Abstract
Reinforced concrete is the most widely used material in the construction of building structures, being noted for its versatility and low cost. However, the durability of reinforced concrete structures can be compromised by the corrosion of steel re-bars, especially in the presence of chlorides. To address this challenge and promote sustainability, the use of corrosion inhibitors has been researched as a way to extend the lifespan of structures. This study assessed the effectiveness of using a commercial corrosion inhibitor on steel re-bars embedded in types of concrete with different chloride percentages, using electrochemical methods to measure the corrosion rate and potential. The results indicate that, in the absence of corrosion inhibitors, corrosion rates become unacceptable with chloride percentages equal to or higher than 0.8% by weight of cement. The application of inhibitors significantly reduced the corrosion rate, particularly at chloride percentages of 0.8% and 1.2%, maintaining the re-bars in a passive state or at moderate levels of corrosion. However, for chloride percentages higher than 1.6%, high levels of corrosion were observed, even in the presence of inhibitors. The findings suggest that the use of inhibitors can be an effective strategy in preventing corrosion in reinforced concrete structures, contributing to their structural integrity and long-term sustainability.
1. Introduction
The widespread use of reinforced concrete in the construction sector is mainly explained by its affordable cost and the flexibility in design it offers [1,2,3,4]. This material provides essential characteristics such as structural integration, adaptability (due to its malleability), and notable fire resistance [3,5,6]. The durability of concrete, resulting in the prolonged lifespan of structures exposed to certain environmental conditions, is another of its highlighted attributes [7]. Reinforced concrete buildings are particularly widely used in coastal areas, where they demonstrate outstanding mechanical resistance and durability against adverse conditions such as freeze–thaw cycles. However, they face difficulties under other conditions, such as exposure to saline environments and carbonation, which can depassivate reinforcing steel [8].
The production of concrete has a considerable environmental impact, primarily due to cement manufacturing [9]. As a result, the industry has become oriented towards the production of concretes with lower ecological impacts in recent years [10,11,12]. Designing and constructing structures that last longer is crucial to minimizing the industry’s carbon footprint, leading to long-term resource savings. The durability of structures can be compromised by several factors, starting with problems in the construction phase and followed by deteriorations in the first ten years of use [13,14]. Therefore, construction regulations establish requirements which ensure the durability of reinforced concrete, with the aim that structures meet their expected lifespan [15]. To mitigate the effects of aggressive environments, different categories of environmental exposure have been established, considering factors such as the levels of humidity, wetting and drying cycles, and the presence of chlorides and other contaminating agents [16].
The corrosion of reinforcing steel is one of the most serious problems affecting the lifespan of reinforced concrete structures. The premature failure of these structures, manifesting in their inability to reach their designed lifespan due to corrosion and the resulting cracks in the concrete, is a constant concern [17,18,19,20]. The Tuutti model [21,22] allows us to analyze the deterioration of concrete, describing corrosion as a process divided into two phases: initiation and propagation. In this model, the presence of chlorides and carbonation are the main deteriorating agents [23,24]. Several concurrent factors are required for the corrosion of steel re-bars by chloride ions: chloride levels above the critical limit, the availability of oxygen and moisture, and geometric irregularities at the interface of steel and concrete [13]. Among the elements that affect the corrosion process in reinforced concrete structures, the quality of the concrete, external environmental conditions, aspects of structural design, and the mechanical loads to which the structures are exposed stand out [25,26].
From a sustainability perspective, protecting structures from corrosion at early stages extends their lifespan and contributes to resource savings [27]. Preventing corrosion is crucial to optimizing the maintenance of structures, with preventive management being preferable over reactive maintenance when seeking to prolong the life of buildings and reduce repair costs. To prevent corrosion, it is essential to use high-quality concrete, with proper curing and sufficient concrete cover, thus improving its physical properties such as density and impermeability. In particularly severe conditions, additional prevention methods can be considered, such as the use of corrosion inhibitors, coatings, corrosion-resistant re-bars, and cathodic protection techniques [28,29,30,31,32,33,34,35].
Corrosion inhibitors are effective in both prevention and in the treatment of structures affected by chloride-induced corrosion or carbonation. Traditionally, two types have been used: inhibitors added at the time of mixing, and migratory inhibitors, which are applied externally on the concrete surface and penetrate inside via a diffusion process to reach the level of the re-bars, thus being ideal for rehabilitation operations. Inhibitors added at the time of mixing are mainly inorganic and have been researched since the 1950s, while migratory inhibitors, developed more recently, have gained attention for their usefulness in the restoration of buildings. These inhibitors act by controlling the anodic or cathodic reaction of corrosion, or both, and by providing a barrier against both the dissolution of metal and the reduction of oxygen [13,36,37]. There is a third type of corrosion inhibitor that is less used in rehabilitation, and which is applied directly to the reinforcement that is intended to be protected [38,39,40].
The research by Batis et al. and other associated studies highlight the significance of migratory corrosion inhibitors and specific coatings like epoxy in improving the corrosion protection of existing concrete structures. It was found that the use of fibre-reinforced mortar-containing corrosion inhibitors introduced at the time of mixing offered superior protection, attributed to the strengthening of the protective passive layer and a reduction in mortar porosity, which decreased the cracking rate thanks to the improved tensile strength provided by the fibres. Additionally, the application of surface coatings, such as epoxy, to reinforcement bars proved to be particularly effective against corrosion in chloride-rich marine environments without compromising adherence to the concrete. These techniques not only prevent the penetration of corrosive agents but also address the issue of the oxidation expansion of steel embedded in reinforced concrete, offering a comprehensive approach to prolonging the lifespan of these structures in the face of environmental aggressiveness [41,42,43,44].
The corrosion of reinforcing steel in reinforced concrete structures is a significant issue that can compromise the integrity and durability of civil infrastructures, such as bridges. In this context, various experimental studies have investigated the corrosion potential of actual reinforced concrete structures, using guidelines established by the ASTM for the evaluation of corrosion and the interpretation of the results obtained. The authors De Domenico, Messina, and Recupero (2023) explore the effects of corrosion on the seismic vulnerability of reinforced concrete bridge piers in their article “Assessment of Seismic Vulnerability of Reinforced Concrete Bridge Piers with Corroded bars”. This study provides valuable insights into how corrosion affects load-bearing capacity and structural resilience in the context of seismic events, utilising experimental data based on ASTM guidelines and additional relevant studies. Additionally, in their article “Recent Advances in the Use of Green Corrosion Inhibitors to Prevent Chloride-Induced Corrosion in Reinforced Concrete”, Casanova et al. (2023) present a review of the latest advances in the development and application of environmentally friendly corrosion inhibitors with which to mitigate chloride-induced corrosion in reinforced concrete structures. This work highlights the importance of seeking sustainable and effective solutions in order to combat corrosion in civil infrastructures. Both studies complement the current understanding of corrosion challenges in reinforced concrete structures, providing valuable insights for the design, maintenance, and management of these key infrastructures [45,46].
A study conducted by Wang et al. [20] shows that the effectiveness of corrosion inhibitors injected at high pressures into hardened concrete is directly influenced by the water/cement ratio of the concrete. It was determined that concrete with a higher water/cement ratio allows for deeper penetration of the inhibitor when a prolonged pressure time is applied. Moreover, additional research indicates that while the inclusion of inhibitors in cement composite materials exposed to chloride-rich environments may decrease their compressive strength, these inhibitors have minimal impact on indirect tensile strength and adherence strength [47]. The aim of this study is to evaluate the efficacy of applying corrosion inhibitors directly to steel re-bars embedded in concrete. Concretes with different chloride ion percentages were studied, and the corrosion of the re-bars was assessed using electrochemical methods, analysing the consistency between corrosion rates and potentials in re-bars with and without inhibitors, and seeking an effective method that can help to reduce the repair costs of corroded structures.
2. Methodology
2.1. Materials Used
To achieve the proposed objectives, an experimental plan was developed that involvds the fabrication of reinforced concrete slabs in the construction-materials laboratory of the Escuela Técnica Superior de Edificación at the Universidad Politécnica de Madrid.
For concrete manufacturing, CEM I 42.5 R cement [48], 0–4 mm of washed river sand–as per EN 13139/AC:2004 [49], and a coarse silicon aggregate of 4–12 mm, in accordance with EN-12620:2003+A1:2009 [50], were selected. The water was sourced from Madrid's municipal supply.
A commercial corrosion inhibitor composed of Portland cement, epoxy resin, selected aggregates, and additives was used. Steel re-bars of B500SD with a diameter of 12 mm were employed. The chemical composition of the steel is shown in Table 1.

Table 1.
Chemical composition of the re-bars used.
2.2. Preparation of the Specimens
Six concrete slabs of 500 × 250 × 100 mm were manufactured, using 275 kg/m3 of cement, 550 kg/m3 of sand, 825 kg/m3 of gravel, and 137.5 kg/m3 of water in a ratio of 1:2:3:0.5. During the mixing of the concrete for each slab, different amounts of chlorides were incorporated by weight of cement: 0.0%, 0.4%, 0.8%, 1.2%, 1.6%, 2.0%. wen steel re-bars were introduced into each slab, with a corrosion inhibitor applied to five of them. The inhibitor application process started with us mixing it at a low speed (<250 rpm) with an electric mixer. After mixing, the solution was left to rest for a period of between 5 and 10 min, ensuring an optimal consistency for its application with a brush. This mixture was applied to the re-bars, using a brush to spread a first layer of approximately 1 mm in thickness. After this layer solidified, which generally takes a period of 2 to 3 h, a second layer was applied and left to dry completely.
