Double-Mode Thermometer Based on Photoluminescence of YbGd2Al2Ga3O12: Cr3+ Operating in the Biological Windows
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure
3.2. Luminescence Properties of YbGAGG: Cr3+
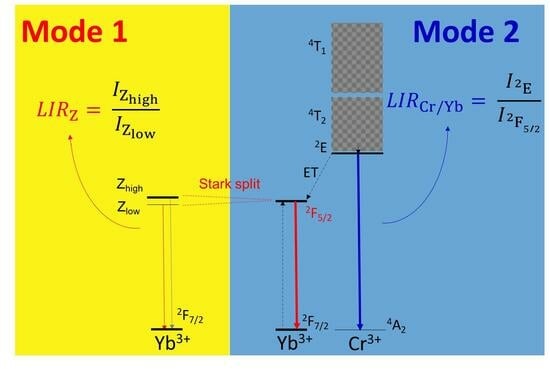
3.3. YbGAGG: Cr3+ Ratiometric Thermometer Based on the Emission of Yb3+ (Mode 1)
3.4. YbGAGG: Cr3+ Thermometer Based on the Photoluminescence Intensity Ratio of Cr3+/Yb3+ (Mode 2)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bunzli, J.C.G. Lanthanide light for biology and medical diagnosis. J. Lumin. 2016, 170, 866–878. [Google Scholar] [CrossRef]
- Bunzli, J.C.G. Rising stars in science and technology: Luminescent lanthanide materials. Eur. J. Inorg. Chem. 2017, 2017, 5058–5063. [Google Scholar] [CrossRef]
- Zhou, J.; Leano, J.L.; Liu, Z.; Jin, D.; Wong, K.L.; Liu, R.S.; Bunzli, J.C.G. Impact of lanthanide nanomaterials on photonic devices and smart applications. Small 2018, 14, 1801854–1801882. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Venkatachalam, N.; Hyodo, H.; Hattori, A.; Ebina, Y.; Kishimoto, H.; Soga, K. Upconverting and NIR emitting rare earth based nanostructures for NIR-bioimaging. Nanoscale 2013, 5, 11339–11361. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Paez, L.; Pedroni, M.; Speghini, A.; Garcia-Sole, J.; Haro-Gonzalez, P.; Jaque, D. Reliability of rare-earth-doped infrared luminescent nanothermometers. Nanoscale 2018, 10, 22319. [Google Scholar] [CrossRef]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Mill, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-based thermometers: At the cutting-edge of luminescence thermometry. Adv. Opt. Mater. 2019, 7, 1801239–1801268. [Google Scholar] [CrossRef]
- Bednarkiewicz, A.; Marciniak, L.; Carlos, L.D.; Jaque, D. Standardizing luminescence nanothermometry for biomedical applications. Nanoscale 2020, 12, 14405–14421. [Google Scholar] [CrossRef] [PubMed]
- Geitenbeek, R.G.; Nieuwelink, A.E.; Jacobs, T.S.; Salzmann, B.B.V.; Goetze, J.; Meijerink, A.; Weckhuysen, B.M. In situ luminescence thermometry to locally measure temperature gradients during catalytic reactions. ACS Catal. 2018, 8, 2397. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gao, X.; Zheng, L.; Zhang, Z.; Cao, W. Short-wavelength upconversion emissions in Ho3+/Yb3+ codoped glass ceramic and the optical thermometry behavior. Opt. Express 2012, 20, 18127–18137. [Google Scholar] [CrossRef]
- Li, X.Y.; Yuan, S.; Hu, F.; Lu, S.; Chen, D.Q.; Yin, M. Near-Infrared to shortwavelength upconversion temperature sensing in transparent bulk glass ceramics containing hexagonal NaGdF4: Yb3+/Ho3+ nanocrystals. Opt. Mater. Express 2017, 7, 3023–3033. [Google Scholar] [CrossRef]
- Allison, S.W.; Gillies, G.T. Remote thermometry with thermographic phosphors: Instrumentation and applications. Rev. Sci. Instrum. 1997, 68, 2615. [Google Scholar] [CrossRef]
- Rocha, U.; Jacinto, C.; Kumar, K.U.; López, F.J.; Bravo, D.; Solé, J.G.; Jaque, D. Real-time deep-tissue thermal sensing with sub-degree resolution by thermally improved Nd3+: LaF3 multifunctional nanoparticles. J. Lumin. 2016, 175, 149–157. [Google Scholar] [CrossRef]
- Skripka, A.; Benayas, A.; Marin, R.; Canton, P.; Hemmer, E.; Vetrone, F. Double rare-earth nanothermometer in aqueous media: Opening the third optical transparency window to temperature sensing. Nanoscale 2017, 9, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Xu, J.; Ueda, J.; Tanabe, S. Neodymium (III)-doped Y3Al2Ga3O12 garnet for multipurpose ratiometric thermometry: From cryogenic to high temperature sensing. J. Ceram. Soc. Jpn. 2023, 131, 57–61. [Google Scholar] [CrossRef]
- Skripka, A.; Morinvil, A.; Matulionyte, M.; Cheng, T.; Vetrone, F. Advancing neodymium single-band nanothermometry. Nanoscale 2019, 11, 11322–11330. [Google Scholar] [CrossRef] [PubMed]
- Kaminskii, A. Crystalline Lasers: Physical Processes and Operating Schemes; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Dramićanin, M. Luminescence Thermometry: Methods; Materials; Applications; Woodhead: Sawston, UK, 2018. [Google Scholar]
