Recent Advances in the Strategies of Simultaneous Enzyme Immobilization Accompanied by Nanocarrier Synthesis
Abstract
1. Introduction
2. Organic–Inorganic Hybrid Nanoflowers
2.1. Conventional HNFs Immobilized Enzymes
2.2. Factors Affecting the Formation of HNFs
2.3. Combination of Hybridized HNFs with Other Immobilization Methods
3. Metal–Organic Frameworks
3.1. MOFs by the Co-Precipitation Method
3.1.1. PVP Co-Precipitating Agent
3.1.2. Alkali Co-Precipitating Agents
3.2. MOFs by Bionic Mineralization
4. Conductive Polymers
4.1. Immobilization by Electrochemical Polymerization of CP Monomers
4.2. Immobilization by Enzymatic Polymerization of CP Monomers
4.3. Other Strategies for Enzyme Immobilization by Electrochemistry
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name | Abbreviations | Full Name |
| HNFs | Organic–inorganic hybrid nanoflowers | HRP | Horseradish peroxidase |
| MOFs | Metal–organic frameworks | GOx | Glucose oxidase |
| ZIF-8 | Zeolitic imidazolate framework-8 | Ur | Urease |
| ZIF-90 | Zeolitic imidazolate framework-90 | Cyt c | Cytochrome c |
| MIL-53 | Materials of Institut Lavoisier-53 | Cys | Cysteine |
| MIL-88 | Materials of Institut Lavoisier-88 | β-Glu | β-glucosidase |
| MIL-100 | Materials of Institut Lavoisier-100 | PyOx | Pyranose oxidase |
| NH2-H2BDC | 2-Aminoterephthalic acid | FDH | Formate dehydrogenase |
| Eu/Tb-BDC | Eu2(1,4-BDC)3(H2O)4/Tb2(1,4-BDC)3(H2O)4 | CPO | Chloroperoxidase |
| MIL-88A | Materials of Institut Lavoisier-88A | QLM | Thermophilic lipase |
| MAF-7 | Metal azolate framework-7 | gRGO | Graphene oxide |
| HKUST-1 | Hong Kong University of Science and Technology-1 | ChOx | Cholesterol oxidase |
| PDA | Polydopamine | LOx | Lactate oxidase |
| EDTA | Ethylenediaminetetraacetic acid | COx | Cholesterol oxidase |
| ABTS | 2, 2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) | FCAT | Fluorescently labeled catalase |
| HAuCl4 | Chloroauric acid | GOD | Glucose oxidase |
| PVP | Polyvinylpyrrolidone | CE | Cholesterol esterase |
| PVP K-30 | Polyvinylpyrrolidone K-30 | Ge | Gelatin |
| CNTs | Carbon nanotubes | CS | Chitosan |
| MNPs | Magnetic nanoparticles | CS-Fc | Chitosan derivatives |
| AuNPs | Gold nanoparticles | CaP | Calcium phosphate |
| Pd-NPs | Palladium nanoparticles | SPase | Sucrose phosphorylase |
| (R)-PEDH | (R)-1-phenylethanol dehydrogenase | ω-ta | Omega-transaminase |
| K4Fe(CN)6 | Potassium ferrocyanide | PLP | 5’-Pyridoxal phosphate |
| NADH | Nicotinamide adenine dinucleotide | SiO2 | Silicon dioxide |
| CPs | Conductive polymers | mIM | 2-Methylimidazole |
| PPy | Conductive polymers | Hmtz | 3-Methyl-1,2,4-triazole |
| PEDOT | Poly(3,4-ethylene-dioxythiophene) | TEA | Triethylamine |
| PANI | Polyaniline | NPG | Nanoporous gold electrode |
| PTH | Polythiophene | Py-NH2 | Amino pyrrole |
References
- Bilal, M.; Asgher, M.; Shah, S.Z.H.; Iqbal, H.M.N. Engineering Enzyme-Coupled Hybrid Nanoflowers: The Quest for Optimum Performance to Meet Biocatalytic Challenges and Opportunities. Int. J. Biol. Macromol. 2019, 135, 677–690. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Tavlasoglu, S.; ÿzdemir, N.; Ocsoy, I. A New Generation Approach in Enzyme Immobilization: Organic-Inorganic Hybrid Nanoflowers with Enhanced Catalytic Activity and Stability. Enzyme Microb. Technol. 2016, 93–94, 105–112. [Google Scholar] [CrossRef]
- Cui, J.; Jia, S. Organic–Inorganic Hybrid Nanoflowers: A Novel Host Platform for Immobilizing Biomolecules. Coord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C. Recent Advances in Nano-Carrier Immobilized Enzymes and Their Applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme Immobilization by Adsorption: A Review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Mo, H.; Qiu, J.; Yang, C.; Zang, L.; Sakai, E. Preparation and Characterization of Magnetic Polyporous Biochar for Cellulase Immobilization by Physical Adsorption. Cellulose 2020, 27, 4963–4973. [Google Scholar] [CrossRef]
- Song, J.; Shen, H.; Yang, Y.; Zhou, Z.; Su, P.; Yang, Y. Multifunctional Magnetic Particles for Effective Suppression of Non-Specific Adsorption and Coimmobilization of Multiple Enzymes by DNA Directed Immobilization. J. Mater. Chem. B 2018, 6, 5718–5728. [Google Scholar] [CrossRef]
- Acet, Ö.; İnanan, T.; Acet, B.Ö.; Dikici, E.; Odabaşı, M. α-Amylase Immobilized Composite Cryogels: Some Studies on Kinetic and Adsorption Factors. Appl. Biochem. Biotechnol. 2021, 193, 2483–2496. [Google Scholar] [CrossRef]
- Zhao, C.-P.; Yin, S.-J.; Chen, G.-Y.; Wang, Y.; Chen, H.; Zhao, J.; Yang, F.-Q. Adsorbed Hollow Fiber Immobilized Tyrosinase for the Screening of Enzyme Inhibitors from Pueraria Lobata Extract. J. Pharm. Biomed. Anal. 2021, 193, 113743. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Kalita, B.J.; Sit, N. Characterization of Cellulase Immobilized by Different Methods of Entrapment and Its Application for Carrot Juice Extraction. Food Sci. Biotechnol. 2024, 33, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yuan, Y.; Salmon, S. Carbonic Anhydrase Immobilized on Textile Structured Packing Using Chitosan Entrapment for CO2 Capture. ACS Sustain. Chem. Eng. 2022, 10, 7772–7785. [Google Scholar] [CrossRef]
