Effects of Equivalent Processing Conditions for Microbial Inactivation by Innovative Nonthermal Technologies on the Safety, Quality, and Shelf-Life of Reineta Parda Apple Puree
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Apple Puree Preparation
2.3. Target Microorganism and Inoculation
2.4. Apple Puree Processing
2.4.1. Conventional Thermal Pasteurization (CTP)
2.4.2. Ultrasound (US)
2.4.3. Pulsed Electric Fields (PEF)
2.4.4. High-Pressure Processing (HPP)
2.5. Impact of the Processing Technologies’ Evaluation
2.5.1. Microbiological Analysis
2.5.2. Colour and Antioxidant Analyses
2.6. Thirty-Day Storage Test
2.7. Data Evaluation
3. Results
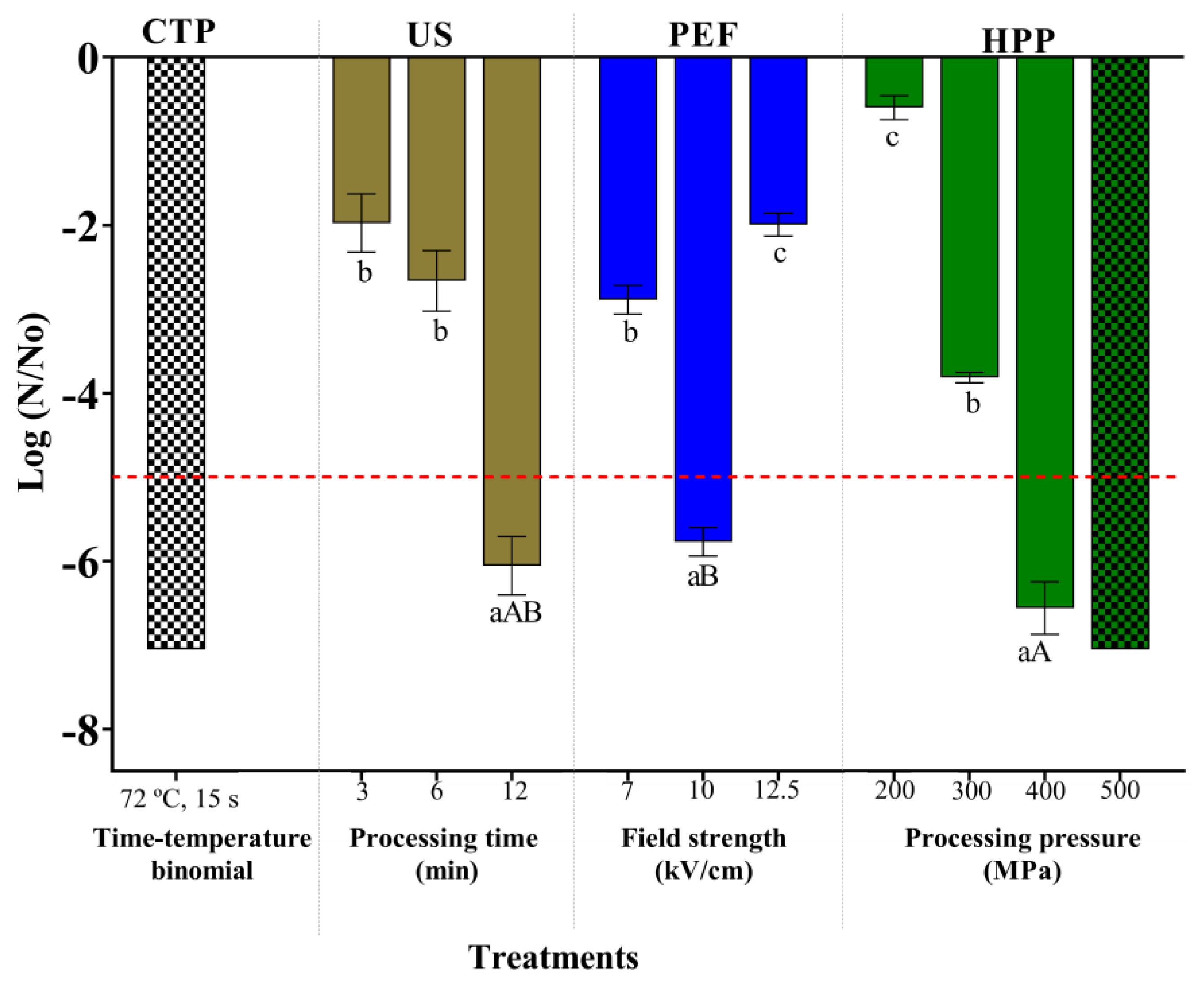
3.1. Inactivation of E. coli and Selection of Equivalent Processing Conditions for Each Technology
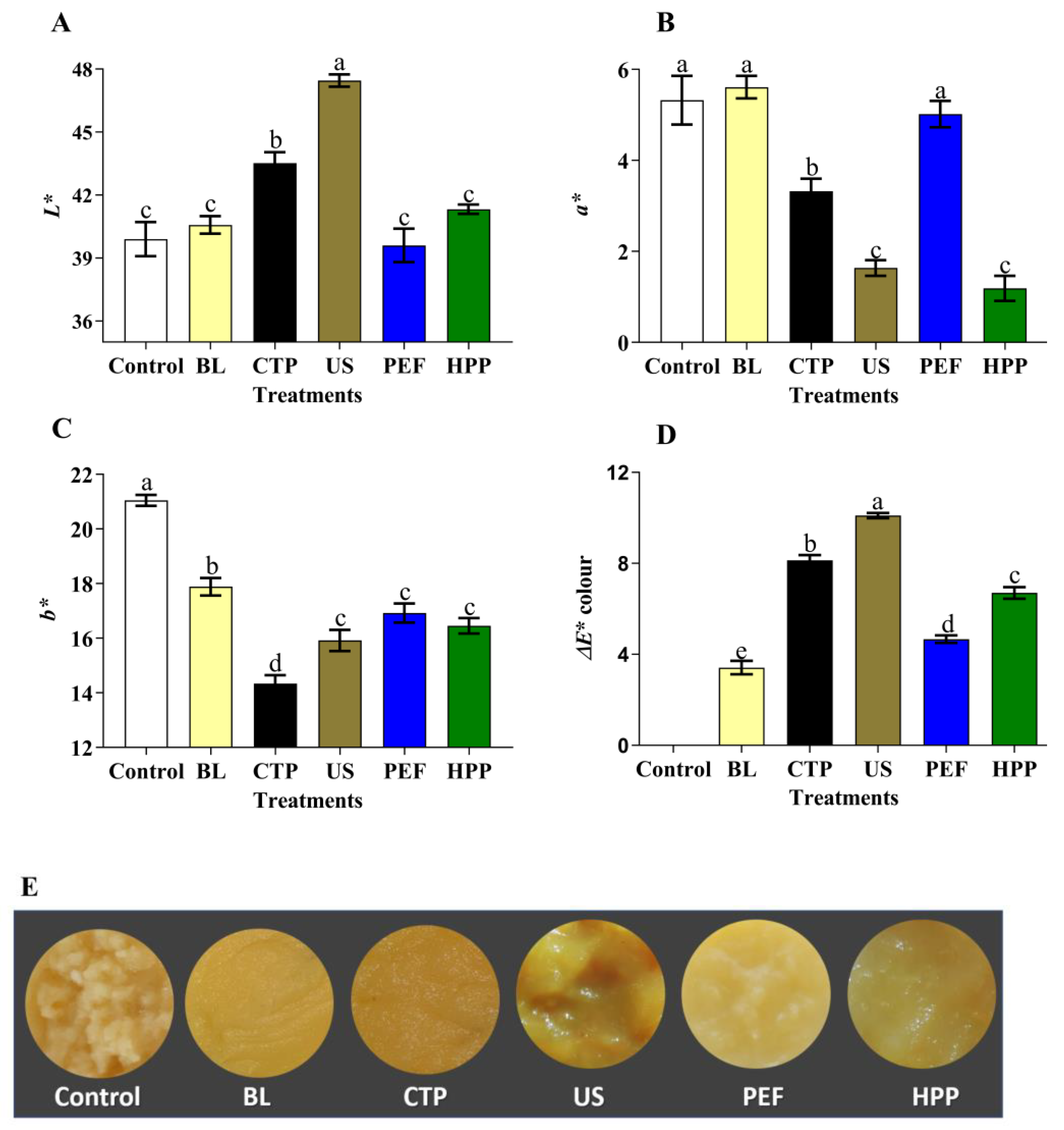
3.2. Impact of the Selected Processing Conditions on the Characteristics of AP
3.2.1. Colour