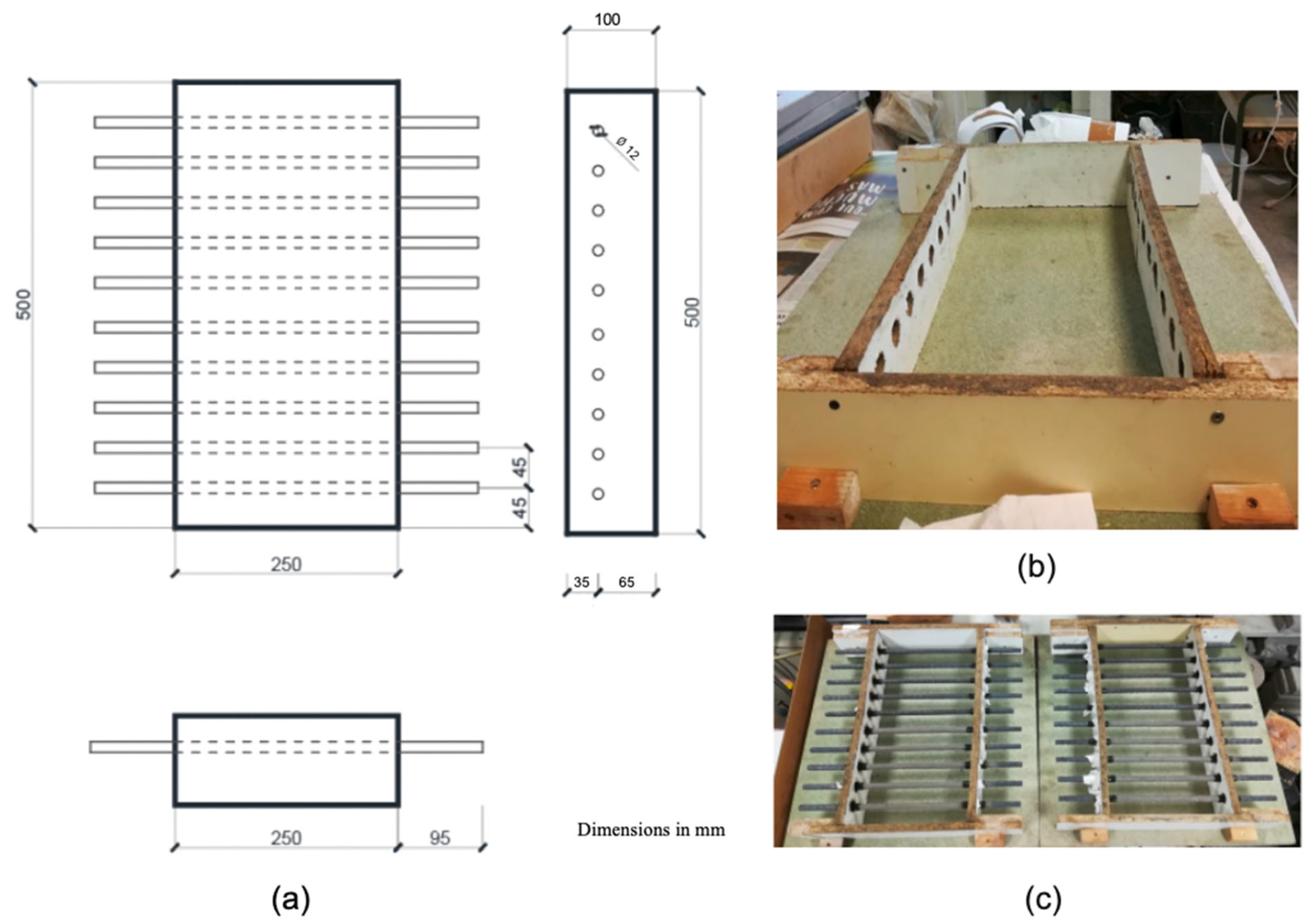
Subsequently, the ends of the re-bars were protected in the area of the interface between concrete, air, and steel with 0.76 mm thick self-amalgamating electrical tape, obtained from the brand 3M. The center of each bar was marked, and from that point, we measured 12.5 cm towards each side (covering a total of 25 cm) to delimit the area that would be embedded in the concrete and within the mould. Finally, the re-bars were placed in the moulds through the prepared holes, ensuring not to damage the inhibitor layer on the treated re-bars. This process is illustrated in Figure 1.
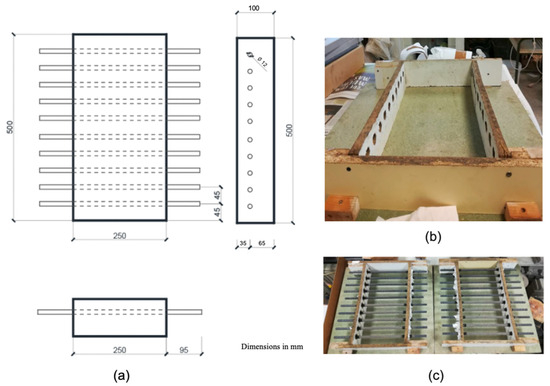
Figure 1.
Scheme and preparation of moulds used. Design of the molds (a), assembly of the molds (b), and full view of the molds with the reinforcement (c).
In Table 2, the nomenclature used to identify each bar and their characteristics is shown.

Table 2.
Nomenclature and characteristics of the re-bars.
To evaluate the effect of the corrosion inhibitors, the samples were kept in a moist state; for this purpose, the slabs were wrapped in cloths that were moistened twice a day, thus allowing for the analysis of the corrosion behavior of the re-bars through electrochemical techniques, such as the measurement of potential and the corrosion rate.
2.3. Measurement
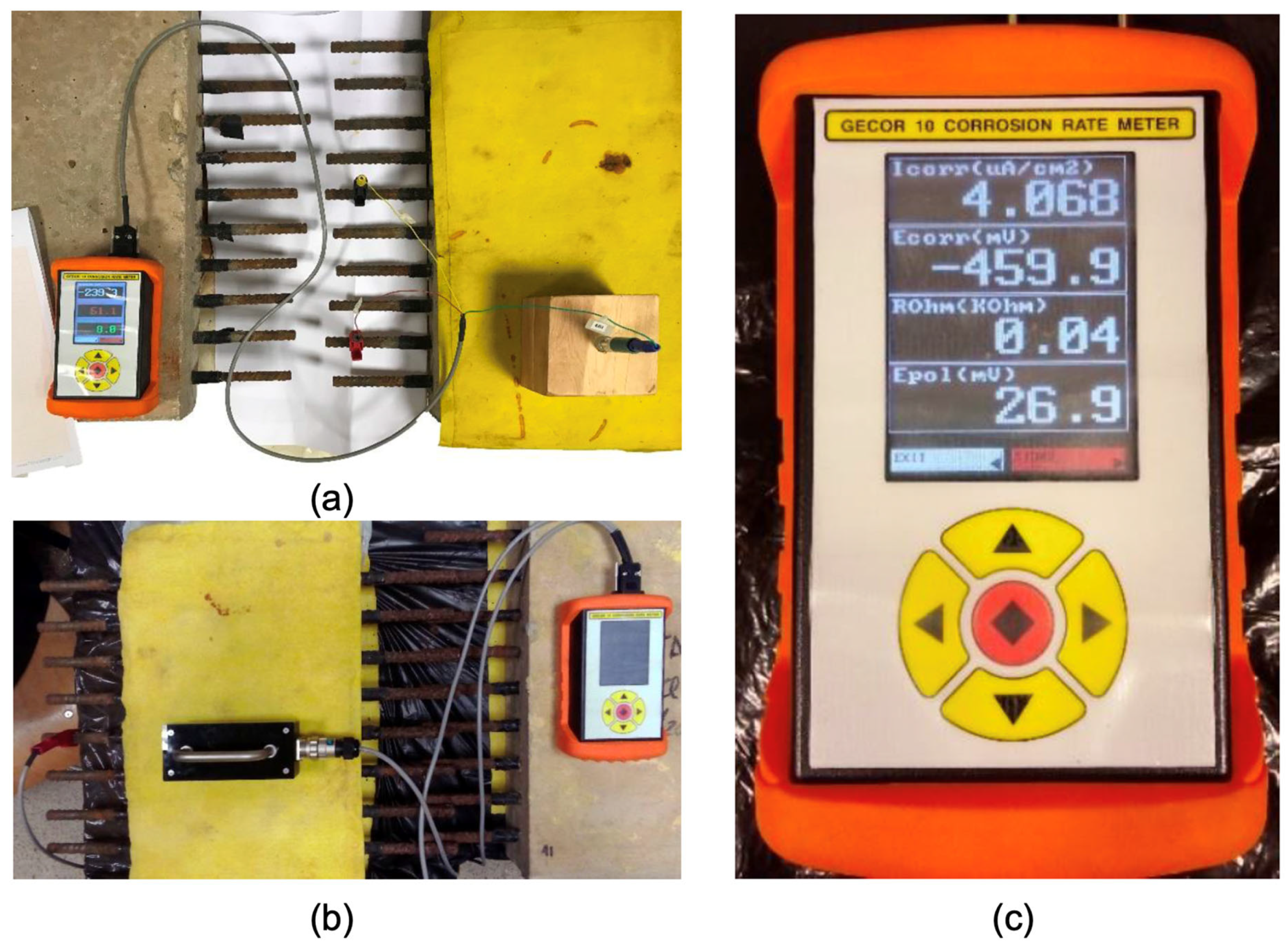
Evaluations were conducted using a Gecor 10 resistivity meter corrosimeter, illustrated in Figure 2a, which is an instrument designed to measure the level of corrosion in re-bars within concrete. This equipment facilitates measurements using sensors that apply potential variations or electric currents to the structure and subsequently examine the response.
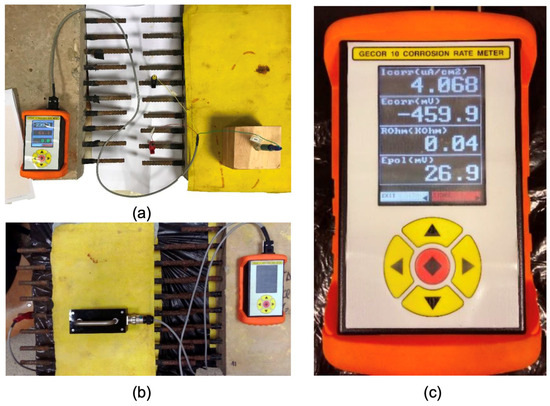
Figure 2.
Gecor 10 device (c) and measurement with an active electrode (a) and four-point sensor (b).
During data collection, each reinforcement was used as an active electrode and to perform the central reinforcement without an inhibitor as the counter electrode, using a silver–silver chloride reference electrode. Effective contact was ensured between the reference electrode and the concrete slab by maintaining a damp cloth between them. The Wenner or four-point method, in accordance with EN 12390-19 [51], was used. This procedure employs a sensor with four probes arranged in line at equidistant intervals, as shown in Figure 2. The outer probes initiate an alternating current while the reaction is measured by the potential difference between the two inner probes based on the induced current.
The corrosion rate (icorr) and the corrosion potential (Ecorr) were measured for each of the 10 re-bars present in every slab. The corrosion potential provides a qualitative measure of the corrosion state of the re-bars [52] and is greatly influenced by the moisture level of the concrete slab, with results dependent on the type of reference electrode used. This measure offers a qualitative indication, which reflects the likelihood of the steel being in a state of corrosion or in a passive state. Table 3 presents the probabilities of corrosion according to ASTM standards using a silver–silver chloride reference electrode [53,54,55]. The corrosion rate (icorr) provides a quantitative measure of the reinforcement deterioration process. Table 4 shows the expected corrosion penetrations (T) based on the corrosion rate [54].

