Physical and Chemical Methods to Assess Performance of TPO-Modified Asphalt Binder
Abstract
:1. Introduction
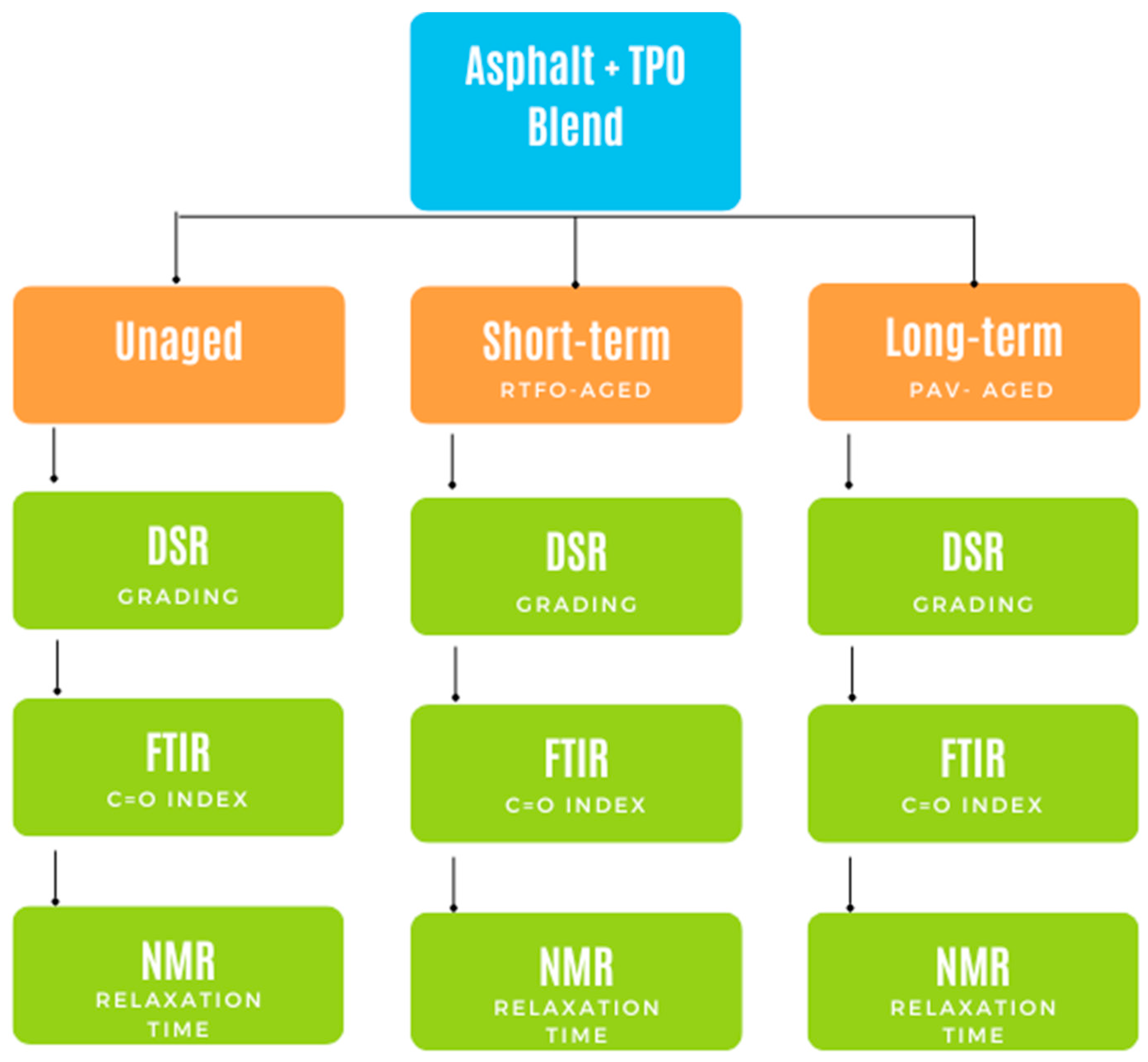
2. Materials and Methods
3. Results
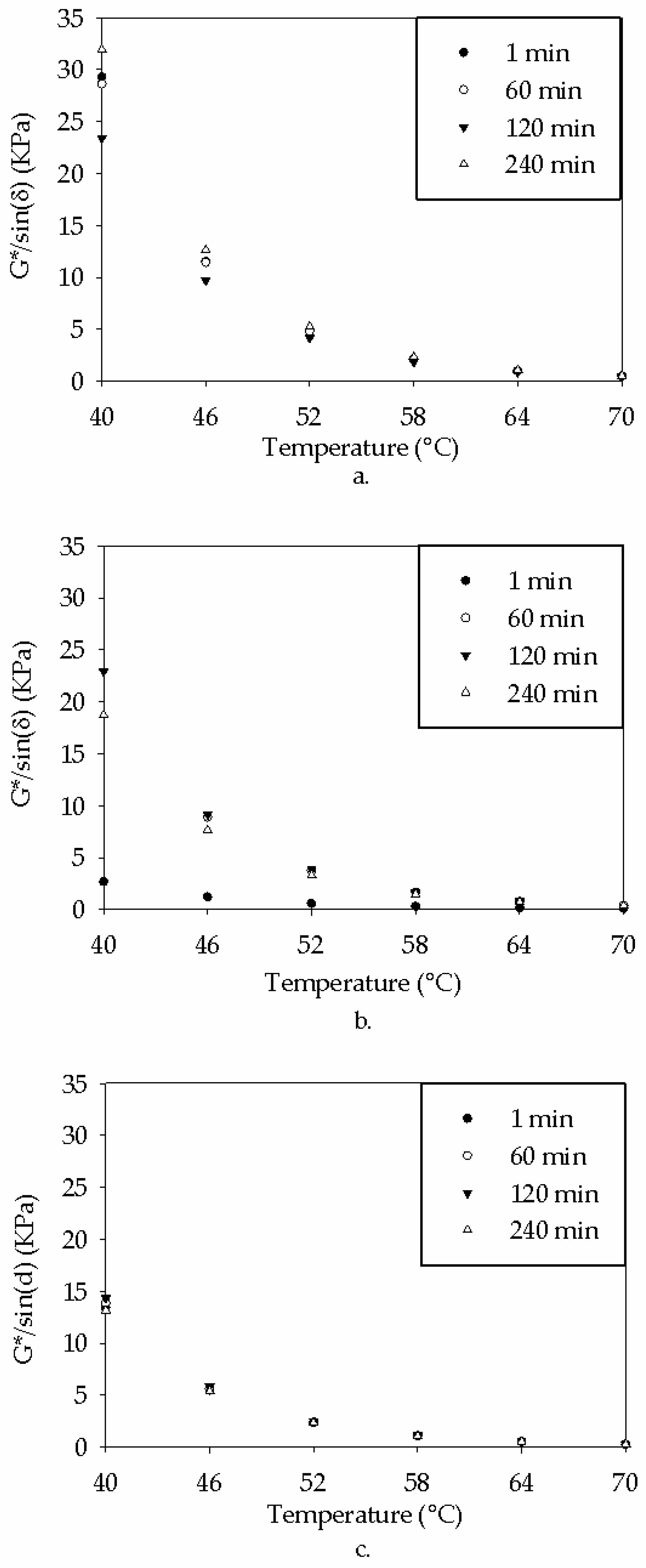
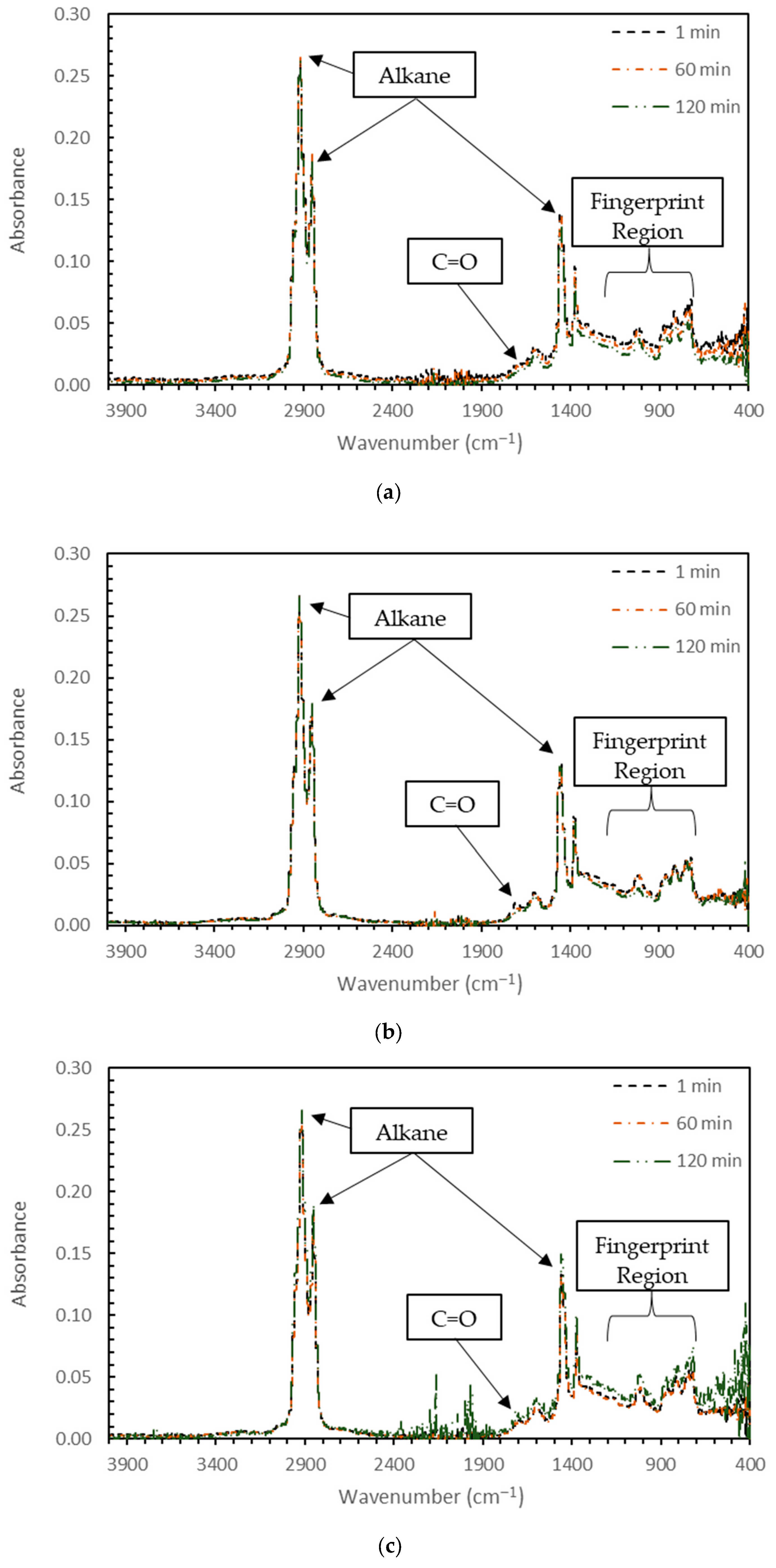
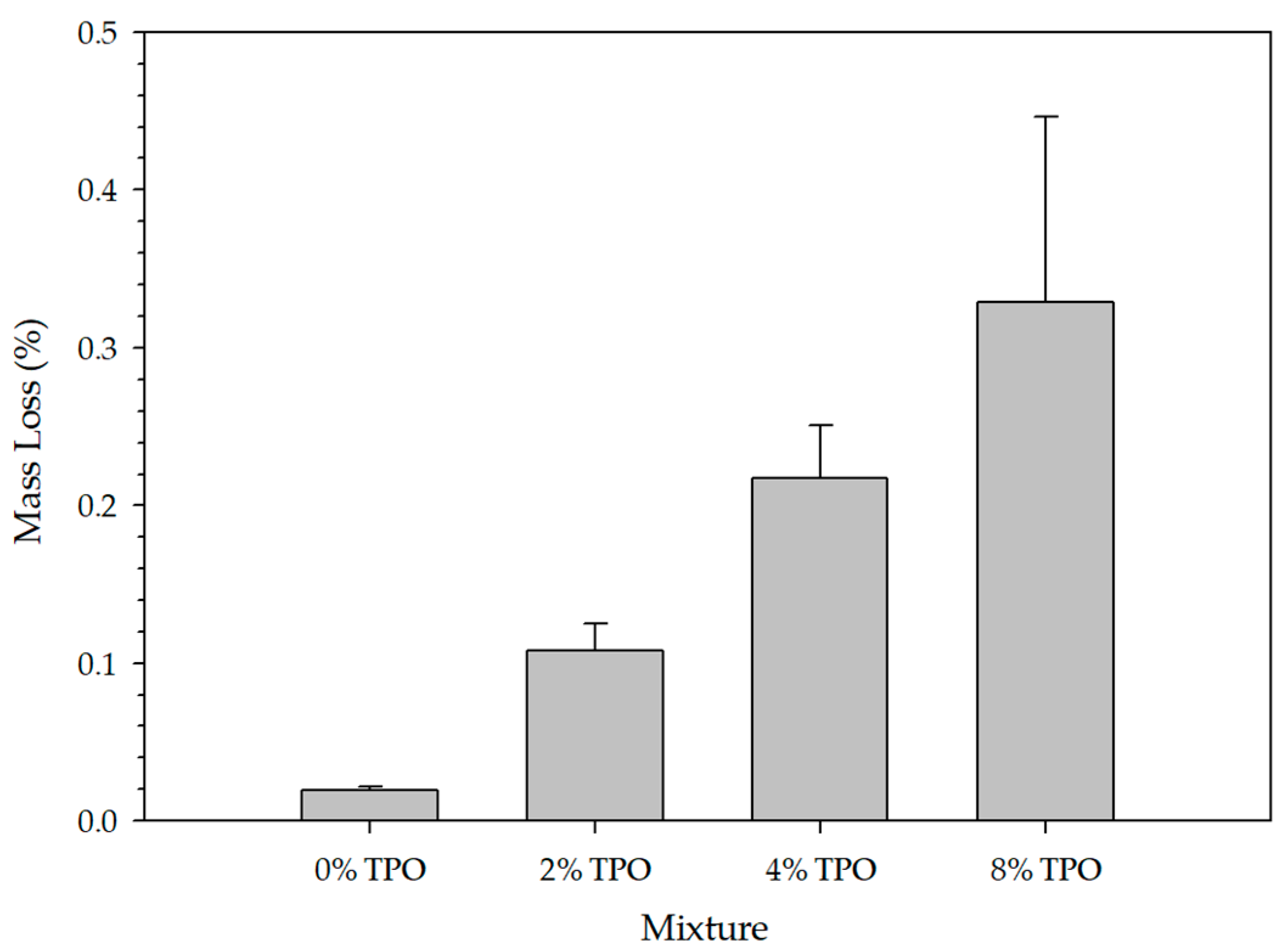
3.1. Blending Homogeneity
3.1.1. Standardized Physical Testing (DSR) on Blending Homogeneity
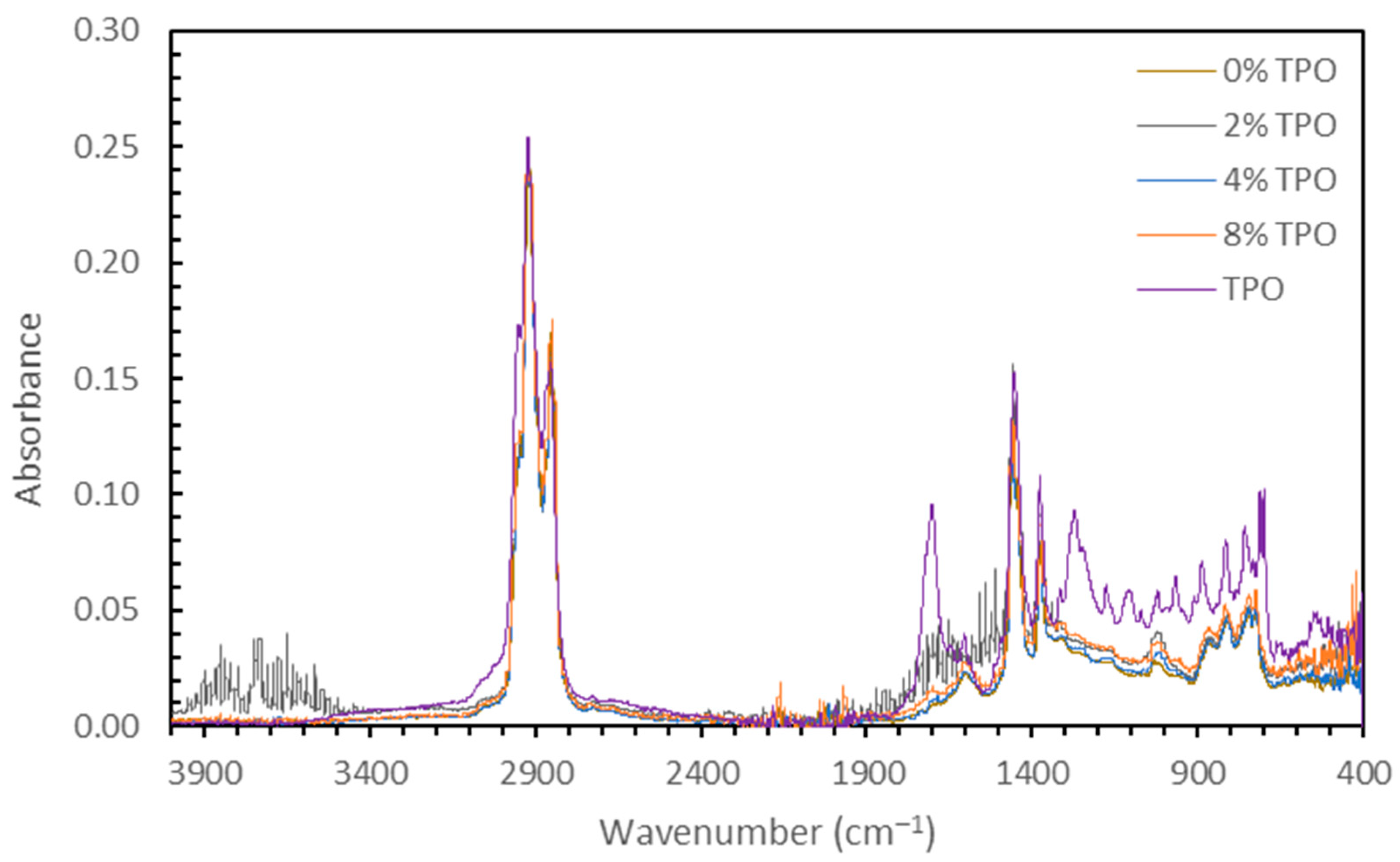
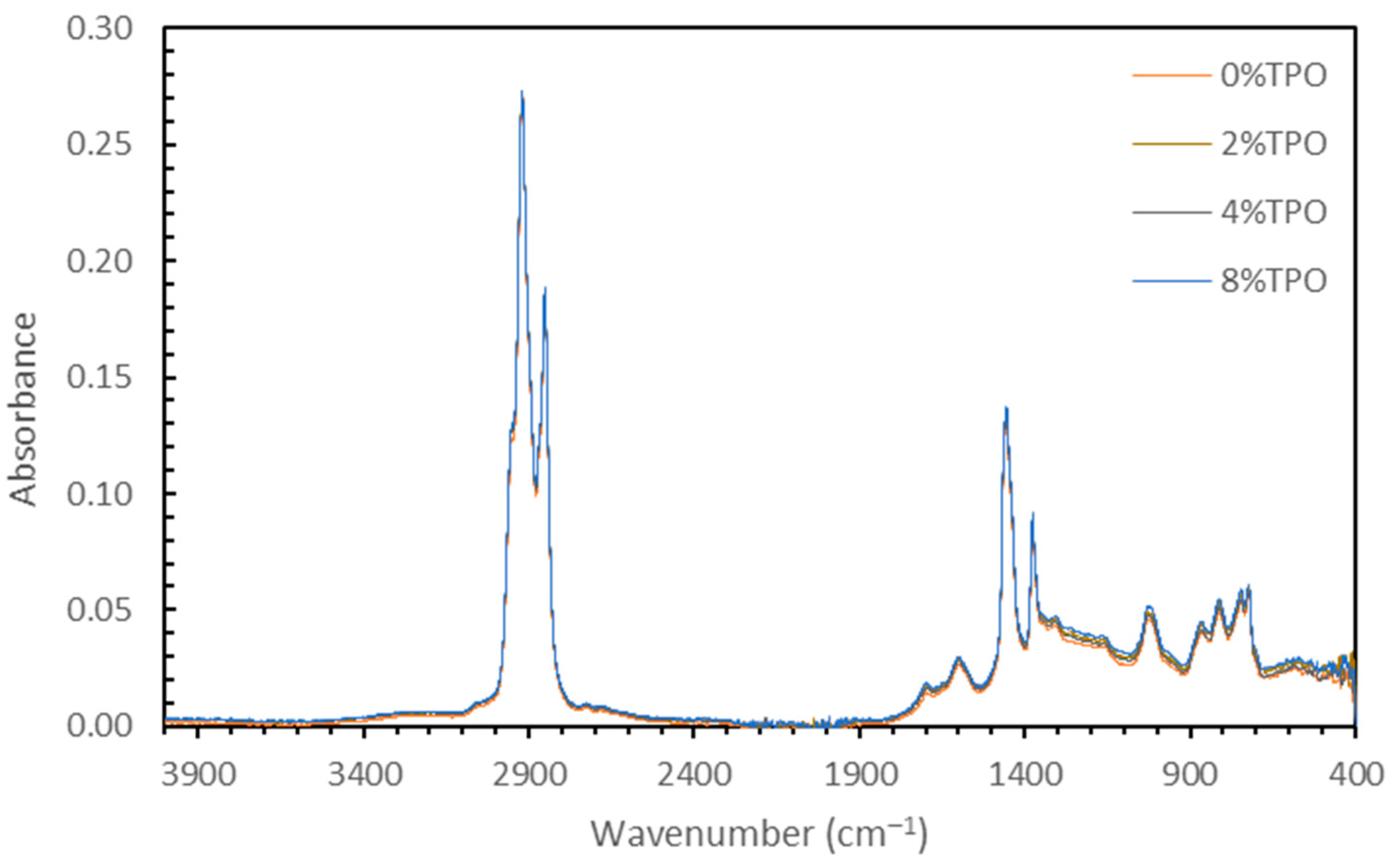
3.1.2. Chemical Testing (FTIR) on Blending Homogeneity
3.2. Unaged Blends
3.2.1. Standardized Physical Testing (DSR) of Unaged Blends
3.2.2. Chemical Testing (FTIR and NMR) of Unaged Blends
3.3. Short-Term Aged Blends
3.3.1. Standardized Physical Testing (DSR) of Short-Term Aged Blends
3.3.2. Chemical Testing (FTIR and NMR) of Short-Term Aged Blends
3.4. Long-Term Aged Blends
3.4.1. Standardized Physical Testing (DSR) of Long-Term Aged Blends
3.4.2. Chemical Testing (FTIR and NMR) of Long-Term Aged Blends
4. Discussion
4.1. DSR Testing Comparison
4.2. FTIR Testing Comparison
4.3. NMR Testing Comparison
5. Conclusions
- -
- Due to the reactivity of TPO, blending the material resulted in physical and chemical variability. The physical variability was reduced as the testing temperature increased. Physical testing temperatures above 58 °C are recommended to homogenize the modified binder matrix. The chemical variability was more subtle, but FTIR analysis indicated that no new IR-sensitive functional groups were formed during mixing.