3.2.2. Antioxidant Activity
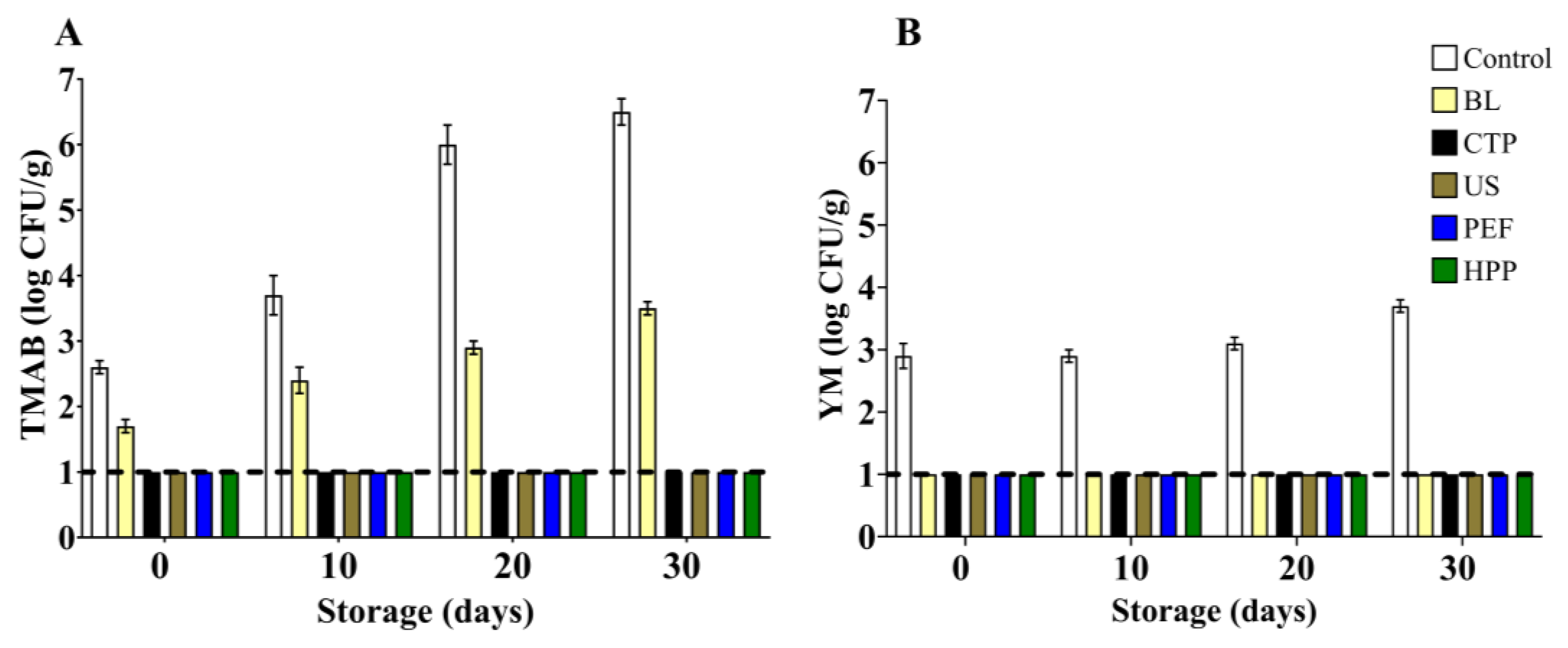
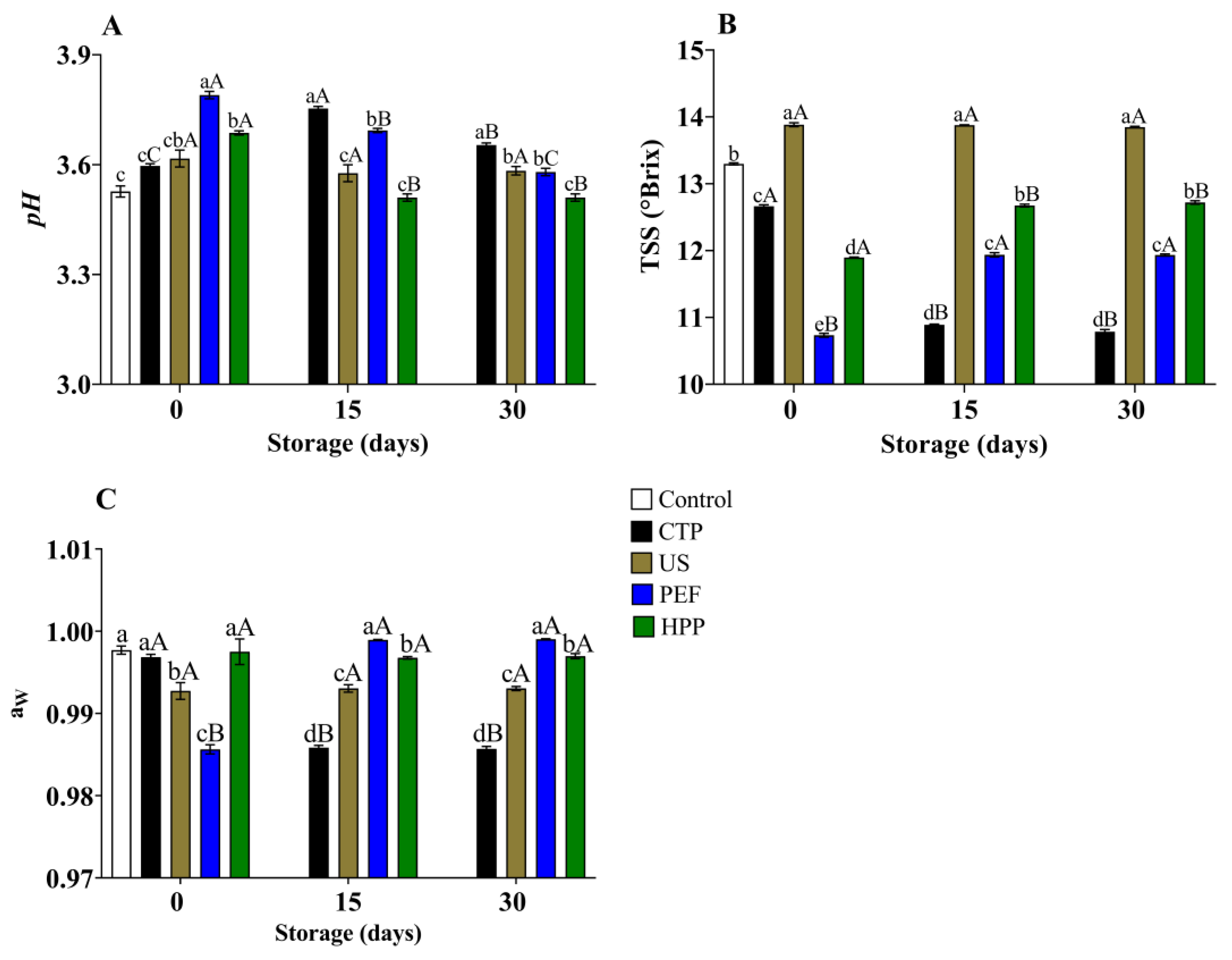
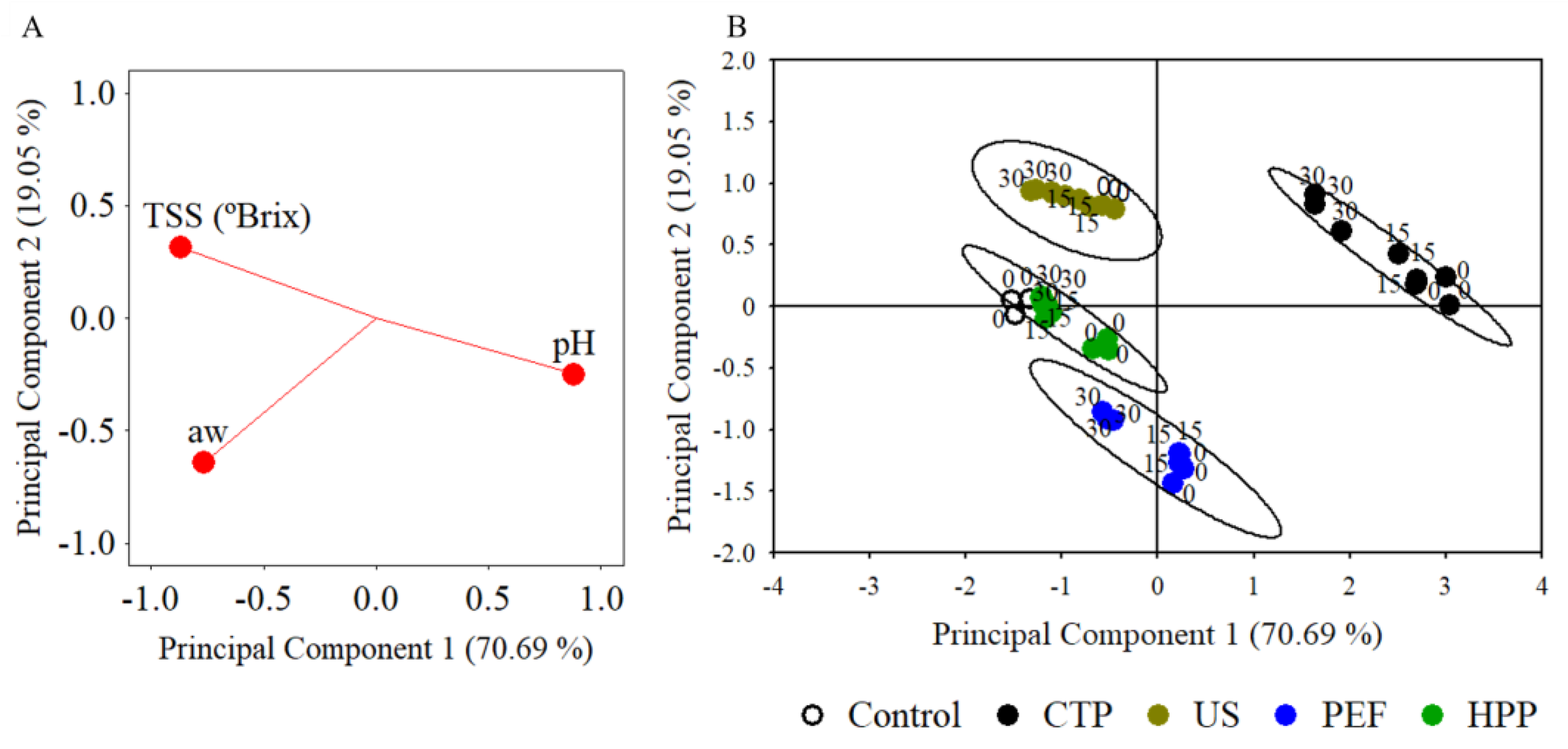
3.3. Thirty-Day Storage Test
3.3.1. Microbial Stability
3.3.2. Physicochemical Quality
4. Discussion
4.1. Inactivation of E. coli and Selection of Equivalent Processing Conditions for Each Technology
4.2. Impact of Each Equivalent Processing Condition on Physicochemical Parameters
4.3. Impact of Each Equivalent Processing Condition on the Antioxidant Activity
4.4. Thirty-Day Storage Test
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#home (accessed on 7 July 2023).
