Effects of Pulsed Electric Field on Oil Extraction Rate and Tocopherol in Peony Seeds
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
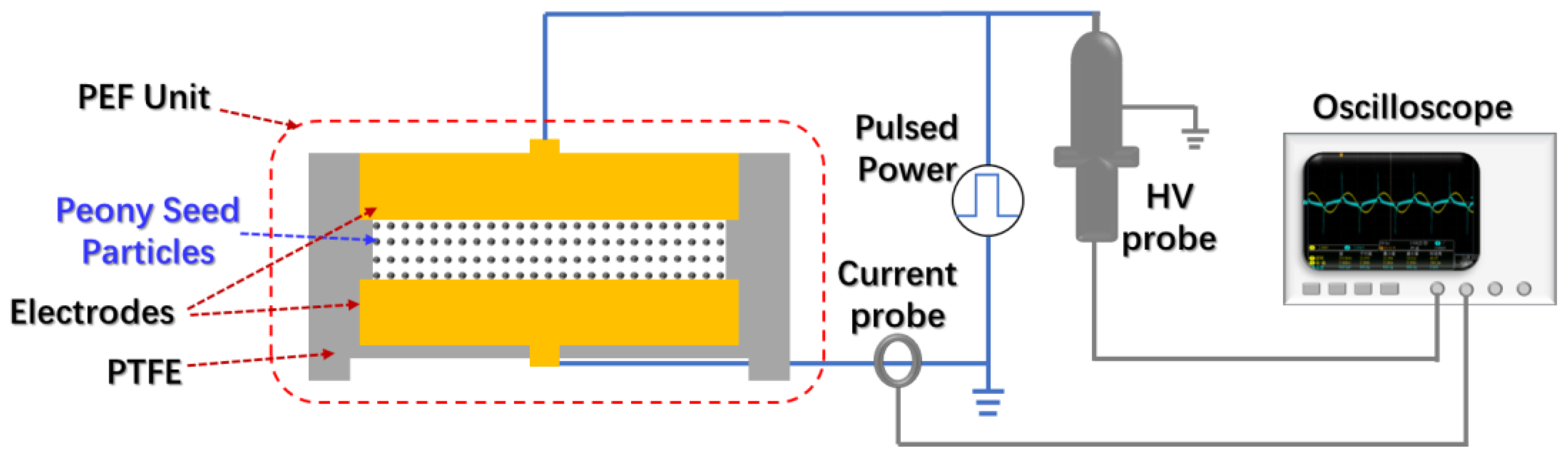
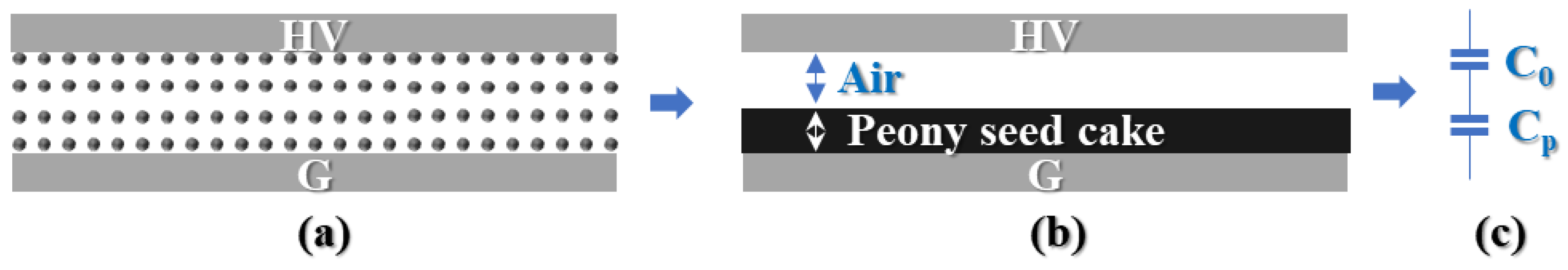
2.1. Pulsed Electric Field System
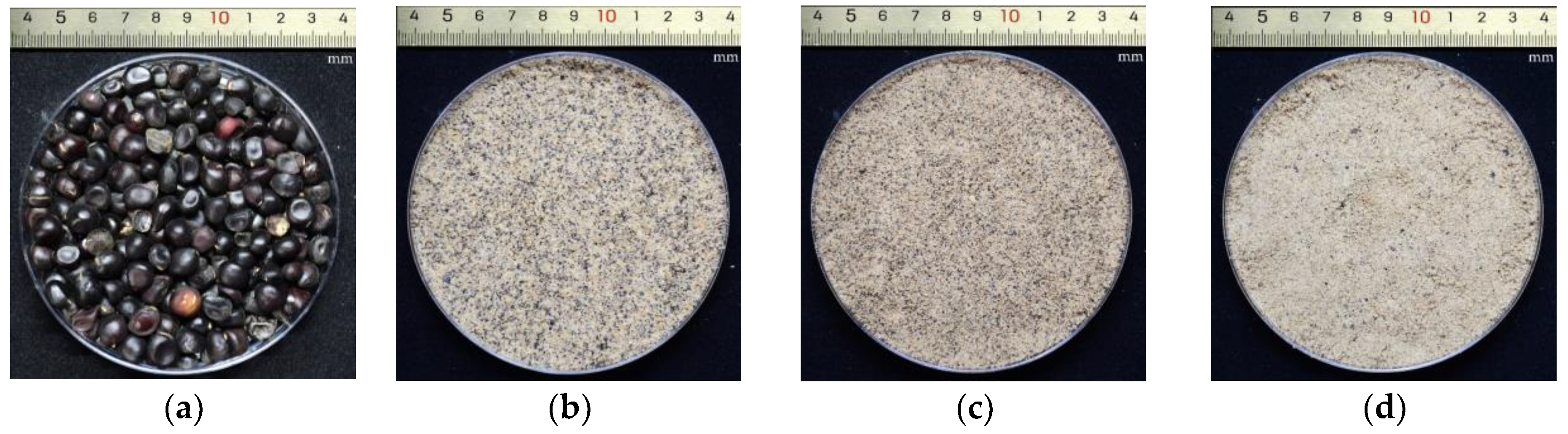
2.2. Peony Seed Sample Preparation
2.3. Peony Seed Oil Extraction
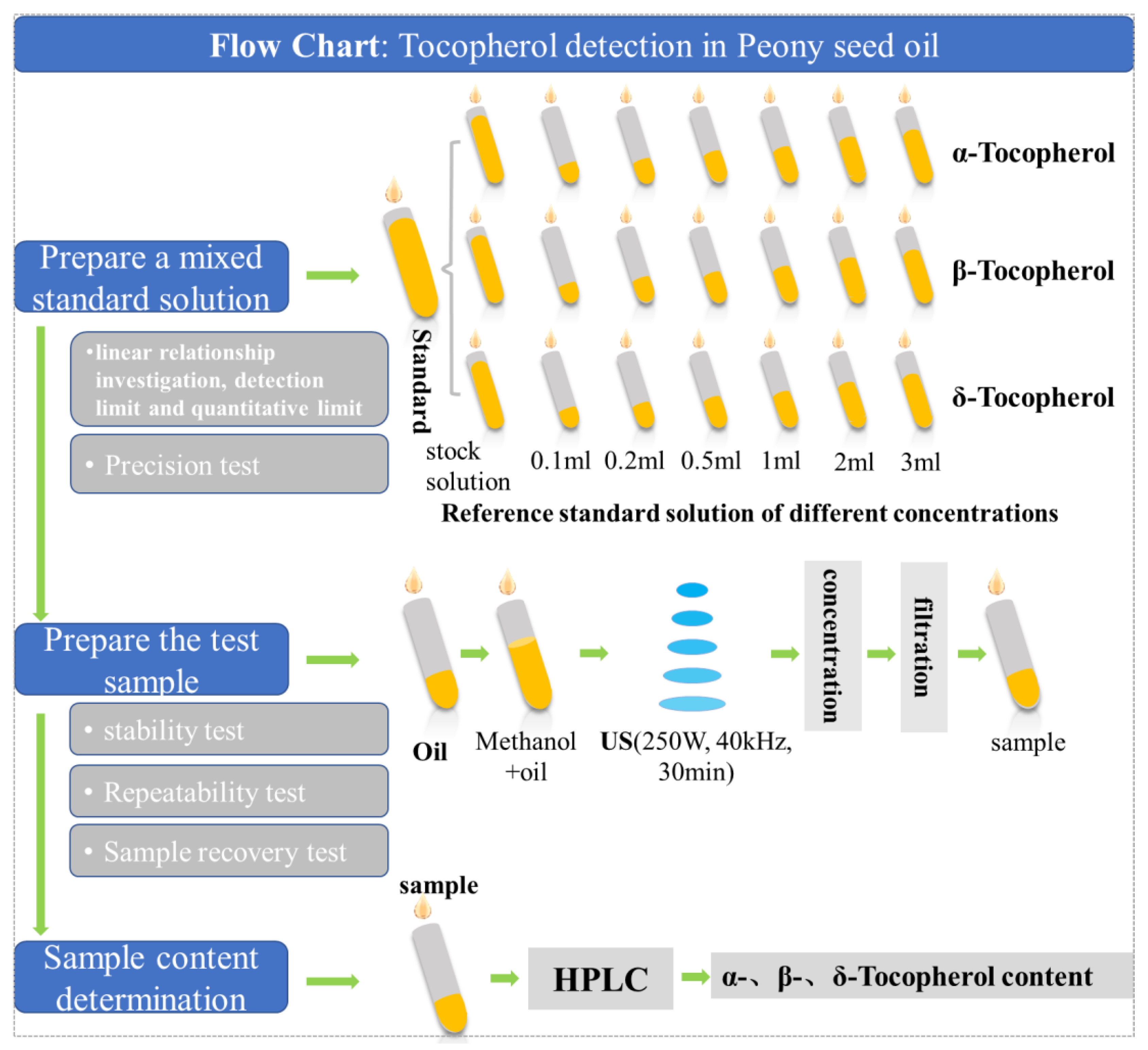
2.4. Tocopherol Detection in Peony Seed Oil
3. Results and Discussion
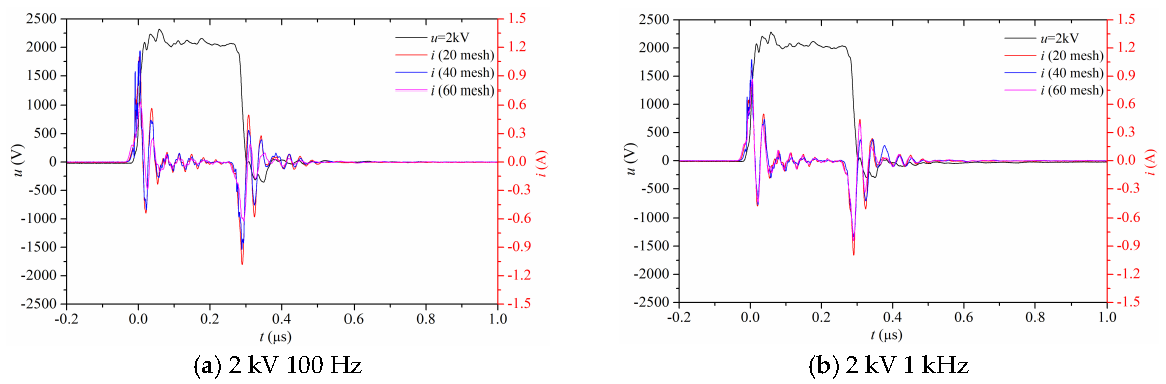
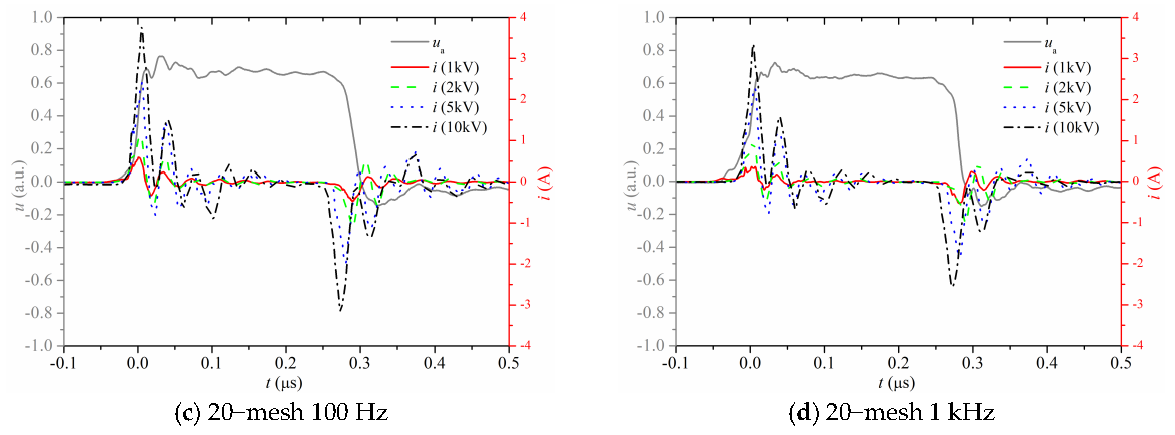
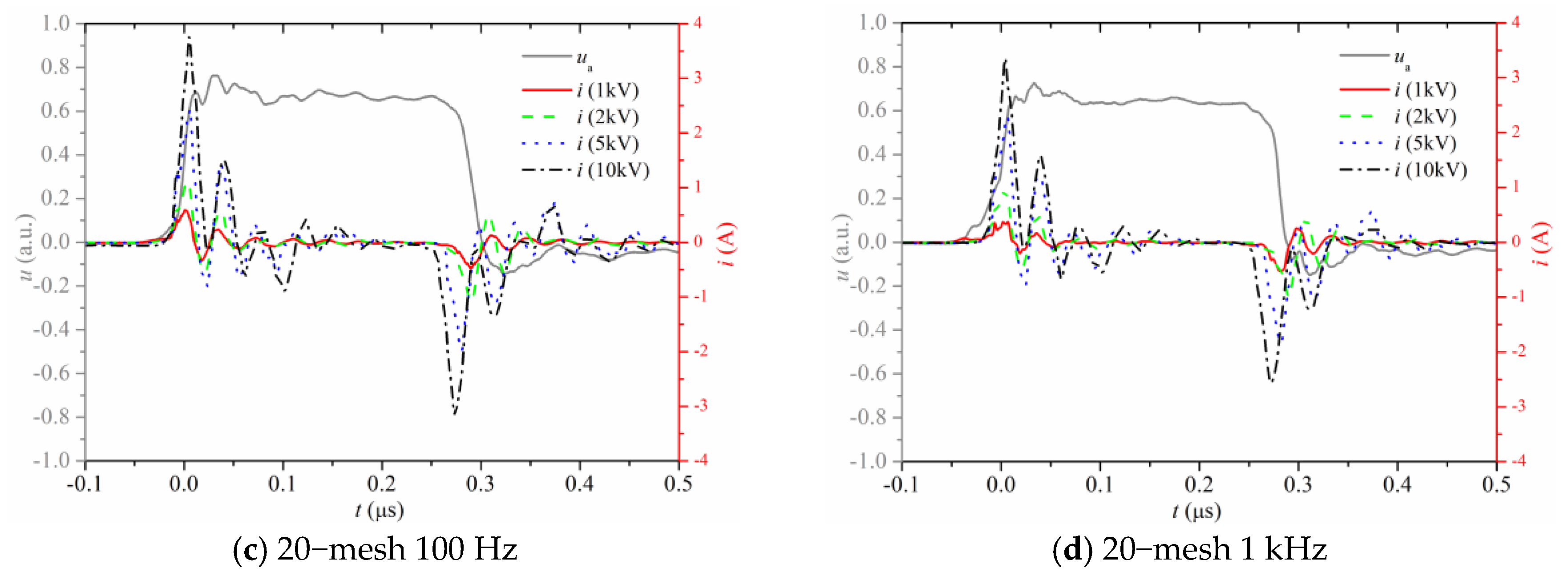
3.1. Electrical Properties
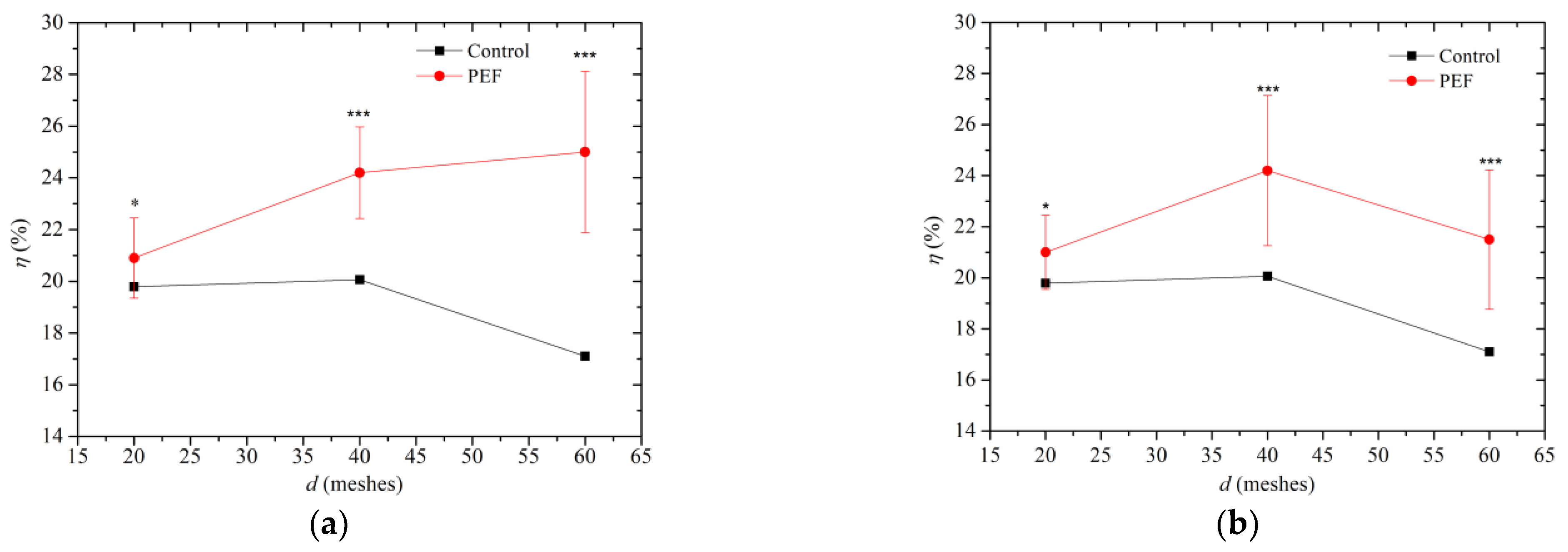