- -
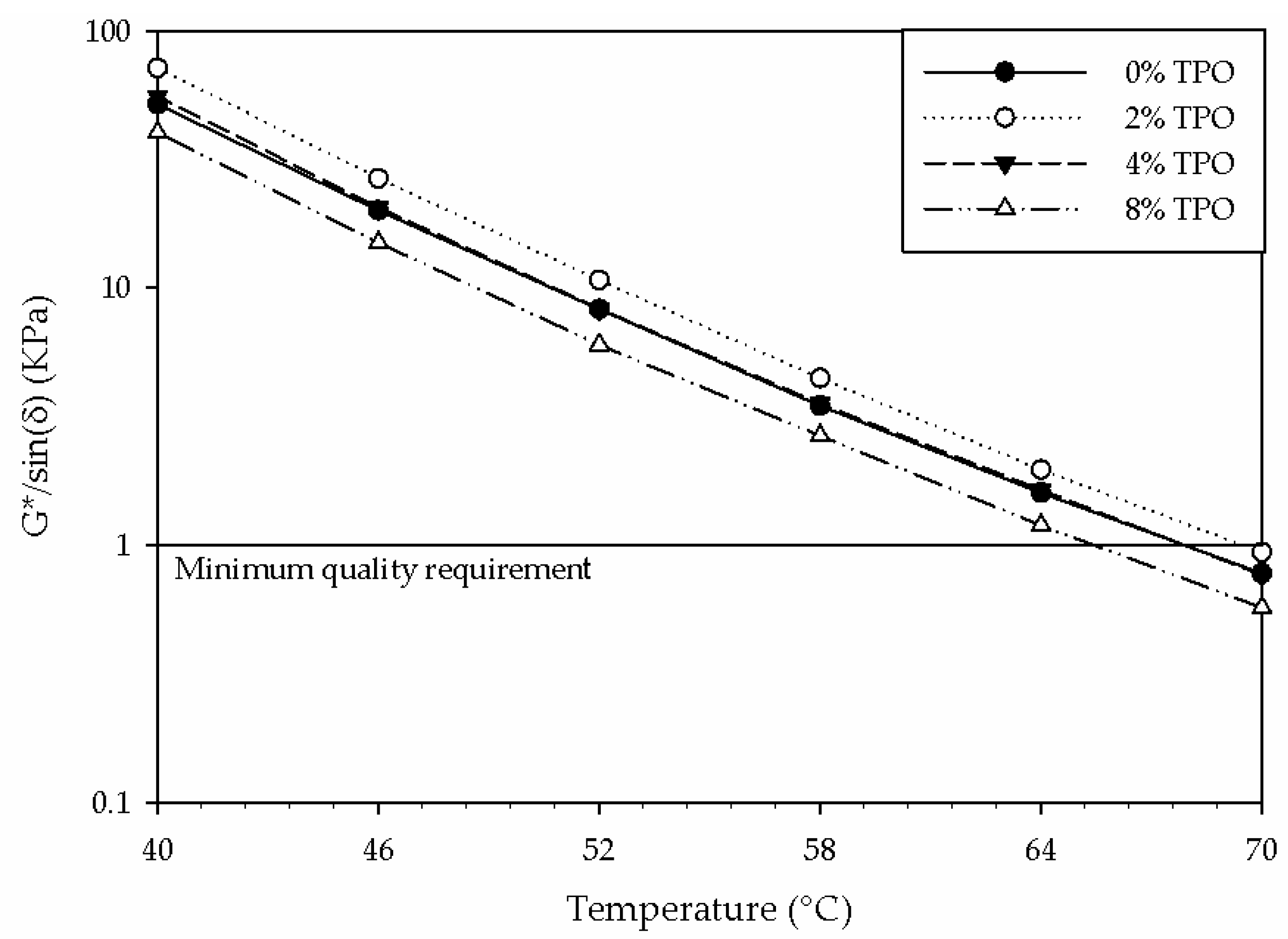
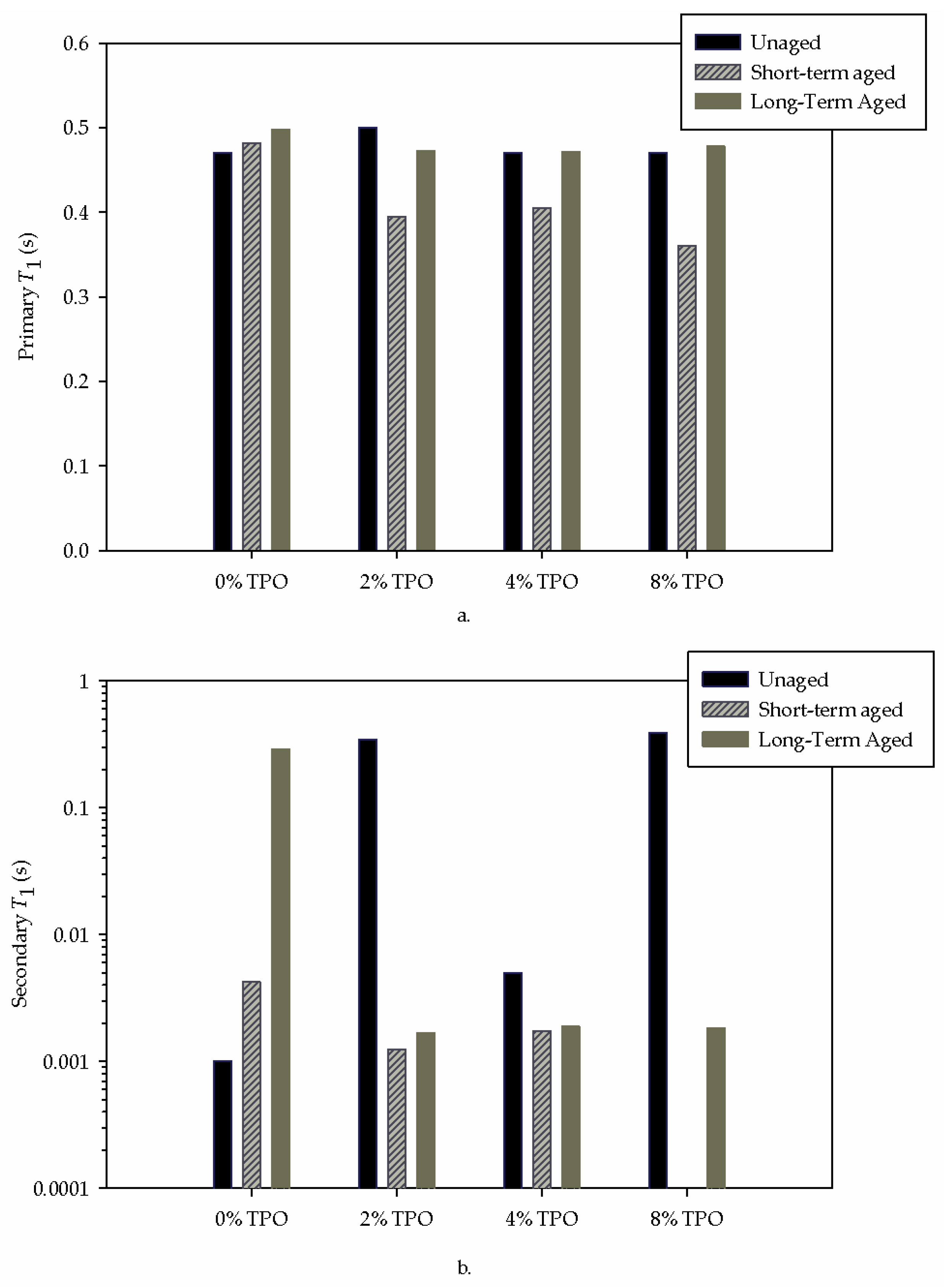
- Physical testing alone could not explain the unexpected stiffening of the 2% sample. FTIR testing indicated a higher amount of water and C=O in the blend. NMR analysis resulted in an increased primary relaxation time, indicating a less mobile system. These chemical results indicate that some TPO components introduced water and stiffened the binder matrix.