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef] [PubMed]
- Maria Helene Giovanetti, C.; Alessandro, N.; Carmen Lúcia de Oliveira, P.; Gilvan, W. Characterization of Apple Pectin—A Chromatographic Approach. In Chromatography; Leonardo de Azevedo, C., Ed.; IntechOpen: Rijeka, Croatia, 2012; p. 14. [Google Scholar]
- Lan, W.; Bureau, S.; Chen, S.; Leca, A.; Renard, C.M.G.C.; Jaillais, B. Visible, near- and mid-infrared spectroscopy coupled with an innovative chemometric strategy to control apple puree quality. Food Control 2021, 120, 107546. [Google Scholar] [CrossRef]
- Techakanon, C.; Sirimuangmoon, C. The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage. Beverages 2020, 6, 43. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Pan, Z.; Deng, L.-Z.; El-Mashad, H.M.; Yang, X.-H.; Mujumdar, A.S.; Gao, Z.-J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P.A. Effect of high pressure processing on the quality of acidified Granny Smith apple purée product. Innov. Food Sci. Emerg. Technol. 2010, 11, 557–564. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal Technologies for Fruit and Vegetable Juices and Beverages: Overview and Advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Tech. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Tian, X.; Yang, Y.; Zhang, W.; Wang, Y. Effect of High Hydrostatic Pressure and Thermal Processing on the Shelf Life and Quality Attributes of Apple-Kiwi-Carrot Puree Blend. J. Food Qual. 2023, 2023, 1631285. [Google Scholar] [CrossRef]
- Sulaiman, A.; Farid, M.; Silva, F.V. Strawberry puree processed by thermal, high pressure, or power ultrasound: Process energy requirements and quality modeling during storage. Food Sci. Technol. Int. 2017, 23, 293–309. [Google Scholar] [CrossRef]
- Geveke, D.J.; Aubuchon, I.; Zhang, H.Q.; Boyd, G.; Sites, J.E.; Bigley, A.B.W. Validation of a pulsed electric field process to pasteurize strawberry purée. J. Food Eng. 2015, 166, 384–389. [Google Scholar] [CrossRef]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Identification of equivalent processing conditions for pasteurization of strawberry juice by high pressure, ultrasound, and pulsed electric fields processing. Innov. Food Sci. Emerg. 2019, 57, 102195. [Google Scholar] [CrossRef]
- National Advisory Committee on Microbiological Criteria for Foods. Requisite Scientific Parameters for Establishing the Equivalence of Alternative Methods of Pasteurization†. J. Food Prot. 2006, 69, 1190–1216. [Google Scholar] [CrossRef]
- Wibowo, S.; Essel, E.A.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Comparing the impact of high pressure, pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- ISO 4833-1:2013/Amd 1:2022; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2022.
- NP 3277-2:1987; Microbiologia Alimentar Contagem de Bolores e Leveduras. Parte 2: Incubação a 37 °C. Norma Portuguesa: Caparica, Portugal, 1988.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-glucuronidase-positive Escherichia coli. Part 2: Colo-ny-count Technique at 44 Degrees C Using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- Kim, A.-N.; Kim, H.-J.; Kerr, W.L.; Choi, S.-G. The effect of grinding at various vacuum levels on the color, phenolics, and antioxidant properties of apple. Food Chem. 2017, 216, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; De Ancos, B. Effect of high-pressure processing on flavonoids, hydroxycinnamic acids, dihydrochalcones and antioxidant activity of apple ‘Golden Delicious’ from different geographical origin. Innov. Food Sci. Emerg. Technol. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zhang, X.; Zeng, X. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.N.; Lee, K.Y.; Rahman, M.S.; Kim, H.J.; Kerr, W.L.; Choi, S.G. Thermal treatment of apple puree under oxygen-free condition: Effect on phenolic compounds, ascorbic acid, antioxidant activities, color, and enzyme activities. Food Biosci. 2021, 39, 100802. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Bellí, G.; Puigpinós, J.; Herrero, E.; Martín-Belloso, O. Screening the Antioxidant Activity of Thermal or Non-Thermally Treated Fruit Juices by In Vitro and In Vivo Assays. Beverages 2022, 8, 36. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Buko, A.; Kusstatscher, P.; Sinkkonen, A.; Laitinen, O.H.; Virtanen, S.M.; Hyöty, H.; Cernava, T.; Berg, G. Modulation of the food microbiome by apple fruit processing. Food Microbiol. 2022, 108, 104103. [Google Scholar] [CrossRef]