3.2. Oil Content
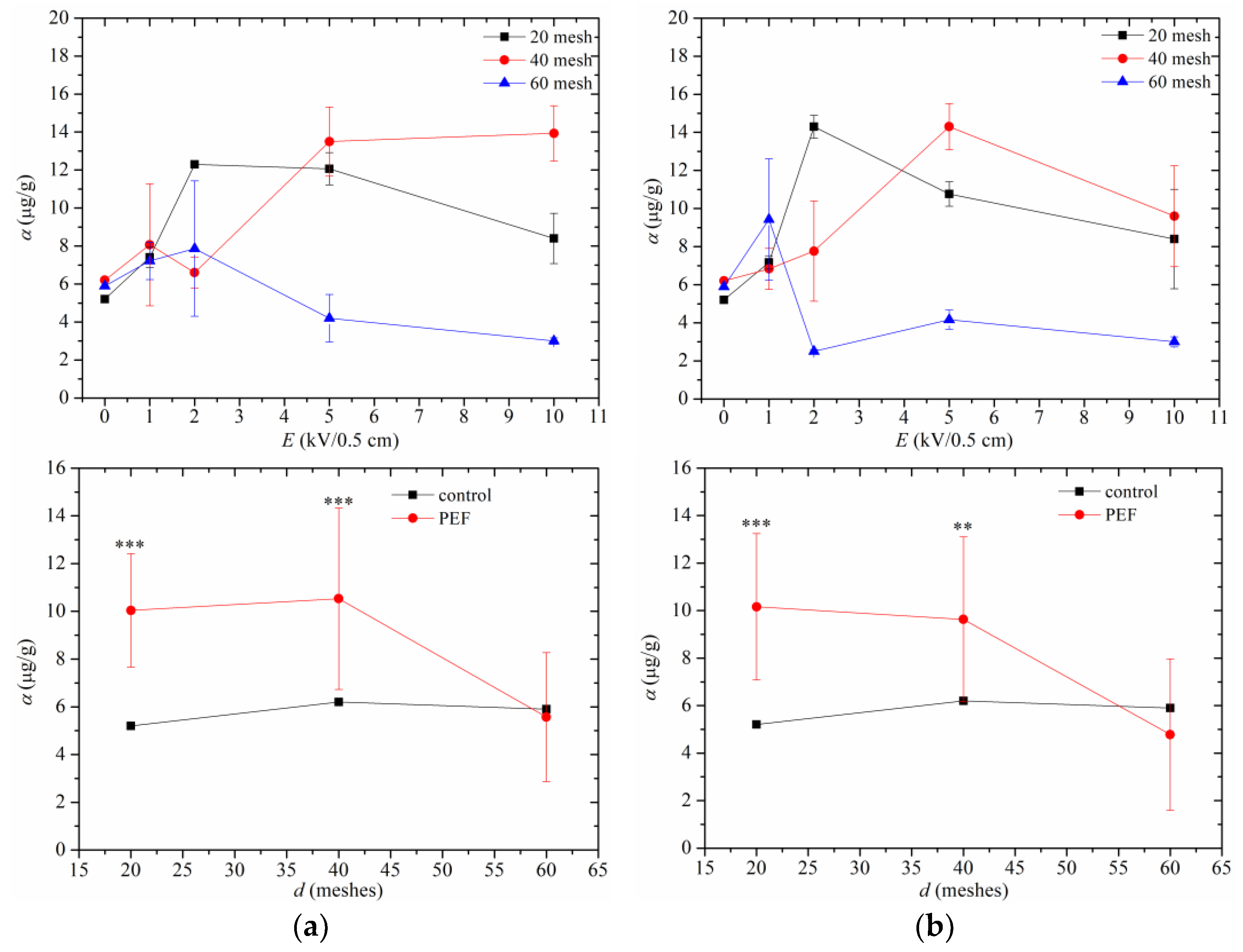
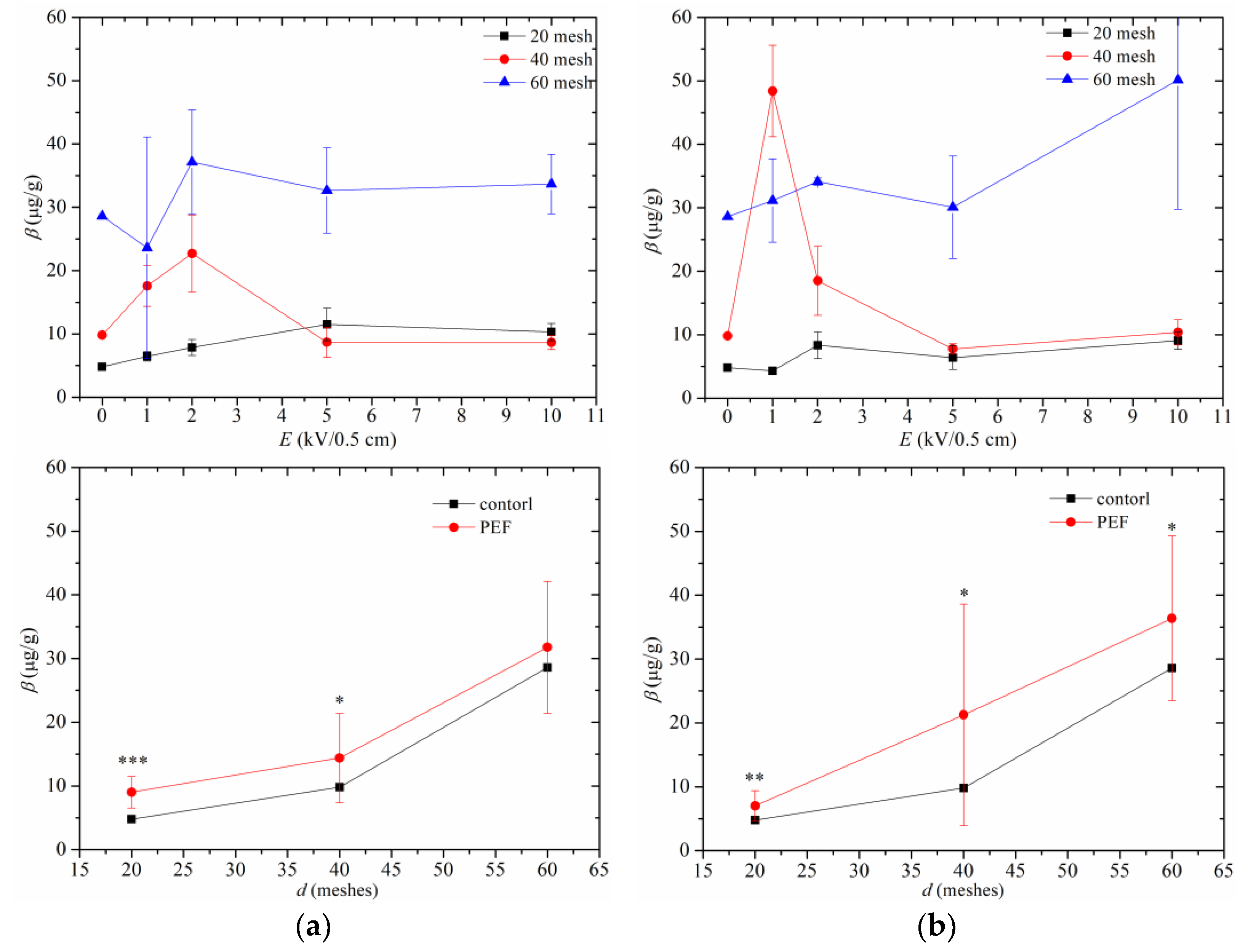
3.3. Tocopherol in Peony Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Tocopherol Detection in Peony Seed Oil
- (1)
- Preparation of mixed standard solution. The α-tocopherol control product, β/γ-tocopherol control product and δ-tocopherol control product were accurately weighed and placed in 25 mL brown volumetric bottle, respectively, dissolved with methanol, and fixed to the scale line. The concentration of α-tocopherol control solution was 0.425 mg·mL−1, the concentration of β/γ-tocopherol control solution was 0.1 mg·mL−1, and