increases glucose transport in skeletal muscle. J. Appl. Physiol. (1985) 1997, 82, 359–363. [Google Scholar] [CrossRef]



| Gene | Forward | Reverse | Size (bp) |
|---|---|---|---|
| 18s | ATGCGGCGGCGTTATTCC | GCTATCAATCTGTCAATCCTGTCC | 204 bp |
| MyoD | GTGAATGAGGCCTTCGAGAC | GAGCCTGCAGACCTTCGATG | 115 bp |
| Myogenin | GAAGAAAAGGGACTGGGGAC | TCTTGAGCCTGCGCTTCTCC | 136 bp |
| MHC1 | TCTTGAGCCTGCGCTTCTCC | GCCACCAGACTCTGCTTCC | 141 bp |
| MHC7 | ACTATGCTGGAGCTGATGCC | CTCTGTGCAGAGCAGACAC | 89 bp |
| mTOR | CGGCACACATTTGAAGAAGC | TCCATGCTGCTGATACGAAC | 130 bp |
| VEGF | AGATCATGCGGATCAAACC | TGACCCTTTCCCTTTCCTC | 139 bp |
| HIF1α | GTGCTGATTTGTGAACCCATTC | AAGTTCTTCCGGCTCATAACC | 150 bp |
| NOS1 | TGGGCGATCCAGCTAATGTGG | GGATCTGAAAGAGTTCAGGGTC | 131 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawlitschek, P.; Klymiuk, M.C.; Eldaey, A.; Wenisch, S.; Arnhold, S.; Elashry, M.I. Modulation of the Cellular Microenvironment by Mechanical Fluid Shear Stress and Hypoxia Alters the Differentiation Capacity of Skeletal Muscle-Derived Stem Cells. Appl. Sci. 2024, 14, 3047. https://doi.org/10.3390/app14073047
Hawlitschek P, Klymiuk MC, Eldaey A, Wenisch S, Arnhold S, Elashry MI. Modulation of the Cellular Microenvironment by Mechanical Fluid Shear Stress and Hypoxia Alters the Differentiation Capacity of Skeletal Muscle-Derived Stem Cells. Applied Sciences. 2024; 14(7):3047. https://doi.org/10.3390/app14073047
Chicago/Turabian StyleHawlitschek, Paula, Michele C. Klymiuk, Asmaa Eldaey, Sabine Wenisch, Stefan Arnhold, and Mohamed I. Elashry. 2024. "Modulation of the Cellular Microenvironment by Mechanical Fluid Shear Stress and Hypoxia Alters the Differentiation Capacity of Skeletal Muscle-Derived Stem Cells" Applied Sciences 14, no. 7: 3047. https://doi.org/10.3390/app14073047





