Magnesium Oxychloride Cement: Development, Opportunities and Challenges
Abstract
:1. Introduction
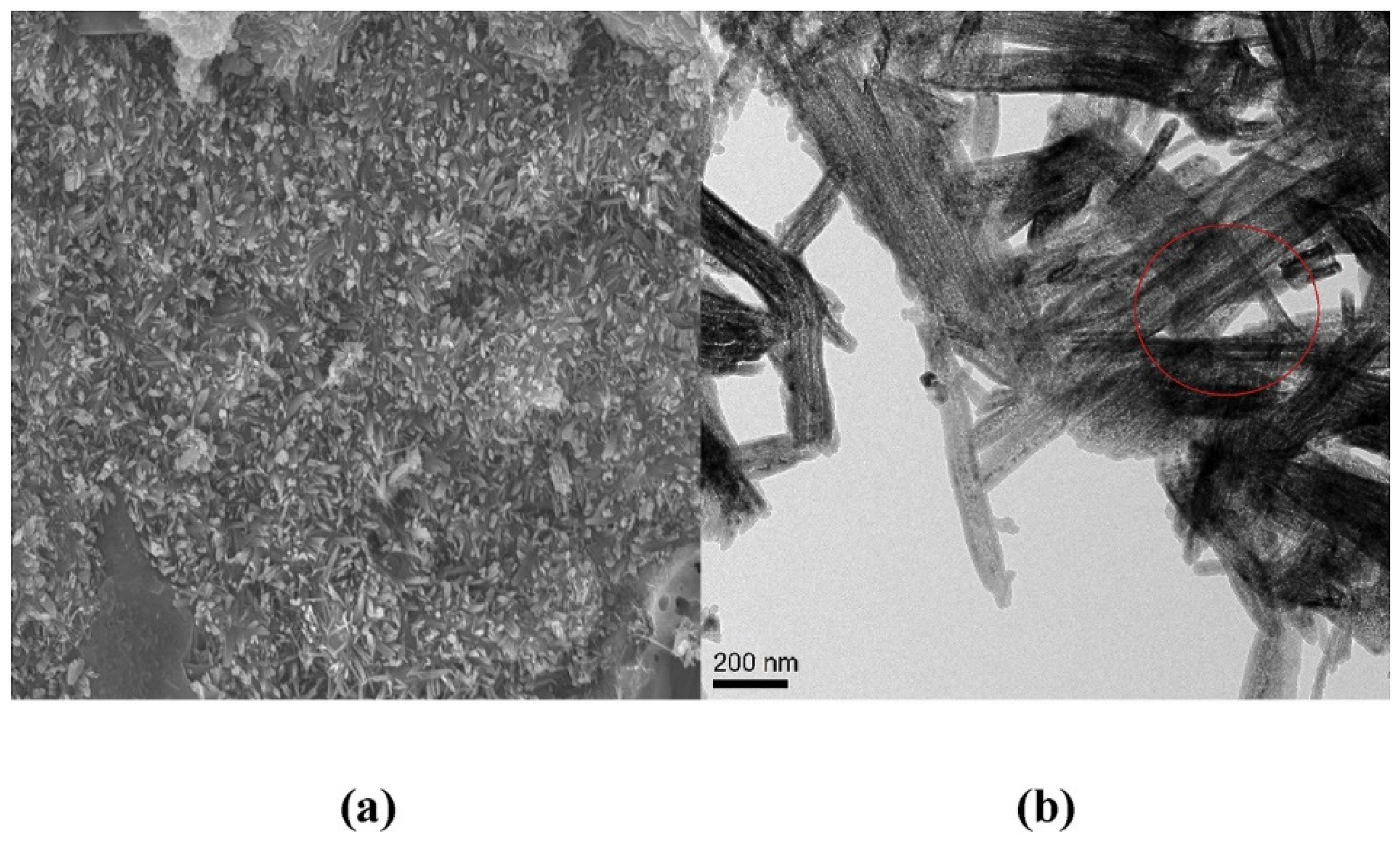
2. Strength Development Mechanism of MOC
- (a)
- Dead-burned magnesia: High-temperature calcination (1500–2000 °C) reduces available surface area, resulting in unreactive MgO.
- (b)
- Hard-burned magnesia: Calcination temperatures ranging from 1000–1500 °C yield MgO with limited reactivity.
- (c)
- Light-burned magnesia: Produced at 600–1000 °C, also known as caustic calcined magnesia, this form exhibits a reactive nature.
3. Curing Mechanism of MOC
3.1. Normal Curing of MOC
3.2. CO2 Curing and Its Effect on MOC-Based Cementitious Composites
4. Influence of SCMs on the Properties of MOC
4.1. Effect of FA on MOC
4.2. Effect of GGBFS on MOC
4.3. Effect of MK on MOC
5. Recent Developments in FRMOC-Based Composites
6. Recent Developments in Water-Resistant MOC
7. Application of MOC as a Fire-Resistive Material
8. Application of MOC in Solid Waste Management
9. Application of MOC and Wood as a Composite Building Material