Table 3.
Corrosion risk, according to Ecorr.

Table 4.
Intervals of corrosion current values associated with the durability of the reinforcement.
This section is divided by subheadings. It provides a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3. Results and Discussion
3.1. Corrosion Potential (Ecorr) Results
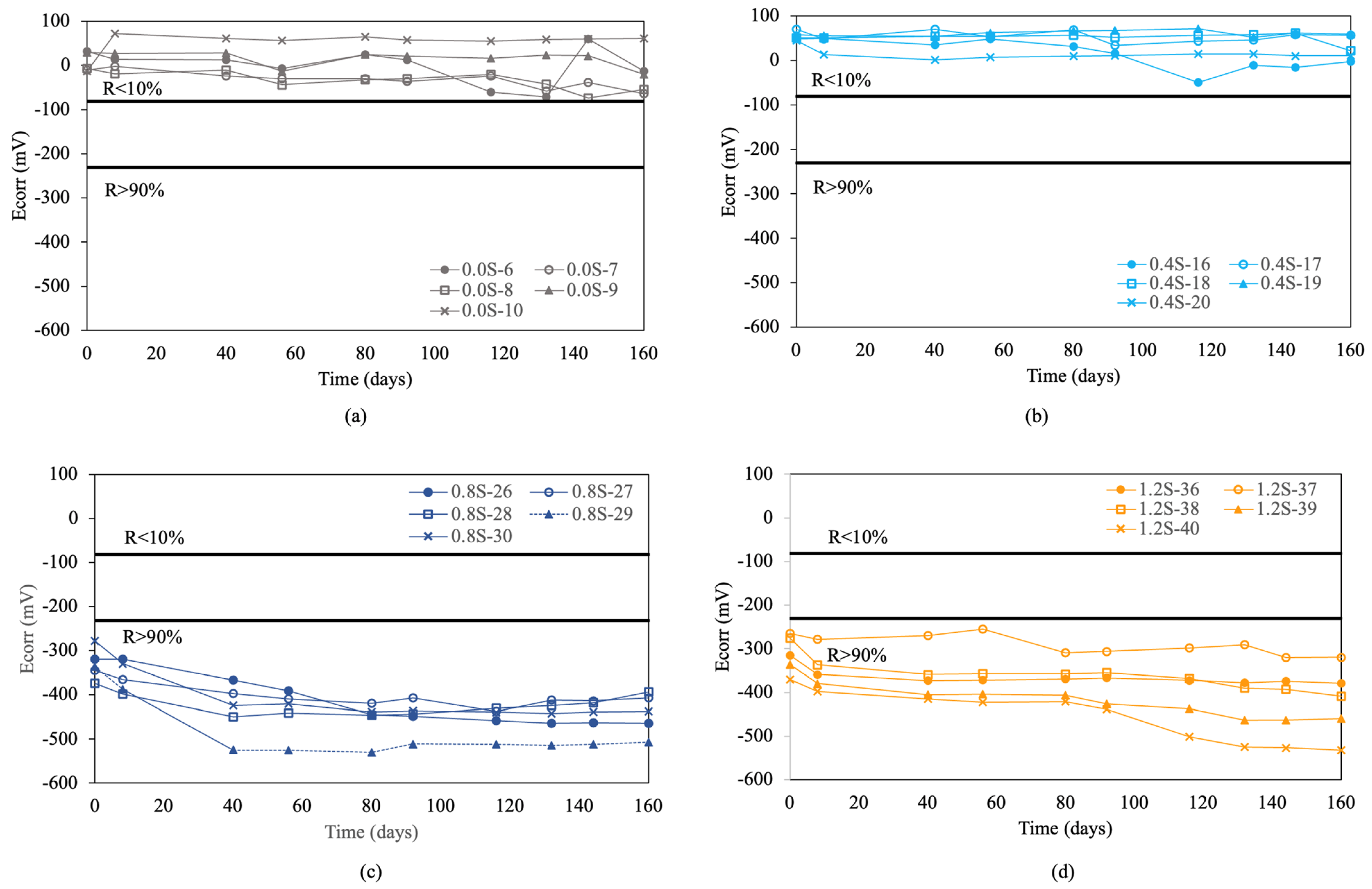
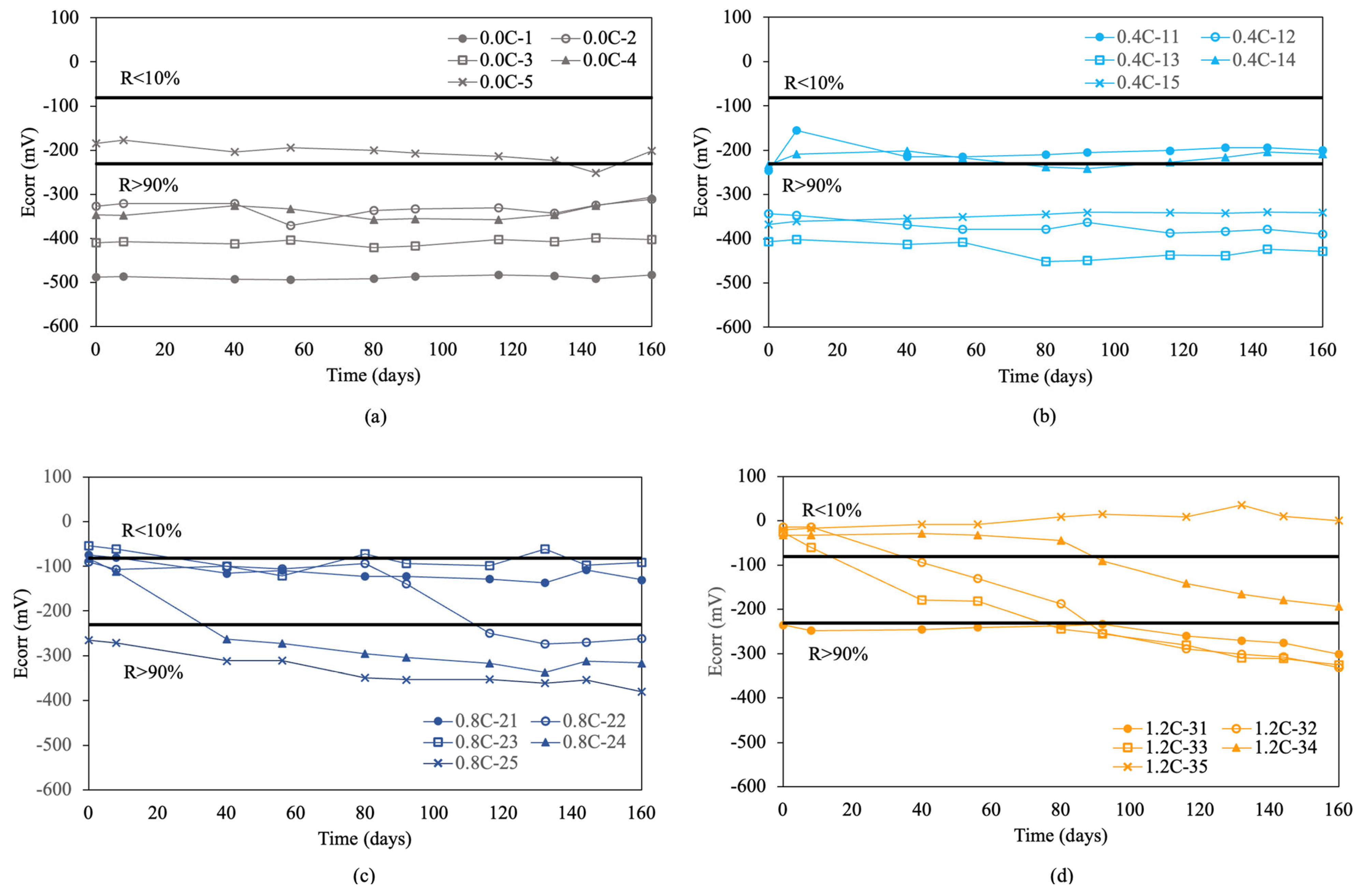
Figure 3 shows the corrosion potential results obtained for the re-bars without inhibitors when embedded in concretes with chloride ion percentages of 0.0%, 0.4%, 0.8%, 1.2%, 1.6%, and 2.0%.
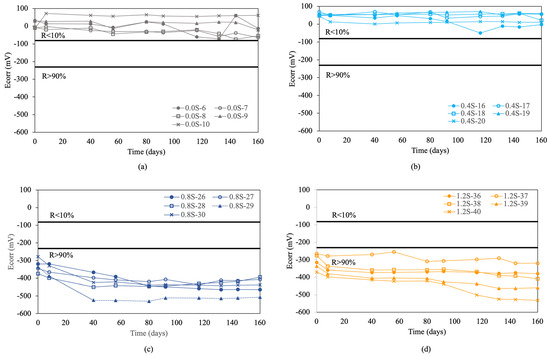
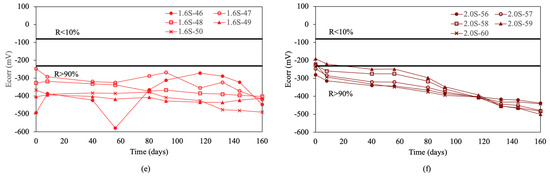
Figure 3.
Corrosion potential (Ecorr) in re-bars without inhibitor and in concretes with different percentages of chloride ion. (a) 0.0%, (b) 0.4%, (c) 0.8%, (d) 1.2%, (e) 1.6%, and (f) 2.0%.
For concretes without chlorides, and with a chloride ion percentage of 0.4% by weight of cement, and containing re-bars without inhibitor, all the analyzed re-bars presented an associated corrosion risk of less than 10.0% throughout the analyzed period, as can be seen in Figure 3a,b. A percentage of 0.4% chloride ion by weight of cement is considered the limit before corrosion is triggered in re-bars [54].
For chloride ion percentages of 0.8% and above, all the re-bars showed an associated corrosion risk of more than 90%, as has been stated by other authors [54]. In most of the re-bars, the potentials evolved towards more negative values over time, reaching corrosion potentials of between −400 and −500 mV at the end of the test, as can be appreciated in Figure 3c–f.