- Tizchang, S.; Khiabani, M.S.; Mokarram, R.R.; Hamishehkar, H.; Mohammadi, N.S.; Chisti, Y. Immobilization of β-Galactosidase by Halloysite-Adsorption and Entrapment in a Cellulose Nanocrystals Matrix. Biochim. Biophys. Acta BBA-Gen. Subj. 2021, 1865, 129896. [Google Scholar] [CrossRef] [PubMed]
- Kadziński, L.; Łyżeń, R.; Bury, K.; Banecki, B. Modeling and Optimization of β-Galactosidase Entrapping in Polydimethylsiloxane-Modified Silica Composites. Int. J. Mol. Sci. 2022, 23, 5395. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Appiah, B.; Zhang, B.-W.; Yang, Z.-H.; Quan, C. Recent Advances in Enzyme Immobilization Based on Nanoflowers. J. Catal. 2023, 418, 31–39. [Google Scholar] [CrossRef]
- Shi, N.; Zheng, M.; Wu, X.; Chen, N.; Jiang, L.; Chang, B.; Lu, F.; Liu, F. Construction and Catalytic Study of Affinity Peptide Orientation and Light Crosslinking Immobilized Sucrose Isomerase. J. Agric. Food Chem. 2023, 71, 13401–13408. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, G.; Liu, N.; Liu, L. Preparation and Properties of Rhizopus Oryzae Lipase Immobilized Using an Adsorption-Crosslinking Method. Int. J. Food Prop. 2016, 19, 1776–1785. [Google Scholar] [CrossRef]
- Ouyang, J.; Pu, S.; Wang, J.; Deng, Y.; Yang, C.; Naseer, S.; Li, D. Enzymatic Hydrolysate of Geniposide Directly Acts as Cross-Linking Agent for Enzyme Immobilization. Process Biochem. 2020, 99, 187–195. [Google Scholar] [CrossRef]
- Guerrieri, A.; Ciriello, R.; Acquavia, M.A.; Bianco, G.; Di Capua, A. Electrophoretic Protein Deposition as a Tool for In Situ Co-Crosslinking Enzyme Immobilization: An Electrochemical/Quartz Crystal Microbalance Study. Appl. Sci. 2023, 14, 212. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic Nanoparticles as Versatile Carriers for Enzymes Immobilization: A Review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, X.-L.; Xiong, J.; Zong, M.-H.; Lou, W.-Y. Metal-Organic Frameworks as Novel Matrices for Efficient Enzyme Immobilization: An Update Review. Coord. Chem. Rev. 2020, 406, 213149. [Google Scholar] [CrossRef]
- Oke, M.A.; Ojo, S.A.; Fasiku, S.A.; Adebayo, E.A. Nanotechnology and Enzyme Immobilization: A Review. Nanotechnology 2023, 34, 385101. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.L.; França, A.D.S.; De Castro, A.M.; Alves De Souza, R.O.M.; Esteves, P.M.; Gonçalves, R.S.B. Enzyme Immobilization in Covalent Organic Frameworks: Strategies and Applications in Biocatalysis. ChemPlusChem 2020, 85, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Lin, Z.-X.; Wu, T.-Y.; Hsu, C.-H.; Lin, H.-P.; Wu, T.-S. Effects of Morphology and Pore Size of Mesoporous Silicas on the Efficiency of an Immobilized Enzyme. RSC Adv. 2021, 11, 10010–10017. [Google Scholar] [CrossRef]
- Samui, A.; Chowdhuri, A.R.; Sahu, S.K. Lipase Immobilized Metal-Organic Frameworks as Remarkably Biocatalyst for Ester Hydrolysis: A One Step Approach for Lipase Immobilization. ChemistrySelect 2019, 4, 3745–3751. [Google Scholar] [CrossRef]
- Wang, N.; Li, Y.; Xie, L.; Zhang, F.; Ma, K.-Q.; Chen, X.-P.; Hu, S.; Yang, C.-T.; Wang, X.-L. Cerium Separation with NaBiO3 Nanoflower Material via an Oxidation Adsorption Strategy. J. Mater. Chem. A 2020, 8, 7907–7913. [Google Scholar] [CrossRef]
- Lee, S.J.; Jang, H.; Lee, D.N. Inorganic Nanoflowers-Synthetic Strategies and Physicochemical Properties for Biomedical Applications: A Review. Pharmaceutics 2022, 14, 1887. [Google Scholar] [CrossRef]
- Rahmah, M.I.; Qasim, H.B. A Novel Method to Prepare Antibacterial ZnO Nanoflowers. Appl. Phys. A 2022, 128, 998. [Google Scholar] [CrossRef]
- Souza, J.S.; Hirata, F.T.H.; Corio, P. Microwave-Assisted Synthesis of Bismuth Vanadate Nanoflowers Decorated with Gold Nanoparticles with Enhanced Photocatalytic Activity. J. Nanoparticle Res. 2019, 21, 35. [Google Scholar] [CrossRef]
- Negrón, L.M.; Díaz, T.L.; Ortiz-Quiles, E.O.; Dieppa-Matos, D.; Madera-Soto, B.; Rivera, J.M. Organic Nanoflowers from a Wide Variety of Molecules Templated by a Hierarchical Supramolecular Scaffold. Langmuir 2016, 32, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; PC, S.; Chaudhary, N.; Ramakrishnan, V. Single Crystal Organic Nanoflowers. Sci. Rep. 2017, 7, 17335. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Ghosh, S.; Sreedhar, R.; Kumari, K.; Thota, S.; Ramakrishnan, V. Anisotropic Ferromagnetic Organic Nanoflowers. J. Phys. Chem. C 2022, 126, 8511–8518. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein-Inorganic Hybrid Nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Gao, C.; Chen, L.; He, Y.; Ma, W.; Lin, Z. Recent Advances in Biomolecule Immobilization Based on Self-Assembly: Organic–Inorganic Hybrid Nanoflowers and Metal-Organic Frameworks as Novel Substrates. J. Mater. Chem. B 2018, 6, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ye, R. One-Pot Synthesis of Enzyme and Antibody/CaHPO4 Nanoflowers for Magnetic Chemiluminescence Immunoassay of Salmonella Enteritidis. Sensors 2023, 23, 2779. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jiang, T.; Lu, T.; Jin, Q.; Xi, Y.; Zhang, W. Biomimetic-Mineralized Bifunctional Nanoflowers for Enzyme-Free and Colorimetric Immunological Detection of Protein Biomarker. Talanta 2022, 238, 123001. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Tang, D.; Wang, J.; Lin, Y. Biomimetic -Mineralized Multifunctional Nanoflowers for Anodic-Stripping Voltammetric Immunoassay of Rehabilitation-Related Proteins. Analyst 2022, 147, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Zhu, J.; Hu, X.; Ji, X.; He, Z. Three-Dimensional Immunosensing Platform Based on a Hybrid Nanoflower for Sensitive Detection of α-Fetoprotein and Enterovirus 71. ACS Appl. Nano Mater. 2018, 1, 4964–4971. [Google Scholar] [CrossRef]
- Zhang, C.; Du, C.; Liu, W.; Guo, T.; Zhou, Y.; Zhou, H.; Zhang, Y.; Liu, X.; Ma, L. A High Sensitivity Electrochemical Immunosensor Based on Monoclonal Antibody Coupled Flower-Shaped Nano-ZnO for Detection of Tenuazonic Acid. Agriculture 2022, 12, 204. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wang, M.; Wang, Z.; Wang, L.; Ma, L.; Huang, X.; Li, Z. Structure Advantage and Peroxidase Activity Enhancement of Deuterohemin-Peptide–Inorganic Hybrid Flowers. RSC Adv. 2016, 6, 104265–104272. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Cao, Y.; Song, Y.; Xu, L.-P.; Zhang, X.; Wang, S. Bioinspired DNA-Inorganic Hybrid Nanoflowers Combined with a Personal Glucose Meter for Onsite Detection of miRNA. ACS Appl. Mater. Interfaces 2018, 10, 42050–42057. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Jiang, H.; Gao, W.; Liu, S.G.; Zhao, Q.; Shi, X. DNA-Dependent Prussian Blue Nanoflowers for Biosensing, Catalysis and Imaging. Adv. Funct. Mater. 2023, 33, 2208897. [Google Scholar] [CrossRef]
- Qi, L.; Yang, M.; Chang, D.; Zhao, W.; Zhang, S.; Du, Y.; Li, Y. A DNA Nanoflower-Assisted Separation-Free Nucleic Acid Detection Platform with a Commercial Pregnancy Test Strip. Angew. Chem. Int. Ed. 2021, 60, 24823–24827. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, D.; Ma, Y.; Liu, J.; Li, Y.; Reza, R.; Zhang, Z.; Liu, J.; Zhang, K. Photoactivated Self-Disassembly of Multifunctional DNA Nanoflower Enables Amplified Autophagy Suppression for Low-Dose Photodynamic Therapy. Small 2021, 17, 2104722. [Google Scholar] [CrossRef]
- Zhang, L.; Abdullah, R.; Hu, X.; Bai, H.; Fan, H.; He, L.; Liang, H.; Zou, J.; Liu, Y.; Sun, Y.; et al. Engineering of Bioinspired, Size-Controllable, Self-Degradable Cancer-Targeting DNA Nanoflowers via the Incorporation of an Artificial Sandwich Base. J. Am. Chem. Soc. 2019, 141, 4282–4290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Su, Z. Enzyme-Based Hybrid Nanoflowers with High Performances for Biocatalytic, Biomedical, and Environmental Applications. Coord. Chem. Rev. 2020, 416, 213342. [Google Scholar] [CrossRef]
- Jafari-Nodoushan, H.; Mojtabavi, S.; Faramarzi, M.A.; Samadi, N. Organic-Inorganic Hybrid Nanoflowers: The Known, the Unknown, and the Future. Adv. Colloid Interface Sci. 2022, 309, 102780. [Google Scholar] [CrossRef] [PubMed]
- Vojdanitalab, K.; Jafari-Nodoushan, H.; Mojtabavi, S.; Shokri, M.; Jahandar, H.; Faramarzi, M.A. Instantaneous Synthesis and Full Characterization of Organic-Inorganic Laccase-Cobalt Phosphate Hybrid Nanoflowers. Sci. Rep. 2022, 12, 9297. [Google Scholar] [CrossRef]
- Zhang, B.; Li, P.; Zhang, H.; Wang, H.; Li, X.; Tian, L.; Ali, N.; Ali, Z.; Zhang, Q. Preparation of Lipase/Zn3(PO4)2 Hybrid Nanoflower and Its Catalytic Performance as an Immobilized Enzyme. Chem. Eng. J. 2016, 291, 287–297. [Google Scholar] [CrossRef]
- Somturk, B.; Hancer, M.; Ocsoy, I.; Özdemir, N. Synthesis of Copper Ion Incorporated Horseradish Peroxidase-Based Hybrid Nanoflowers for Enhanced Catalytic Activity and Stability. Dalton Trans. 2015, 44, 13845–13852. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, L.; He, Q.; Zhu, Q.; Zhang, C.; Cui, X.; Zheng, J.; Zhao, S. Bifunctional Hybrid Enzyme-Catalytic Metal Organic Framework Reactors for α-Glucosidase Inhibitor Screening. ACS Appl. Mater. Interfaces 2019, 11, 32769–32777. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.S.; Nadar, S.S.; Rathod, V.K. A Rapid Self-Assembled Hybrid Bio-Microflowers of Alpha-Amylase with Enhanced Activity. J. Biotechnol. 2020, 317, 27–33. [Google Scholar] [CrossRef]
