Surface Chemistry Study of Normal and Diseased Human Meibum Films Prior to and after Supplementation with Tear Mimetic Eyedrop Formulation
Abstract
1. Introduction
2. Materials and Methods
3. Results
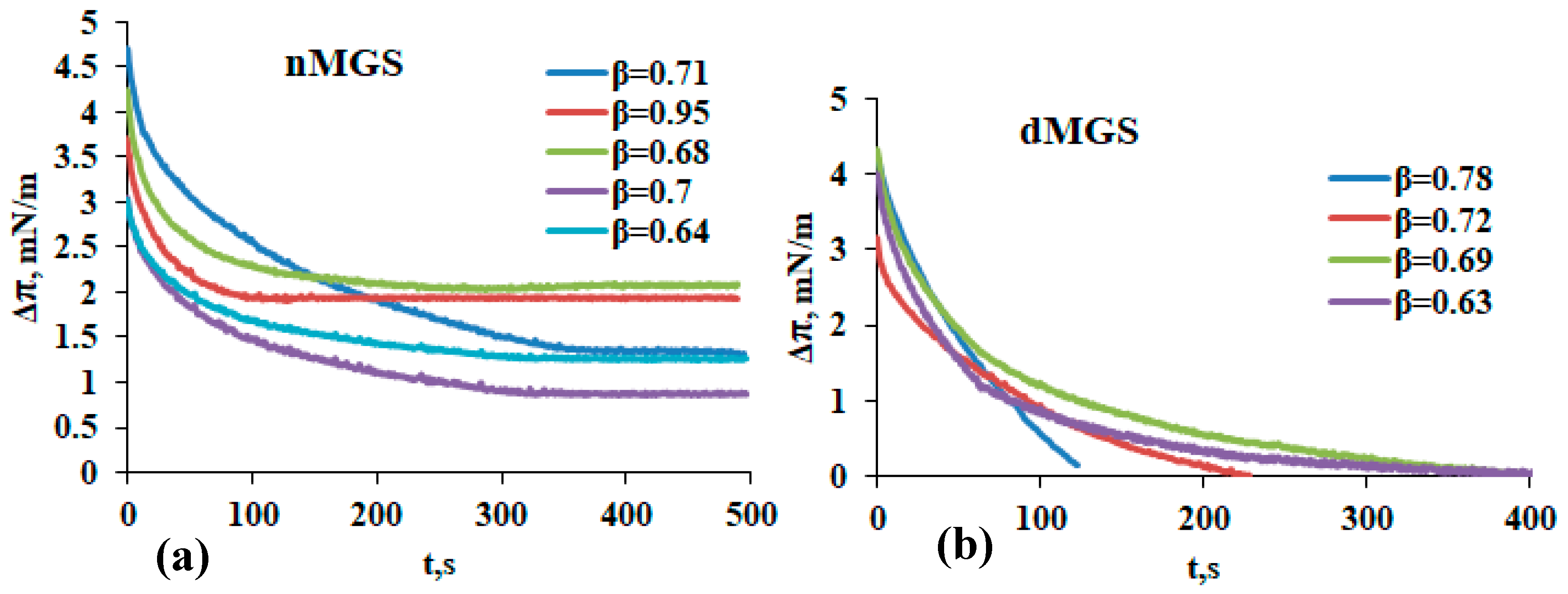
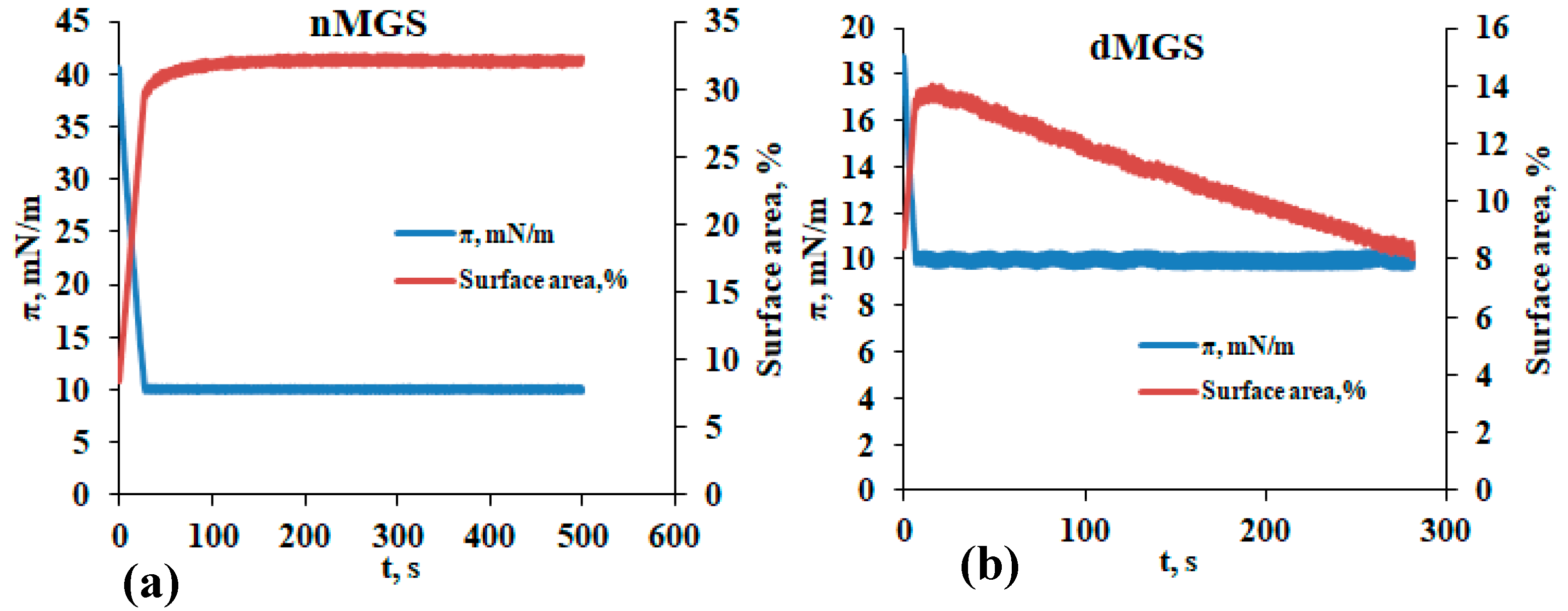
3.1. Surface Pressure Area/Isocycles of nMGS and dMGS Films and Their Morphology as Visualized by Brewster Angle Microscopy
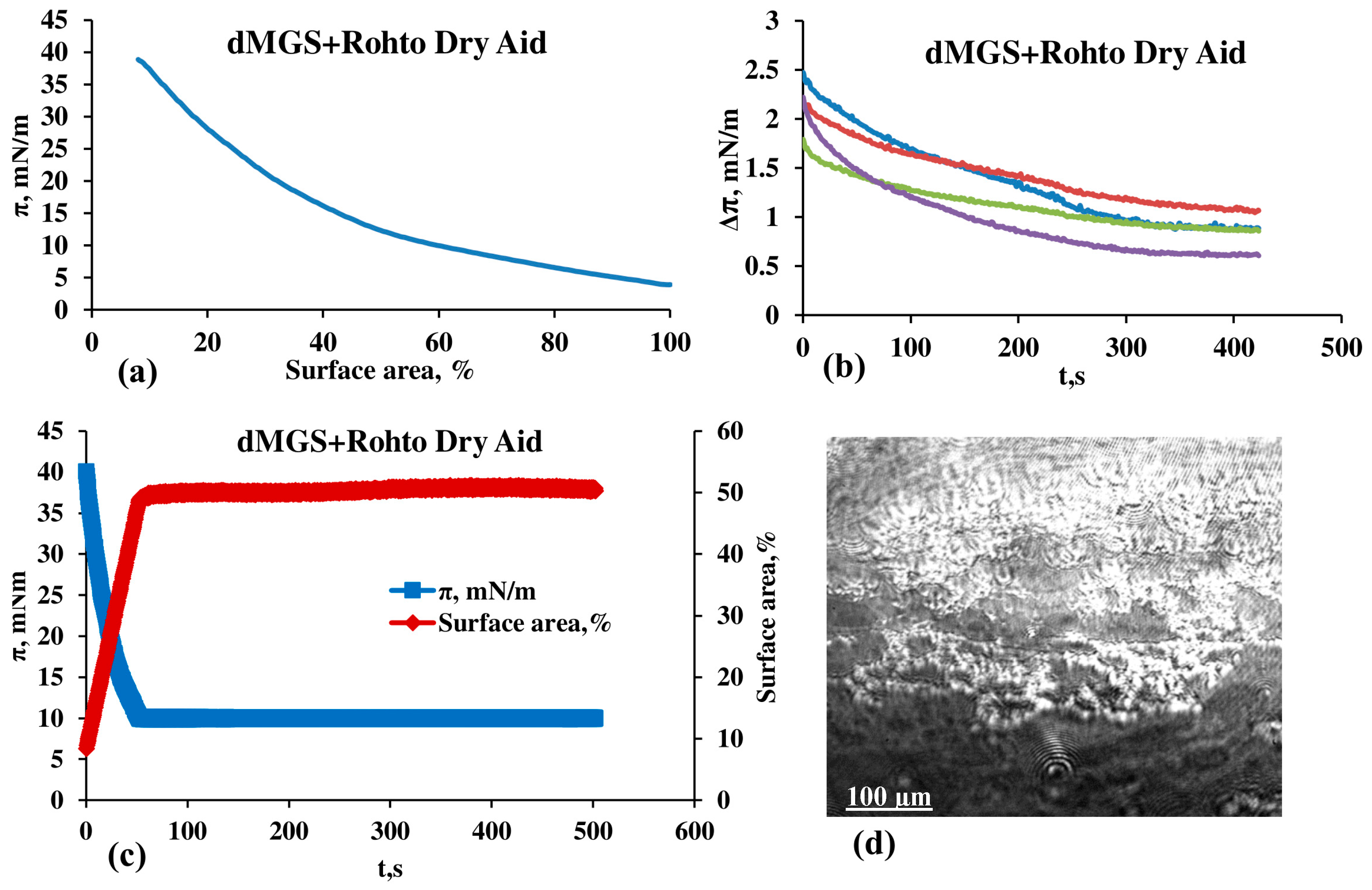
3.2. Effect of Rohto Dry Aid on dMGS Films
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | Aqueous tears |
| BAM | Brewster angle microscopy |
| MGS | Meibomian gland secretion (or simply meibum) |
| nMGS | Meibum from healthy individuals |
| dMGS | Meibum from MGD individuals |
| MGD | Meibomian gland disfunction (or disease) |
| CO-10 | Polyoxyethylene Castor Oil 10 |
| MGD | Meibomian gland disease |
| MNT | Menthol |
| MYS-40 | Polyoxyl 40 stearate |
| NPL | Nonpolar lipids |
| OP | Oil phase |
| PL | Polar lipids |
| RDA | Rohto Dry Aid |
| SO | Sesame oil |
| TF | Tear film |
| TFLL | Tear film lipid layer |
| π | Surface pressure |
| Cs−1 | Reciprocal compressibility (or rigidity) modulus |
| A | Surface film area |
| Δπ | Transient increase of surface pressure |
| Am | Amplitude of the stress relaxation transient |
| t | Time |
| τ | Characteristic relaxation time |
| β | Stretched exponential |
| ΔπEQ | Equilibrium increase of surface pressure after completion of the relaxation |
References
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017, 163, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human Meibum Lipid Conformation and Thermodynamic Changes with Meibomian-Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A. Tear film lipids. Exp. Eye Res. 2013, 117, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Tonchev, V.; Nencheva, Y.; Krastev, R. Surface relaxations as a tool to distinguish the dynamic interfacial properties of films formed by normal and diseased meibomian lipids. Soft Matter 2014, 10, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, L.; Cerretani, C.; Leiske, D.L.; Toney, M.F.; Radke, C.J.; Fuller, G.G. Structural and Rheological Properties of Meibomian Lipid. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2720–2732. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Donthineni, P.R.; Kammari, P.; Shanbhag, S.S.; Singh, V.; Das, A.V.; Basu, S. Incidence, demographics, types and risk factors of dry eye disease in India: Electronic medical records driven big data analytics report I. Ocul. Surf. 2019, 17, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Eftimov, P.; Yokoi, N.; Georgiev, G.A. Surface Chemistry Study of the Interactions of Sesame Oil with Meibomian Films. Molecules 2022, 27, 464. [Google Scholar] [CrossRef]
- Torrent-Burgués, J. Tear Film Constituents and Medicines for Eyes Investigated as Langmuir Films. BioNanoScience 2023, 13, 1324–1338. [Google Scholar] [CrossRef]
- Torrent-Burgués, J. Langmuir films study on lipid-containing artificial tears. Colloids Surf. B Biointerfaces 2016, 140, 185–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eftimov, P.; Yokoi, N.; Tsuji, K.; Peev, N.; Georgiev, G.A. Langmuir Trough Study of the Interactions of Tear Mimetic Eyedrop Formulation with Human Meibum Films. Appl. Sci. 2022, 12, 12095. [Google Scholar] [CrossRef]
- Mudgil, P.; Millar, T.J. Surfactant Properties of Human Meibomian Lipids. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena; Academic Press: Cambridge, MA, USA, 1961; p. 64. [Google Scholar]
- Tsang, K.Y.; Ngai, K.L. Relaxation in interacting arrays of oscillators. Phys. Rev. E 1996, 54, R3067–R3070. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.; Minkov, I.; Panaiotov, I.; Saulnier, P.; Proust, J.E. Dilatational properties and morphology of surface films spread from clinically used lung surfactants. Colloid Polym. Sci. 2004, 282, 1258–1267. [Google Scholar] [CrossRef]