- Xiang, B.; Sundararajan, S.; Mis Solval, K.; Espinoza-Rodezno, L.; Aryana, K.; Sathivel, S. Effects of Pulsed Electric Fields on Physicochemical Properties and Microbial Inactivation of Carrot Juice. J. Food Process. Pres. 2014, 38, 1556–1564. [Google Scholar] [CrossRef]
- Fishburn, J.D.; Tang, Y.; Frank, J. Efficacy of various consumer-friendly produce washing technologies in reducing pathogens on fresh produce. Food Prot. Trends 2012, 32, 456–466. [Google Scholar]
- Ling, B.; Tang, J.; Kong, F.Y.; Mitcham, E.J.; Wang, S. Kinetics of Food Quality Changes during Thermal Processing: A Review. Food Bioprocess Technol. 2015, 8, 343–358. [Google Scholar] [CrossRef]
- Rocha, C.d.S.; Magnani, M.; Ramos, G.L.d.P.A.; Bezerril, F.F.; Freitas, M.Q.; Cruz, A.G.; Pimentel, T.C. Emerging technologies in food processing: Impacts on sensory characteristics and consumer perception. Curr. Opin. Food Sci. 2022, 47, 100892. [Google Scholar] [CrossRef]
- Price, S.B.; Wright, J.C.; DeGraves, F.J.; Castanie-Cornet, M.P.; Foster, J.W. Acid resistance systems required for survival of Escherichia coli O157:H7 in the bovine gastrointestinal tract and in apple cider are different. Appl. Environ. Microbiol. 2004, 70, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Barrientos, J.U.; Wang, Q.; Markland, S.M.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W.; Kniel, K.E.; Moraru, C.I. Efficient Reduction of Pathogenic and Spoilage Microorganisms from Apple Cider by Combining Microfiltration with UV Treatment. J. Food Prot. 2015, 78, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Romero, E.; Feng, H.; Martin, S.E.; Cadwallader, K.R.; Robinson, S.J. Inactivation of Escherichia coli with Power Ultrasound in Apple Cider. J. Food Sci. 2006, 71, E102–E108. [Google Scholar] [CrossRef]
- Baboli, Z.M.; Williams, L.; Chen, G. Rapid Pasteurization of Apple Juice Using a New Ultrasonic Reactor. Foods 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, L.; Hao, C.; Liu, K.; Qiu, J. Synergistic effect of pulsed electric fields and temperature on the inactivation of microorganisms. AMB Exp. 2021, 11, 47. [Google Scholar] [CrossRef]
- Picouet, P.A.; Landl, A.; Abadias, M.; Castellari, M.; Viñas, I. Minimal processing of a Granny Smith apple purée by microwave heating. Innov. Food Sci. Emerg. Technol. 2009, 10, 545–550. [Google Scholar] [CrossRef]
- Rinaldi, M.; Langialonga, P.; Dhenge, R.; Aldini, A.; Chiavaro, E. Quality traits of apple puree treated with conventional, ohmic heating and high-pressure processing. Eur. Food Res. Technol. 2021, 247, 1679–1688. [Google Scholar] [CrossRef]
- Keenan, D.F.; Brunton, N.; Butler, F.; Wouters, R.; Gormley, R. Evaluation of thermal and high hydrostatic pressure processed apple purees enriched with prebiotic inclusions. Innov. Food Sci. Emerg. 2011, 12, 261–268. [Google Scholar] [CrossRef]
- Salazar-Orbea, G.L.; García-Villalba, R.; Bernal, M.J.; Hernández, A.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. Stability of phenolic compounds in apple and strawberry: Effect of different processing techniques in industrial set up. Food Chem. 2023, 401, 134099. [Google Scholar] [CrossRef] [PubMed]