- Somturk, B.; Yilmaz, I.; Altinkaynak, C.; Karatepe, A.; Özdemir, N.; Ocsoy, I. Synthesis of Urease Hybrid Nanoflowers and Their Enhanced Catalytic Properties. Enzyme Microb. Technol. 2016, 86, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Fei, X.; Li, Y.; Tian, J.; Xu, L.; Wang, X.; Wang, Y. Hierarchical Assembly of Enzyme-Inorganic Composite Materials with Extremely High Enzyme Activity. RSC Adv. 2015, 5, 96997–97002. [Google Scholar] [CrossRef]
- Cao, G.; Gao, J.; Zhou, L.; He, Y.; Li, J.; Jiang, Y. Enrichment and Coimmobilization of Cofactors and His-Tagged ω-Transaminase into Nanoflowers: A Facile Approach to Constructing Self-Sufficient Biocatalysts. ACS Appl. Nano Mater. 2018, 1, 3417–3425. [Google Scholar] [CrossRef]
- Zheng, L.; Xie, X.; Wang, Z.; Zhang, Y.; Wang, L.; Cui, X.; Huang, H.; Zhuang, H. Fabrication of a Nano-Biocatalyst for Regioselective Acylation of Arbutin. Green Chem. Lett. Rev. 2018, 11, 55–61. [Google Scholar] [CrossRef]
- Rai, S.K.; Narnoliya, L.K.; Sangwan, R.S.; Yadav, S.K. Self-Assembled Hybrid Nanoflowers of Manganese Phosphate and L-Arabinose Isomerase: A Stable and Recyclable Nanobiocatalyst for Equilibrium Level Conversion of D-Galactose to D-Tagatose. ACS Sustain. Chem. Eng. 2018, 6, 6296–6304. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Yilmaz, I.; Koksal, Z.; Özdemir, H.; Ocsoy, I.; Özdemir, N. Preparation of Lactoperoxidase Incorporated Hybrid Nanoflower and Its Excellent Activity and Stability. Int. J. Biol. Macromol. 2016, 84, 402–409. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Wang, A.; Li, N.; Chen, X.; Pei, X.; Zhang, P.; Wu, S.G. Dual-Cycle Immobilization to Reuse Both Enzyme and Support by Reblossoming Enzyme-Inorganic Hybrid Nanoflowers. RSC Adv. 2018, 8, 16088–16094. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Zhang, H.; Xin, Y.; Shi, Y.; Gu, Z.; Zhang, L.; Zhong, J.; Guo, X.; Li, Y.; et al. Development of a Multimetal-Based Phosphotriesterase Hybrid Nanoflowers for Decontamination of Environmental Organophosphorus Compounds Pollution. Chem. Eng. J. 2022, 446, 136933. [Google Scholar] [CrossRef]
- Sharma, N.; Parhizkar, M.; Cong, W.; Mateti, S.; Kirkland, M.A.; Puri, M.; Sutti, A. Metal Ion Type Significantly Affects the Morphology but Not the Activity of Lipase-Metal-Phosphate Nanoflowers. RSC Adv. 2017, 7, 25437–25443. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Wang, A.; Li, H.; Wang, C.; Pei, X.; Zhang, P.; Wu, S.G. Efficient Synthesis of the Key Chiral Alcohol Intermediate of Crizotinib Using Dual-enzyme@CaHPO4 Hybrid Nanoflowers Assembled by Mimetic Biomineralization: Efficient Synthesis of Crizotinib Intermediate Using Dual-enzyme@CaHPO4 Hybrid Nanoflowers. J. Chem. Technol. Biotechnol. 2019, 94, 236–243. [Google Scholar] [CrossRef]
- Cheng, P.; Tang, M.; Chen, Z.; Liu, W.; Jiang, X.; Pei, X.; Su, W. Dual-Enzyme and NADPH Co-Embedded Organic-Inorganic Hybrid Nanoflowers Prepared Using Biomimetic Mineralization for the Asymmetric Synthesis of (R)-(-)-Pantolactone. React. Chem. Eng. 2020, 5, 1973–1980. [Google Scholar] [CrossRef]
- Li, P.; Jia, J.; Geng, Z.; Pang, S.; Wang, R.; Bilal, M.; Bian, H.; Cui, J.; Jia, S. A Dual Enzyme-Phosphate Hybrid Nanoflower for Glutamate Detection. Particuology 2023, 83, 63–70. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Li, M.; Liu, Y.; Bai, X.; Shi, K.; Chen, M.; Yao, H. Construction of Novel Bienzyme-Inorganic Hybrid Nanoflowers Beads and Their Application in the Efficient Degradation of Acridine. Arab. J. Chem. 2023, 16, 104770. [Google Scholar] [CrossRef]
- Lou, L.; Li, Z.; Li, Z. Rational Design to Enhance Enzyme Activity for the Establishment of an Enzyme-Inorganic Hybrid Nanoflower Co-Immobilization System for Efficient Nucleotide Production. J. Agric. Food Chem. 2022, 70, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Y.; Wang, C.; Wang, Z.; Chen, X.; Li, M.; Zhao, R.; Wang, L. Application of Dual-Enzyme Nanoflower in the Epoxidation of Alkenes. Process Biochem. 2018, 74, 103–107. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Du, M.; Wang, X.; Ji, X.; He, Z. The Preparation of Dual-Functional Hybrid Nanoflower and Its Application in the Ultrasensitive Detection of Disease-Related Biomarker. Biosens. Bioelectron. 2017, 92, 68–73. [Google Scholar] [CrossRef]
- Li, Y.; Xie, G.; Qiu, J.; Zhou, D.; Gou, D.; Tao, Y.; Li, Y.; Chen, H. A New Biosensor Based on the Recognition of Phages and the Signal Amplification of Organic-Inorganic Hybrid Nanoflowers for Discriminating and Quantitating Live Pathogenic Bacteria in Urine. Sens. Actuators B Chem. 2018, 258, 803–812. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of Cross-Linked Protein-Metal Hybrid Nanoflowers and Its Application in Repeated Batch Decolorization of Synthetic Dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Chung, Y.; Kwon, Y. Co-Immobilization of Glucose Oxidase and Catalase for Enhancing the Performance of a Membraneless Glucose Biofuel Cell Operated under Physiological Conditions. Nanoscale 2017, 9, 1993–2002. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wu, J.; Li, Y.; Zhou, Y.; Wang, L.; Luo, P.; Wang, Y. Construction of Multienzyme Co-Immobilized Hybrid Nanoflowers for an Efficient Conversion of Cellulose into Glucose in a Cascade Reaction. J. Agric. Food Chem. 2021, 69, 7910–7921. [Google Scholar] [CrossRef]
