Influence of DMSO Non-Toxic Solvent on the Mechanical and Chemical Properties of a PVDF Thin Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
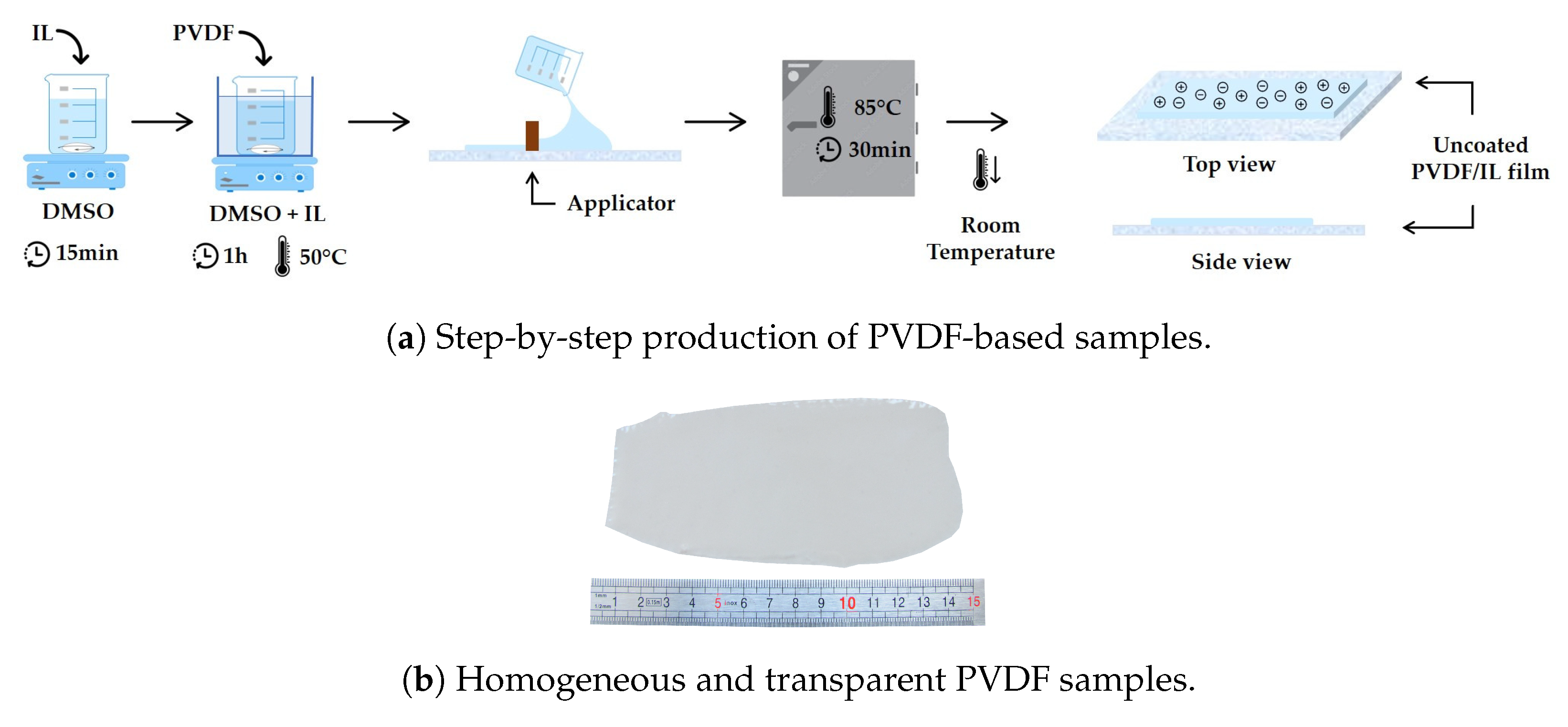
2.2. Sample Preparation
2.3. Optical Analysis
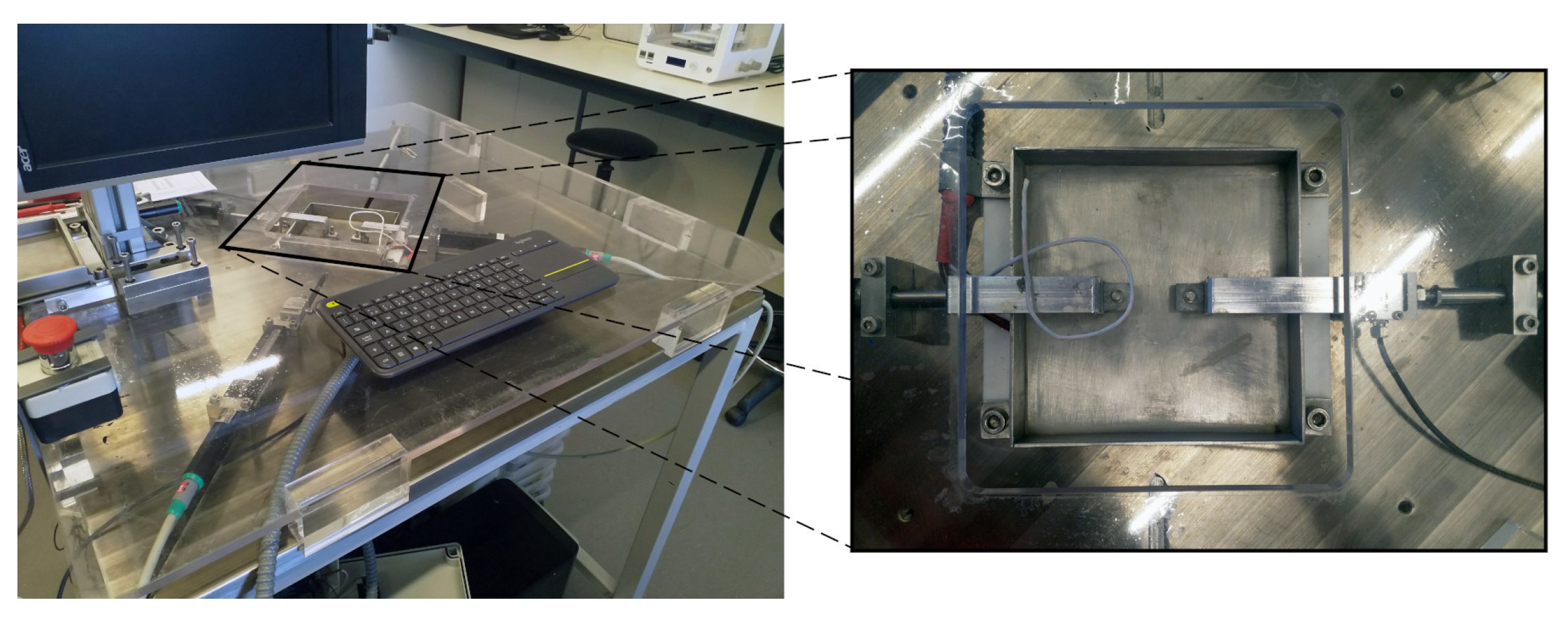
2.4. Mechanical Characterization
2.5. Chemical Characterization
3. Results and Discussion
3.1. Optical Analysis
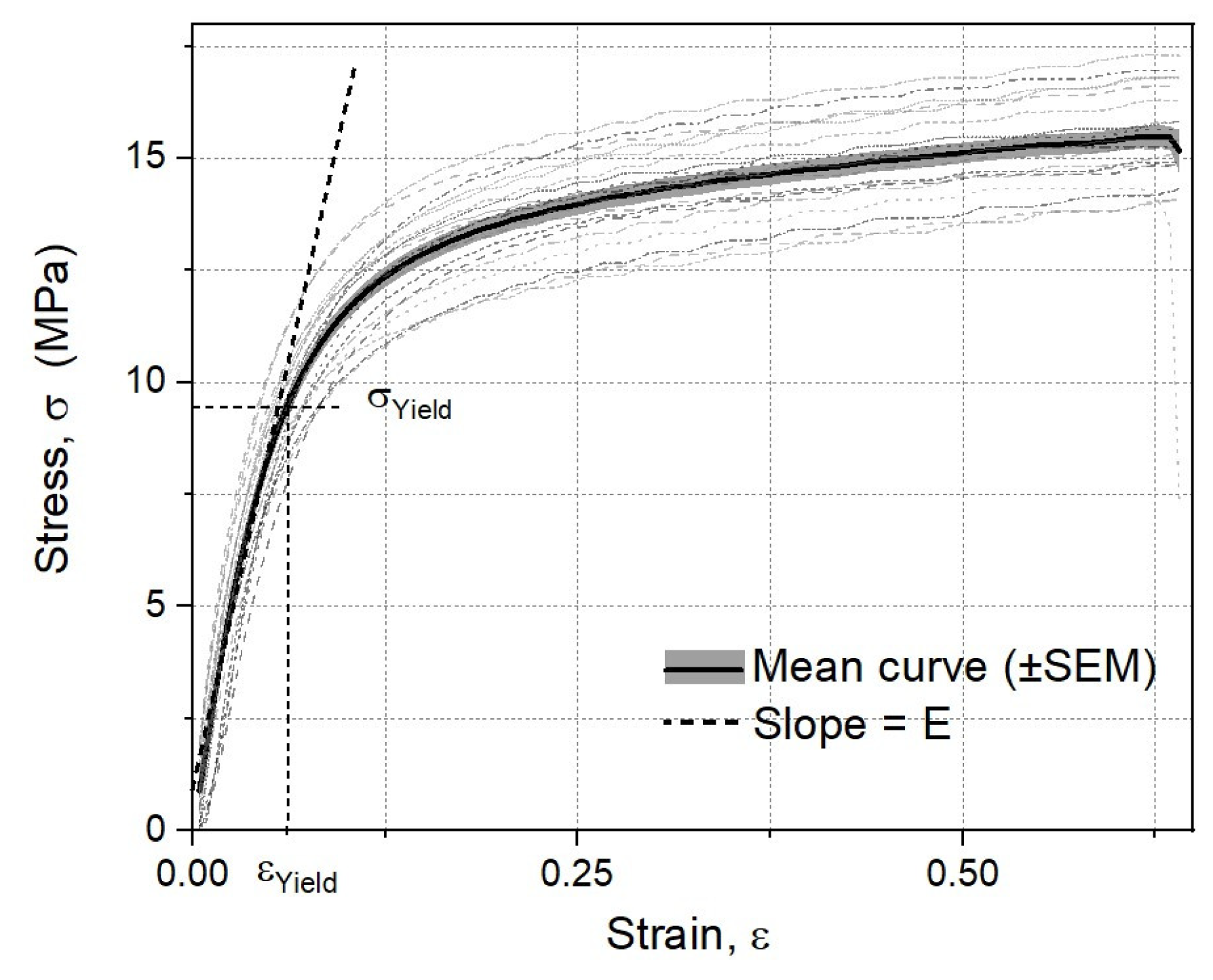
3.2. Mechanical Characterization
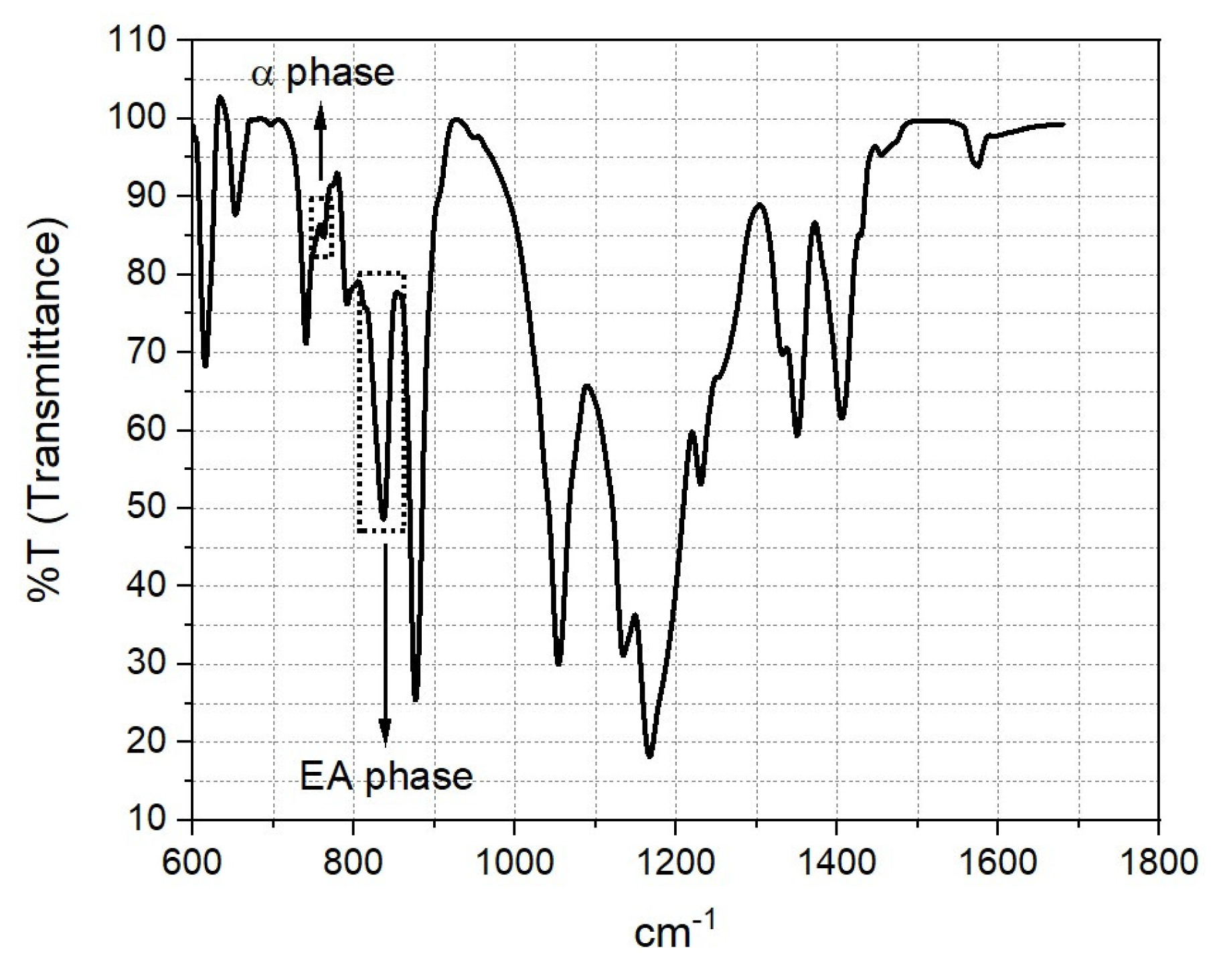
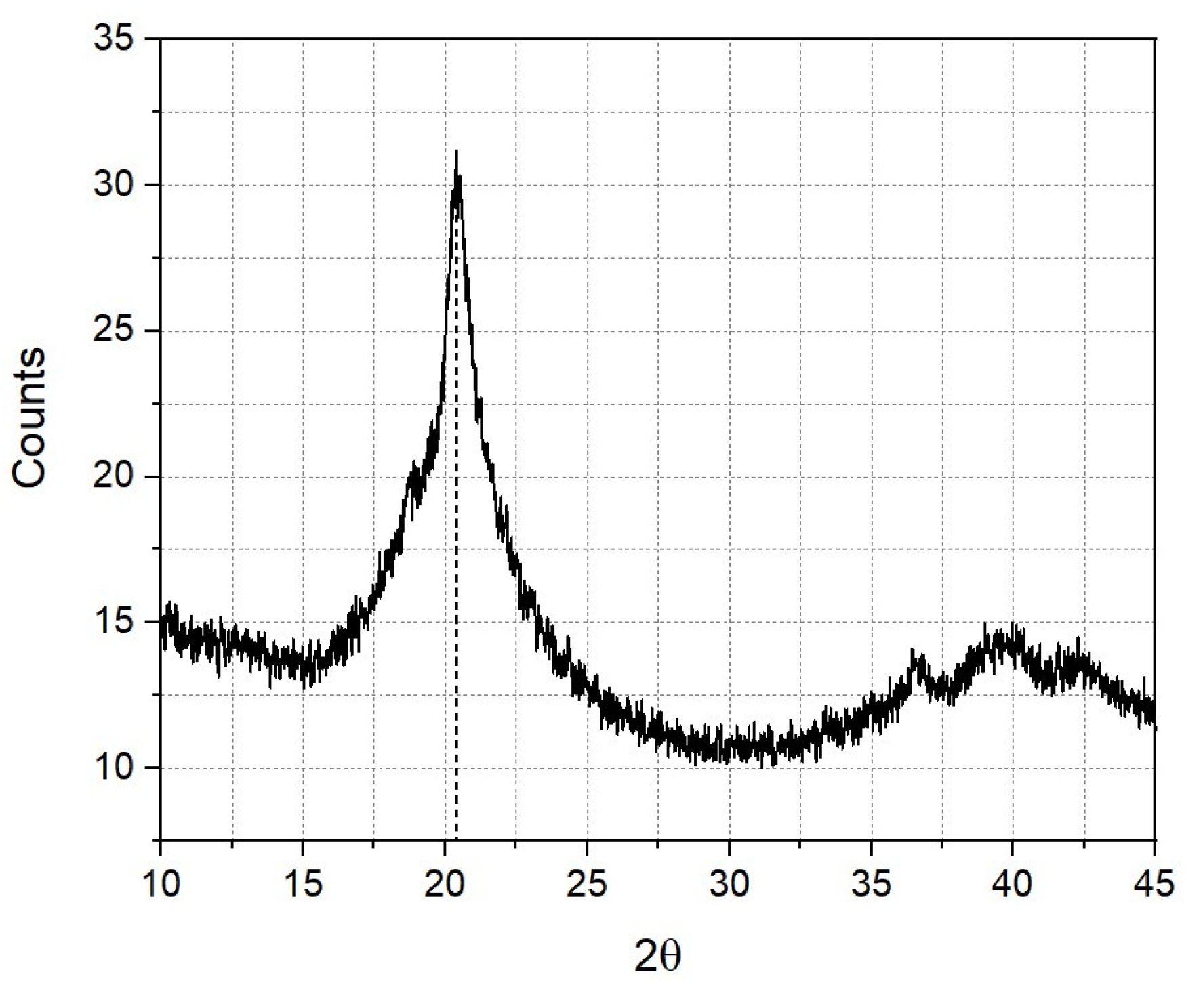
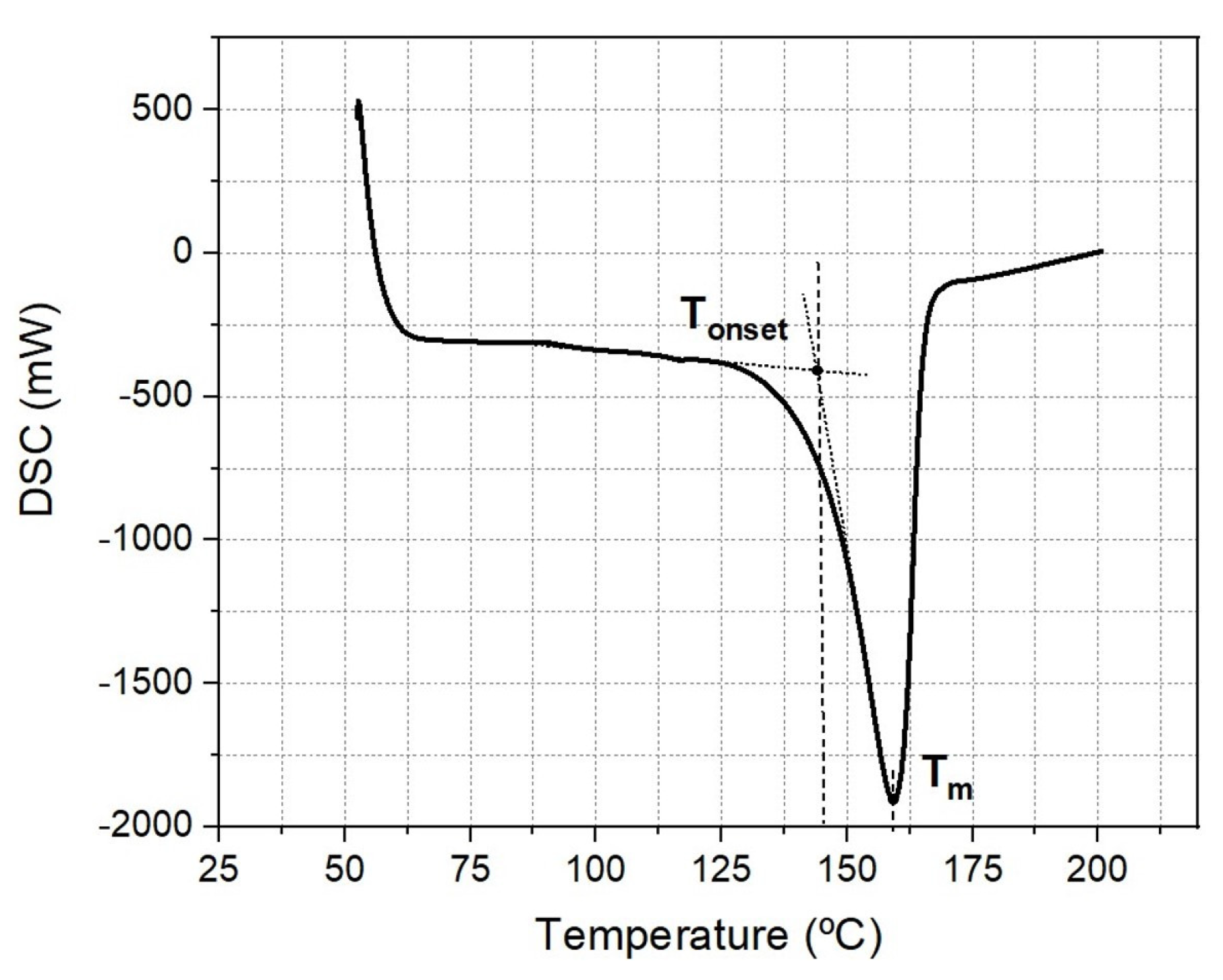
3.3. Chemical Characterization
4. Conclusions and Future Works
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMAC | Dimethylacetamide |
| DMF | Dimethylformamide |
| DMSO | Dymethilsulfoxide |
| EA | Electroactive |
| DSC | Differential scanning calorimetry |
| FEUP | Faculty of Engineering of the University of Porto |
| FTIR | Fourier-transform infrared spectroscopy |
| i3A | Aragón Institute for Engineering Research |
| IL | Ionic liquid |
| INEGI | Institute of Science and Innovation in Mechanical and Industrial Engineering |
| LAETA | Associated Laboratory of Energy, Transport and Aeronautics |
| MEK | Methyl ethyl ketone |
| MWCNTs | Multi-walled carbon nanotubes |
| PVDF | Polyvinylidene fluoride |
| SEM | Standard error of mean |
| STD | Standard deviation |
| UBS | Biomechanic and Health Unit |
| XRD | X-ray diffraction |
References
- Elahi, H.; Munir, K.; Eugeni, M.; Abrar, M.; Khan, A.; Arshad, A.; Gaudenzi, P. A Review on Applications of Piezoelectric Materials in Aerospace Industry. Integr. Ferroelectr. 2020, 211, 25–44. [Google Scholar] [CrossRef]
- Sharma, K.; Srinivas, G. Flying smart: Smart materials used in aviation industry. Mater. Today Proc. 2020, 27, 244–250. [Google Scholar] [CrossRef]