10. Conclusions
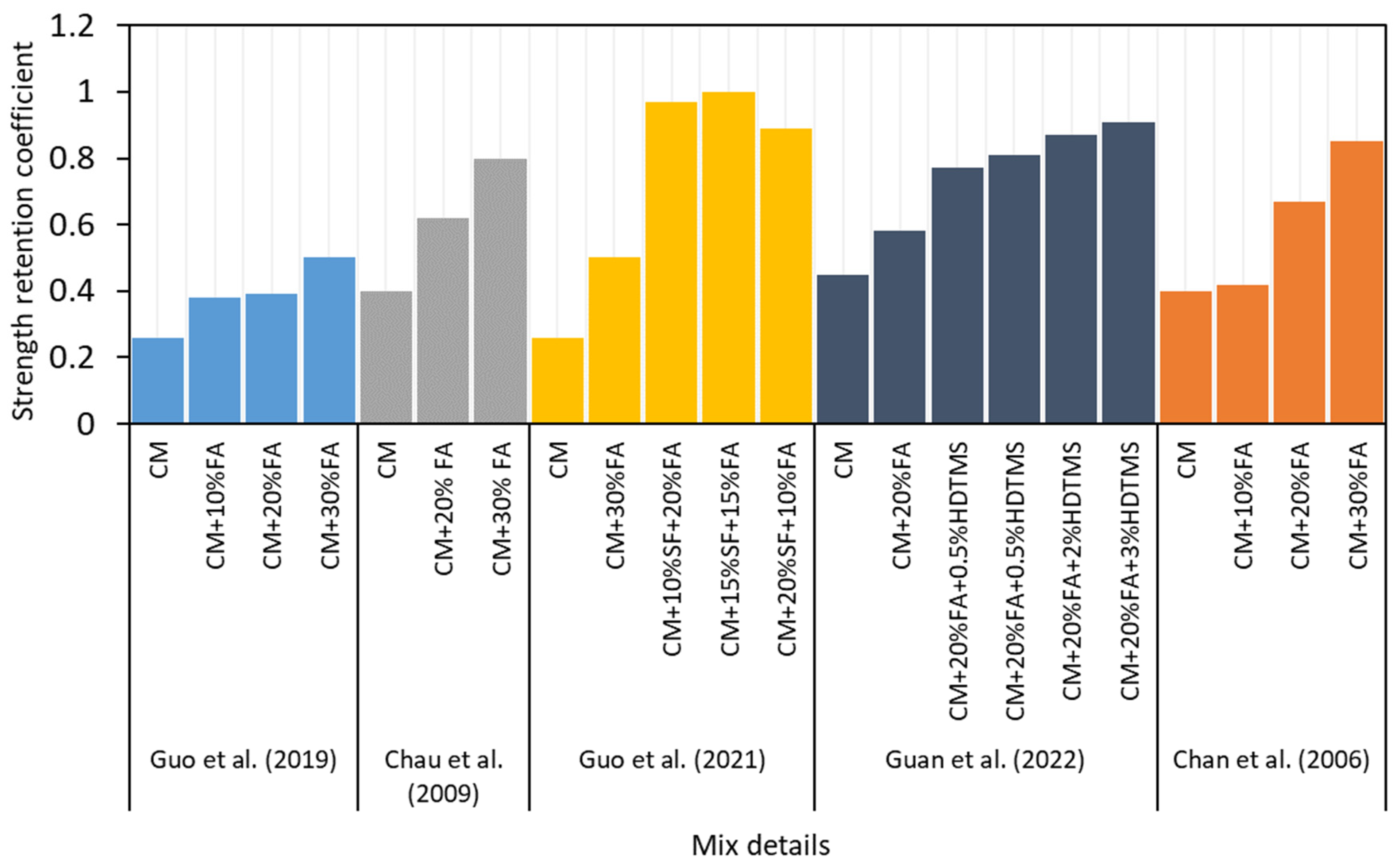
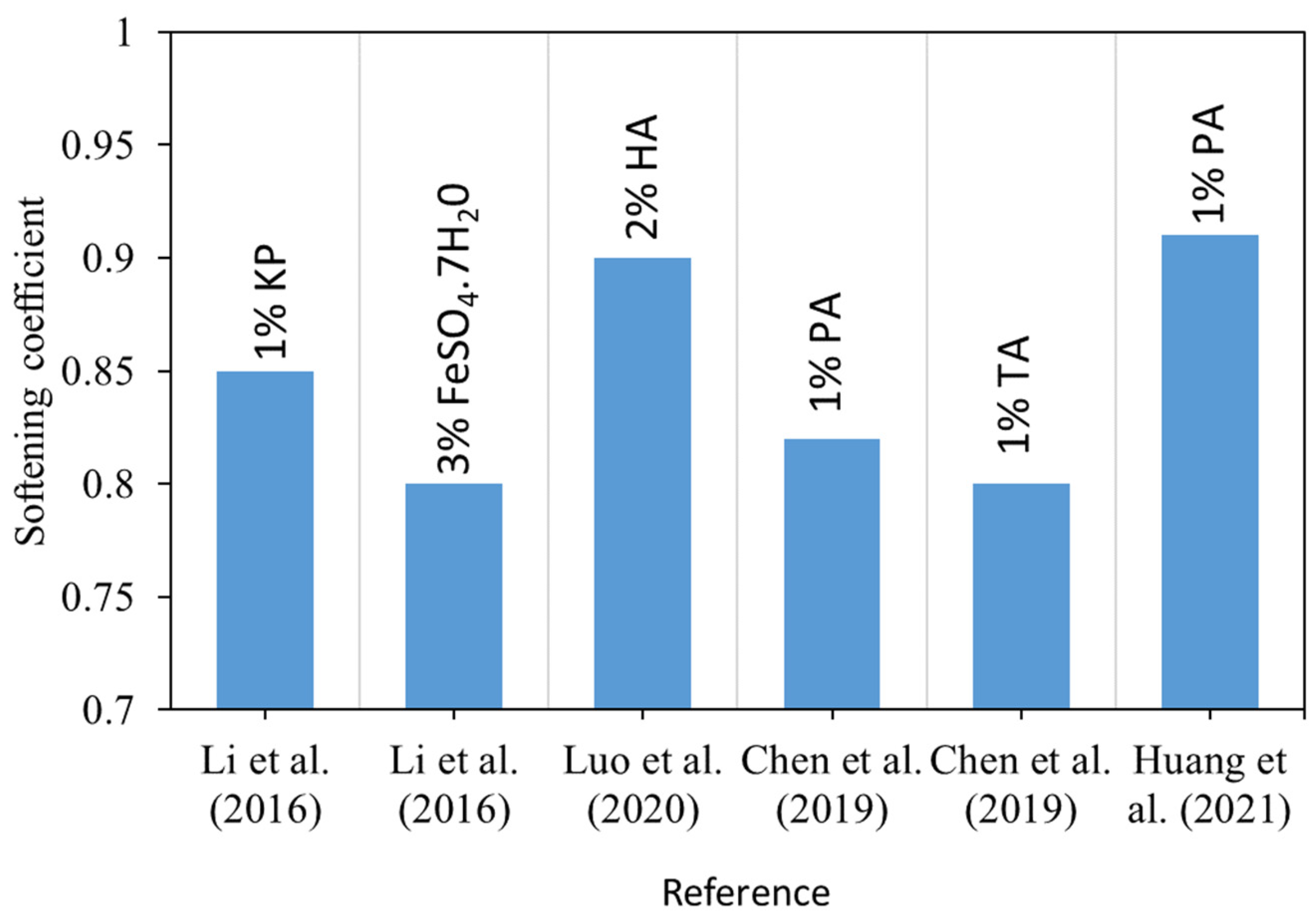
- Different mineral admixtures and chemical reagents such as acids and soluble phosphates were added to the MOC over the past few decades, and it was found that they improved the water resistance performance up to a certain level. Moreover, recently, a water-resistant MOC mix (molar ratios of MgO/MgCl2-9 and H2O/MgCl2-13, 30% FA, 0.5% PA, and 0.5% MFP) was discovered with a strength retention co-efficient of 1.08 and 0.9, respectively, at normal (25 °C) and warm water (60 °C) immersion. This discovery potentially broadens the scope of MOC to outdoor applications.
- Controlled curing conditions such as a temperature of 24 ± 1 °C and relative humidity of 60 ± 5% were identified as suitable for curing MOC composites. Additionally, accelerated CO2 curing demonstrated significant enhancements in the strength and water-resistant characteristics of MOC. This improvement was attributed to the formation of an insoluble amorphous gel and the densification of the microstructure.