- Aydemir, D.; Gecili, F.; Özdemir, N.; Nuray Ulusu, N. Synthesis and Characterization of a Triple Enzyme-Inorganic Hybrid Nanoflower (TrpE@ihNF) as a Combination of Three Pancreatic Digestive Enzymes Amylase, Protease and Lipase. J. Biosci. Bioeng. 2020, 129, 679–686. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Chen, R.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y. Multilayer Petal-like Enzymatic-Inorganic Hybrid Micro-Spheres [CPO-(Cu/Co/Cd)3(PO4)2] with High Bio-Catalytic Activity. Chem. Eng. Res. Des. 2018, 134, 52–61. [Google Scholar] [CrossRef]
- Hua, X.; Xing, Y.; Zhang, X. Controlled Synthesis of an Enzyme–Inorganic Crystal Composite Assembled into a 3D Structure with Ultrahigh Enzymatic Activity. RSC Adv. 2016, 6, 46278–46281. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, H.; Lee, J.-K. Multimetal-Based Inorganic–Protein Hybrid System for Enzyme Immobilization. ACS Sustain. Chem. Eng. 2019, 7, 13633–13638. [Google Scholar] [CrossRef]
- Batule, B.S.; Park, K.S.; Gautam, S.; Cheon, H.J.; Kim, M.I.; Park, H.G. Intrinsic Peroxidase-like Activity of Sonochemically Synthesized Protein Copper Nanoflowers and Its Application for the Sensitive Detection of Glucose. Sens. Actuators B Chem. 2019, 283, 749–754. [Google Scholar] [CrossRef]
- An, S.S.; Park, H.G.; Kim, M.I.; Batule, B.; Park, K.S. Ultrafast Sonochemical Synthesis of Protein-Inorganic Nanoflowers. Int. J. Nanomedicine 2015, 10, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Subramani, I.G.; Perumal, V.; Gopinath, S.C.B.; Fhan, K.S.; Mohamed, N.M. Organic-Inorganic Hybrid Nanoflower Production and Analytical Utilization: Fundamental to Cutting-Edge Technologies. Crit. Rev. Anal. Chem. 2022, 52, 1488–1510. [Google Scholar] [CrossRef]
- Lee, H.R.; Chung, M.; Kim, M.I.; Ha, S.H. Preparation of Glutaraldehyde-Treated Lipase-Inorganic Hybrid Nanoflowers and Their Catalytic Performance as Immobilized Enzymes. Enzyme Microb. Technol. 2017, 105, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liang, H. Chitosan-Regulated Biomimetic Hybrid Nanoflower for Efficiently Immobilizing Enzymes to Enhance Stability and by-Product Tolerance. Int. J. Biol. Macromol. 2022, 220, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Dadi, S.; Temur, N.; Gul, O.T.; Yilmaz, V.; Ocsoy, I. In Situ Synthesis of Horseradish Peroxidase Nanoflower@Carbon Nanotube Hybrid Nanobiocatalysts with Greatly Enhanced Catalytic Activity. Langmuir 2023, 39, 4819–4828. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.J.; Adhikari, M.D.; Chung, M.; Tran, T.D.; Kim, J.; Kim, M.I. Magnetic Nanoparticles-Embedded Enzyme-Inorganic Hybrid Nanoflowers with Enhanced Peroxidase-Like Activity and Substrate Channeling for Glucose Biosensing. Adv. Healthc. Mater. 2019, 8, 1801507. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wen, M.; Wen, W.; Du, D.; Zhang, X.; Wang, S.; Lin, Y. Recent Progress in Biosensors Based on Organic-Inorganic Hybrid Nanoflowers. Biosens. Bioelectron. 2018, 120, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, Y.; Wen, K.; Jiang, W.; Li, Q. Immobilized Enzymes in Inorganic Hybrid Nanoflowers for Biocatalytic and Biosensing Applications. J. Mater. Chem. B 2021, 9, 7597–7607. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; He, Z. Organic-Inorganic Nanoflowers: From Design Strategy to Biomedical Applications. Nanoscale 2019, 11, 17179–17194. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ren, S.; Sun, B.; Jia, S. Optimization Protocols and Improved Strategies for Metal-Organic Frameworks for Immobilizing Enzymes: Current Development and Future Challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W.; Wang, Y. Progress & Prospect of Metal-Organic Frameworks (MOFs) for Enzyme Immobilization (Enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar] [CrossRef]
- Chen, Y.; Lykourinou, V.; Vetromile, C.; Hoang, T.; Ming, L.-J.; Larsen, R.W.; Ma, S. How Can Proteins Enter the Interior of a MOF? Investigation of Cytochrome c Translocation into a MOF Consisting of Mesoporous Cages with Microporous Windows. J. Am. Chem. Soc. 2012, 134, 13188–13191. [Google Scholar] [CrossRef]
- Li, F.-G.; Liu, C.; Yuan, D.; Dai, F.; Wang, R.; Wang, Z.; Lu, X.; Sun, D. Ultrahigh Hydrogen Uptake in an Interpenetrated Zn4O-Based Metal-Organic Framework. CCS Chem. 2022, 4, 832–837. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Hu, M.-C.; Li, S.-N.; Jiang, Y.-C.; Qu, P.; Zhai, Q.-G. Assembly of [Cu2(COO)4] and [M3(μ3-O)(COO)6] (M = Sc, Fe, Ga, and In) Building Blocks into Porous Frameworks towards Ultra-High C2H2/CO2 and C2H2/CH4 Separation Performance. Chem. Commun. 2018, 54, 2012–2015. [Google Scholar] [CrossRef]