the concentration of δ-tocopherol control solution was 0.1 mg·mL−1. We precisely absorbed 0.1 mL, 0.2 mL, 0.5 mL, 1.0 mL, 2 mL and 3 mL of each reference solution into a 25 mL volumetric bottle, filled it with methanol to the scale line, and shook well as a mixed reference solution with different concentrations.
- (2)
- Preparation of test samples. The appropriate amount of peony seed oil extracted from different samples was taken, and 0.1 g was taken from each sample. As for the graduated centrifuge tube, methanol was used as the extraction agent, the ratio of solid to liquid was 1:1.2 (g/mL), ultrasonic extraction (power 250 W, frequency 40 kHz) was carried out for 30 min, and the methanol extract was extracted twice. After concentration by nitrogen blowing instrument, the volume of peony seed oil was fixed with methanol to 0.15 mL. The sample was filtrated, then the further filtrated liquid was taken, and then obtain the sample liquid to be measured.
- (3)
- Determination of samples. High performance liquid chromatography (HPLC) was performed by AgiLent1260, including high pressure four-element pump (G1311A), column temperature chamber (AT-950) and diode array detector (G1315D), and Agilent ZORBAX Eclipse Plus C18 column (4.6 mm× 250 mm, 5 µm) was selected. The volume ratio of mobile phase methanol to water was 98:2, the volume flow rate was 1.0 mL·min−1, the column temperature was 35 °C, the detection wavelength of DAD detector was 295 nm, and the sample size was 20 μL. All components could be separated well under the chromatographic conditions. We precisely absorbed the mixed reference solution in step (1) and the sample solution to be measured in step (2), and, respectively, injected the samples for determination according to the chromatographic conditions in step (3). The liquid chromatographic spectra of several tocopherols in the mixed reference product and the sample to be measured are shown in Figure A1.