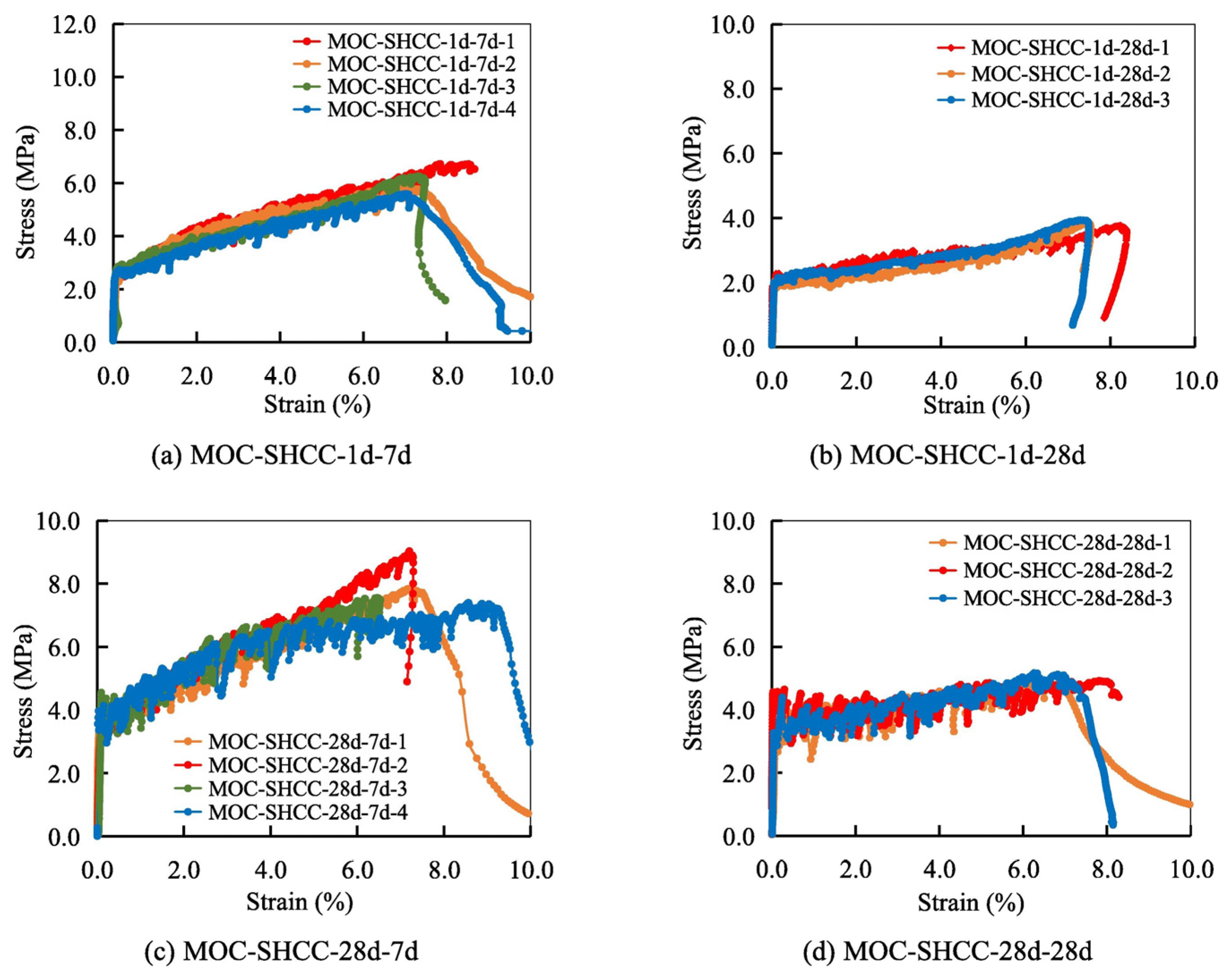
- The addition of 2% PE fiber was effective in improving MOC composites’ ductility with tensile strength ranging from 5 to 10.95 MPa and tensile strain capacity within the range of 4.41 to 8% without compromising the overall performance of MOC. This demonstrates the potential of FRMOC for utilization in structural applications.
- MOC exhibits promising potential in non-structural applications such as cladding or coating considering its superior ability to withstand high temperatures without compromising the integrity of the underlying substrate. However, existing literature revealed a complete loss in mechanical properties for MOC exposed to temperatures between 600–800 °C, highlighting the need for further research to enhance its fire resistance capabilities, especially for structural applications.
- MOC also exhibits strong bonding potential along with rapid hardening and a significant early strength gain with various industrial byproducts such as sewage sludge ash, pulverized fuel ash, phosphogypsum, and flue gas desulfurization gypsum. Hence, MOC may serve as an environmentally friendly alternative, reducing waste accumulation and contributing to a more sustainable environment.
- MOC also demonstrates good compatibility with wood as opposed to OPC, and this further establishes MOC as a green and sustainable material for the development of lightweight wood-based composite building materials.
11. Recommendation and Future Research Directions
- Though past studies have reported that MOC is inherently fire resistant, the extent of strength retention post fire exposure remains unclear. Therefore, a systematic investigation into the fire performance of MOC is needed to broaden the understanding in this area and further improve the performance as needed.
- Concerning the reactivity of MgO, it has been determined that MgO calcined within the range of 800–900 °C yields optimal results for MOC production. Nevertheless, further investigation is needed to elucidate the underlying mechanism of the impact of varying reactivities on MOC performance.
- The effect of CO2 curing on MOC performance has not been extensively investigated and requires more in-depth study to elucidate the underlying mechanisms effectively and its potential to improve water resistance.
- The lightweight, high-strength, and ductile nature of FRMOC suggests the potential to be used for lightweight infrastructural applications. However, the performance needs to be further improved before it can be implemented and hence, more research efforts should be directed towards evaluating the potential of MOC in attaining high tensile performance. Additionally, the use of FRMOC as cladding panels necessitates further large-scale testing studies to ensure its suitability and efficacy.