- Yang, X.; Yan, C.; Li, Z.; Li, X.; Yu, Q.; Sang, T.; Gai, Y.; Zhang, Q.; Xiong, K. Viologen-Based Cationic Metal-Organic Framework for Efficient Cr2O72– Adsorption and Dye Separation. Inorg. Chem. 2021, 60, 5988–5995. [Google Scholar] [CrossRef]
- Wei, L.-Q.; Ye, B.-H. Efficient Conversion of CO2 via Grafting Urea Group into a [Cu2(COO)4]-Based Metal-Organic Framework with Hierarchical Porosity. Inorg. Chem. 2019, 58, 4385–4393. [Google Scholar] [CrossRef]
- Majewski, M.B.; Howarth, A.J.; Li, P.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Enzyme Encapsulation in Metal-Organic Frameworks for Applications in Catalysis. CrystEngComm 2017, 19, 4082–4091. [Google Scholar] [CrossRef]
- Liang, K.; Coghlan, C.J.; Bell, S.G.; Doonan, C.; Falcaro, P. Enzyme Encapsulation in Zeolitic Imidazolate Frameworks: A Comparison between Controlled Co-Precipitation and Biomimetic Mineralisation. Chem. Commun. 2016, 52, 473–476. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Iqbal, H.M.N. Multifunctional Metal-Organic Frameworks-Based Biocatalytic Platforms: Recent Developments and Future Prospects. J. Mater. Res. Technol. 2019, 8, 2359–2371. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Kumar, V.; Samaddar, P.; Kumar, P.; Ramakrishnappa, T.; Kim, K.-H. Biomolecule-Embedded Metal-Organic Frameworks as an Innovative Sensing Platform. Biotechnol. Adv. 2018, 36, 467–481. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. One Pot Synthesis of α-Amylase Metal Organic Framework (MOF)-Sponge via Dip-Coating Technique. Int. J. Biol. Macromol. 2019, 138, 1035–1043. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Rathod, V.K. Enzyme Embedded Metal Organic Framework (Enzyme-MOF): De Novo Approaches for Immobilization. Int. J. Biol. Macromol. 2020, 149, 861–876. [Google Scholar] [CrossRef]
- Song, J.; He, W.; Shen, H.; Zhou, Z.; Li, M.; Su, P.; Yang, Y. Construction of Multiple Enzyme Metal-Organic Frameworks Biocatalyst via DNA Scaffold: A Promising Strategy for Enzyme Encapsulation. Chem. Eng. J. 2019, 363, 174–182. [Google Scholar] [CrossRef]
- Wang, C.; Liao, K. Recent Advances in Emerging Metal- and Covalent-Organic Frameworks for Enzyme Encapsulation. ACS Appl. Mater. Interfaces 2021, 13, 56752–56776. [Google Scholar] [CrossRef]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-Pot Synthesis of Protein-Embedded Metal-Organic Frameworks with Enhanced Biological Activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Wu, X.; Ge, J.; Yang, C.; Hou, M.; Liu, Z. Facile Synthesis of Multiple Enzyme-Containing Metal-Organic Frameworks in a Biomolecule-Friendly Environment. Chem. Commun. 2015, 51, 13408–13411. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, J.; Hou, J.; Zhang, Y. Enzyme-Embedded Metal-Organic Framework Membranes on Polymeric Substrates for Efficient CO2 Capture. J. Mater. Chem. A 2017, 5, 19954–19962. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic Mineralization of Metal-Organic Frameworks as Protective Coatings for Biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef]
- He, H.; Han, H.; Shi, H.; Tian, Y.; Sun, F.; Song, Y.; Li, Q.; Zhu, G. Construction of Thermophilic Lipase-Embedded Metal-Organic Frameworks via Biomimetic Mineralization: A Biocatalyst for Ester Hydrolysis and Kinetic Resolution. ACS Appl. Mater. Interfaces 2016, 8, 24517–24524. [Google Scholar] [CrossRef]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.-H.; Wu, K.C.-W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal-Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef]
- Gascón, V.; Castro-Miguel, E.; Díaz-García, M.; Blanco, R.M.; Sanchez-Sanchez, M. In Situ and Post-Synthesis Immobilization of Enzymes on Nanocrystalline MOF Platforms to Yield Active Biocatalysts: In Situ and Post-Synthesis Immobilization of Enzymes on Nanocrystalline MOFs. J. Chem. Technol. Biotechnol. 2017, 92, 2583–2593. [Google Scholar] [CrossRef]
- Jeong, G.-Y.; Ricco, R.; Liang, K.; Ludwig, J.; Kim, J.-O.; Falcaro, P.; Kim, D.-P. Bioactive MIL-88A Framework Hollow Spheres via Interfacial Reaction in-Droplet Microfluidics for Enzyme and Nanoparticle Encapsulation. Chem. Mater. 2015, 27, 7903–7909. [Google Scholar] [CrossRef]
- Nobakht, N.; Faramarzi, M.A.; Shafiee, A.; Khoobi, M.; Rafiee, E. Polyoxometalate-Metal Organic Framework-Lipase: An Efficient Green Catalyst for Synthesis of Benzyl Cinnamate by Enzymatic Esterification of Cinnamic Acid. Int. J. Biol. Macromol. 2018, 113, 8–19. [Google Scholar] [CrossRef]
- Gascón, V.; Carucci, C.; Jiménez, M.B.; Blanco, R.M.; Sánchez-Sánchez, M.; Magner, E. Rapid In Situ Immobilization of Enzymes in Metal-Organic Framework Supports under Mild Conditions. ChemCatChem 2017, 9, 1182–1186. [Google Scholar] [CrossRef]