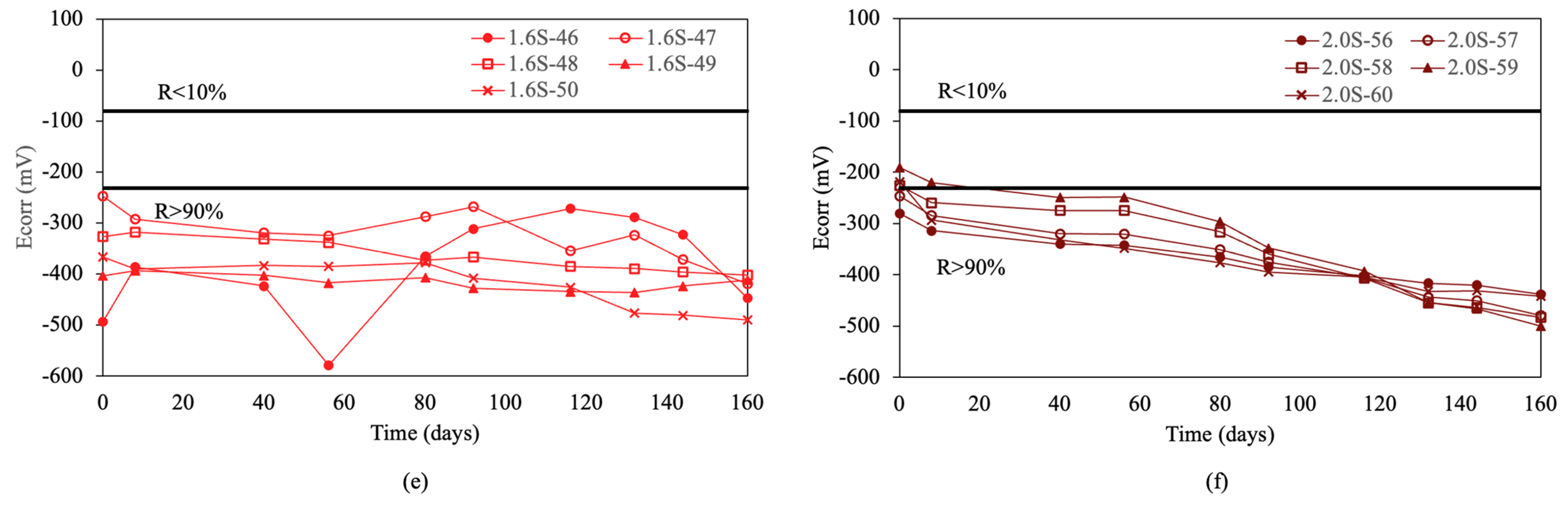
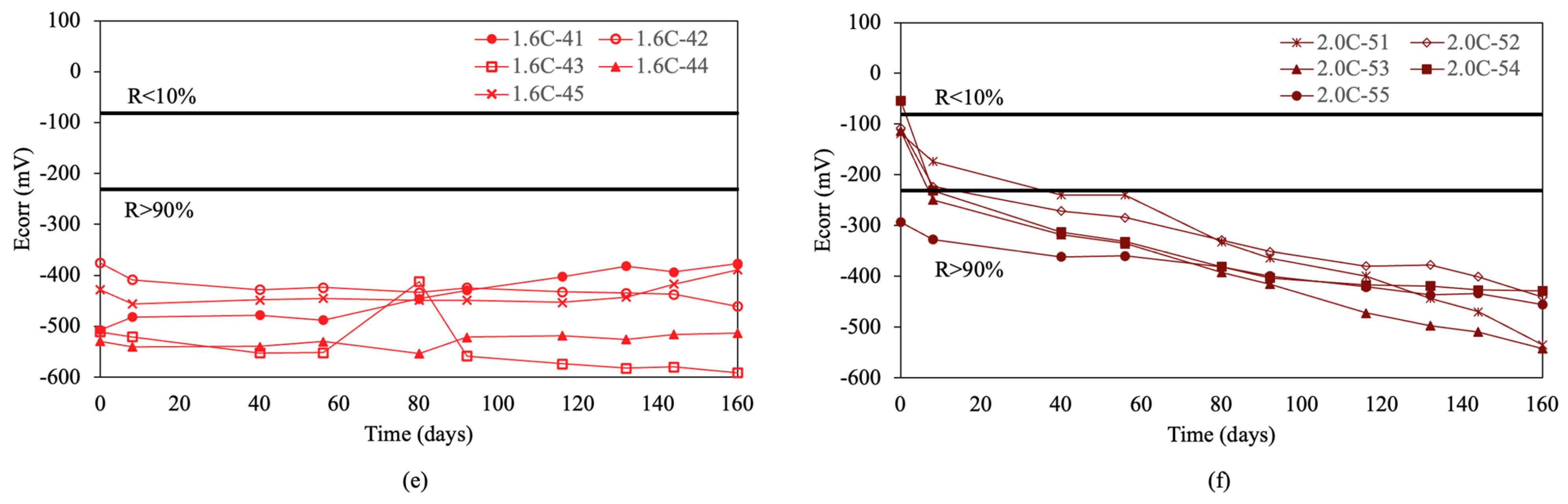
Figure 4 shows the results of the corrosion potentials for re-bars with inhibitors when embedded in concretes with chloride ion percentages of 0.0%, 0.4%, 0.8%, 1.2%, 1.6%, and 2.0%.
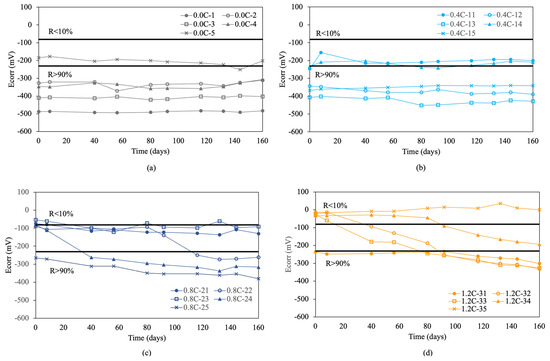
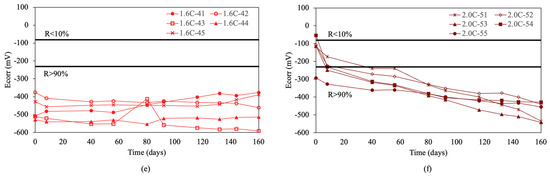
Figure 4.
Corrosion potential (Ecorr) in re-bars with inhibitor and in concretes with different percentages of chloride ion. (a) 0.0%, (b) 0.4%, (c) 0.8%, (d) 1.2%, (e) 1.6%, and (f) 2.0%.
For chloride ion percentages of 0.0% and 0.4%, shown in Figure 4a,b, practically all re-bars exhibit a constant corrosion potential over time, which is characteristic of corrosion probabilities above 90%. For chloride ion percentages of 0.8% and 1.2%, the corrosion potentials evolve towards more negative values over time, with about half of the re-bars showing potentials that indicate an uncertain risk of corrosion by the end of the test.
For chloride ion percentages of 1.6%, shown in Figure 4e, and 2.0%, shown in Figure 4f, all re-bars at the end of the test show corrosion potentials which indicate that all re-bars are at a risk of corrosion above 90%.
Comparing the results obtained for corrosion potentials in re-bars with and without inhibitor, it is evident that for chloride ion percentages of 1.6% and 2.0%, the results are very similar. For chloride ion percentages of 0.0% and 0.4%, the re-bars without inhibitor exhibit fewer negative potentials than those with inhibitor. For chloride ion percentages of 0.8% and 1.2%, the potentials of the re-bars with inhibitor are less negative than in the case of re-bars without inhibitor.
3.2. Corrosion Rate Results (icorr) in Re-Bars with and without Inhibitor, Incorporated into Concretes with Different Percentages of Chloride Ion
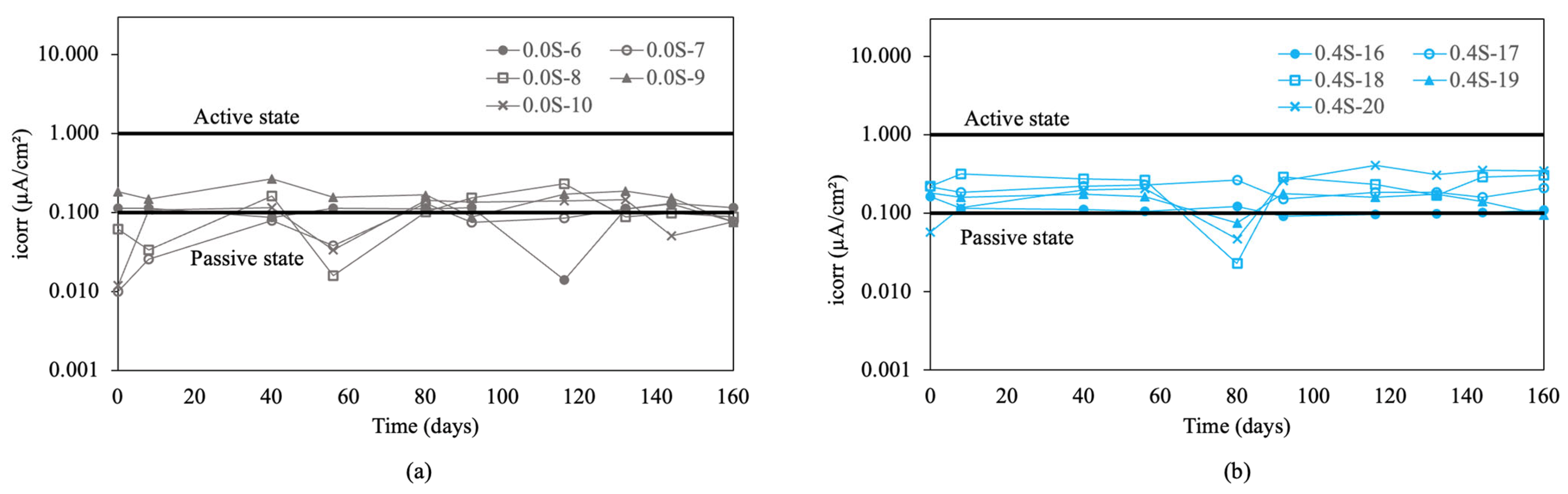
Figure 5 presents the corrosion rate results for re-bars without inhibitor embedded in concretes with 0.0%, 0.4%, 0.8%, 1.2%, and 1.6% chloride.
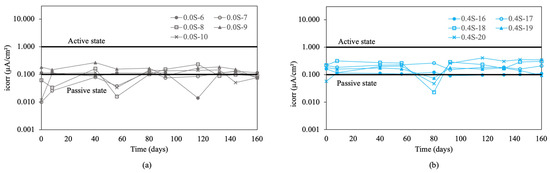
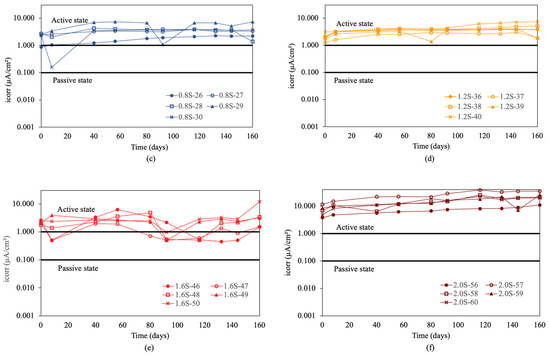
Figure 5.