- Holman, H.; Kavarana, M.N.; Rajab, T.K. Smart materials in cardiovascular implants: Shape memory alloys and shape memory polymers. Artif. Organs 2020, 45, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C. Smart Materials for Drug Delivery, 1st ed.; Number v.2 in ISSN; Description based on publisher supplied metadata and other sources; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- André, A.; Martins, P. Soft materials for exoskeleton development. In Advances and Current Trends in Biomechanics; CRC Press: Boca Raton, FL, USA, 2021; pp. 5–8. [Google Scholar] [CrossRef]
- Wang, Q. Smart Materials for Tissue Engineering; Description based on publisher supplied metadata and other sources; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric Scaffolds as Smart Materials for Neural Tissue Engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. A Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Addington, D.M. Smart Materials and Technologies, First Issued in Hardback ed.; Routledge: New York, NY, USA, 2016. [Google Scholar]
- Akhras, G. Smart materials and smart systems for the future. Can. Mil. J. 2000, 1, 25–31. [Google Scholar]
- Bahl, S.; Nagar, H.; Singh, I.; Sehgal, S. Smart materials types, properties and applications: A review. Mater. Today Proc. 2020, 28, 1302–1306. [Google Scholar] [CrossRef]
- Bogue, R. Smart materials: A review of capabilities and applications. Assem. Autom. 2014, 34, 16–22. [Google Scholar] [CrossRef]
- Bengisu, M. Materials That Move; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- El-Atab, N.; Mishra, R.B.; Al-Modaf, F.; Joharji, L.; Alsharif, A.A.; Alamoudi, H.; Diaz, M.; Qaiser, N.; Hussain, M.M. Soft Actuators for Soft Robotic Applications: A Review. Adv. Intell. Syst. 2020, 2, 2000128. [Google Scholar] [CrossRef]