- Salgaonkar, M.; Nadar, S.S.; Rathod, V.K. Combi-Metal Organic Framework (Combi-MOF) of α-Amylase and Glucoamylase for One Pot Starch Hydrolysis. Int. J. Biol. Macromol. 2018, 113, 464–475. [Google Scholar] [CrossRef]
- Velásquez-Hernández, M.d.J.; Linares-Moreau, M.; Astria, E.; Carraro, F.; Alyami, M.Z.; Khashab, N.M.; Sumby, C.J.; Doonan, C.J.; Falcaro, P. Towards Applications of bioentities@MOFs in Biomedicine. Coord. Chem. Rev. 2021, 429, 213651. [Google Scholar] [CrossRef]
- Chen, G.; Huang, S.; Kou, X.; Wei, S.; Huang, S.; Jiang, S.; Shen, J.; Zhu, F.; Ouyang, G. A Convenient and Versatile Amino-Acid-Boosted Biomimetic Strategy for the Nondestructive Encapsulation of Biomacromolecules within Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 1463–1467. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.-K.; Falcaro, P.; Doonan, C.J. Metal-Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Liang, W.; Xu, H.; Carraro, F.; Maddigan, N.K.; Li, Q.; Bell, S.G.; Huang, D.M.; Tarzia, A.; Solomon, M.B.; Amenitsch, H.; et al. Enhanced Activity of Enzymes Encapsulated in Hydrophilic Metal-Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 2348–2355. [Google Scholar] [CrossRef]
- Maddigan, N.K.; Tarzia, A.; Huang, D.M.; Sumby, C.J.; Bell, S.G.; Falcaro, P.; Doonan, C.J. Protein Surface Functionalisation as a General Strategy for Facilitating Biomimetic Mineralisation of ZIF-8. Chem. Sci. 2018, 9, 4217–4223. [Google Scholar] [CrossRef]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Entrapment of Surfactant Modified Lipase within Zeolitic Imidazolate Framework (ZIF)-8. Int. J. Biol. Macromol. 2020, 146, 678–686. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J. Green Synthesis of Enzyme/Metal-Organic Framework Composites with High Stability in Protein Denaturing Solvents. Bioresour. Bioprocess. 2017, 4, 24. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Bai, S.; Shao, X.; Jiang, L.; Li, Q. Immobilized Lipase in Bio-Based Metal-Organic Frameworks Constructed by Biomimetic Mineralization: A Sustainable Biocatalyst for Biodiesel Synthesis. Colloids Surf. B Biointerfaces 2020, 188, 110812. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Han, J.; Wu, J.; Li, C.; Ni, L.; Wang, Y. Improving Laccase Activity and Stability by HKUST-1 with Cofactor via One-Pot Encapsulation and Its Application for Degradation of Bisphenol A. J. Hazard. Mater. 2020, 383, 121130. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, Z.; Sentosun, K.; Pérez-Juste, I.; Bals, S.; Liz-Marzán, L.M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Hong, M. Shape Control in ZIF-8 Nanocrystals and Metal nanoparticles@ZIF-8 Heterostructures. Nanoscale 2017, 9, 16645–16651. [Google Scholar] [CrossRef]
- Pu, S.; Zhang, X.; Yang, C.; Naseer, S.; Zhang, X.; Ouyang, J.; Li, D.; Yang, J. The Effects of NaCl on Enzyme Encapsulation by Zeolitic Imidazolate Frameworks-8. Enzyme Microb. Technol. 2019, 122, 1–6. [Google Scholar] [CrossRef]
- Karadeniz, B.; Howarth, A.J.; Stolar, T.; Islamoglu, T.; Dejanović, I.; Tireli, M.; Wasson, M.C.; Moon, S.-Y.; Farha, O.K.; Friščić, T.; et al. Benign by Design: Green and Scalable Synthesis of Zirconium UiO-Metal-Organic Frameworks by Water-Assisted Mechanochemistry. ACS Sustain. Chem. Eng. 2018, 6, 15841–15849. [Google Scholar] [CrossRef]
- Wei, T.-H.; Wu, S.-H.; Huang, Y.-D.; Lo, W.-S.; Williams, B.P.; Chen, S.-Y.; Yang, H.-C.; Hsu, Y.-S.; Lin, Z.-Y.; Chen, X.-H.; et al. Rapid Mechanochemical Encapsulation of Biocatalysts into Robust Metal-Organic Frameworks. Nat. Commun. 2019, 10, 5002. [Google Scholar] [CrossRef]
- Chen, W.-H.; Vázquez-González, M.; Zoabi, A.; Abu-Reziq, R.; Willner, I. Biocatalytic Cascades Driven by Enzymes Encapsulated in Metal-Organic Framework Nanoparticles. Nat. Catal. 2018, 1, 689–695. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Self-Encapsulation of Oxidases as a Basic Approach to Tune the Upper Detection Limit of Amperometric Biosensors. Analyst 2008, 133, 1083. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Fotouhi, L.; Ehsani, A. Recent Progress in the Development of Conducting Polymer-Based Nanocomposites for Electrochemical Biosensors Applications: A Mini-Review. Chem. Rec. 2018, 18, 599–618. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ryskevic, N.; Kausaite-Minkstimiene, A.; Bubniene, U.; Baleviciute, I.; Oztekin, Y.; Ramanaviciene, A. Fluorescence Study of Glucose Oxidase Self-Encapsulated within Polypyrrole. Sens. Actuators B Chem. 2012, 171–172, 753–759. [Google Scholar] [CrossRef]
- Solanki, P.R.; Arya, S.K.; Singh, S.P.; Pandey, M.K.; Malhotra, B.D. Application of Electrochemically Prepared Poly-N-Methylpyrrole-p-Toluene Sulphonate Films to Cholesterol Biosensor. Sens. Actuators B Chem. 2007, 123, 829–839. [Google Scholar] [CrossRef]