- Currently, the investigation into the advancement of MOC–wood composite is very scarce despite the potential it has shown in comparison to the OPC-based alternatives. Therefore, detailed investigations are needed to further enhance the industrial applications of MOC-based wood composites, aiming to establish them as eco-friendly alternative to OPC-based composites.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, F.; Rawat, S.; Yang, R.; Zhang, L.; Guo, Y.; Fanna, D.; Zhang, Y.X. Effect of Hybrid Fibres on Mechanical Behaviour of Magnesium Oxychloride Cement-Based Composites. Constr. Build. Mater. 2024, 424, 135937. [Google Scholar] [CrossRef]
- Yu, K.; Guo, Y.; Zhang, Y.; Soe, K. Magnesium oxychloride cement-based strain-hardening cementitious composite: Mechanical property and water resistance. Constr. Build. Mater. 2020, 261, 119970. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A.; Salim, M.U.; Ahmad, N.; Ahmad, F.; Ahmad, A. Reusing biochar as a filler or cement replacement material in cementitious composites: A review. Constr. Build. Mater. 2021, 300, 124295. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.S.; Yao, N.; Zhang, A. Recent progress of magnesium oxychloride cement: Manufacture, curing, structure and performance. Constr. Build. Mater. 2020, 255, 119381. [Google Scholar] [CrossRef]
- Nie, Y.; Lu, J.; Liu, Z.; Meng, D.; He, Z.; Shi, J. Mechanical, water resistance and environmental benefits of magnesium oxychloride cement incorporating rice husk ash. Sci. Total Environ. 2022, 849, 157871. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Li, Z. Influence of cenospheres on properties of magnesium oxychloride cement-based composites. Mater. Struct. 2015, 49, 1319–1326. [Google Scholar] [CrossRef]
- Montle, J.; Mayhan, K. Magnesium oxychloride as a fire retardant material. J. Fire Flam/Fire Retard Chem. 1974, 1, 243–254. [Google Scholar]
- Guo, Y.Y.; Zhang, Y.X.; Soe, K.; Pulham, M. Recent development in magnesium oxychloride cement. Struct. Concr. 2018, 19, 1290–1300. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Yuan, Y. Strength improvement and micromechanism of inorganic/organic additive-modified magnesium oxychloride cement solidified sludge. Constr. Build. Mater. 2023, 366, 130159. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.; Gao, X. Sustainable Improvement of Magnesium Oxychloride Cement Solidified Waste Sludge with Fly-Ash Inclusion. J. Mater. Civ. Eng. 2022, 34, 04022317. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chau, C. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Chau, C.K.; Li, Z. Accelerated Reactivity Assessment of Light Burnt Magnesium Oxide. J. Am. Ceram. Soc. 2008, 91, 1640–1645. [Google Scholar] [CrossRef]
- Xu, B.W.; Ma, H.Y.; Hu, C.L.; Yang, S.Q.; Li, Z.J. Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Constr. Build. Mater. 2016, 102, 613–619. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, W.; Dong, J.; Wen, J.; Chang, C.; Xiao, X. Influences of different bischofite on the properties of magnesium oxychloride cement. J. Build. Eng. 2022, 57, 104923. [Google Scholar] [CrossRef]
- Li, Z.; Qiao, F.; Chau, C.K. Recent development of magnesium-based cements-magnesium phosphate cement and mag-nesium oxychloride cement. Adv. Sci. Technol. 2010, 69, 21–30. [Google Scholar]
- An, L.; Chang, C.; Yan, F.; Peng, J. Study on the Deterioration Mechanism of Magnesium Oxychloride Cement under an Alkaline Environment. Materials 2023, 16, 5924. [Google Scholar] [CrossRef]
- Chen, D.; Mahadevan, S. Chloride-induced reinforcement corrosion and concrete cracking simulation. Cem. Concr. Compos. 2008, 30, 227–238. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Soe, K.; Wuhrer, R.; Hutchison, W.D.; Timmers, H. Development of magnesium oxychloride cement with enhanced water resistance by adding silica fume and hybrid fly ash-silica fume. J. Clean. Prod. 2021, 313, 127682. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Soe, K.; Hutchison, W.D.; Timmers, H.; Poblete, M.R. Effect of fly ash on mechanical properties of magnesium oxychloride cement under water attack. Struct. Concr. 2019, 21, 1181–1199. [Google Scholar] [CrossRef]
- Yu, H.; Liu, P.; Wang, W. The properties of silica fume magnesium oxychloride cement materials. Acta Metall. Sin. (Engl. Lett.) 1999, 12, 1038. [Google Scholar]
- Chau, C.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Pei, H.; Yu, H. The influence of FeSO4 and KH2PO4 on the performance of magnesium oxychloride cement. Constr. Build. Mater. 2016, 102, 233–238. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, W.; Li, Y.; Chang, C.; Wen, J.; Dong, J.; Xiao, X. The Effect of Doping High Volume Magnesium Sulfate on Properties of Magnesium Oxychloride Cement. Crystals 2022, 12, 857. [Google Scholar] [CrossRef]
- Lauermannová, A.-M.; Lojka, M.; Záleská, M.; Pavlíková, M.; Pivák, A.; Pavlík, Z.; Růžička, K.; Jankovský, O. Magnesium oxychloride cement-based composites for latent heat storage: The effect of the in-troduction of multi-walled carbon nanotubes. J. Build. Eng. 2023, 72, 106604. [Google Scholar] [CrossRef]