- Bilodeau, R.A.; Miriyev, A.; Lipson, H.; Kramer-Bottiglio, R. All-soft material system for strong soft actuators. In Proceedings of the 2018 IEEE International Conference on Soft Robotics (RoboSoft), Livorno, Italy, 24–28 April 2018; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2016, 29, 1603483. [Google Scholar] [CrossRef]
- Verpaalen, R.C.P.; da Cunha, M.P.; Engels, T.A.P.; Debije, M.G.; Schenning, A.P.H.J. Liquid Crystal Networks on Thermoplastics: Reprogrammable Photo-Responsive Actuators. Angew. Chem. Int. Ed. 2020, 59, 4532–4536. [Google Scholar] [CrossRef] [PubMed]
- Florea, L.; Diamond, D.; Benito-Lopez, F. Photo-Responsive Polymeric Structures Based on Spiropyran. Macromol. Mater. Eng. 2012, 297, 1148–1159. [Google Scholar] [CrossRef]
- Schwartz, M.M. (Ed.) Smart Materials; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Ju, G.; Cheng, M.; Shi, F. A pH-responsive smart surface for the continuous separation of oil/water/oil ternary mixtures. NPG Asia Mater. 2014, 6, e111. [Google Scholar] [CrossRef]
- Yang, M.; Wang, S.Q.; Liu, Z.; Chen, Y.; Zaworotko, M.J.; Cheng, P.; Ma, J.G.; Zhang, Z. Fabrication of Moisture-Responsive Crystalline Smart Materials for Water Harvesting and Electricity Transduction. J. Am. Chem. Soc. 2021, 143, 7732–7739. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, E.; Plamthottam, R.; Pei, Q. Dielectric Elastomer Artificial Muscle: Materials Innovations and Device Explorations. Accounts Chem. Res. 2019, 52, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.; Hajra, S.; Sahu, M.; Jung, S.I.; Jang, I.R.; Kim, H.J. PVDF-bismuth titanate based self-powered flexible tactile sensor for biomechanical applications. Mater. Lett. 2022, 309, 131308. [Google Scholar] [CrossRef]
- Thomas, J.; Jose, J.; Kalarikkal, N.; Thomas, S. (Eds.) Nanoparticles in Polymer Systems for Biomedical Applications; Apple Academic Press: Oakville, ON, Canada, 2019. [Google Scholar]
- Park, J.; Kim, S.J.; Kyeong, S.; Kim, J.; Na, Y. Feasibility of proportional EMG control for a hand exoskeleton: A Fitts’ Law approach. IFAC-PapersOnLine 2018, 51, 214–219. [Google Scholar] [CrossRef]