- -
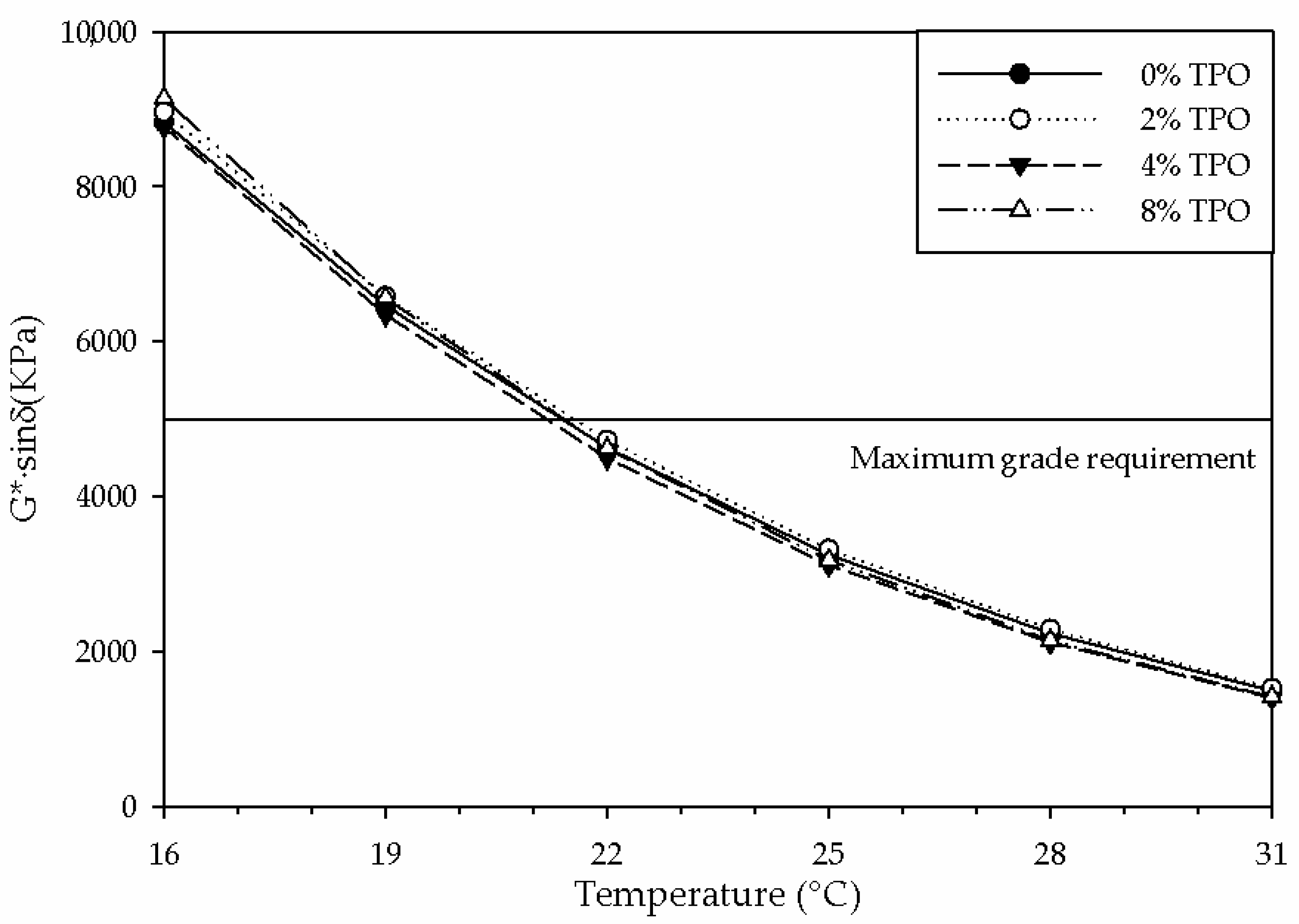
- Adding 8% TPO hindered the high PG from 64 °C to 58 °C, reducing the resistance to rutting. This physical testing was supported by the decrease in the NMR primary relaxation time, indicating a more mobile system.
- -
- None of the TPO blends enhanced the intermediate PG. The similar physical properties were supported by the similarities in chemical testing.