Corrosion rate (icorr) in re-bars without inhibitor and in concretes with different percentages of chloride ion. (a) 0.0%, (b) 0.4%, (c) 0.8%, (d) 1.2%, (e) 1.6%, and (f) 2.0%.
In the initial condition without chlorides, shown in Figure 5a, the measured values are in a passive state. Increasing the chloride percentage to 0.4% resulted in an increase in corrosion rate, exceeding the threshold of 0.1 μA/cm2. This increase marked a clear transition to an active corrosion state, demonstrating the detrimental effect of chlorides on the stability of the passive layer.
In concretes with 0.8%, 1.2%, and 1.6% chloride ion, shown in Figure 5c–e, it was observed that the re-bars were clearly in an active corrosion state, maintaining uniform values over time [2,3,55,56]. For concretes with 2.0% chloride ion, shown in Figure 5f, the results indicated that all values remained above 1 μA/cm2, indicating high corrosion, especially for the sample with 1.6% chlorides. Initial measurements in concretes with 2.0% chloride ion showed very high initial corrosion rate values above 3 μA/cm2. These values exceeded 30 μA/cm2 at the end of the experiment.
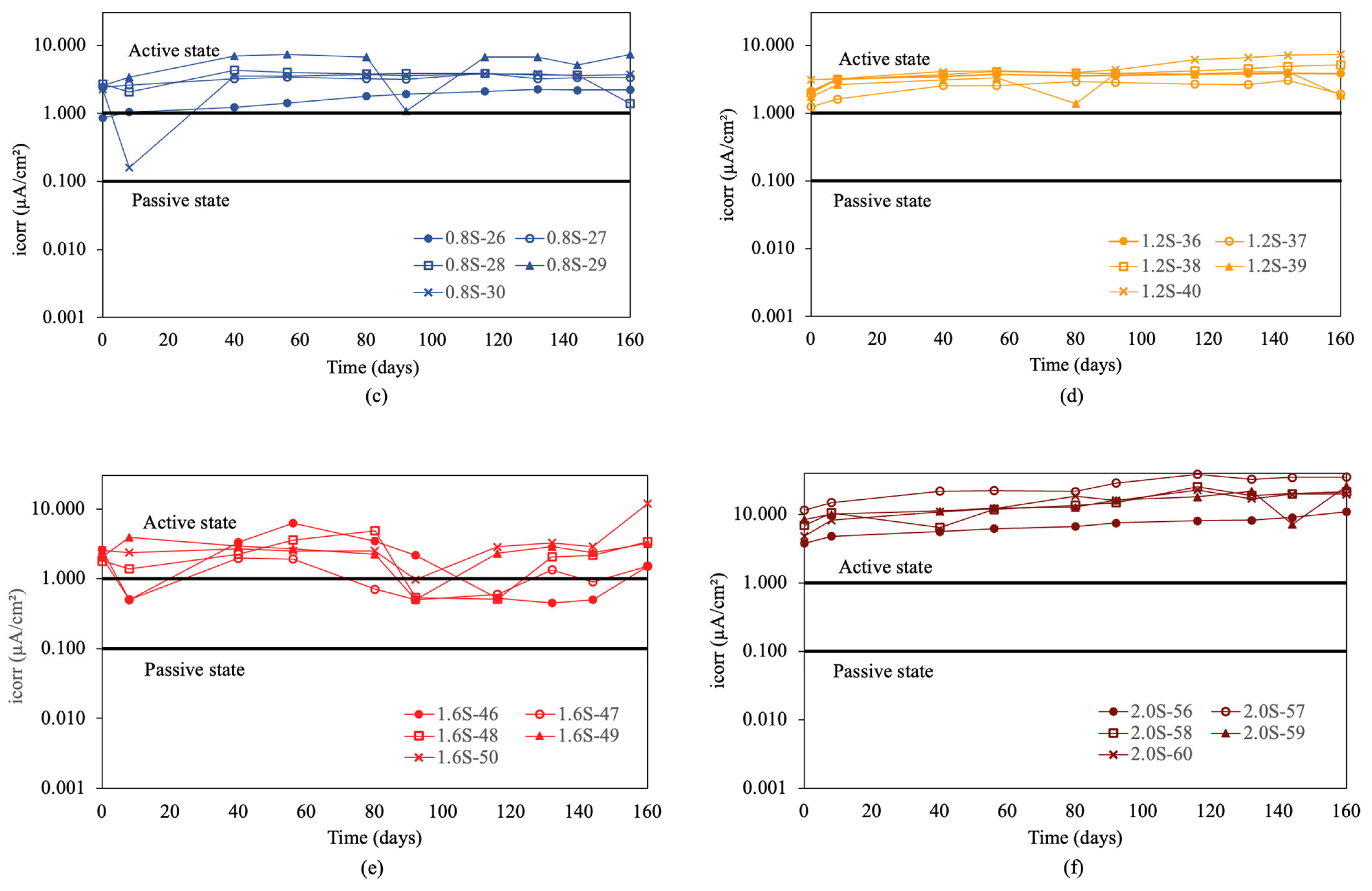
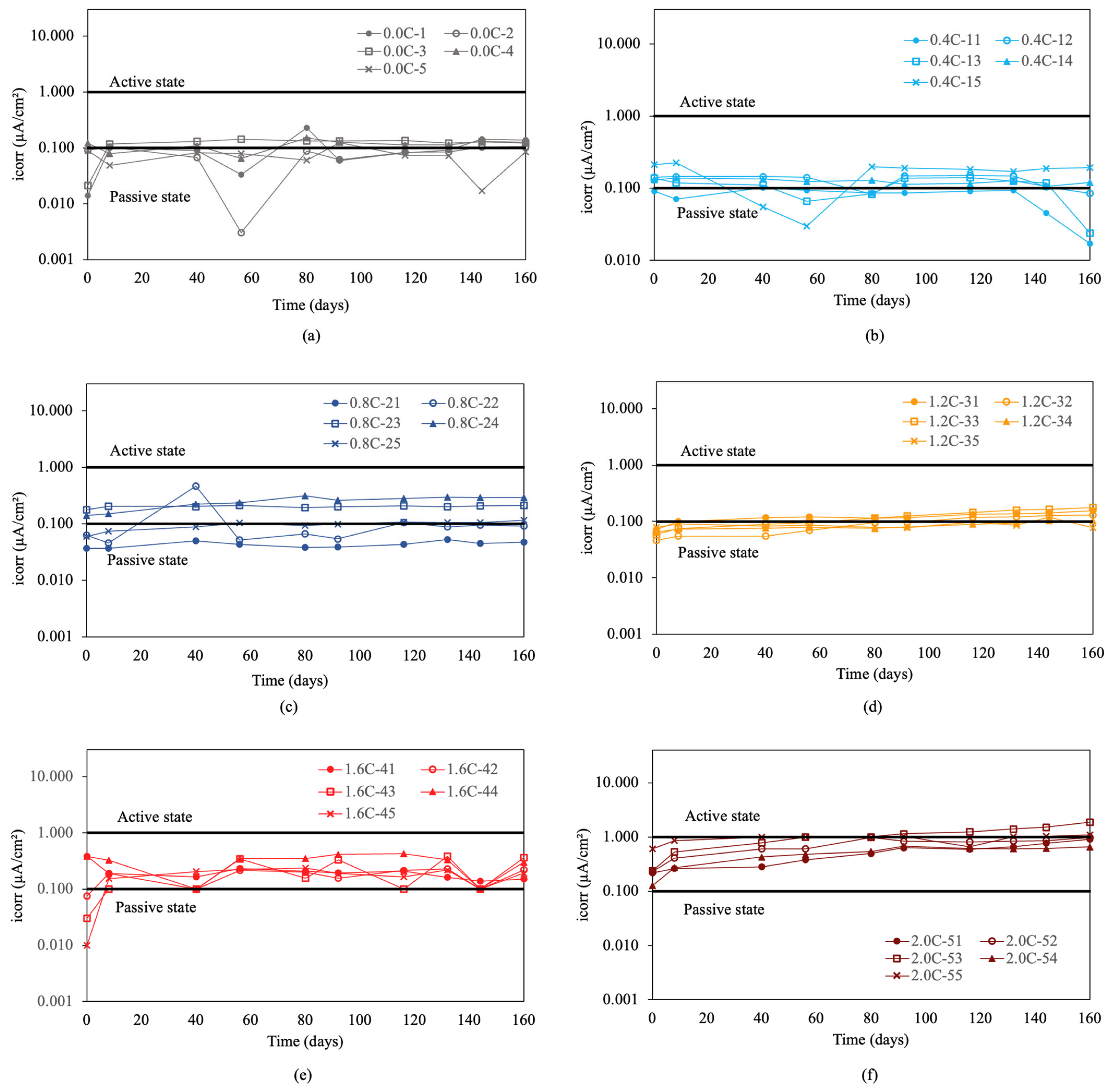
The corrosion rate results for re-bars with inhibitor are shown in Figure 6. For chloride ion percentages of 0.0%, and 0.4%, shown in Figure 6a and b, it is observed that in both cases, the values are approximately 0.1 μA/cm2, indicating a negligible level of corrosion [57]. For chloride ion percentages of 0.8%, and 1.2%, shown in Figure 6c,d, the values are around the level expected for a passive state.
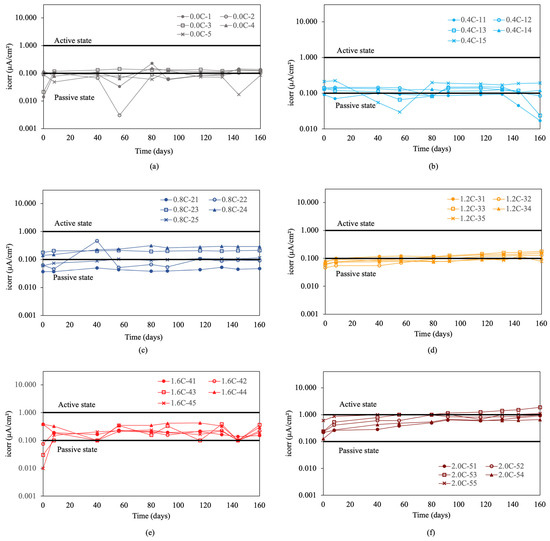
Figure 6.