- Cardoso, V.; Correia, D.; Ribeiro, C.; Fernandes, M.; Lanceros-Méndez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef]
- Harper, C.A.; Baker, A.M.M.; Mead, J.; Wright, R.E.; Margolis, J.M.; Kattas, L.; Gastrock, F.; Levin, I.; Cacciatore, A.; Barry, C.M.F.; et al. (Eds.) Modern Plastics Handbook, 1st ed.; McGraw-Hill’s AccessEngineering, McGraw-Hill Education: New York, NY, USA, 2000. [Google Scholar]
- Bar-Cohen, Y.; Anderson, I.A. Electroactive polymer (EAP) actuators—Background review. Mech. Soft Mater. 2019, 1, 5. [Google Scholar] [CrossRef]
- Sessler, G.M. Piezoelectricity in polyvinylidenefluoride. J. Acoust. Soc. Am. 1981, 70, 1596–1608. [Google Scholar] [CrossRef]
- Sekhon, S.; Singh, H.P. Ionic conductivity of PVdF-based polymer gel electrolytes. Solid State Ionics 2002, 152–153, 169–174. [Google Scholar] [CrossRef]
- Soin, N.; Boyer, D.; Prashanthi, K.; Sharma, S.; Narasimulu, A.A.; Luo, J.; Shah, T.H.; Siores, E.; Thundat, T. Exclusive self-aligned β-phase PVDF films with abnormal piezoelectric coefficient prepared via phase inversion. Chem. Commun. 2015, 51, 8257–8260. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Barbosa, J.C.; Costa, C.M.; Reis, P.M.; Esperança, J.M.S.S.; de Zea Bermudez, V.; Lanceros-Méndez, S. Ionic Liquid Cation Size-Dependent Electromechanical Response of Ionic Liquid/Poly(vinylidene fluoride)-Based Soft Actuators. J. Phys. Chem. C 2019, 123, 12744–12752. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Lopes, A.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A. Analysis Method. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Pan, H.; Na, B.; Lv, R.; Li, C.; Zhu, J.; Yu, Z. Polar phase formation in poly(vinylidene fluoride) induced by melt annealing. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1433–1437. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Ribelles, J.L.G.; Lanceros-Méndez, S. Influence of Processing Conditions on Polymorphism and Nanofiber Morphology of Electroactive Poly(vinylidene fluoride) Electrospun Membranes. Soft Mater. 2010, 8, 274–287. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y. β-phase formation of poly(vinylidene fluoride) from the melt induced by quenching. J. Mater. Sci. Lett. 1987, 6, 599–603. [Google Scholar] [CrossRef]
- Sencadas, V.; Filho, R.G.; Lanceros-Mendez, S. Processing and characterization of a novel nonporous poly(vinilidene fluoride) films in the β phase. J. Non-Cryst. Solids 2006, 352, 2226–2229. [Google Scholar] [CrossRef]
- Rinaldo Gregorio, J.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Benz, M.; Euler, W.B.; Gregory, O.J. The Role of Solution Phase Water on the Deposition of Thin Films of Poly(vinylidene fluoride). Macromolecules 2002, 35, 2682–2688. [Google Scholar] [CrossRef]
- Zhu, H. Interfacial preparation of ferroelectric polymer nanostructures for electronic applications. Polym. J. 2021, 53, 877–886. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of Hydrophilic PVDF Membrane Using Vapour Induced Phase Separation Method for Produced Water Treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef]
- Wang, F.; Tanaka, M.; Chonan, S. Development of a PVDF Piezopolymer Sensor for Unconstrained In-Sleep Cardiorespiratory Monitoring. J. Intell. Mater. Syst. Struct. 2003, 14, 185–190. [Google Scholar] [CrossRef]
- Hu, C.; Behdinan, K.; Moradi-Dastjerdi, R. PVDF Energy Harvester for Prolonging the Battery Life of Cardiac Pacemakers. Actuators 2022, 11, 187. [Google Scholar] [CrossRef]
- Correia, D.; Fernandes, L.; Pereira, N.; Barbosa, J.; Serra, J.; Pinto, R.; Costa, C.; Lanceros-Méndez, S. All printed soft actuators based on ionic liquid/polymer hybrid materials. Appl. Mater. Today 2021, 22, 100928. [Google Scholar] [CrossRef]