- Zhang, B.T.; He, J.L.; Jia, Z.T.; Li, Y.B.; Liu, S.D.; Wang, Z.W.; Wang, R.H.; Liu, X.M.; Tao, X.T. Spectroscopy and laser properties of Yb-doped Gd3AlxGa5-xO12 crystal. Appl. Phys. Express 2013, 6, 082702. [Google Scholar] [CrossRef]
- Novoselov, A.; Kagamitani, Y.; Kasamoto, T.; Guyot, Y.; Ohta, H.; Shibata, H.; Yoshikawa, A.; Boulon, G.; Fukuda, T. Crystal growth and characterization of Yb3+-doped Gd3Ga5O12. Mater. Res. Bull. 2007, 42, 27. [Google Scholar] [CrossRef]
- Xu, J.; Ueda, J.; Tanabe, S. Toward tunable and bright deep-red persistent luminescence of Cr3+ in garnets. J. Am. Ceram. Soc. 2017, 100, 4033–4044. [Google Scholar] [CrossRef]
- Fang, Z.; Li, Y.; Zhang, F.; Ma, Z.; Dong, G.; Qiu, J. Enhanced sunlight excited 1-μm emission in Cr3+–Yb3+ codoped transparent glass-ceramics containing Y3Al5O12 nanocrystals. J. Am. Ceram. Soc. 2015, 98, 1105–1110. [Google Scholar] [CrossRef]
- Solarz, P.; Głowacki, M.; Lisiecki, R.; Sobczyk, M.; Komar, J.; Macalik, B.; Ryba-Romanowski, W. Impact of temperature on excitation, emission and cross-relaxation processes of terbium ions in GGAG single crystal. J. Alloys Compd. 2019, 789, 409. [Google Scholar] [CrossRef]
- Mironova, N.; Brik, M.G.; Grube, J.; Krieke, G.; Kemere, M.; Antuzevics, A.; Gabrusenoks, E.; Skvortsova, V.; Elsts, E.; Sarakovskis, A.; et al. EPR, optical and thermometric studies of Cr3+ ions in the α-Al2O3 synthetic single crystal. Opt. Mater. 2022, 132, 112859–112865. [Google Scholar] [CrossRef]
- Niedźwiedzki, T.; Ryba-Romanowski, W.; Komar, J.; Głowacki, M.; Berkowski, M. Excited state relaxation dynamics and up-conversion phenomena in Gd3(Al, Ga)5O12 single crystals co-doped with erbium and ytterbium. J. Lumin 2016, 177, 219–227. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Yoshiasa, A.; Yamanaka, T. Cation distribution and crystal chemistry of Y3Al5−xGaxO12 (0 ≤ x ≤ 5) garnet solid solutions. Acta Crystallogr. Sect. B Struct. Sci. 1999, 55, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Laguta, V.; Zorenko, Y.; Gorbenko, V.; Iskaliyeva, A.; Zagorodniy, Y.; Sidletskiy, O.; Bilski, P.; Twardak, A.; Nikl, M.; Chem, J.P. Aluminum and gallium substitution in yttrium and lutetium aluminum–gallium garnets: Investigation by single-crystal NMR and TSL methods. J. Phys. Chem. C 2016, 120, 24400–24408. [Google Scholar] [CrossRef]
- Wei, G.; Li, P.; Li, R.; Wang, Y.; He, S.; Li, J.; Shi, Y.; Suo, H.; Wang, Z. How to Achieve Excellent Luminescence Properties of Cr Ion-Doped Near-Infrared Phosphors. Adv. Opt. Mater. 2023, 11, 2301794–2301814. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M. Critical review—A review of the electronic structure and optical properties of ions with d3 electron configuration (V2+, Cr3+, Mn4+, Fe5+) and main related misconceptions. ECS J. Solid State Sci. Technol. 2017, 7, R3079. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Brik, M.G.; Grube, J.; Krieke, G.; Antuzevics, A.; Skvortsova, V.; Kemere, M.; Elsts, E.; Sarakovskis, A.; Piasecki, M.; et al. Spectroscopic studies of Cr3+ ions in natural single crystal of magnesium aluminate spinel MgAl2O4. Opt. Mater. 2021, 121, 111496. [Google Scholar] [CrossRef]
- Henderson, B.; Imbusch, G.F. Optical Spectroscopy of Inorganic Solids; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Xu, D.; Zhang, Q.; Wu, X.; Li, W.; Meng, J. Synthesis, luminescence properties and energy transfer of Ca2MgWO6: Cr3+, Yb3+ phosphors. Mater. Res. Bull. 2019, 110, 135–140. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Ueda, J.; Tanabe, S. Double-Mode Thermometer Based on Photoluminescence of YbGd2Al2Ga3O12: Cr3+ Operating in the Biological Windows. Appl. Sci. 2024, 14, 3357. https://doi.org/10.3390/app14083357
Zhang Q, Ueda J, Tanabe S. Double-Mode Thermometer Based on Photoluminescence of YbGd2Al2Ga3O12: Cr3+ Operating in the Biological Windows. Applied Sciences. 2024; 14(8):3357. https://doi.org/10.3390/app14083357
Chicago/Turabian StyleZhang, Qixuan, Jumpei Ueda, and Setsuhisa Tanabe. 2024. "Double-Mode Thermometer Based on Photoluminescence of YbGd2Al2Ga3O12: Cr3+ Operating in the Biological Windows" Applied Sciences 14, no. 8: 3357. https://doi.org/10.3390/app14083357