- Wüstneck, R.; Enders, P.; Wüstneck, N.; Pison, U.; Miller, R.; Vollhardt, D. Surface dilational behaviour of spread dipalmitoyl phosphatidyl glycerol monolayers. PhysChemComm 1999, 2, 50–61. [Google Scholar] [CrossRef]
- Wüstneck, R.; Wüstneck, N.; Grigoriev, D.O.; Pison, U.; Miller, R. Stress relaxation behaviour of dipalmitoyl phosphatidylcholine monolayers spread on the surface of a pendant drop. Colloids Surf. B Biointerfaces 1999, 15, 275–288. [Google Scholar] [CrossRef]
- Dimitrov, D.S.; Panaiotov, I.; Richmond, P.; Ter-Minassian-Saraga, L. Dynamics of insoluble monolayers: I. Dilatational or elastic modulus, friction coefficient, and Marangoni effect for dipalmitoyl lecithin monolayers. J. Colloid Interface Sci. 1978, 65, 483–494. [Google Scholar] [CrossRef]
- Panaiotov, I.; Dimitrov, D.S.; Ter-Minassian-Saraga, L. Dynamics of insoluble monolayers: II. Viscoelastic behavior and marangoni effect for mixed protein phospholipid films. J. Colloid Interface Sci. 1979, 72, 49–53. [Google Scholar] [CrossRef]
- Tiffany, J.M.; Winter, N.; Bliss, G. Tear film stability and tear surface tension. Curr. Eye Res. 1989, 8, 507–515. [Google Scholar] [CrossRef]
- Goto, E.; Tseng, S.C. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch. Ophthalmol. 2003, 121, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Baron, D.F.; Herman, J.P.; Finnemore, V.M.; Exford, J.M.; Hermosa, J.L.; Leahy, C.D.; Glonek, T.; Greiner, J.V. Tear film lipid layer thickness as a function of blinking. Cornea 1994, 13, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.G.; Thompson, J.M.; Rahman, I.B.; Ellis, R.E.; Green, E.M.; Miano, F.; Winlove, C.P. Two-dimensional order in mammalian pre-ocular tear film. Exp. Eye Res. 2007, 84, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Smaby, J.M.; Brockman, H.L. Novel surface phase containing cholesteryl esters. 