- Singh, R.; Janakiraman, S.; Khalifa, M.; Anandhan, S.; Ghosh, S.; Venimadhav, A.; Biswas, K. An electroactive β-phase polyvinylidene fluoride as gel polymer electrolyte for magnesium–ion battery application. J. Electroanal. Chem. 2019, 851, 113417. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, H.; Xie, G.; Jiang, Y.; Chen, C.; Su, Y.; Wang, Y.; Tai, H. Flexible piezoelectric pressure sensor based on polydopamine-modified BaTiO3/PVDF composite film for human motion monitoring. Sens. Actuators A Phys. 2020, 301, 111789. [Google Scholar] [CrossRef]
- Aronson, J.K. Meyler’s Side Effects of Drugs The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier Science and Technology Books: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility parameters of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the Solubility and Stability of Polyvinylidene Fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Ursino, C.; Avruscio, E.; Desiderio, G.; Perrone, A.; Santoro, S.; Galiano, F.; Figoli, A. Innovative Poly (Vinylidene Fluoride) (PVDF) Electrospun Nanofiber Membrane Preparation Using DMSO as a Low Toxicity Solvent. Membranes 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dong, X.; Escobar, I.C.; Cheng, Y.T. Lithium Ion Battery Electrodes Made Using Dimethyl Sulfoxide (DMSO)—A Green Solvent. ACS Sustain. Chem. Eng. 2020, 8, 11046–11051. [Google Scholar] [CrossRef]
- Venault, A.; Aini, H.N.; Galeta, T.A.; Chang, Y. Using the dimethyl sulfoxide green solvent for the making of antifouling PEGylated membranes by the vapor-induced phase separation process. J. Membr. Sci. Lett. 2022, 2, 100025. [Google Scholar] [CrossRef]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive poly(vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef]
- ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Gaihre, B.; Alici, G.; Spinks, G.M.; Cairney, J.M. Effect of electrolyte storage layer on performance of PPy-PVDF-PPy microactuators. Sens. Actuators B Chem. 2011, 155, 810–816. [Google Scholar] [CrossRef]
- Poudel, A.; Fernandez, M.A.; Tofail, S.A.M.; Biggs, M.J.P. Boron Nitride Nanotube Addition Enhances the Crystallinity and Cytocompatibility of PVDF-TrFE. Front. Chem. 2019, 7, 448246. [Google Scholar] [CrossRef]
- Chen, D.; Chen, K.; Brown, K.; Hang, A.; Zhang, J.X.J. Liquid-phase tuning of porous PVDF-TrFE film on flexible substrate for energy harvesting. Appl. Phys. Lett. 2017, 110, 153902. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Ribeiro, S.; Ribeiro, C.; Gombek, C.; Gama, F.; Gomes, A.; Patterson, D.; Lanceros-Mendez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym. Test. 2015, 44, 234–241. [Google Scholar] [CrossRef]
- Ali, I.; Bamaga, O.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.; Soliman, M.; Drioli, E.; Albeirutty, M. Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Ma, Y.; Sun, J.; Zhao, Q.; Yang, L.; Hou, Y.; He, H.; Huang, H.; Ji, H.; Qiu, J. Flexible and Transparent Ionic Liquids/Poly(vinylidene fluoride) Composition-Gradient Piezo-Active Composites for Highly Sensitive Pressure Sensor. Adv. Electron. Mater. 2022, 9, 2201013. [Google Scholar] [CrossRef]
- Kong, F.; Chang, M.; Wang, Z. Comprehensive Analysis of Mechanical Properties of CB/SiO2/PVDF Composites. Polymers 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fernández, I.; Raghibi, M.; Bouzina, A.; Timperman, L.; Bigarré, J.; Anouti, M. Protic ionic liquids/poly(vinylidene fluoride) composite membranes for fuel cell application. J. Energy Chem. 2021, 53, 197–207. [Google Scholar] [CrossRef]
- Varposhti, A.M.; Yousefzadeh, M.; Kowsari, E.; Latifi, M. Enhancement of β-Phase Crystalline Structure and Piezoelectric Properties of Flexible PVDF/Ionic Liquid Surfactant Composite Nanofibers for Potential Application in Sensing and Self-Powering. Macromol. Mater. Eng. 2020, 305, 1900796. [Google Scholar] [CrossRef]