- Nistor, O.V.; Stãnciuc, N.; Andronoiu, D.G.; Mocanu, G.D.ţ.; Botez, E. Ohmic treatment of apple puree (Golden Delicious variety) in relation to product quality. Food Technol. Biotechnol. 2015, 24, 51–59. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wolniak, M.; Wojdyło, A.; Wawer, I. Influence of apple purée preparation and storage on polyphenol contents and antioxidant activity. Food Chem. 2008, 107, 1473–1484. [Google Scholar] [CrossRef]
- Lara-Valderrama, G.; Nagaya, M.; Uemura, K. Quality characterization of apple puree processed by high frequency pulse continuous heating. Food Chem. Adv. 2023, 2, 100228. [Google Scholar] [CrossRef]
- Geicu, M.; Popa, M.; Mitelut, A.; Niculiţă, P.; Cramariuc, R. Study of Color Evolution during Shelf Life of Apple Puree Treated with Pulsed Electric Field. In Proceedings of the 2nd International Symposium “New research in Biotechnology”, USAMV, Bucharest, Romania, 19–20 November 2009; pp. 212–218. [Google Scholar]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of thermal and non-thermal treatments on the color of citrus juice: A review. Food Rev. Int. 2021, 39, 3555–3577. [Google Scholar] [CrossRef]
- Lan, W.; Baeten, V.; Jaillais, B.; Renard, C.M.G.C.; Arnould, Q.; Chen, S.; Leca, A.; Bureau, S. Comparison of near-infrared, mid-infrared, Raman spectroscopy and near-infrared hyperspectral imaging to determine chemical, structural and rheological properties of apple purees. J. Food Eng. 2022, 323, 111002. [Google Scholar] [CrossRef]
- Ibarz, A.; Pagán, J.; Garza, S. Kinetic models of non-enzymatic browning in apple puree. J. Sci. Food Agric. 2000, 80, 1162–1168. [Google Scholar] [CrossRef]
- Lan, W.; Jaillais, B.; Leca, A.; Renard, C.M.G.C.; Bureau, S. A new application of NIR spectroscopy to describe and predict purees quality from the non-destructive apple measurements. Food Chem. 2020, 310, 125944. [Google Scholar] [CrossRef]
- Pellegrino, D. Antioxidants and Cardiovascular Risk Factors. Diseases 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S. Variation in Polyphenolics and Antioxidant Activity of Traditional Apple Cultivars from West Himalaya, Uttarakhand. Hortic. Plant J. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Lončarić, A.; Kopjar, M.; Piližota, V. Improving the quality of apple purée. J. Food Sci. Technol. 2017, 54, 3201–3207. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Li, F.; Chen, G.; Zhang, B.; Fu, X. Current applications and new opportunities for the thermal and non-thermal processing technologies to generate berry product or extracts with high nutraceutical contents. Food Res. Int. 2017, 100, 19–30. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino-Hernández, E.; Alves, M.; Moreira, N.; Lima, V.; Pinto, C.A.; Saraiva, J.A. Effects of Equivalent Processing Conditions for Microbial Inactivation by Innovative Nonthermal Technologies on the Safety, Quality, and Shelf-Life of Reineta Parda Apple Puree. Appl. Sci. 2024, 14, 3088. https://doi.org/10.3390/app14073088
Pino-Hernández E, Alves M, Moreira N, Lima V, Pinto CA, Saraiva JA. Effects of Equivalent Processing Conditions for Microbial Inactivation by Innovative Nonthermal Technologies on the Safety, Quality, and Shelf-Life of Reineta Parda Apple Puree. Applied Sciences. 2024; 14(7):3088. https://doi.org/10.3390/app14073088
Chicago/Turabian StylePino-Hernández, Enrique, Marco Alves, Nicole Moreira, Vasco Lima, Carlos A. Pinto, and Jorge A. Saraiva. 2024. "Effects of Equivalent Processing Conditions for Microbial Inactivation by Innovative Nonthermal Technologies on the Safety, Quality, and Shelf-Life of Reineta Parda Apple Puree" Applied Sciences 14, no. 7: 3088. https://doi.org/10.3390/app14073088
APA StylePino-Hernández, E., Alves, M., Moreira, N., Lima, V., Pinto, C. A., & Saraiva, J. A. (2024). Effects of Equivalent Processing Conditions for Microbial Inactivation by Innovative Nonthermal Technologies on the Safety, Quality, and Shelf-Life of Reineta Parda Apple Puree. Applied Sciences, 14(7), 3088. https://doi.org/10.3390/app14073088