2. Nonequivalence of cholesteryl arachidonate and those with 18-carbon, cis-unsaturated acyl groups. Biochemistry 1981, 20, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Yamada, M.; Hatou, S.; Tsubota, K. Turnover rate of tear-film lipid layer determined by fluorophotometry. Br. J. Ophthalmol. 2009, 93, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Bron, A.J.; Georgiev, G.A. The Precorneal Tear Film as a Fluid Shell: The Effect of Blinking and Saccades on Tear Film Distribution and Dynamics. Ocul. Surf. 2014, 12, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Yamada, H.; Mizukusa, Y.; Bron, A.J.; Tiffany, J.M.; Kato, T.; Kinoshita, S. Rheology of tear film lipid layer spread in normal and aqueous tear-deficient dry eyes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5319–5324. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Trokel, S.; Arieli, Y.; Epshtien, S.; Gefen, R.; Harris, A. Mapping the Lipid Layer of the Human Tear Film. Cornea 2020, 39, 132–135. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; Lu, H.; Butovich, I.A. The spectrophotometric sulfo-phospho-vanillin assessment of total lipids in human meibomian gland secretions. Lipids 2013, 48, 513–525. [Google Scholar] [CrossRef]
- Weisenberger, K.; Fogt, N.; Fogt, J.S. Comparison of nanoemulsion and non-emollient artificial tears on tear lipid layer thickness and symptoms. J. Optom. 2021, 14, 20–27. [Google Scholar] [CrossRef]
- Frey, S.L.; Lee, K.Y. Temperature dependence of poloxamer insertion into and squeeze-out from lipid monolayers. Langmuir 2007, 23, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Morishige, N.; Sakamoto, I.; Imai, N.; Shimada, Y.; Igaki, M.; Suzuki, A.; Itoh, K.; Tsubota, K. Effects of a warm compress containing menthol on the tear film in healthy subjects and dry eye patients. Sci. Rep. 2017, 7, 45848. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.I.; Dervichian, D.G. The Oils of the Meibomian Glands: Physical and Surface Characteristics. Arch. Ophthalmol. 