Corrosion rate (icorr) in re-bars with inhibitor and in concretes with different percentages of chloride ion. (a) 0.0%, (b) 0.4%, (c) 0.8%, (d) 1.2%, (e) 1.6%, and (f) 2.0%.
For chloride ion percentages of 1.6%, shown in Figure 6e, it was observed that the corrosion rates increased to values corresponding to a medium level of corrosion, ranging from 0.1 to 1 μA/cm2. For chloride ion percentages of 2.0%, shown in Figure 6f, the corrosion rates increased over time from initial values between 0.1 and 1 μA/cm2 to final values between 0.6 and 2 μA/cm2. The joint analysis of Figure 6a–f revealed that, while in the first five cases the corrosion rates remained constant over time throughout the experiment, for chloride ion percentages of 2.0% in the five measured bars, there was a significant and steady increase in the corrosion rate over the course of the experiment.
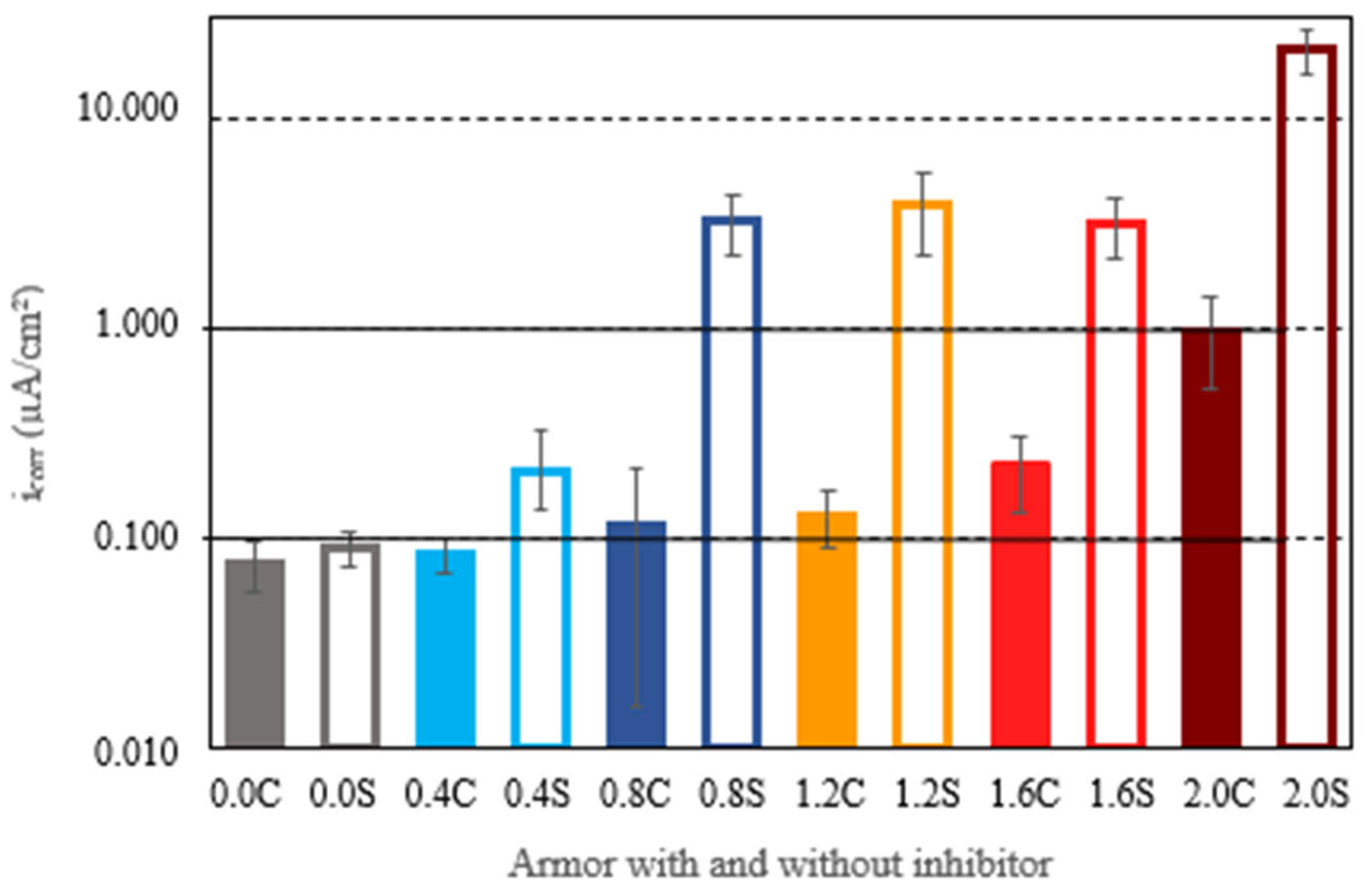
The comparison of results in armored structures without an inhibitor and with an inhibitor allowed us to verify that with 0.4% chloride ion, the effect of the inhibitor was not significant, offering very similar results. However, for the rest of the chloride ion percentages, the differences were very significant, with higher levels of corrosion being reached in structures without the inhibitor. Figure 7 shows the average corrosion rates achieved by the armored structures at the end of experimentation. Again, it can be observed that, at the end of experimentation, in all cases, the corrosion rates in structures without the inhibitor were higher than the corrosion rates with the inhibitor. For structures free of chlorides or embedded in concretes with a percentage of 0.4% chloride ion, the corrosion rates were very low or negligible. With chloride ion percentages of 0.8%, 1.2%, and 1.6%, the armored structures without inhibitor exhibited corrosion rates exceeding 1 μA/cm2, as is typical of the active state. For these chloride ion percentages, the presence of the inhibitor was shown to be very effective, as the corrosion rates in armored structures with the inhibitor were significantly reduced, falling to values corresponding to low levels of corrosion. In concretes with a chloride ion percentage of 2%, the presence of the inhibitor significantly reduced the corrosion rate but did not prevent corrosion rates of 1 μA/cm2, typical of the active state, from being reached.
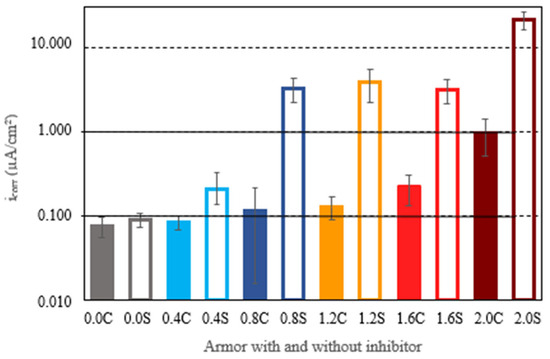
Figure 7.
The average corrosion rates (icorr) reached by all armored structures at the end of the test. The filled lines represent the re-bars with inhibitor, and the unfilled ones are those without inhibitor.
3.3. Relationship between Icorr and Ecorr in Armored Structures with and without Inhibitor
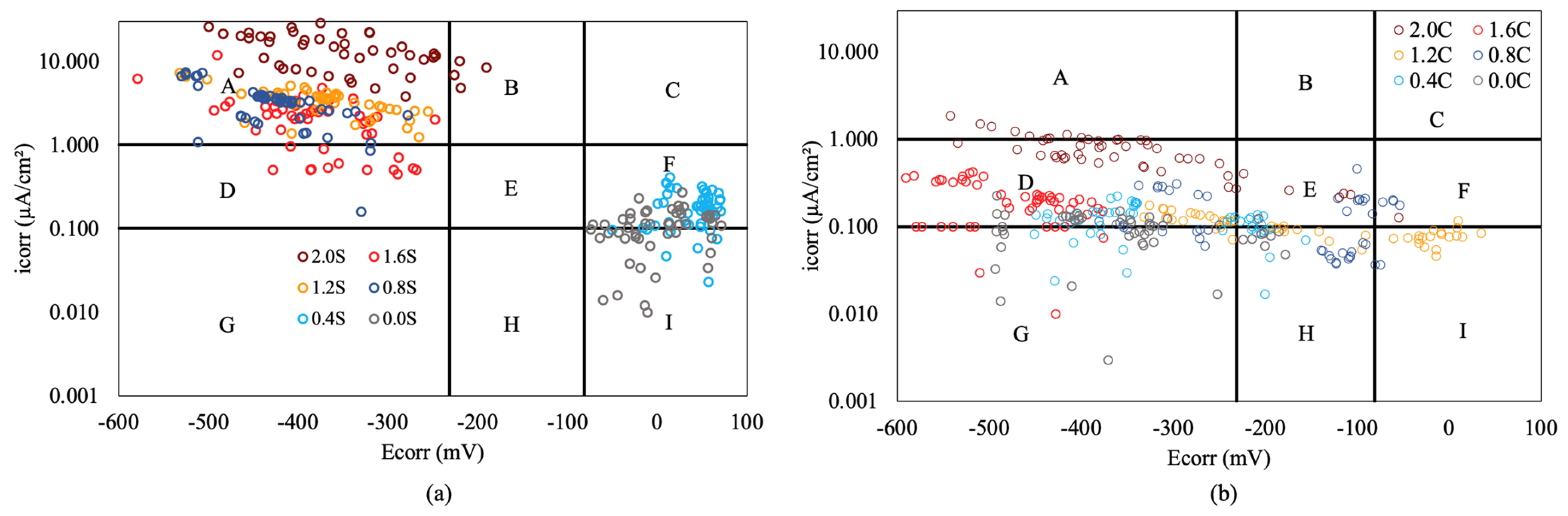
The joint analysis of corrosion rate measurements and corrosion potential allows us to verify the validity of the latter in assessing the corrosion state of steel [57]. Figure 8 displays the values reached by the corrosion potential and corrosion rate of all armored structures at the end of the test. The combined analysis of these values allows us to determine if the qualitative assessment based on the corrosion potential, with the limits set by ASTM [57] standard, corresponds to the quantitative assessment of the corrosion process based on the corrosion rate values obtained during the tests.
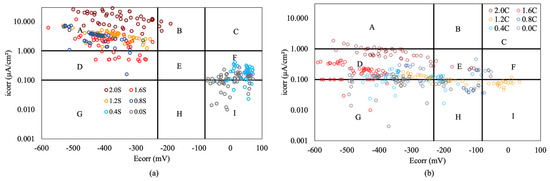
Figure 8.
Comparison of (icorr) and (Ecorr) with different percentages of chloride in armored structures, without inhibitor (a) and with inhibitor (b).