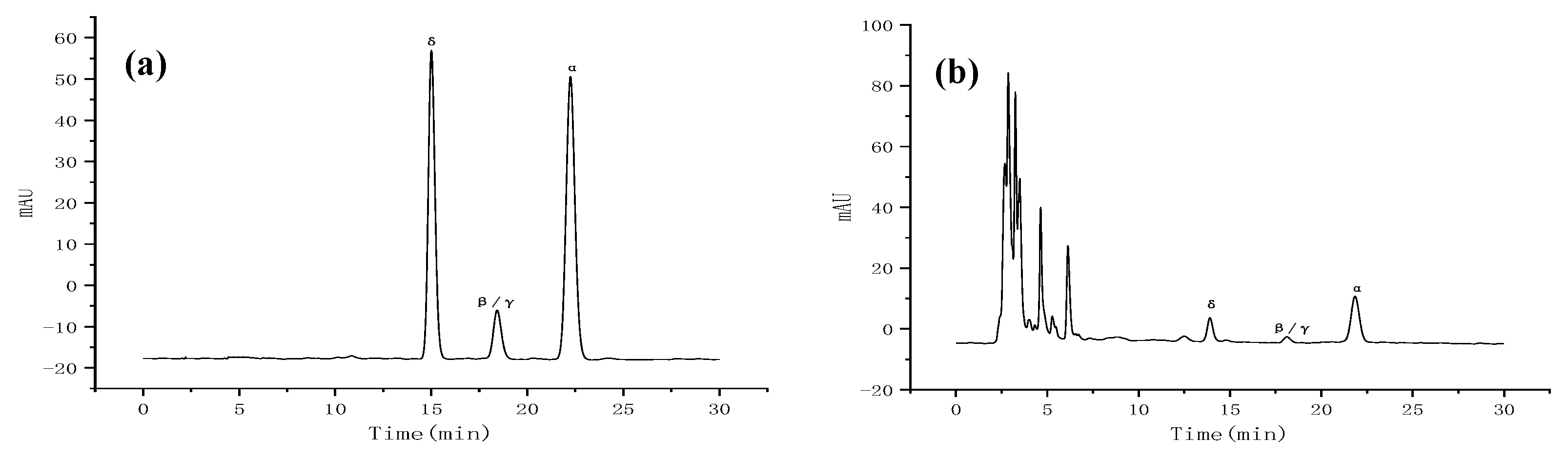
- (4)
- Investigation of linear relationship, determination of detection limit and quantitative limit. The mixed reference solution (20 μL each) prepared in step (1) was precision absorbed and injected into the liquid chromatograph. The peak areas of α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol were measured according to the chromatographic conditions in (3). The peak areas were repeated 3 times, and the average values were taken. The standard curve was calculated with injection concentration X as the horizontal coordinate and peak area and Y as the vertical coordinate. When the signal-to-noise ratio (S/N) is 3, the concentration of the measured substance is the limit of detection, and when the signal-to-noise ratio (S/N) is 10, the concentration of the measured substance is the limit of quantification. The results are listed in Table A1. From Table A1, it can be seen that each component has a good linear relationship within its own range.
| Tocopherol | Linear Equation | R | Linearity Range/μg·mL−1 | LOD (Limit of Detection)/μg | LOQ (Limit of Quantitation)/μg |
|---|---|---|---|---|---|
| α | y = 6102.3x − 2.8202 | R = 0.9999 | 2.65–212 | 0.18 | 0.54 |
| β/γ | y = 11,459x − 0.625 | R = 0.9997 | 6.25–100 | 0.24 | 0.72 |
| δ | y = 33,365x − 6.0833 | R = 0.9999 | 6.25–100 | 0.27 | 0.82 |
- (5)
- Precision test. A total of 20 μL of α-tocopherol, β-tocopherol, delta-tocopherol mixed reference solution was accurately absorbed, and the samples were continuously injected for 6 times according to the chromatographic conditions in step (3). The peak area was recorded, and the RSD value of the peak area of each compound was calculated. The results are shown in Table A2.
| Number of Times | α-Tocopherol/A | β-Tocopherol/A | δ-Tocopherol/A |
|---|---|---|---|
| 1st | 736 | 613 | 805 |
| 2nd | 728 | 602 | 813 |
| 3rd | 738 | 616 | 814 |
| 4th | 739 | 634 | 818 |
| 5th | 748 | 624 | 812 |
| 6th | 716 | 602 | 804 |
| RSD (%) | 1.49 | 2.03 | 0.67 |
- (6)
- Stability test. The same sample solution was taken and placed at room temperature, and the peak areas of α-tocopherol, β-tocopherol and δ-tocopherol were injected at 0, 2, 4, 6, 8, and 24 h, respectively. The peak areas were recorded, and the RSD values of α-tocopherol, β-tocopherol and δ-tocopherol were calculated, respectively. The results are shown in Table A3. The RSD% of the 7 indexes was less than 3.0%, indicating that the solution was stable within 24 h after preparation and had no significant effect on the determination results.
| Time | α-Tocopherol/A | β-Tocopherol/A | δ-Tocopherol/A |
|---|---|---|---|
| 0 h | 106.2 | 32.3 | 159.7 |
| 2 h | 102.1 | 32.1 | 159.0 |
| 4 h | 109.7 | 31.0 | 154.6 |
| 6 h | 110.2 | 32.7 | 156.0 |
| 8 h | 105.3 | 32.1 | 161.2 |
| 24 h | 107.5 | 32.0 | 160.0 |
| RSD (%) | 2.81 | 1.75 | 1.58 |
- (7)
- Repeatability test. The test samples were prepared according to the above-mentioned preparation method, and 6 samples were taken in parallel to prepare the test product solution with a certain concentration. According to the chromatographic conditions described in (4), the peak areas of α-tocopherol, β/γ-tocopherol and δ-tocopherol were, respectively, injected to determine the peak areas of α-tocopherol, β-tocopherol and δ-tocopherol, which were recorded, and the RSD values of the peak areas of α-tocopherol, β-tocopherol and δ-tocopherol were calculated, respectively. The results are shown in Table A4. The RSD of α-tocopherol, β-tocopherol and δ-tocopherol were 1.97%, 0.67% and 0.35%, respectively, and RSD% of the peak area of the three components was less than 3.0%, indicating good reproducibility of the method.