- Jiříčková, A.; Lauermannová, A.-M.; Jankovský, O.; Lojka, M.; Záleská, M.; Pivák, A.; Pavlíková, M.; Merglová, A.; Pavlík, Z. Impact of nano-dopants on the mechanical and physical properties of magnesium oxychloride cement composites—Experimental assessment. J. Build. Eng. 2024, 87, 108981. [Google Scholar] [CrossRef]
- Brichni, A.; Hammi, H.; Aggoun, S.; Mnif, A. Optimisation of magnesium oxychloride cement properties by silica glass. Adv. Cem. Res. 2016, 28, 654–663. [Google Scholar] [CrossRef]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Yu, W.; Yu, H.; Ma, H.; Shi, T.; Wen, J.; Ma, H. Long-term phase composition and microstructure characteristics of phosphoric acid modified magnesium ox-ychloride cement exposed to natural environment. Mater. Today Commun. 2023, 36, 106399. [Google Scholar] [CrossRef]
- Li, C.; Yu, H. Influence of fly ash and silica fume on water-resistant property of magnesium oxychloride cement. J. Wuhan Univ. Technol. Sci. Ed. 2010, 25, 721–724. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Y.; Yu, J.; Xiao, J.; Xu, S. Feasibility study of strain hardening magnesium oxychloride cement-based composites. Constr. Build. Mater. 2018, 165, 750–760. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Moraitou, G. Sorel’s cement mortars: Decay susceptibility and effect on Pentelic marble. Cem. Concr. Res. 1999, 29, 1929–1935. [Google Scholar] [CrossRef]
- Sorre, C.A.; Armstrong, C.R. Reactions and Equi ibria in Magnesium Oxych oride Cements. J. Am. Ceram. Soc. 1976, 59, 51–54. [Google Scholar] [CrossRef]
- Aiken, T.A.; McPolin, D.; Russell, M.; Nanukuttan, S.; Bagnall, L. Exposure of magnesium oxide boards to various conditions for extended durations. Constr. Build. Mater. 2021, 302, 124429. [Google Scholar] [CrossRef]
- Rawat, S.; Saliba, P.; Estephan, P.C.; Ahmad, F.; Zhang, Y. Mechanical Performance of Hybrid Fibre Reinforced Magnesium Oxychloride Cement-Based Composites at Ambient and Elevated Temperature. Buildings 2024, 14, 270. [Google Scholar] [CrossRef]
- Montle, J.F.; Mayhan, K.G. The role of magnesium oxychloride as a fire-resistive material. Fire Technol. 1974, 10, 201–210. [Google Scholar] [CrossRef]
- Góchez, R.; Wambaugh, J.; Rochner, B.; Kitchens, C.L. Kinetic study of the magnesium oxychloride cement cure reaction. J. Mater. Sci. 2017, 52, 7637–7646. [Google Scholar] [CrossRef]
- Misra, A.K.; Mathur, R. Magnesium oxychloride cement concrete. Bull. Mater. Sci. 2007, 30, 239–246. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.; Grover, L. Effect of phosphoric acid on the properties of magnesium oxychloride cement as a biomaterial. Cem. Concr. Res. 2014, 56, 69–74. [Google Scholar] [CrossRef]
- Ba, H.; Guan, H. Influence of MgO/MgCl2 molar ratio on phase stability of magnesium oxychloride cement. J. Wuhan Univ. Technol. Sci. Ed. 2009, 24, 476–481. [Google Scholar] [CrossRef]
- Chau, C.K.; Li, Z. Microstructures of magnesium oxychloride. Mater. Struct. 2007, 41, 853–862. [Google Scholar] [CrossRef]
- Karimi, Y.; Monshi, A. Investigations on the properties of magnesium oxychloride cement produced by in situ and classic methods. Int. J. Metall. Mater. Sci. Eng. 2012, 2, 1–11. [Google Scholar]
- Sglavo, V.M.; De Genua, F.; Conci, A.; Ceccato, R.; Cavallini, R. Influence of curing temperature on the evolution of magnesium oxychloride cement. J. Mater. Sci. 2011, 46, 6726–6733. [Google Scholar] [CrossRef]
- Matkovic, B.; Young, J.F. Microstructure of Magnesium Oxychloride Cements. Nat. Phys. Sci. 1973, 246, 79–80. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, R.; Goel, P. Factors influencing strength of magnesium oxychloride cement. Constr. Build. Mater. 2021, 303, 124571. [Google Scholar] [CrossRef]
- Shand, M.A. The Chemistry and Technology of Magnesia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Harper, F.C. Effect of calcination temperature on the properties of magnesium oxides for use in magnesium oxychloride cements. J. Appl. Chem. 1967, 17, 5–10. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Soe, K. Effect of sodium monofluorophosphate and phosphates on mechanical properties and water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2022, 129, 104472. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of Hydration of Calcium Silicates by Carbon Dioxide Treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, S.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2013, 67, 3–7. [Google Scholar] [CrossRef]
- Shi, C.; Liu, M.; Zou, Q.; He, F. A study on factors influencing compressive strength of CO2-cured concrete. In Proceedings of the International RILEM Conference on Advances in Construction Materials through Science and Engineering, Hong Kong, 5–7 September 2011. [Google Scholar]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef]