Figure 8a,b show the limits that define active and passive states for the corrosion rate and probabilities greater than 90% or less than 10% for the corrosion potential. These limits divide the figures into 9 quadrants, labeled from “A” to “I”. Zone “A” corresponds to values where there is coherence between the corrosion rate and corrosion potential, indicating an active state of the armored structures. Zone “I” also shows coherence between the results, corresponding to the passive state. Zones “C” and “G” exhibit no coherence between the results obtained, showing a total discrepancy between qualitative and quantitative measurements. In zones “B”, “D”, “E”, “F”, and “H”, the correlation between both types of measurements cannot be ensured [58].
The analysis of Figure 8a,b reveals a higher coherence in the results obtained in structures without inhibitors (Figure 8a), where there are numerous measurements in zones “A” and “I”. However, with the inhibitor present (Figure 8b), these two zones barely have measurements, often resulting in very negative corrosion potentials and average corrosion rates. This is because the corrosion potential heavily depends on the amount of moisture present in the concrete rather than the corrosion state of the steel. Since all slabs had the same moisture level regardless of the inhibitor presence in the armored structures, a large number of measurements with very negative potentials were recorded in all cases. In the case of structures without inhibitors, these measurements were located in quadrant "A" for chloride ion percentages above 0.8%, while in structures with inhibitors, the corrosion rates significantly decreased, placing the measurements in zones “D” and “G”. This suggests that corrosion potential measurements, taken in isolation in structures with inhibitor-containing armored structures, are not indicative of the steel's corrosion state [58]. Therefore, in concretes with reinforcement without a corrosion inhibitor, measuring corrosion potentials is an effective method of determining the corrosion process of the reinforcement. However, in concretes with reinforcement containing an inhibitor, measuring the corrosion potential is not a good method for determining the state of corrosion of the reinforcement, making it necessary to perform corrosion rate measurements. The findings obtained will serve as a basis for other researchers in studying the effectiveness of this type of inhibitors, starting directly from the premise of measuring corrosion rates, in order to determine the corrosion process of the reinforcement.
3.4. Statistical Analysis of icorr and Ecorr in Re-Bars with and without Inhibitor
To assess whether there were significant differences in the corrosion behaviour of reinforcements embedded in concretes with various chloride percentages, both with and without inhibitor, a statistical analysis was conducted using the SPSS software, focusing on the measurement of corrosion rate (icorr) and corrosion potential (Ecorr) [59]. This analysis included a normality test, the results of which are presented in Table 5 and Table 6.

Table 5.
Normality test for icorr.

Table 6.
Normality test for Ecorr.
In both cases, the assumption of normality is not met according to the Kolmogorov–Smirnov–Lilliefors p-value. Therefore, from a non-parametric viewpoint, we proceeded with tests comparing median or average ranks, such as the Mann–Whitney U test, as shown in Table 7.

Table 7.
Normality test for icorr Ecorr.
The results showed significant differences in median values between the response variable measurements of icorr regarding the 2 groups of inhibitor use, Yes/No (U = 16,260.5, p < 0.001). The median was significantly higher for the case of re-bars without inhibitor (2.27 vs. 0.12). In the case of the response variable Ecorr, statistically significant differences in median or average ranks between the 2 groups were observed (H = 39,999.5, p = 0.019). The median was slightly higher for the case of re-bars without inhibitor (−336.3 vs. −332.25), conclusively demonstrating that the use of the inhibitor for any percentage of chlorides performs well and helps the re-bars to reduce the corrosion rate, thereby increasing the lifespan of the structures.
Subsequently, the corrosion behaviour of reinforcement embedded in concretes with different chloride percentages will be statistically studied, using measurements of corrosion rate and corrosion potential, as detailed in Table 8.

Table 8.
Test statistics icorr Ecorr.
As observed, statistically significant differences were noted in the median between at least one pair of groups (H = 232.48, p < 0.001) for icorr and (H = 177.01, p < 0.001) for Ecorr.
Using the Bonferroni post hoc method for multiple comparisons, statistically significant differences were identified in the corrosion rate in most comparisons, as shown in Table 9. However, no significant differences were observed between concentrations of 1.2% and 0.8%, nor between 1.2% and 1.6% and between 0.8% and 1.6%, where both groups exhibited similar median behaviors. This analysis corroborates the results shown in Figure 7. Regarding the corrosion potential, statistically significant differences were also found in most comparisons, except for the pairs showing 2% with 0.8% and 0% with 0.4%. The correlation between the corrosion potentials for concretes with 0.8 and 2.0% chloride ion, indicating a corrosion probability above 90%, is consistent with the results obtained in the corrosion rate measurements, which show values typical of the active state. The percentages of 0 and 0.4% chloride ion, where the corrosion potentials are correlated, showing a corrosion probability below 10%, display a result consistent with that obtained from the corrosion rates, where low or very low corrosion levels were observed. Therefore, in a structure without inhibitor, it is possible to determine the corrosion process of a structure through measurements of the corrosion potential.

Table 9.
Pairwise Chloride Comparisons icorr Ecorr.
4. Conclusions
The following conclusions can be drawn from the analysis of the results obtained in the present study:
- Reinforcement bars without inhibitor reach corrosion speeds typical of the active state for chloride ion percentages equal to or greater than 0.8%. In reinforcement bars with inhibitor, a chloride ion percentage of 2.0% in the concrete is necessary for the reinforcement bars to reach corrosion values typical of the active state.
- In all cases, the presence of corrosion inhibitor significantly reduces the corrosion speed achieved by the reinforcement bars.
- The correlation between corrosion potential measurements and corrosion speed is only consistent in reinforcement bars without inhibitor.
- Corrosion potential measurements taken in isolation in structures with reinforcement bars with inhibitor are not indicative of the steel corrosion state.
- The direct application of inhibitors on the reinforcement bars emerges as a viable strategy, both for new constructions and rehabilitation, being particularly effective in concretes with chloride ion percentages between 0.8% and 1.6%, where their application reduces the corrosion speed to low levels, increasing the lifespan of the structures.
As future research directions, the adherence of the reinforcement with and without inhibitor will be studied, in addition to comparing their efficacy with respect to other types of inhibitors (applied superficially and added during mixing).
Author Contributions
Conceptualization, A.L.M. and J.P.F.; methodology, A.L.M. and J.P.F.; validation, A.L.M., J.P.F., A.C.E. and M.I.P.B.; formal analysis, A.L.M., J.P.F., A.C.E., M.I.P.B. and T.G.P.; investigation, A.L.M., J.P.F. and M.I.P.B.; resources, A.C.E. and M.I.P.B.; data curation, J.P.F.; writing—original draft preparation, A.L.M. and J.P.F.; writing—review and editing, A.L.M., A.C.E., M.I.P.B. and T.G.P.; visualization, A.L.M.; supervision, A.C.E. and M.I.P.B.; project administration, A.C.E. and M.I.P.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prieto Barrio, M.I. Análisis del Comportamiento a Corrosión de Armaduras Embebidas en Probetas de Mortero. Con Sustitución Parcial de Áridos y Cemento por Escorias Blancas de Horno Cuchara. Ph.D. Thesis, Arquitectura_Tecnica, Escuela Universitaria de Arquitectura Técnica, La Coruña, Spain, 2012. [Google Scholar]
- Al-Amoudi, O.S.B.; Maslehuddin, M.; Lashari, A.N.; Almusallam, A.A. Effectiveness of corrosion inhibitors in contaminated concrete. Cem. Concr. Compos. 2003, 25, 439–449. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Purnima; Goyal, S.; Luxami, V. Influence of corrosion inhibitors on two different concrete systems under combined chloride and carbonated environment. Structures 2023, 48, 717–735. [Google Scholar] [CrossRef]
- Tian, Y.; Bao, J.; Xie, D.; Wang, B.; Zhang, P.; Zhao, T.; Lei, D. The effects of organic corrosion inhibitor on concrete properties and frost resistance. J. Build. Eng. 2023, 65, 105762. [Google Scholar] [CrossRef]
- Medina, E. Construcción de Estructuras de Hormigón Armado; Bellisco Ediciones: Madrid, Spain, 2009. [Google Scholar]
- Aguirre, A.M.; De Gutiérrez, R.M. Durabilidad del hormigón armado expuesto a condiciones agresivas. Mater. Construcción 2013, 63, 7–38. [Google Scholar] [CrossRef]
- BOE-A-2021-13681 Real Decreto 470/2021. de 29 de Junio. Por el que se Aprueba el Código Estructural. (s. f.); Ministerio de la Presidencia: Madrid, Spain, 2021.