| Samples | α-Tocopherol/A | β-Tocopherol/A | β-Tocopherol/A |
|---|---|---|---|
| 1st | 18.7 | 103.0 | 169.0 |
| 2nd | 18.2 | 104.0 | 168.3 |
| 3rd | 18.3 | 103.5 | 167.8 |
| 4th | 19.0 | 103.7 | 168.0 |
| 5th | 19.1 | 102.6 | 169.2 |
| 6th | 18.5 | 102.2 | 169.0 |
| RSD (%) | 1.97 | 0.67 | 0.35 |
- (8)
- Sample recovery experiment. The appropriate amount of the test sample was accurately weighed and added to the volumetric bottle, 6 parts of each index component were parallel, and a certain amount of α-tocopherol, β-tocopherol, δ-tocopherol control products were added to the corresponding volumetric bottle. According to the above-mentioned preparation methods, the test samples with added recovery rates were prepared, respectively, and the results were shown in Table A5. It can be seen from Table A5 that the recoveries were between 96.36% and 99.93% after standard substances were added with different proportions, and the recoveries of the three index components were RSDS% < 3.0%, indicating a good recovery rate of the test method.
| Ingredient | Sample Content/mg | Addition/mg | Measured Amount/mg | Sample Recovery/% | Average Recovery Rate/% | RSD/% |
|---|---|---|---|---|---|---|
| α-tocopherol | 0.122 | 0.10 | 0.219 | 98.65 | 98.57 | 1.15 |
| 0.123 | 0.10 | 0.221 | 99.10 | |||
| 0.120 | 0.10 | 0.212 | 96.36 | |||
| 0.124 | 0.10 | 0.222 | 99.11 | |||
| 0.121 | 0.10 | 0.218 | 98.64 | |||
| 0.123 | 0.10 | 0.222 | 99.55 | |||
| β-tocopherol | 0.0762 | 0.07 | 0.1458 | 99.43 | 99.33 | 0.17 |
| 0.0815 | 0.07 | 0.1512 | 99.57 | |||
| 0.0682 | 0.07 | 0.1376 | 99.14 | |||
| 0.0716 | 0.07 | 0.1412 | 99.43 | |||
| 0.0626 | 0.07 | 0.1321 | 99.28 | |||
| 0.0738 | 0.07 | 0.1432 | 99.14 | |||
| δ-tocopherol | 0.0325 | 0.03 | 0.0622 | 99.00 | 99.72 | 0.36 |
| 0.0326 | 0.03 | 0.0626 | 99.93 | |||
| 0.0312 | 0.03 | 0.0612 | 99.90 | |||
| 0.0324 | 0.03 | 0.0623 | 99.87 | |||
| 0.0335 | 0.03 | 0.0634 | 99.80 | |||
| 0.0325 | 0.03 | 0.0624 | 99.83 |
- (9)
- Sample content determination. The appropriate amount of peony seed oil (about 0.1 g) of the above-mentioned prepared samples was moved into a graduated centrifuge tube. The samples were extracted for 30 min by using ultrasonic extraction (power 250 W, frequency 40 kHz) in where methanol as the extraction agent, and the materials-liquid ratio of 1:1.2 (g/mL). The above extraction process was proceeded twice. The extraction liquid after traction twice were combined and concentrated with nitrogen blowing apparatus, and then fixed volume with methanol to 0.15 mL. The samples were filtered as a sample liquid to be tested. The sample liquid was absorbed to be measured according to the chromatographic conditions described in step (3). The peak area of the target peak was recorded, and the content of α-tocopherol, β-tocopherol and δ-tocopherol in the sample were calculated.
References
- GBT 40622-2021; Peony Seed Oil. Standardization Administration of China: Beijing, China, 2021.
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Romaniello, R.; Juliano, P.; Tamborrino, A. Use of a mixing-coil heat exchanger combined with microwave and ultrasound technology in an olive oil extraction process. Innov. Food Sci. Emerg. Technol. 2018, 50, 66–72. [Google Scholar] [CrossRef]
- Iqdiam, B.M.; Abuagela, M.O.; Marshall, S.M.; Yagiz, Y.; Goodrich-Schneider, R.; Baker, G.L., IV; Welt, B.A.; Marshall, M.R. Combining high power ultrasound pre-treatment with malaxation oxygen control to improve quantity and quality of extra virgin olive oil. J. Food Eng. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Tamborrino, A.; Romaniello, R.; Caponio, F.; Squeo, G.; Leone, A. Combined industrial olive oil extraction plant using ultrasounds, microwave, and heat exchange: Impact on olive oil quality and yield. J. Food Eng. 2019, 245, 124–130. [Google Scholar] [CrossRef]
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical characterization of virgin olive oil obtained using an ultrasound-assisted extraction at an industrial scale: Influence of olive maturity index and malaxation time. Food Chem. 2019, 289, 7–15. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Zagaria, R.; Sabella, E.; Romaniello, R. Plant innovation in the olive oil extraction process: A comparison of efficiency and energy consumption between microwave treatment and traditional malaxation of olive pastes. J. Food Eng. 2015, 146, 44–52. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Tamborrino, A.; Xu, X.-Q.; Juliano, P. Microwave and megasonics combined technology for a continuous olive oil process with enhanced extractability. Innov. Food Sci. Emerg. Technol. 2017, 42, 56–63. [Google Scholar] [CrossRef]
- Amarillo, M.; Pérez, N.; Blasina, F.; Gambaro, A.; Leone, A.; Romaniello, R.; Xu, X.-Q.; Juliano, P. Impact of sound attenuation on ultrasound-driven yield improvements during olive oil extraction. Ultrason. Sonochem. 2019, 53, 142–151. [Google Scholar] [CrossRef]
- Tamborrino, A.; Urbani, S.; Servili, M.; Romaniello, R.; Perone, C.; Leone, A. Pulsed Electric Fields for the Treatment of Olive Pastes in the Oil Extraction Process. Appl. Sci. 2020, 10, 114. [Google Scholar] [CrossRef]
- Knorr, D.; Ade-Omowaye, B.I.O.; Heinz, V. Nutritional improvement of plant foods by non-thermal processing. Proc. Nutr. Soc. 2009, 61, 311–318. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, W.; Lu, W.; Zhang, G.; Chang, Z. Structure optimization of pulsed electric field processor and its sterilization effect. Food Qual. Saf. 2024, 8, fyad044. [Google Scholar] [CrossRef]