1969, 82, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Arshinoff, S.A.; Hofmann, I.; Nae, H. Role of rheology in tears and artificial tears. J. Cataract. Refract. Surg. 2021, 47, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Arshinoff, S.; Hofmann, I.; Nae, H. Rheological behavior of commercial artificial tear solutions. J. Cataract. Refract. Surg. 2021, 47, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Mehdaoui, H.; Ait Abderrahmane, H.; de Loubens, C.; Nait Bouda, F.; Hamani, S. Dynamics of a Gel-Based Artificial Tear Film with an Emphasis on Dry Disease Treatment Applications. Gels 2021, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Martinez, A.; Mateo-Orobia, A.; Blasco-Alberto, J.; Pablo-Julvez, L. Rheological Behavior Patterns in Artificial Tears. Optom. Vis. Sci. 2022, 99, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Recchioni, A.; Mocciardini, E.; Ponzini, E.; Tavazzi, S. Viscoelastic properties of the human tear film. Exp Eye Res. 2022, 219, 109083. [Google Scholar] [CrossRef]
- Fatt, I. Observations of tear film break up on model eyes. CLAO J. Off. Publ. Contact Lens Assoc. Ophthalmol. Inc. 1991, 17, 267–281. [Google Scholar]
- McDonnell, A.; Lee, J.H.; Makrai, E.; Yeo, L.Y.; Downie, L.E. Tear Film Extensional Viscosity Is a Novel Potential Biomarker of Dry Eye Disease. Ophthalmology 2019, 126, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.Y.; Chen, A.; Fang, P.C.; Yu, H.J.; Kuo, M.T. Comparing Tear Film Viscosity between Sjögren and Non-Sjögren Dry Eye Disease. Life 2023, 13, 1484. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Ross, M.; Fahmy, K.; McEwen, B.; Hofmann, I.; Chan, V.W.Y.; Clark-Baba, C.; Jones, L. Evaluating Viscosity and Tear Breakup Time of Contemporary Commercial Ocular Lubricants on an In Vitro Eye Model. Transl. Vis. Sci. Technol. 2023, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-based ocular drug delivery systems: Recent advances and future prospects. J. Nanobiotechnology 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Dhahir, R.K.; Al-Nima, A.M.; Al-Bazzaz, F.Y. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turk. J. Pharm. Sci. 2021, 18, 652–664. [Google Scholar] [CrossRef]