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Yan, H.; Shu, H.; Wang, J. Study on the influence of compound rust inhibitor on corrosion of steel bars in chloride concrete by electrical parameters. Constr. Build. Mater. 2020, 262, 120763. [Google Scholar] [CrossRef]
- Balaji, C.R.; De Azevedo, A.R.G.; Madurwar, M.V. Sustainable perspective of ancillary construction materials in infrastructure industry: An overview. J. Clean. Prod. 2022, 365, 132864. [Google Scholar] [CrossRef]
- Maury-Ramírez, A.; De Belie, N. Environmental and Economic Assessment of Eco-Concrete for Residential Buildings: A Case Study of Santiago de Cali (Colombia). Sustainability 2023, 15, 12032. [Google Scholar] [CrossRef]
- Da Silva, S.R.; De Oliveira Andrade, J.J. A Review on the Effect of Mechanical Properties and Durability of Concrete with Construction and Demolition Waste (CDW) and Fly Ash in the Production of New Cement Concrete. Sustainability 2022, 14, 6740. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J.; Al-Nuaimi, N. Recent Advancements in the Nanomaterial Application in Concrete and Its Ecological Impact. Materials 2021, 14, 6387. [Google Scholar] [CrossRef]
- James, A.; Bazarchi, E.; Chiniforush, A.A.; Aghdam, P.P.; Hosseini, M.R.; Akbarnezhad, A.; Martek, I.; Ghodoosi, F. Rebar corrosion detection. protection. and rehabilitation of reinforced concrete structures in coastal environments: A review. Constr. Build. Mater. 2019, 224, 1026–1039. [Google Scholar] [CrossRef]
- Wittocx, L.; Buyle, M.; Audenaert, A.; Seuntjens, O.; Renne, N.; Craeye, B. Revamping corrosion damaged reinforced concrete balconies: Life cycle assessment and life cycle cost of life-extending repair methods. J. Build. Eng. 2022, 52, 104436. [Google Scholar] [CrossRef]
- Gandía-Romero, J.M. Sensores Electroquímicos Aplicados al Estudio de la Corrosión en Estructuras de Hormigón Armado. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2015. [Google Scholar] [CrossRef]
- Crişan, M.; Muntean, C.; Chumakov, Y.; Pleşu, N. Investigating the Corrosion Inhibition Mechanisms of Alkanolammonium Salts: A Case Study with Ethylethanolammonium 4-Nitrobenzoate on Carbon Steel in Saline Solution. Appl. Sci. 2024, 14, 1832. [Google Scholar] [CrossRef]
- Bonilla Mieles, A.F. Efectividad de los Inhihidores de Corrosión en el Hormigón Armado; Universidad Politécnica de Madrid: Madrid, Spain, 2023. [Google Scholar]
- Bellal, Y.; Benghanem, F.; Keraghel, S. A new corrosion inhibitor for steel rebar in concrete: Synthesis. electrochemical and theoretical studies. J. Mol. Struct. 2021, 1225, 129257. [Google Scholar] [CrossRef]
- Wei, A.; Tan, M.Y.; Koay, Y.C.; Hu, X.; Al-Ameri, R. Effect of carbon fiber waste on steel corrosion of reinforced concrete structures exposed to the marine environment. J. Clean. Prod. 2021, 316, 128356. [Google Scholar] [CrossRef]
- Qu, L.; Wang, Q.; Xu, S.; Wang, N.; Shi, Z. Chloride corrosion resistance of double-layer anticorrosive coating in simulated concrete pore solution. Constr. Build. Mater. 2021, 295, 123682. [Google Scholar] [CrossRef]
- Deza, A. Análisis del Comportamiento Frente a la Corrosión del Acero Embebido en Hormigón en Ambiente Marino. Ph.D. Thesis, E.T.S.I. Navales (UPM), Madrid, Spain, 2017. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete. Ph.D. Thesis, Swedish Cement and Concrete Research Institute, Stockholm, Sweden, 1982. [Google Scholar]
- Rodrigues, R.; Gaboreau, S.; Gance, J.; Ignatiadis, I.; Betelu, S. Reinforced concrete structures: A review of corrosion mechanisms and advances in electrical methods for corrosion monitoring. Constr. Build. Mater. 2021, 269, 121240. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Khalid, M.A.; Miyazato, S.; Mizuguchi, H.; Miyaguchi, K. Performance Evaluation of Lithium Nitrite-Based Gel against Corrosion of Rebar with Partial Short Cover Depth in Chloride Environment. Eng. Proc. 2023, 55, 35. [Google Scholar] [CrossRef]
- González-Parra, J.R.; Di Turo, F. The Use of Plant Extracts as Sustainable Corrosion Inhibitors for Cultural Heritage Alloys: A Mini-Review. Sustainability 2024, 16, 1868. [Google Scholar] [CrossRef]
- Zhang, Y.; Ayyub, B.M.; Fung, J.F. Projections of corrosion and deterioration of infrastructure in United States coasts under a changing climate. Resilient Cities Struct. 2022, 1, 98–109. [Google Scholar] [CrossRef]
- Anterrieu, O.; Giroux, B.; Gloaguen, E.; Carde, C. Non-destructive data assimilation as a tool to diagnose corrosion rate in reinforced concrete structures. J. Build. Eng. 2019, 23, 193–206. [Google Scholar] [CrossRef]
- Bouteiller, V.; Tissier, Y.; Marie-Vic, E.; Chaussadent, T.; Joiret, S. The application of electrochemical chloride extraction to reinforced concrete—A review. Constr. Build. Mater. 2022, 351, 128931. [Google Scholar] [CrossRef]
- Carmona, J.; Garcés, P.; Climent, M. Efficiency of a conductive cement-based anodic system for the application of cathodic protection. cathodic prevention and electrochemical chloride extraction to control corrosion in reinforced concrete structures. Corros. Sci. 2015, 96, 102–111. [Google Scholar] [CrossRef]
- Helene, P.R.; Pereira, F. Manual de Rehabilitación de Estructuras de Hormigón Reparación, Refuerzo y Protección; Ciencia y Tecnología para Desarrollo: Buenos Aires, Argentina, 2003. [Google Scholar]
- Liu, Y.; Ding, W.; Zhao, P.; Qin, L.; Shiotani, T. Research on in-situ corrosion process monitoring and evaluation of reinforced concrete via ultrasonic guided waves. Constr. Build. Mater. 2022, 321, 126317. [Google Scholar] [CrossRef]
- Andrade, C. Steel corrosion rates in concrete in contact to sea water. Cem. Concr. Res. 2023, 165, 107085. [Google Scholar] [CrossRef]
- Suárez Quintero, J.A. Evaluación de Inhibidores de Corrosión Aplicados en la Industria Mediante Pruebas Electroquímicas. Ph.D. Thesis, Universidad Pedagógica y Tecnológica de Colombia, Tunja, Colombia, 2018. [Google Scholar]
- Al-Akhras, N.M.; Mashaqbeh, Y. Potential use of eucalyptus leaves as green corrosion inhibitor of steel reinforcement. J. Build. Eng. 2021, 35, 101848. [Google Scholar] [CrossRef]
- Coppola, L.; Beretta, S.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Caputo, D.; Carsana, M.; Cioffi, R.; et al. The Improvement of Durability of Reinforced Concretes for Sustainable Structures: A Review on Different Approaches. Materials 2023, 15, 2728. [Google Scholar] [CrossRef] [PubMed]
- Tsouli, S.; Nikolaidis, C.; Kleftakis, S.; Lekatou, A.G. The Effect of pH and Fly Ash on the Electrochemical Performance of Stainless-Steel Concrete Reinforcement in Harsh Environments. Mater. Proc. 2021, 6, 25. [Google Scholar] [CrossRef]
- Bijapur, K.; Vandana, M.; Shetty, A.; Toghan, A.; De Padova, P.; Hegde, G. Recent Trends and Progress in Corrosion Inhibitors and Electrochemical Evaluation. Appl. Sci. 2023, 13, 10107. [Google Scholar] [CrossRef]
- Martín, D.; Seyhan, E.C. Protection of Reinforced Concrete Steel Exposed to a Marine Environment: A Preliminary Onsite Study of the Performance of a New Generation of Surface-Applied Corrosion Inhibitors. Corros. Mater. Degrad. 2022, 3, 628–645. [Google Scholar] [CrossRef]
- Chaudhari, B.; Panda, B.; Šavija, B.; Paul, S.C. Microbiologically Induced Concrete Corrosion: A Concise Review of Assessment Methods, Effects, and Corrosion-Resistant Coating Materials. Materials 2022, 15, 4279. [Google Scholar] [CrossRef] [PubMed]
- Batis, G.; Routoulas, A.; Rakanta, E. Effects of migrating inhibitors on corrosion of reinforcing steel covered with repair mortar. Cem. Concr. Compos. 2003, 25, 109–115. [Google Scholar] [CrossRef]
- Al-Tholaia, M.M.H.; Azad, A.K.; Ahmad, S.; Baluch, M.H. A comparative study of corrosion resistance of different coatings for mortar–embedded steel plates. Constr. Build. Mater. 2014, 56, 74–80. [Google Scholar] [CrossRef]
- Fernández Cánovas, M.; Gálvez Ruiz, J.; Goicolea Marin, P. Estudio del comportamiento mecánico y frente a corrosión de armaduras pasivas de acero revestidas con resina epoxi. Hormigón Acero 2010, 61, 91–104. [Google Scholar]
- Wang, Y.; Wang, C.; Zhou, S.; Liu, K. Influence of cationic epoxy resin type on electrophoretic deposition effect on repair of rust-cracked reinforced concrete. Constr. Build. Mater. 2022, 324, 126714. [Google Scholar] [CrossRef]
- De Domenico, D.; Messina, D.; Recupero, A. Seismic vulnerability assessment of reinforced concrete bridge piers with corroded bars. Struct. Concr. 2023, 24, 56–83. [Google Scholar] [CrossRef]
- Casanova, L.; Ceriani, F.; Messinese, E.; Paterlini, L.; Beretta, S.; Bolzoni, F.M.; Brenna, A.; Diamanti, M.V.; Ormellese, M.; Pedeferri, M. Recent Advances in the Use of Green Corrosion Inhibitors to Prevent Chloride-Induced Corrosion in Reinforced Concrete. Materials 2023, 16, 7462. [Google Scholar] [CrossRef]
- Shehnazdeep; Pradhan, B. A study on effectiveness of inorganic and organic corrosion inhibitors on rebar corrosion in concrete: A review. Mater. Today Proc. 2022, 65, 1360–1366. [Google Scholar] [CrossRef]
- BOE-A-2016-6167 Real Decreto 256/2016. de 10 de Junio. por el que se Aprueba la Instrucción Para la Recepción de Cementos (RC-16). (s. f.). Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2016-6167 (accessed on 21 January 2024).
- UNE-EN 13139:2003 Áridos Para Morteros. (s. f.). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0028815 (accessed on 21 January 2024).
- UNE-EN 12620:2003+A1:2009 Áridos Para Hormigón. (s. f.). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0043155 (accessed on 21 January 2024).
- UNE-EN 12390-19:2023 Ensayos de Hormigón Endurecido. Parte 19: … (s. f.). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0071389 (accessed on 21 January 2024).
- Polder, R. Test methods for on site measurement of resistivity of concrete—A RILEM TC-154 technical recommendation. Constr. Build. Mater. 2001, 15, 125–131. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, S.; Chen, E.; Li, W. A review on corrosion detection and protection of existing reinforced concrete (RC) structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Robles, K.P.V.; Yee, J.; Kee, S. Electrical Resistivity Measurements for Nondestructive Evaluation of Chloride-Induced Deterioration of Reinforced Concrete—A Review. Materials 2022, 15, 2725. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, D.; Alonso, C.; Andrade, C.; Castellote, M. Potentiostatic determination of chloride threshold values for rebar depassivation. Electrochim. Acta 2004, 49, 2731–2739. [Google Scholar] [CrossRef]
- Harilal, M.; Rathish, V.; Anandkumar, B.; George, R.; Mohammed, M.S.; Philip, J.; Amarendra, G. High performance green concrete (HPGC) with improved strength and chloride ion penetration resistance by synergistic action of fly ash. nanoparticles and corrosion inhibitor. Constr. Build. Mater. 2019, 198, 299–312. [Google Scholar] [CrossRef]
- ASTM C876-22b; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshehoken, PA, USA, 2022. Available online: https://www.astm.org (accessed on 21 January 2024).
- González, M.R.; Barrio, M.I.P.; Escamilla, A.C.; Olmedo, F.I. Electrochemical Study of Clean and Pre-Corroded Reinforcements Embedded in Mortar Samples with Variable Amounts of Chloride Ions. Materials 2021, 14, 6883. [Google Scholar] [CrossRef]
- IBM. (n.d.). IBM SPSS Statistics. Available online: https://www.ibm.com/es-es/products/spss-statistics (accessed on 25 March 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).