- -
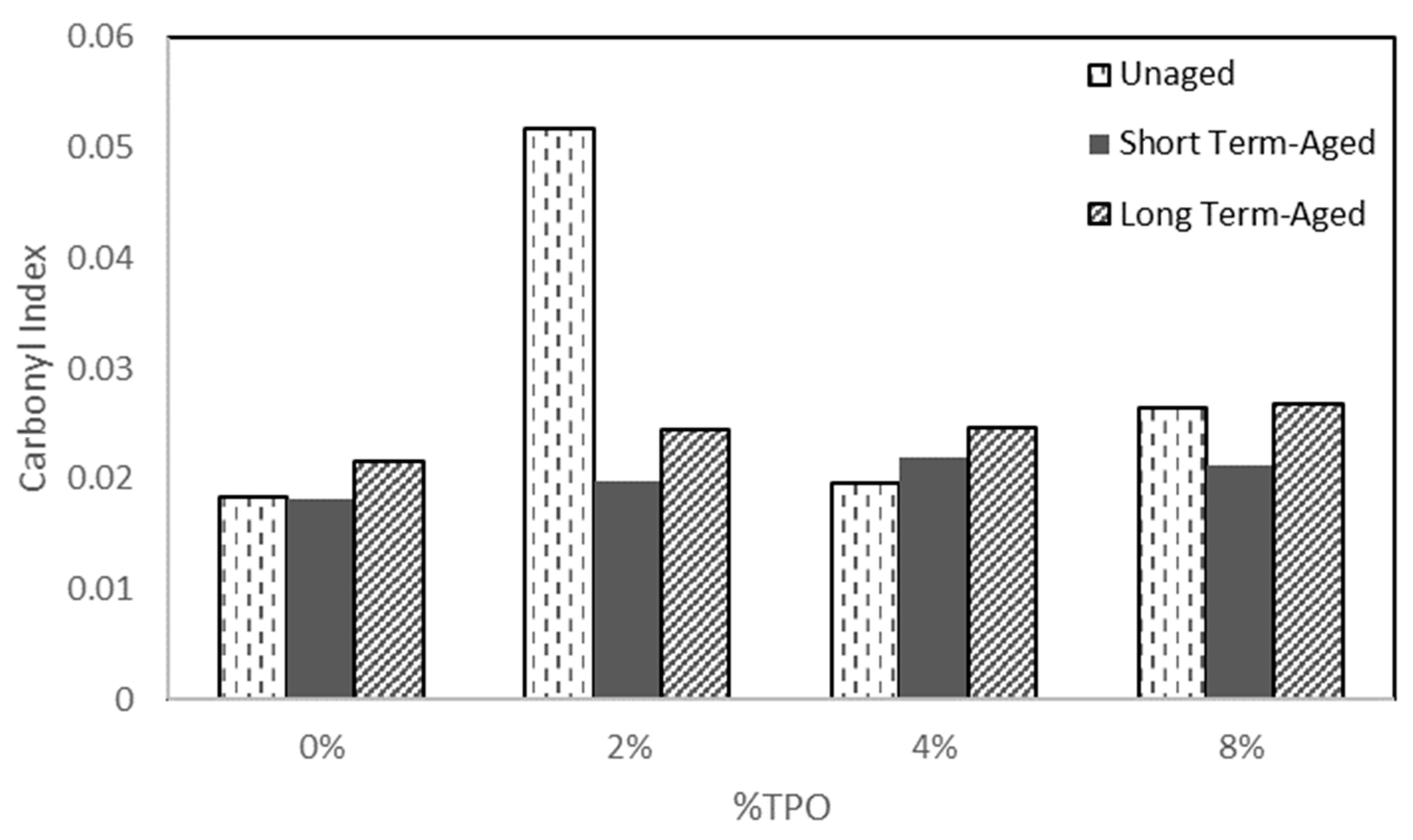
- FTIR analysis showed little variation from aging. The carbonyl indices indicated that long-term aging had the largest carbonyl content across most samples. Further analysis was complicated due to the carbonyl groups present in the TPO.
- -
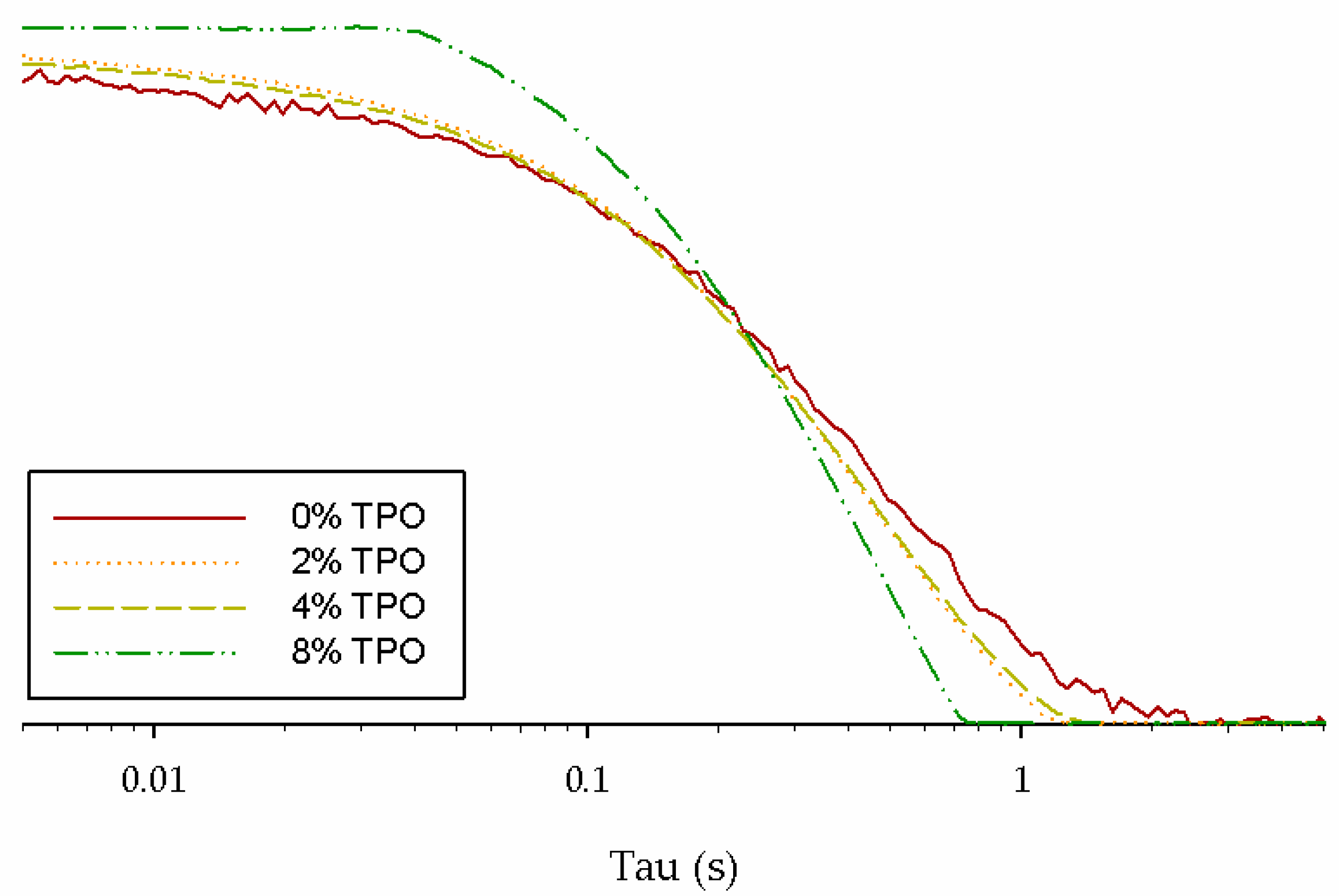
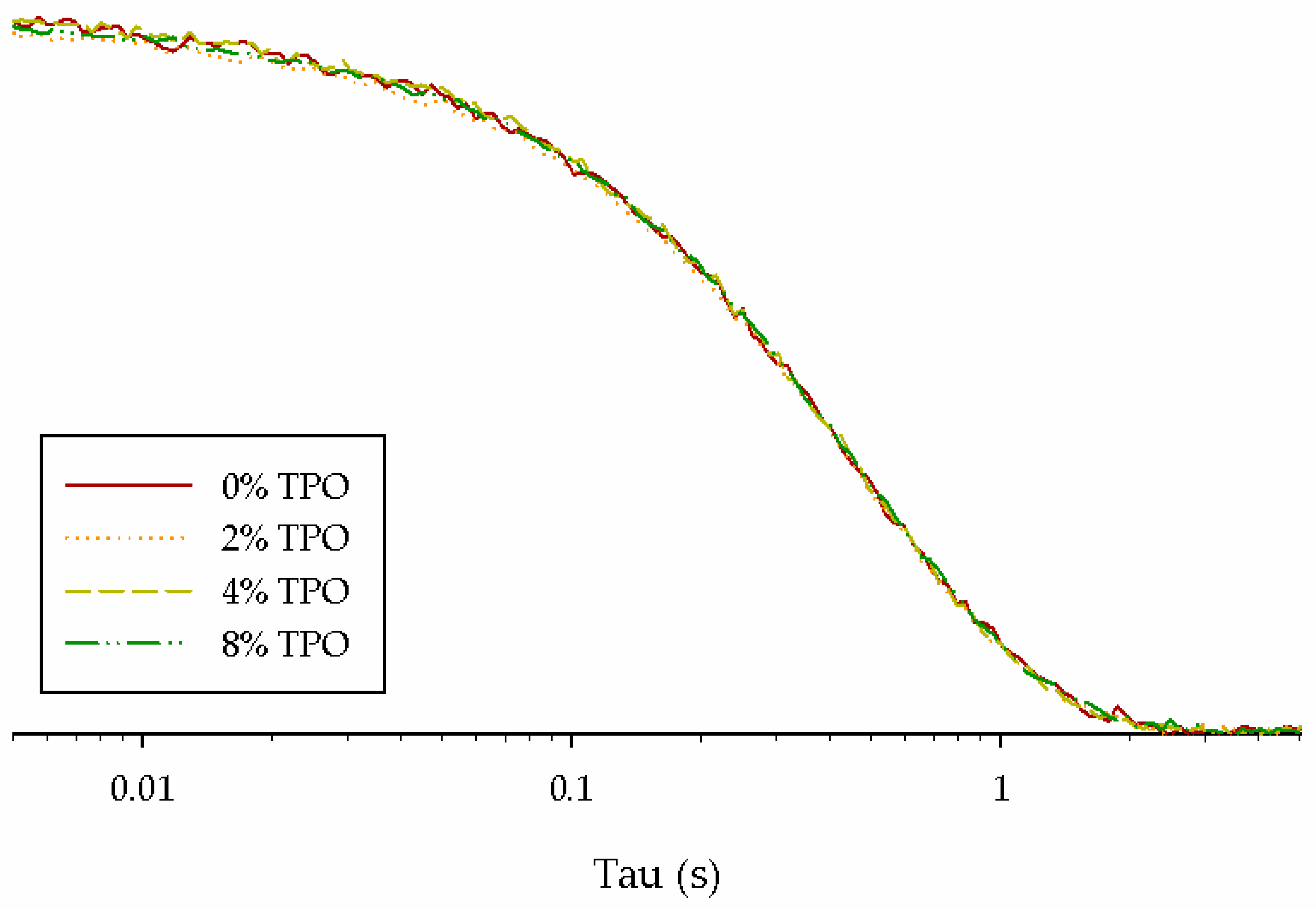
- The primary NMR relaxation times indicated a connection to physical testing. Since NMR depends on the binder matrix, the impact of the TPO and aging could be seen. Some connections could be made between the secondary relaxation times, but more testing is needed to determine a correlation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colunga-Sánchez, L.M.; Salazar-Cruz, B.A.; Rivera-Armenta, J.L.; Morales-Cepeda, A.B.; Ramos-Gálvan, C.E.; Chávez-Cinco, M.Y. Evaluation of Chicken Feather and Styrene-Butadiene/Chicken Feather Composites as Modifier for Asphalts Binder. Appl. Sci. 2019, 9, 5188. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, M.; Liu, Z.; He, B.; Yu, J.; Xue, L. Effect of Different Rejuvenators on the Rheological Properties of Aged SBS Modified Bitumen in Long Term Aging. Constr. Build. Mater. 2019, 215, 709–717. [Google Scholar] [CrossRef]
- Hemida, A.; Abdelrahman, M. Component Analysis of Bio-Asphalt Binder Using Crumb Rubber Modifier and Guayule Resin as an Innovative Asphalt Replacer. Resour. Conserv. Recycl. 2021, 169, 105486. [Google Scholar] [CrossRef]
- Flávia Justino Uchoa, A.; da Silva Rocha, W.; Peter Macedo Feitosa, J.; Lopes Nogueira, R.; Hellen Almeida de Brito, D.; Barbosa Soares, J.; de Aguiar Soares, S. Bio-Based Palm Oil as an Additive for Asphalt Binder: Chemical Characterization and Rheological Properties. Constr. Build. Mater. 2021, 285, 122883. [Google Scholar] [CrossRef]
- Alattieh, S.A.; Al-Khateeb, G.G.; Zeiada, W.; Shanableh, A. Performance Assessment of Bio-Modified Asphalt Binder Using Extracted Bio Oil from Date Seeds Waste. Int. J. Syst. Assur. Eng. Manag. 2020, 11, 1260–1270. [Google Scholar] [CrossRef]
- Banar, M.; Akyildiz, V.; Özkan, A.; Çokaygil, Z.; Onay, Ö. Characterization of Pyrolytic Oil Obtained from Pyrolysis of TDF (Tire Derived Fuel). Energy Convers. Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Campuzano, F.; Abdul Jameel, A.G.; Zhang, W.; Emwas, A.H.; Agudelo, A.F.; Martínez, J.D.; Sarathy, S.M. Fuel and Chemical Properties of Waste Tire Pyrolysis Oil Derived from a Continuous Twin-Auger Reactor. Energy Fuels 2020, 34, 12688–12702. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of Waste Tyres: A Review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, R.; Kumar, A. Evaluation of Waste Tire Pyrolytic Oil as a Rejuvenation Agent for Unmodified, Polymer-Modified, and Rubber-Modified Aged Asphalt Binders. J. Mater. Civil. Eng. 2022, 34, 04022246. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, F.; Zhang, Y.; Zhao, L.; Chen, J.; Cao, L.; Gao, J.; Xu, C. Properties and Utilization of Waste Tire Pyrolysis Oil: A Mini Review. Fuel Process. Technol. 2021, 211, 106582. [Google Scholar] [CrossRef]