- Han, S.-F.; Jin, W.; Yang, Q.; El-Fatah Abomohra, A.; Zhou, X.; Tu, R.; Chen, C.; Xie, G.-J.; Wang, Q. Application of pulse electric field pretreatment for enhancing lipid extraction from Chlorella pyrenoidosa grown in wastewater. Renew. Energy 2019, 133, 233–239. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Eissa, A.H.A. Pulsed Electric Fields for Food Processing Technology. In Structure and Function of Food Engineering; InTech: Houston, TX, USA, 2012. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Silve, A.; Papachristou, I.; Wüstner, R.; Sträßner, R.; Schirmer, M.; Leber, K.; Guo, B.; Interrante, L.; Posten, C.; Frey, W. Extraction of lipids from wet microalga Auxenochlorella protothecoides using pulsed electric field treatment and ethanol-hexane blends. Algal Res. 2018, 29, 212–222. [Google Scholar] [CrossRef]
- Guderjan, M.; Elez-Martínez, P.; Knorr, D. Application of pulsed electric fields at oil yield and content of functional food ingredients at the production of rapeseed oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 55–62. [Google Scholar] [CrossRef]
- Puértolas, E.; Koubaa, M.; Barba, F.J. An overview of the impact of electrotechnologies for the recovery of oil and high-value compounds from vegetable oil industry: Energy and economic cost implications. Food Res. Int. 2016, 80, 19–26. [Google Scholar] [CrossRef]
- Shorstkii, I.; Mirshekarloo, M.S.; Koshevoi, E. Application of Pulsed Electric Field for Oil Extraction from Sunflower Seeds: Electrical Parameter Effects on Oil Yield. J. Food Process Eng. 2015, 40, e12281. [Google Scholar] [CrossRef]
- Haji-Moradkhani, A.; Rezaei, R.; Moghimi, M. Optimization of pulsed electric field-assisted oil extraction from cannabis seeds. J. Food Process Eng. 2019, 42, e13028. [Google Scholar] [CrossRef]
- Rábago-Panduro, L.M.; Morales-de la Peña, M.; Martín-Belloso, O.; Welti-Chanes, J. Application of Pulsed Electric Fields PEF on Pecan Nuts Carya illinoinensis Wangenh. K. Koch: Oil Extraction Yield and Compositional Characteristics of the Oil and Its By-product. Food Eng. Rev. 2021, 13, 676–685. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Esposto, S.; Berardi, A.; Servili, M. Investigation on the Effects of a Pulsed Electric Field (PEF) Continuous System Implemented in an Industrial Olive Oil Plant. Foods 2022, 11, 2758. [Google Scholar] [CrossRef]
- Tamborrino, A.; Mescia, L.; Taticchi, A.; Berardi, A.; Lamacchia, C.M.; Leone, A.; Servili, M. Continuous pulsed electric field pilot plant for olive oil extraction process. Innov. Food Sci. Emerg. Technol. 2022, 82, 103192. [Google Scholar] [CrossRef]
- Martínez-Beamonte, R.; Ripalda, M.; Herrero-Continente, T.; Barranquero, C.; Dávalos, A.; López de las Hazas, M.C.; Álvarez-Lanzarote, I.; Sánchez-Gimeno, A.C.; Raso, J.; Arnal, C.; et al. Pulsed electric field increases the extraction yield of extra virgin olive oil without loss of its biological properties. Front. Nutr. 2022, 9, 1065543. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.-L.; Wang, X.-Y.; He, X.-D.; Qian, J.-Y. Safety Assessment of Canola Oil Extracted by Aid of Pulsed Electric Field: Genetic, Acute and Subacute Toxicity. J. Oleo Sci. 2022, 71, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Mazroei Seydani, L.; Gharachorloo, M.; Asadi, G. Use of pulsed electric field to extract rapeseed oil and investigation of the qualitative properties of oils. J. Food Process Eng. 2022, 45, e14149. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Calcio Gaudino, E.; Binello, A.; Rego, D.; Pereira, M.; Martínez, M.; Cravotto, G. Combined Ultrasound and Pulsed Electric Fields in Continuous-Flow Industrial Olive-Oil Production. Foods 2022, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Andreou, V.; Kourmbeti, E.; Dimopoulos, G.; Psarianos, M.; Katsaros, G.; Taoukis, P. Optimization of Virgin Olive Oil Yield and Quality Applying Nonthermal Processing. Food Bioprocess Technol. 2022, 15, 891–903. [Google Scholar] [CrossRef]
- Li, N.; Fan, C.; Li, X.; Long, L.; Cao, X. Optimization of determination method and analysis on tocopherol content in edible vegetable oils. J. Food Sci. Technol. 2015, 33, 59–63. [Google Scholar]
- Li, H. Study on the Preparation of High Content of Natural α-Tocopherol. Master’s Thesis, Jiangnan University, Wuxi, China, 2013. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Liu, J.; Tian, Y.; Liu, J.; Chang, Z. Effects of Pulsed Electric Field on Oil Extraction Rate and Tocopherol in Peony Seeds. Appl. Sci. 2024, 14, 3299. https://doi.org/10.3390/app14083299
Xu W, Liu J, Tian Y, Liu J, Chang Z. Effects of Pulsed Electric Field on Oil Extraction Rate and Tocopherol in Peony Seeds. Applied Sciences. 2024; 14(8):3299. https://doi.org/10.3390/app14083299
Chicago/Turabian StyleXu, Wei, Jianfei Liu, Ye Tian, Junchi Liu, and Zhengshi Chang. 2024. "Effects of Pulsed Electric Field on Oil Extraction Rate and Tocopherol in Peony Seeds" Applied Sciences 14, no. 8: 3299. https://doi.org/10.3390/app14083299
APA StyleXu, W., Liu, J., Tian, Y., Liu, J., & Chang, Z. (2024). Effects of Pulsed Electric Field on Oil Extraction Rate and Tocopherol in Peony Seeds. Applied Sciences, 14(8), 3299. https://doi.org/10.3390/app14083299