- Vandeperre, L.; Liska, M.; Al-Tabbaa, A. Microstructures of reactive magnesia cement blends. Cem. Concr. Compos. 2008, 30, 706–714. [Google Scholar] [CrossRef]
- Vandeperre, L.J.; Al-Tabbaa, A. Accelerated carbonation of reactive MgO cements. Adv. Cem. Res. 2007, 19, 67–79. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem. Concr. Res. 2012, 42, 769–777. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Accelerated carbonation—A potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Characterization of light and heavy hydrated magnesium carbonates using thermal analysis. J. Therm. Anal. Calorim. 2013, 115, 595–607. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—Effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Xiong, Y.; Lord, A.S. Experimental investigations of the reaction path in the MgO–CO2–H2O system in solutions with various ionic strengths, and their applications to nuclear waste isolation. Appl. Geochem. 2008, 23, 1634–1659. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Bi, W.; Tan, Y.; Zhang, N.; Wu, C.; Ma, H.; Hua, S. Effects of sodium citrate and citric acid on the properties of magnesium oxysulfate cement. Constr. Build. Mater. 2018, 169, 697–704. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Li, W.; Ye, H. Modification of Magnesium Oxysulfate Cement by Incorporating Weak Acids. J. Mater. Civ. Eng. 2018, 30, 04018209. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Zhang, H.; Yu, H.; Zhang, W.; Jiang, N.; Liu, L. The hydration mechanism and performance of Modified magnesium oxysulfate cement by tartaric acid. Constr. Build. Mater. 2017, 144, 516–524. [Google Scholar] [CrossRef]
- de Castellar, M.D.; Lorente, J.C.; Traveria, A.; Tura, J.M. Cracks in Sorel’s cement polishing bricks as a result of magnesium oxychloride carbonatation. Cem. Concr. Res. 1996, 26, 1199–1202. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Effect of pulverized fuel ash and CO2 curing on the water resistance of magnesium oxychloride cement (MOC). Cem. Concr. Res. 2017, 97, 115–122. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Pavlík, Z.; Sedmidubský, D. Carbon Dioxide Uptake by MOC-Based Materials. Appl. Sci. 2020, 10, 2254. [Google Scholar] [CrossRef]
- Guan, B.; He, Z.; Wei, F.; Wang, F.; Yu, J. Effects of fly ash and hexadecyltrimethoxysilane on the compressive properties and water resistance of mag-nesium oxychloride cement. Polymers 2022, 15, 172. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, W.; Xiao, X.; Dong, J.; Wen, J.; Chang, C. Effects of fly ash, phosphoric acid, and nano-silica on the properties of magnesium oxychloride cement. Ceram. Int. 2021, 47, 34341–34351. [Google Scholar] [CrossRef]
- Chan, J.; Li, Z. Influence of fly ash on the properties of magnesium oxychloride cement. In Measuring, Monitoring and Modeling Concrete Properties; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Sharma, A.K.; Sivapullaiah, P. Ground granulated blast furnace slag amended fly ash as an expansive soil stabilizer. Soils Found. 2016, 56, 205–212. [Google Scholar] [CrossRef]
- Liu, Z.; Takasu, K.; Koyamada, H.; Suyama, H. A study on engineering properties and environmental impact of sustainable concrete with fly ash or GGBS. Constr. Build. Mater. 2022, 316, 125776. [Google Scholar] [CrossRef]
- Qureshi, L.A.; Ali, B.; Ali, A. Combined effects of supplementary cementitious materials (silica fume, GGBS, fly ash and rice husk ash) and steel fiber on the hardened properties of recycled aggregate concrete. Constr. Build. Mater. 2020, 263, 120636. [Google Scholar] [CrossRef]
- Tanwar, V.; Bisht, K.; Kabeer, K.S.A.; Ramana, P. Experimental investigation of mechanical properties and resistance to acid and sulphate attack of GGBS based concrete mixes with beverage glass waste as fine aggregate. J. Build. Eng. 2021, 41, 102372. [Google Scholar] [CrossRef]
- Virgalitte, S.J.; Luther, M.D.; Rose, J.H.; Mather, B.; Bell, L.W.; Ehmke, B.A.; Klieger, P.; Roy, D.M.; Call, B.M.; Hooton, R.D.; et al. Ground Granulated Blast-Furnace Slag as a Cementitious Constituent in Concrete Reported by ACI Committee 233 ACI 233 R-95; American Concrete Institute: Farmington Hills, MI, USA, 1995. [Google Scholar]
- Jin, Y.J.; Xiao, L.G.; Luo, F. Influence of Calcium Added Slag on the Strength and Water-Repellency of Magnesium Oxychloride Cement. Appl. Mech. Mater. 2013, 278–280, 437–439. [Google Scholar] [CrossRef]
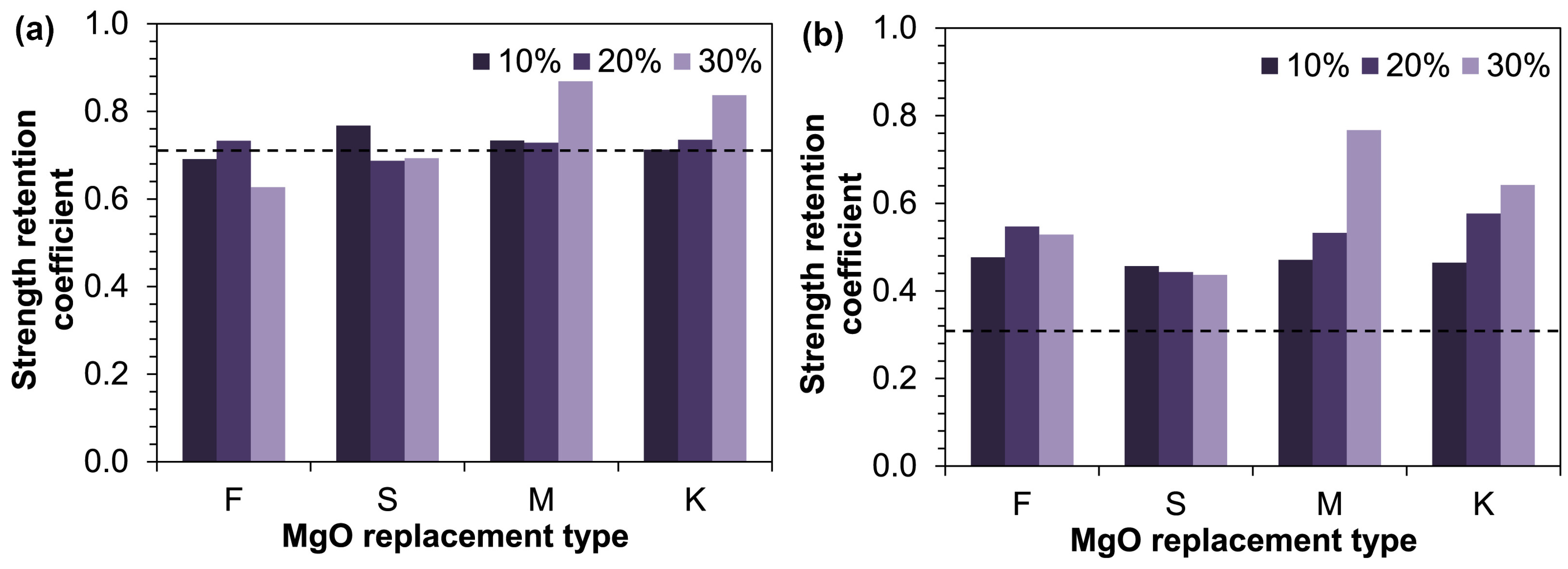
- Aiken, T.A.; Kwasny, J.; Russell, M.; McPolin, D.; Bagnall, L. Effect of partial MgO replacement on the properties of magnesium oxychloride cement. Cem. Concr. Compos. 2022, 134, 104791. [Google Scholar] [CrossRef]
- Qiao, H.X.; Zhu, B.R.; Shi, Y.Y.; Dong, J.M.; Wanjiru, M.E. Strength development and micro-mechanism of magnesium oxychloride cement concrete. Mater. Res. Innov. 2015, 19, S1-185–S1-190. [Google Scholar] [CrossRef]
- Wang, D.; Gao, X.; Liu, X.; Zeng, G. Strength, durability and microstructure of granulated blast furnace slag-modified magnesium oxychloride cement solidified waste sludge. J. Clean. Prod. 2021, 292, 126072. [Google Scholar] [CrossRef]