- Al-Sabaeei, A.M.; Napiah, M.B.; Sutanto, M.H.; Alaloul, W.S.; Yusoff, N.I.M.; Khairuddin, F.H.; Memon, A.M. Evaluation of the High-Temperature Rheological Performance of Tire Pyrolysis Oil-Modified Bio-Asphalt. Int. J. Pavement Eng. 2022, 23, 4007–4022. [Google Scholar] [CrossRef]
- Ržek, L.; Ravnikar Turk, M.; Tušar, M. Increasing the Rate of Reclaimed Asphalt in Asphalt Mixture by Using Alternative Rejuvenator Produced by Tire Pyrolysis. Constr. Build. Mater. 2020, 232, 117177. [Google Scholar] [CrossRef]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef]
- Campuzano, F.; Abdul Jameel, A.G.; Zhang, W.; Emwas, A.H.; Agudelo, A.F.; Martínez, J.D.; Sarathy, S.M. On the Distillation of Waste Tire Pyrolysis Oil: A Structural Characterization of the Derived Fractions. Fuel 2021, 290, 120041. [Google Scholar] [CrossRef]
- Kebritchi, A.; Firoozifar, H.; Shams, K.; Jalali-Arani, A. Effect of Pre-Devulcanization and Temperature on Physical and Chemical Properties of Waste Tire Pyrolytic Oil Residue. Fuel 2013, 112, 319–325. [Google Scholar] [CrossRef]
- Benallal, B.; Roy, C.; Pakdel, H.; Chabot, S.; Poirier, M.A. Characterization of Pyrolytic Light Naphtha from Vacuum Pyrolysis of Used Tyres Comparison with Petroleum Naphtha. Fuel 1995, 74, 1589–1594. [Google Scholar] [CrossRef]
- Jan Kole, P.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Lin, P.; Gao, Y.; Erkens, S. Insight into the Compatibility Behaviors between Various Rejuvenators and Aged Bitumen: Molecular Dynamics Simulation and Experimental Validation. Mater. Des. 2022, 223, 111141. [Google Scholar] [CrossRef]
- Hofko, B.; Alavi, M.Z.; Grothe, H.; Jones, D.; Harvey, J. Repeatability and Sensitivity of FTIR ATR Spectral Analysis Methods for Bituminous Binders. Mater. Struct./Mater. Constr. 2017, 50, 187. [Google Scholar] [CrossRef]
- Durrieu, F.; Farcas, F.; Mouillet, V. The Influence of UV Aging of a Styrene/Butadiene/Styrene Modified Bitumen: Comparison between Laboratory and on Site Aging. Fuel 2007, 86, 1446–1451. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Lin, P.; Jing, R.; Erkens, S. Toward the Long-Term Aging Influence and Novel Reaction Kinetics Models of Bitumen. Int. J. Pavement Eng. 2022, 24, 2024188. [Google Scholar] [CrossRef]
- Caputo, P.; Loise, V.; Ashimova, S.; Teltayev, B.; Vaiana, R.; Oliviero Rossi, C. Inverse Laplace Transform (ILT)NMR: A Powerful Tool to Differentiate a Real Rejuvenator and a Softener of Aged Bitumen. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 154–161. [Google Scholar] [CrossRef]
- He, X.; Hochstein, D.; Ge, Q.; Ali, A.W.; Chen, F.; Yin, H. Accelerated Aging of Asphalt by UV Photo-Oxidation Considering Moisture and Condensation Effects. J. Mater. Civil. Eng. 2018, 30, 04017261. [Google Scholar] [CrossRef]