- Lv, Z.; Li, S.; Zeng, Z.; Yao, K.; Han, H. Recent progress of nanomedicine in managing dry eye disease. Adv. Ophthalmol. Pract. Res. 2024, 4, 24–33. [Google Scholar] [CrossRef]


| Active Ingredients * | Oil Phase ** (9.12 × 10−3 M) |
|---|---|
| Povidone (3.96 × 10−5 M), Propylene Glycol (3.9 × 10−2 M) | Sesame Oil/Menthol/Polyoxyethylene 10 Castor Oil/Polyoxyl 40 Stearate/Poloxamer 407 = 1.15/0.12/3.2/4.6/0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eftimov, P.; Yokoi, N.; Tsuji, K.; Takahashi, K.; Nishiyama, M.; Peev, N.; Koeva, A.S.; Georgiev, G.A. Surface Chemistry Study of Normal and Diseased Human Meibum Films Prior to and after Supplementation with Tear Mimetic Eyedrop Formulation. Appl. Sci. 2024, 14, 3339. https://doi.org/10.3390/app14083339
Eftimov P, Yokoi N, Tsuji K, Takahashi K, Nishiyama M, Peev N, Koeva AS, Georgiev GA. Surface Chemistry Study of Normal and Diseased Human Meibum Films Prior to and after Supplementation with Tear Mimetic Eyedrop Formulation. Applied Sciences. 2024; 14(8):3339. https://doi.org/10.3390/app14083339
Chicago/Turabian StyleEftimov, Petar, Norihiko Yokoi, Kazuhiro Tsuji, Kyoko Takahashi, Miho Nishiyama, Nikola Peev, Ani S. Koeva, and Georgi As. Georgiev. 2024. "Surface Chemistry Study of Normal and Diseased Human Meibum Films Prior to and after Supplementation with Tear Mimetic Eyedrop Formulation" Applied Sciences 14, no. 8: 3339. https://doi.org/10.3390/app14083339
APA StyleEftimov, P., Yokoi, N., Tsuji, K., Takahashi, K., Nishiyama, M., Peev, N., Koeva, A. S., & Georgiev, G. A. (2024). Surface Chemistry Study of Normal and Diseased Human Meibum Films Prior to and after Supplementation with Tear Mimetic Eyedrop Formulation. Applied Sciences, 14(8), 3339. https://doi.org/10.3390/app14083339