- Gomes, J.; Serrado Nunes, J.; Sencadas, V.; Lanceros-Mendez, S. Influence of the β-phase content and degree of crystallinity on the piezo- and ferroelectric properties of poly(vinylidene fluoride). Smart Mater. Struct. 2010, 19, 065010. [Google Scholar] [CrossRef]
- Liu, X.; Shang, Y.; Zhang, J.; Zhang, C. Ionic Liquid-Assisted 3D Printing of Self-Polarized β-PVDF for Flexible Piezoelectric Energy Harvesting. ACS Appl. Mater. Interfaces 2021, 13, 14334–14341. [Google Scholar] [CrossRef]
- Begum, S.; Ullah, H.; Ahmed, I.; Zhan, Y.; Kausar, A.; Aleem, M.A.; Ahmad, S. Investigation of morphology, crystallinity, thermal stability, piezoelectricity and conductivity of PVDF nanocomposites reinforced with epoxy functionalized MWCNTs. Compos. Sci. Technol. 2021, 211, 108841. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, A.D.; Teixeira, A.M.; Martins, P. Influence of DMSO Non-Toxic Solvent on the Mechanical and Chemical Properties of a PVDF Thin Film. Appl. Sci. 2024, 14, 3356. https://doi.org/10.3390/app14083356
André AD, Teixeira AM, Martins P. Influence of DMSO Non-Toxic Solvent on the Mechanical and Chemical Properties of a PVDF Thin Film. Applied Sciences. 2024; 14(8):3356. https://doi.org/10.3390/app14083356
Chicago/Turabian StyleAndré, António Diogo, Ana Margarida Teixeira, and Pedro Martins. 2024. "Influence of DMSO Non-Toxic Solvent on the Mechanical and Chemical Properties of a PVDF Thin Film" Applied Sciences 14, no. 8: 3356. https://doi.org/10.3390/app14083356
APA StyleAndré, A. D., Teixeira, A. M., & Martins, P. (2024). Influence of DMSO Non-Toxic Solvent on the Mechanical and Chemical Properties of a PVDF Thin Film. Applied Sciences, 14(8), 3356. https://doi.org/10.3390/app14083356