- Zhang, M.; Malhotra, V. Characteristics of a thermally activated alumino-silicate pozzolanic material and its use in concrete. Cem. Concr. Res. 1995, 25, 1713–1725. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition–A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N. Effect of fly ash and metakaolin on the macroscopic and microscopic characterizations of magnesium oxychloride cement. Constr. Build. Mater. 2020, 267, 120957. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Yu, J.; Yu, K. Mechanical properties of high ductile magnesium oxychloride cement-based composites after water soaking. Cem. Concr. Compos. 2019, 97, 248–258. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z. Light-weight wood–magnesium oxychloride cement composite building products made by extrusion. Constr. Build. Mater. 2012, 27, 382–389. [Google Scholar] [CrossRef]
- Sheng, G.; Zheng, L.; Li, P.; Sun, B.; Li, X.; Zuo, Y. The water resistance and mechanism of FeSO4 enhancing bamboo scraps/magnesium oxychloride cement composite. Constr. Build. Mater. 2021, 317, 125942. [Google Scholar] [CrossRef]
- Lu, H.; Wang, P.; Jiang, N. Design of additives for water-resistant magnesium oxychloride cement using pattern recognition. Mater. Lett. 1994, 20, 217–223. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, D. The effect of aluminate minerals on the phases in magnesium oxychloride cement. Cem. Concr. Res. 1996, 26, 1203–1211. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, H.; Yu, H. The effects of alumina-leached coal fly ash residue on magnesium oxychloride cement. Adv. Cem. Res. 2013, 25, 254–261. [Google Scholar] [CrossRef]
- Karimi, Y.; Monshi, A. Effect of magnesium chloride concentrations on the properties of magnesium oxychloride cement for nano SiC composite purposes. Ceram. Int. 2011, 37, 2405–2410. [Google Scholar] [CrossRef]
- Xu, K.; Xi, J.; Guo, Y.; Dong, S. Effects of a new modifier on the water-resistance of magnesite cement tiles. Solid State Sci. 2012, 14, 10–14. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N. Effect of Metakaolin on Water Resistance of Magnesium Oxychloride Cement. ACI Mater. J. 2022, 119, 47–57. [Google Scholar]
- Li, G.; Yu, Y.; Li, J.; Wang, Y.; Liu, H. Experimental study on urban refuse/magnesium oxychloride cement compound floor tile. Cem. Concr. Res. 2003, 33, 1663–1668. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Yu, Y. The influence of compound additive on magnesium oxychloride cement/urban refuse floor tile. Constr. Build. Mater. 2008, 22, 521–525. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Using incinerated sewage sludge ash to improve the water resistance of magnesium ox-ychloride cement (MOC). Constr. Build. Mater. 2017, 147, 519–524. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–7. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Richardson, I.G.; Tsang, D.C.W. The mechanism of supplementary cementitious materials enhancing the water resistance of magnesium ox-ychloride cement (MOC): A comparison between pulverized fuel ash and incinerated sewage sludge ash. Cem. Concr. Compos. 2020, 109, 103562. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Sun, X.-Y.; Li, X.; Zhang, D.; Xie, W.J.; Liu, J.; Oh, W.-C. Study on Water Resistance of Environmentally Friendly Magnesium Oxychloride Cement for Waste Wood Solidification. J. Korean Ceram. Soc. 2018, 55, 446–451. [Google Scholar] [CrossRef]
- He, Z.; Han, X.; Zhang, Y.; Zhang, Z.; Shi, J.; Gencel, O. Development of a new magnesium oxychloride cement board by recycling of waste wood, rice husk ash and flue gas desulfurization gypsum. J. Build. Eng. 2022, 61, 105206. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Antončík, F.; Pivák, A.; Pavlík, Z. Influence of Waste Plastic Aggregate and Water-Repellent Additive on the Properties of Lightweight Magnesium Oxychloride Cement Composite. Appl. Sci. 2019, 9, 5463. [Google Scholar] [CrossRef]
- Chen, X.-F.; Kou, S.-C.; Xing, F. Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder. Materials 2018, 12, 115. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B.; Bi, W.; Guan, Y. Recycling of waste gypsum in preparation of magnesium oxychloride cement (MOC). J. Clean. Prod. 2021, 313, 127958. [Google Scholar] [CrossRef]
- Ma, C.; Chen, G.; Cao, L.; Zhou, H.; Ren, W. Effects and mechanisms of waste gypsum influencing the mechanical properties and durability of magnesium oxychloride cement. J. Clean. Prod. 2022, 339, 130679. [Google Scholar] [CrossRef]