- Yu, X.; Zaumanis, M.; Dos Santos, S.; Poulikakos, L.D. Rheological, Microscopic, and Chemical Characterization of the Rejuvenating Effect on Asphalt Binders. Fuel 2014, 135, 162–171. [Google Scholar] [CrossRef]
- Abe, A.A.; Rossi, C.O.; Eskandarsefat, S.; Porto, M.; Loise, V.; Venturini, L.; Caputo, P. Reclaimed Asphalt Recycling Agents: Looking into the Blueprint of Their Mechanisms of Action. Constr. Build. Mater. 2023, 363, 129843. [Google Scholar] [CrossRef]
- Hu, Y.; Si, W.; Kang, X.; Xue, Y.; Wang, H.; Parry, T.; Airey, G.D. State of the Art: Multiscale Evaluation of Bitumen Ageing Behaviour. Fuel 2022, 326, 125045. [Google Scholar] [CrossRef]
- Mazumder, M.; Ahmed, R.; Wajahat Ali, A.; Lee, S.J. SEM and ESEM Techniques Used for Analysis of Asphalt Binder and Mixture: A State of the Art Review. Constr. Build. Mater. 2018, 186, 313–329. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, K.; Han, S.; Liang, Z.; Kong, W.; Zhan, X. Method for the Evaluation of the Homogeneity of Asphalt Mixtures by 2-Dimensional Image Analysis. Materials 2022, 15, 4265. [Google Scholar] [CrossRef]
- Lamontagne, J.; Dumas, P.; Mouillet, V.; Kister, J. Comparison by Fourier Transform Infrared (FTIR) Spectroscopy of Different Ageing Techniques: Application to Road Bitumens. Fuel 2001, 80, 483–488. [Google Scholar] [CrossRef]
- Mayes, Z.G.; Rice, W.H.; Chi, L.; Woelk, K. A Robust Freeman-Hill-Inspired Pulse Protocol for Ringdown-Free T1 Relaxation Measurements. J. Magn. Reson. 2023, 352, 107490. [Google Scholar] [CrossRef]
- Keeler, J. Chapter 8. In Understanding NMR Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- OChemOnline Infrared Spectroscopy Absorption Table. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Reference_Tables/Infrared_Spectroscopy_Absorption_Table (accessed on 14 February 2024).
- MilliporeSigma IR Spectrum Table. Available online: https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 14 February 2024).



| Property | TPO |
|---|---|
| Production Temp (°F) | 400 |
| Flash Point (°C) | 130 |
| Viscosity (cP) | 53 |
| Sample | Unaged Grade (°C) | RTFOT Grade (°C) | High PG (°C) | Intermediate PG (°C) |
|---|---|---|---|---|
| 0% TPO | 64 | 64 | 64 | 22 |
| 2% TPO | 64 | 64 | 64 | 22 |
| 4% TPO | 64 | 64 | 64 | 22 |
| 8% TPO | 64 | 58 | 58 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herndon, R.M.; Balasubramanian, J.; Woelk, K.; Abdelrahman, M. Physical and Chemical Methods to Assess Performance of TPO-Modified Asphalt Binder. Appl. Sci. 2024, 14, 3300. https://doi.org/10.3390/app14083300
Herndon RM, Balasubramanian J, Woelk K, Abdelrahman M. Physical and Chemical Methods to Assess Performance of TPO-Modified Asphalt Binder. Applied Sciences. 2024; 14(8):3300. https://doi.org/10.3390/app14083300
Chicago/Turabian StyleHerndon, Rebecca M., Jay Balasubramanian, Klaus Woelk, and Magdy Abdelrahman. 2024. "Physical and Chemical Methods to Assess Performance of TPO-Modified Asphalt Binder" Applied Sciences 14, no. 8: 3300. https://doi.org/10.3390/app14083300