- Wen, J.; Yu, H.-F.; Li, Y.; Wu, C.-Y.; Dong, J.-M.; Zheng, L.-N. Effects of H3PO4 and Ca(H2PO4)2 on mechanical properties and water resistance of thermally decomposed magnesium oxychloride cement. J. Central South Univ. 2013, 20, 3729–3735. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Bi, W.; Cheeseman, C. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, S.; Wang, H.; Chen, R. Effect of 5-phase seed crystal on the mechanical properties and microstructure of magnesium oxychloride cement. Constr. Build. Mater. 2017, 150, 409–417. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Li, Z.; Li, H. Simulation of the properties of MgO-MgfCl2-H2O system by thermodynamic method. Cem. Concr. Res. 2015, 68, 105–111. [Google Scholar] [CrossRef]
- Luo, X.; Fan, W.; Li, C.; Wang, Y.; Yang, H.; Liu, X.; Yang, S. Effect of hydroxyacetic acid on the water resistance of magnesium oxychloride cement. Constr. Build. Mater. 2020, 246, 118428. [Google Scholar] [CrossRef]
- Guan, B.; Tian, H.; Ding, D.; Wu, J.; Xiong, R.; Xu, A.; Chen, H. Effect of Citric Acid on the Time-Dependent Rheological Properties of Magnesium Oxychloride Cement. J. Mater. Civ. Eng. 2018, 30, 04018275. [Google Scholar] [CrossRef]
- Sun, J.; Song, Z.; Zhang, Y.; Zhang, Y.; Zhao, S.; Guo, M.-Z.; Jiang, L. Effect of red mud and phosphate on water resistance and hydration mechanism of magnesium oxychloride cement. Constr. Build. Mater. 2024, 413, 134844. [Google Scholar] [CrossRef]
- Guo, Y. Development of Water-Resistant Magnesium Oxychloride Cement; UNSW: Sydney, Australia, 2020. [Google Scholar]
- Xia, S.; Xing, P.; Gao, S. Studies on the basic compounds of magnesia cement: The thermal behaviour of magnesium ox-ychlorides. Thermochim. Acta 1991, 183, 349–363. [Google Scholar] [CrossRef]
- Chang, C.; An, L.; Lin, R.; Wen, J.; Dong, J.; Zheng, W.; Yan, F.; Xiao, X. Effect of Calcination Temperature on Mechanical Properties of Magnesium Oxychloride Cement. Materials 2022, 15, 607. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.H.C. Magnesium Oxychloride Fireproofing. US3778304A, 1 November 1971. [Google Scholar]
- Jianli, M.; Youcai, Z.; Jinmei, W.; Li, W. Effect of magnesium oxychloride cement on stabilization/solidification of sewage sludge. Constr. Build. Mater. 2010, 24, 79–83. [Google Scholar] [CrossRef]
- Jin, S.; Li, K.; Li, J.; Chen, H. A Low-Cost, Formaldehyde-Free and High Flame Retardancy Wood Adhesive from Inorganic Adhesives: Properties and Performance. Polymers 2017, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Hossain, U.; Poon, C.S.; Tsang, D.C. Mechanical, durability and environmental aspects of magnesium oxychloride cement boards incorporating waste wood. J. Clean. Prod. 2018, 207, 391–399. [Google Scholar] [CrossRef]
- Maier, A.; Manea, D.L. Perspective of Using Magnesium Oxychloride Cement (MOC) and Wood as a Composite Building Material: A Bibliometric Literature Review. Materials 2022, 15, 1772. [Google Scholar] [CrossRef]
- Simatupang, M.H.; Geimer, R.L. Inorganic binder for wood composites: Feasibility and limitations. In Proceedings of the Wood Adhesive Symposium, Forest Product Resources Society, Portland, OR, USA, 11 May 1990. [Google Scholar]
- He, P.; Poon, C.S.; Tsang, D.C. Water resistance of magnesium oxychloride cement wood board with the incorporation of supplementary cementitious materials. Constr. Build. Mater. 2020, 255, 119145. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, Q.; Shi, S.Q.; Fang, Z.; Gao, Q.; Li, J. A strong magnesium oxychloride cement wood adhesive via organic–inorganic hybrid. Constr. Build. Mater. 2021, 297, 123776. [Google Scholar] [CrossRef]
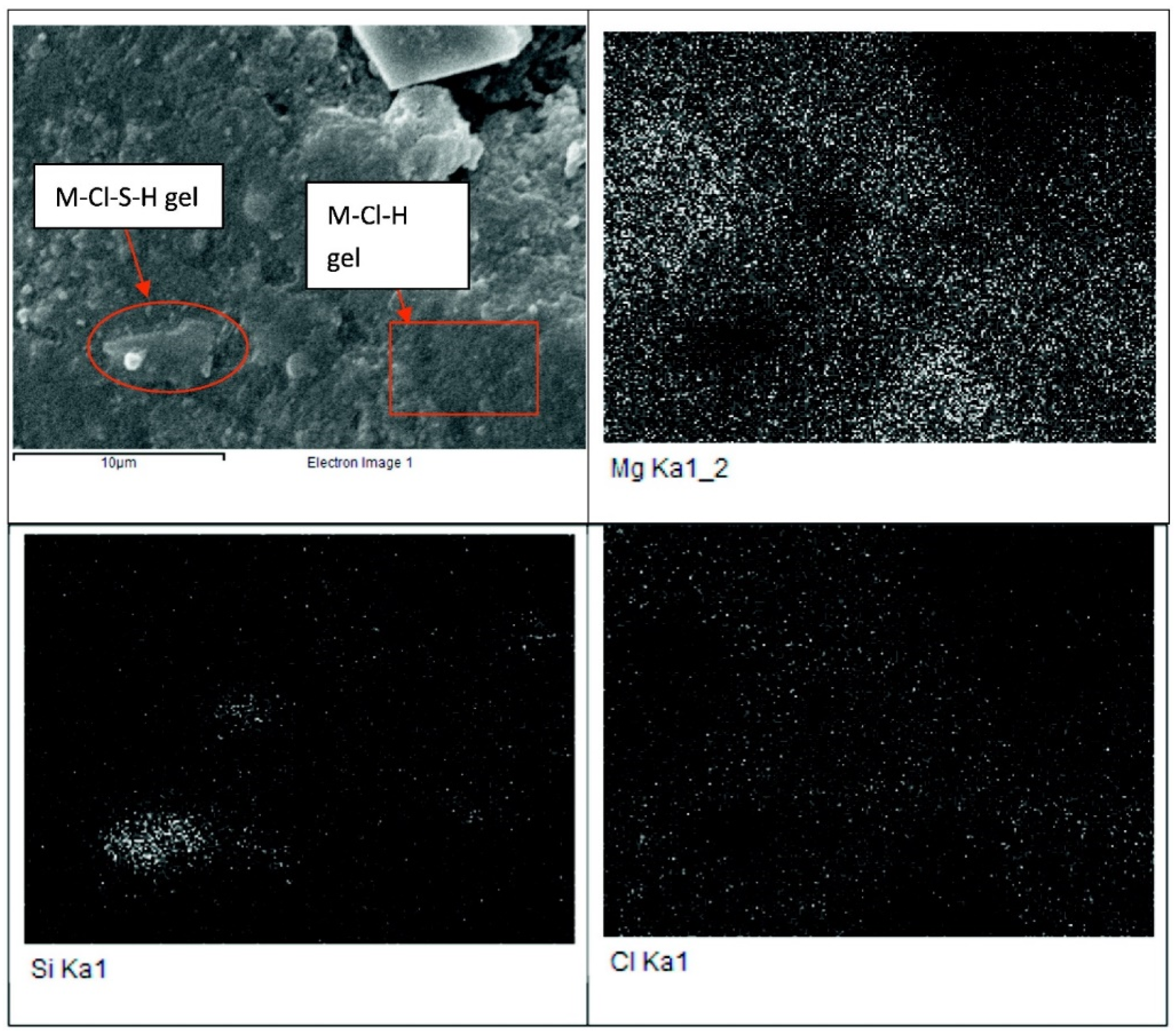


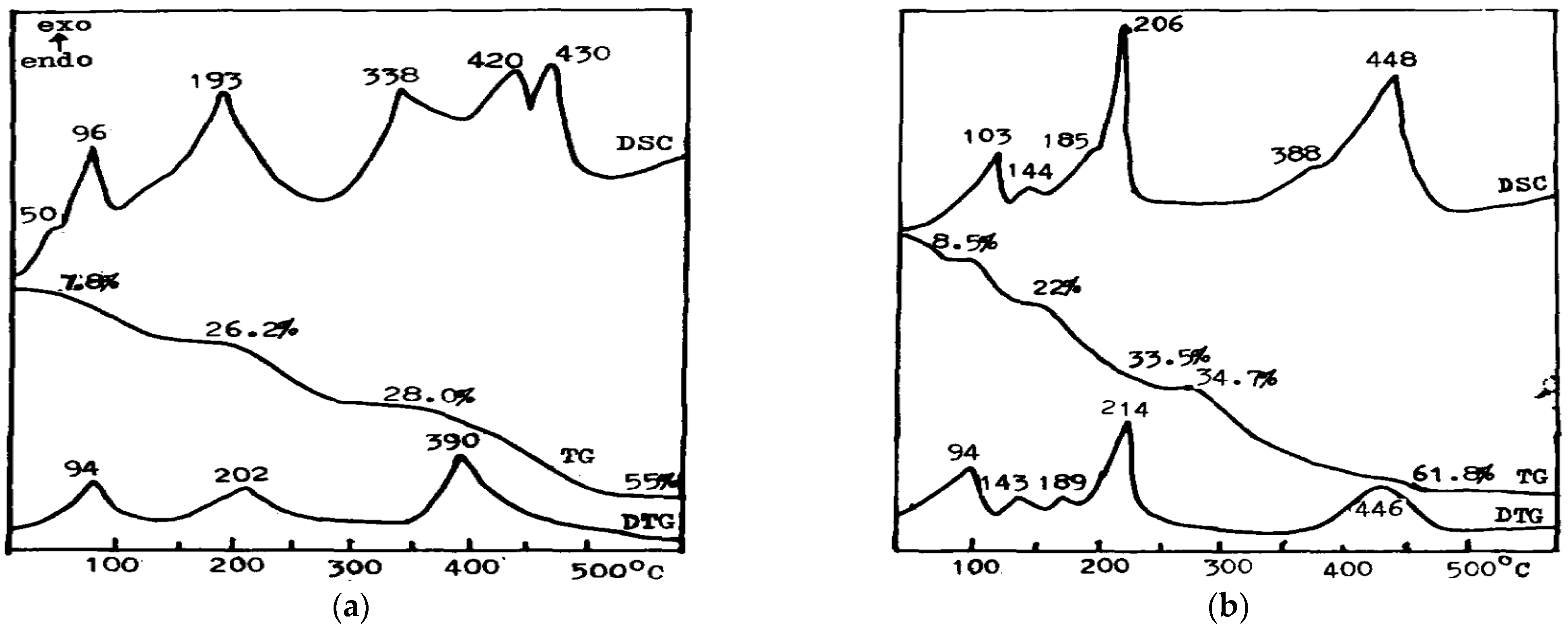
| No | DSC | Enthalpy | DTA | TG | Products |
|---|---|---|---|---|---|
| (°C) | (kJ mol−1) | (°C) | (wt.%) | ||
| Phase 5 (5Mg(OH)2·MgCl2·8H2O) | |||||
| 1 | 96 | 76.58 | 90 | 7.8 | 5Mg(OH)2·MgCl2·6H2O |
| 2 | 145 | 140 | 13.5 | 5Mg(OH)2·MgCl2·4H2O | |
| 3 | 193 | 363.4 | 202 | 26.4 | 5Mg(OH)2·MgCl2 |
| 4 | 250 | 27.0 | 5Mg(OH)2·MgCl2 | ||
| 5 | 378 | 390 | 44.0 | 5Mg(OH)2·MgCl2 + MgO | |
| 6 | 420 | 607.0 | 430 | 54.5 | MgO |
| Phase 3 (3Mg(OH)2·MgCl2·8H2O) | |||||
| 1 | 103 | 78.36 | 94 | 8.5 | 3Mg(OH)2·MgCl2·6H2O |
| 2 | 144 | 149 | 19.0 | 3Mg(OH)2·MgCl2·4H2O | |
| 3 | 185 | 189 | 22.0 | 3Mg(OH)2·MgCl2·4H2O + 3Mg(OH)2·MgCl2 | |
| 4 | 206 | 286.9 | 216 | 33.5 | 3Mg(OH)2·MgCl2 |
| 5 | 250 | 34.7 | 3Mg(OH)2·MgCl2 | ||
| 6 | 388 | 3Mg(OH)2·MgCl2 + MgO | |||
| 7 | 448 | 666.4 | 446 | 61.8 | MgO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, F.; Rawat, S.; Zhang, Y. Magnesium Oxychloride Cement: Development, Opportunities and Challenges. Appl. Sci. 2024, 14, 3074. https://doi.org/10.3390/app14073074
Ahmad F, Rawat S, Zhang Y. Magnesium Oxychloride Cement: Development, Opportunities and Challenges. Applied Sciences. 2024; 14(7):3074. https://doi.org/10.3390/app14073074
Chicago/Turabian StyleAhmad, Farhan, Sanket Rawat, and Yixia Zhang. 2024. "Magnesium Oxychloride Cement: Development, Opportunities and Challenges" Applied Sciences 14, no. 7: 3074. https://doi.org/10.3390/app14073074
APA StyleAhmad, F., Rawat, S., & Zhang, Y. (2024). Magnesium Oxychloride Cement: Development, Opportunities and Challenges. Applied Sciences, 14(7), 3074. https://doi.org/10.3390/app14073074