- Ozdemir, C.; Yeni, F.; Odaci, D.; Timur, S. Electrochemical Glucose Biosensing by Pyranose Oxidase Immobilized in Gold Nanoparticle-Polyaniline/AgCl/Gelatin Nanocomposite Matrix. Food Chem. 2010, 119, 380–385. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Mulato, M. Enzymatically Functionalized Polyaniline Thin Films Produced with One-Step Electrochemical Immobilization and Its Application in Glucose and Urea Potentiometric Biosensors. Biomed. Microdevices 2020, 22, 22. [Google Scholar] [CrossRef]
- Marzuki, N.; Bakar, F.; Salleh, A.; Heng, L.; Yusof, N.; Siddiquee, S. Electrochemical Biosensor Immobilization of Formaldehyde Dehydrogenase with Nafion for Determination of Formaldehyde from Indian Mackerel (Rastrelliger Kanagurta) Fish. Curr. Anal. Chem. 2012, 8, 534–542. [Google Scholar] [CrossRef]
- Liang, R.-P.; Fan, L.-X.; Wang, R.; Qiu, J.-D. One-Step Electrochemically Deposited Nanocomposite Film of CS-Fc/MWNTs/GOD for Glucose Biosensor Application. Electroanalysis 2009, 21, 1685–1691. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Jang, M.; Park, H.S.; Lee, J.Y. One-Pot Electrochemical Fabrication of High Performance Amperometric Enzymatic Biosensors Using Polypyrrole and Polydopamine. J. Ind. Eng. Chem. 2021, 97, 316–325. [Google Scholar] [CrossRef]
- Iqbal, S.; Ahmad, S. Recent Development in Hybrid Conducting Polymers: Synthesis, Applications and Future Prospects. J. Ind. Eng. Chem. 2018, 60, 53–84. [Google Scholar] [CrossRef]
- Schuhmann, W. Conducting Polymer Based Amperometric Enzyme Electrodes. Mikrochim. Acta 1995, 121, 1–29. [Google Scholar] [CrossRef]
- Shen, F.; Arshi, S.; Magner, E.; Ulstrup, J.; Xiao, X. One-Step Electrochemical Approach of Enzyme Immobilization for Bioelectrochemical Applications. Synth. Met. 2022, 291, 117205. [Google Scholar] [CrossRef]
- Ahuja, T.; Mir, I.; Kumar, D.; Rajesh. Biomolecular Immobilization on Conducting Polymers for Biosensing Applications. Biomaterials 2007, 28, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Killard, A.J.; Morrin, A.; Smyth, M.R. In Situ Electropolymerised Silica-Polyaniline Core-Shell Structures: Electrode Modification and Enzyme Biosensor Enhancement. Electrochimica Acta 2007, 52, 1865–1870. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Sarkar, P.; Bhattacharyay, D.; Majumdar, P. One Step Electrode Fabrication for Direct Electron Transfer Cholesterol Biosensor Based on Composite of Polypyrrole, Green Reduced Graphene Oxide and Cholesterol Oxidase. Electroanalysis 2018, 30, 2719–2730. [Google Scholar] [CrossRef]
- Singh, M.; Kathuroju, P.K.; Jampana, N. Polypyrrole Based Amperometric Glucose Biosensors. Sens. Actuators B Chem. 2009, 143, 430–443. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, J.; Chen, T.; Yu, Z.; Li, G. Enlargement of Gold Nanoparticles on the Surface of a Self-Assembled Monolayer Modified Electrode: A Mode in Biosensor Design. Anal. Chem. 2006, 78, 5227–5230. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.Z.A.; Govender-Hondros, G.; Adeloju, S.B. A Single Step Electrochemical Integration of Gold Nanoparticles, Cholesterol Oxidase, Cholesterol Esterase and Mediator with Polypyrrole Films for Fabrication of Free and Total Cholesterol Nanobiosensors. Talanta 2018, 189, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Deng, X.; Li, J.; Huang, S.; Jin, X.; Zhu, X. Self-Encapsulated Enzyme through in-Situ Growth of Polypyrrole for High-Performance Enzymatic Biofuel Cell. Chem. Eng. J. 2022, 429, 132148. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Enzymatic Formation of Polyaniline, Polypyrrole, and Polythiophene Nanoparticles with Embedded Glucose Oxidase. Nanomaterials 2019, 9, 806. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Characterisation of Enzyme-Assisted Formation of Polypyrrole and Polyaniline Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. J. Electrochem. Soc. 2020, 167, 165501. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization Strategies to Develop Enzymatic Biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Yuqing, M.; Jianrong, C.; Xiaohua, W. Using Electropolymerized Non-Conducting Polymers to Develop Enzyme Amperometric Biosensors. Trends Biotechnol. 2004, 22, 227–231. [Google Scholar] [CrossRef]
- Semenova, D.; Gernaey, K.V.; Morgan, B.; Silina, Y.E. Towards One-Step Design of Tailored Enzymatic Nanobiosensors. Analyst 2020, 145, 1014–1024. [Google Scholar] [CrossRef]
- Jia, W.-Z.; Wang, K.; Zhu, Z.-J.; Song, H.-T.; Xia, X.-H. One-Step Immobilization of Glucose Oxidase in a Silica Matrix on a Pt Electrode by an Electrochemically Induced Sol-Gel Process. Langmuir 2007, 23, 11896–11900. [Google Scholar] [CrossRef] [PubMed]
- Otero, F.; Mandal, T.; Leech, D.; Magner, E. An Electrochemical NADH Biosensor Based on a Nanoporous Gold Electrode Modified with Diaphorase and an Osmium Polymer. Sens. Actuators Rep. 2022, 4, 100117. [Google Scholar] [CrossRef]

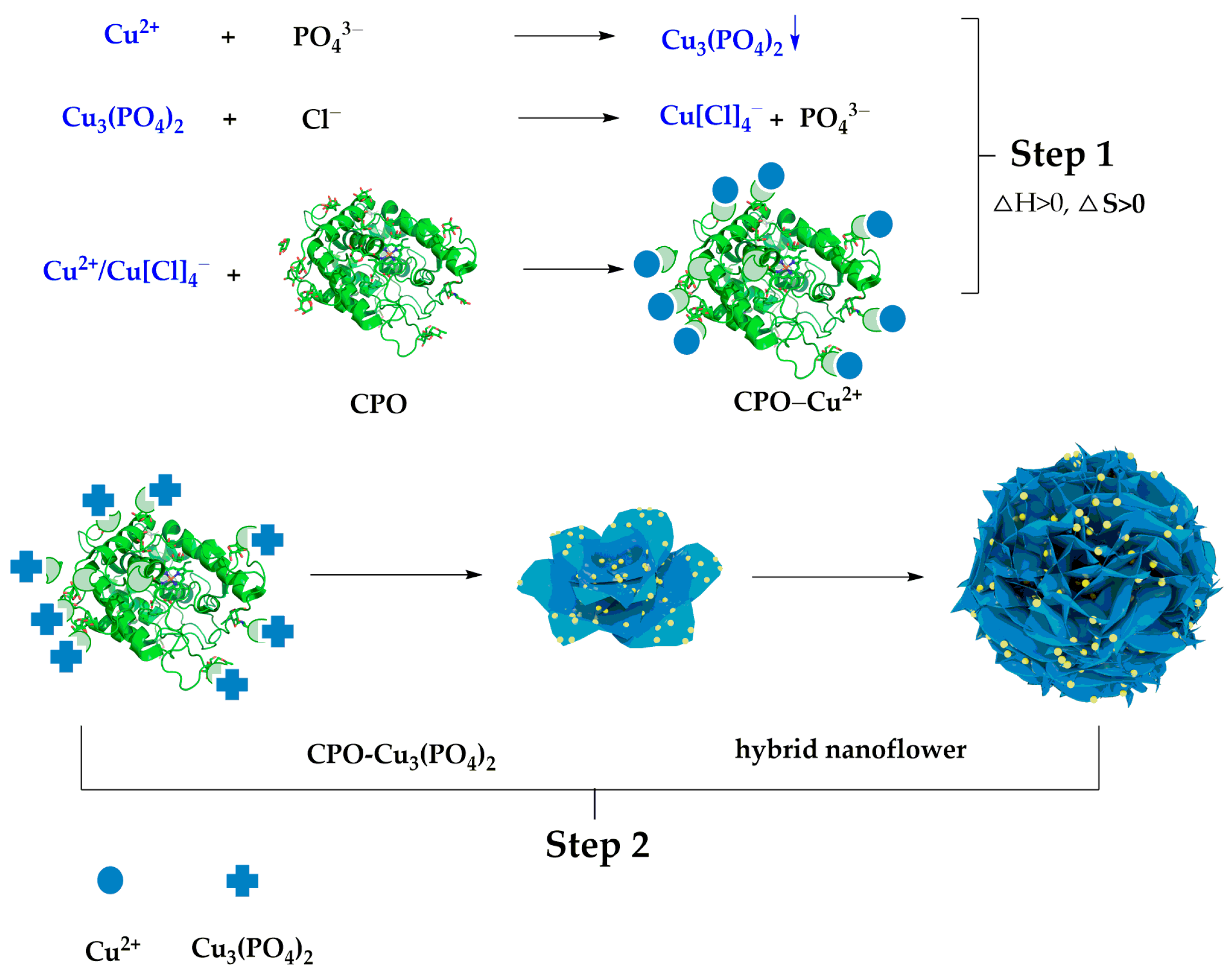
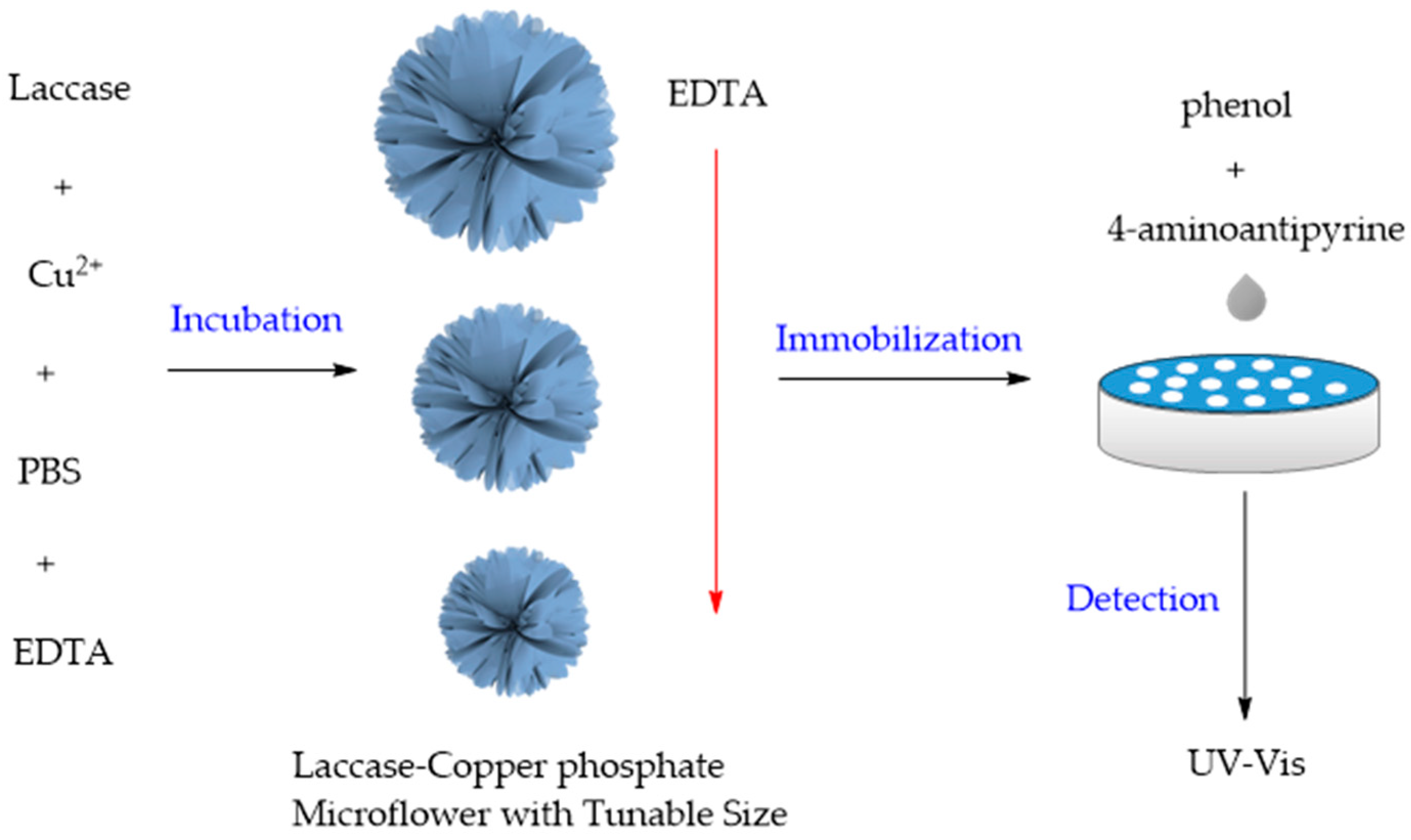


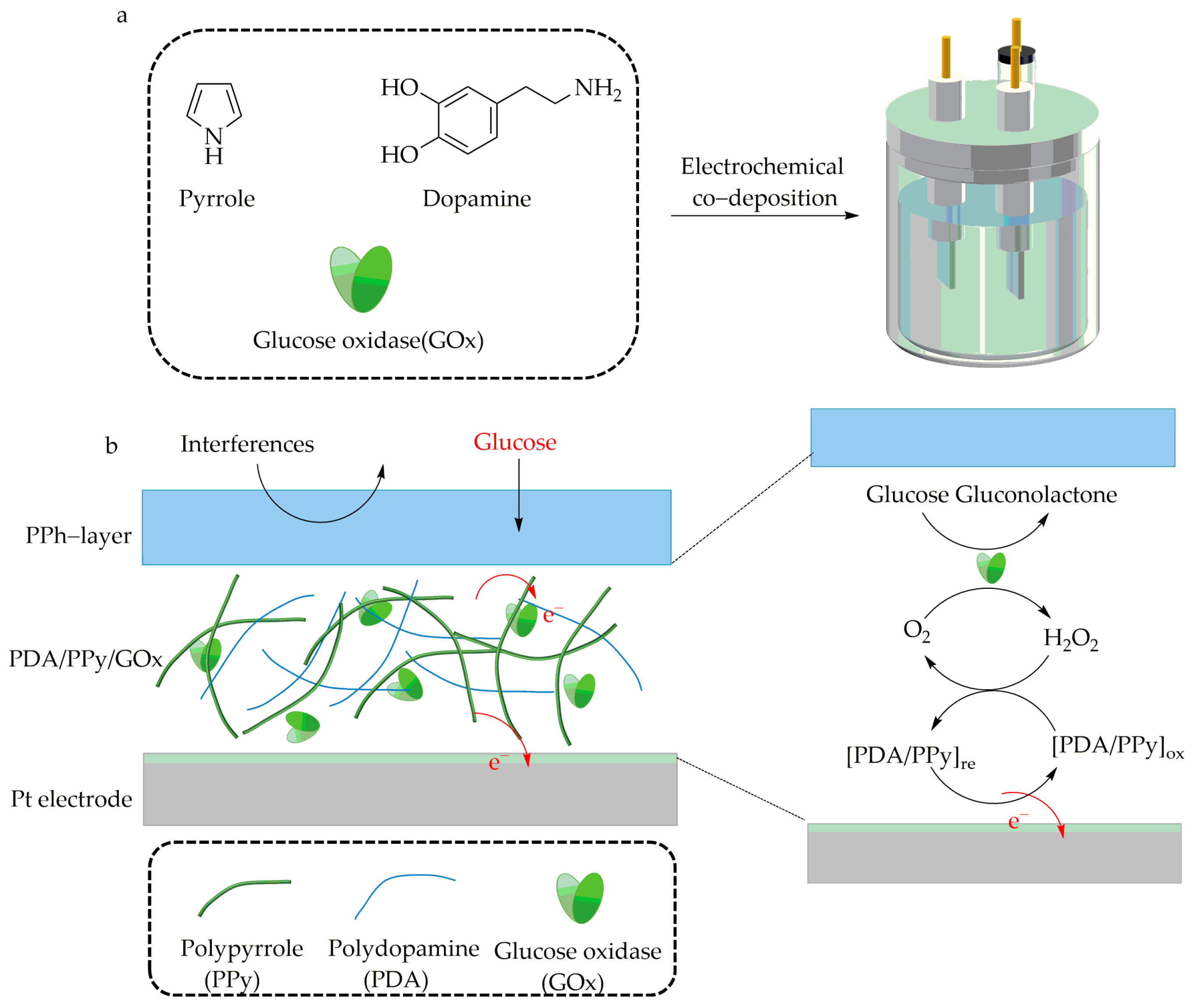
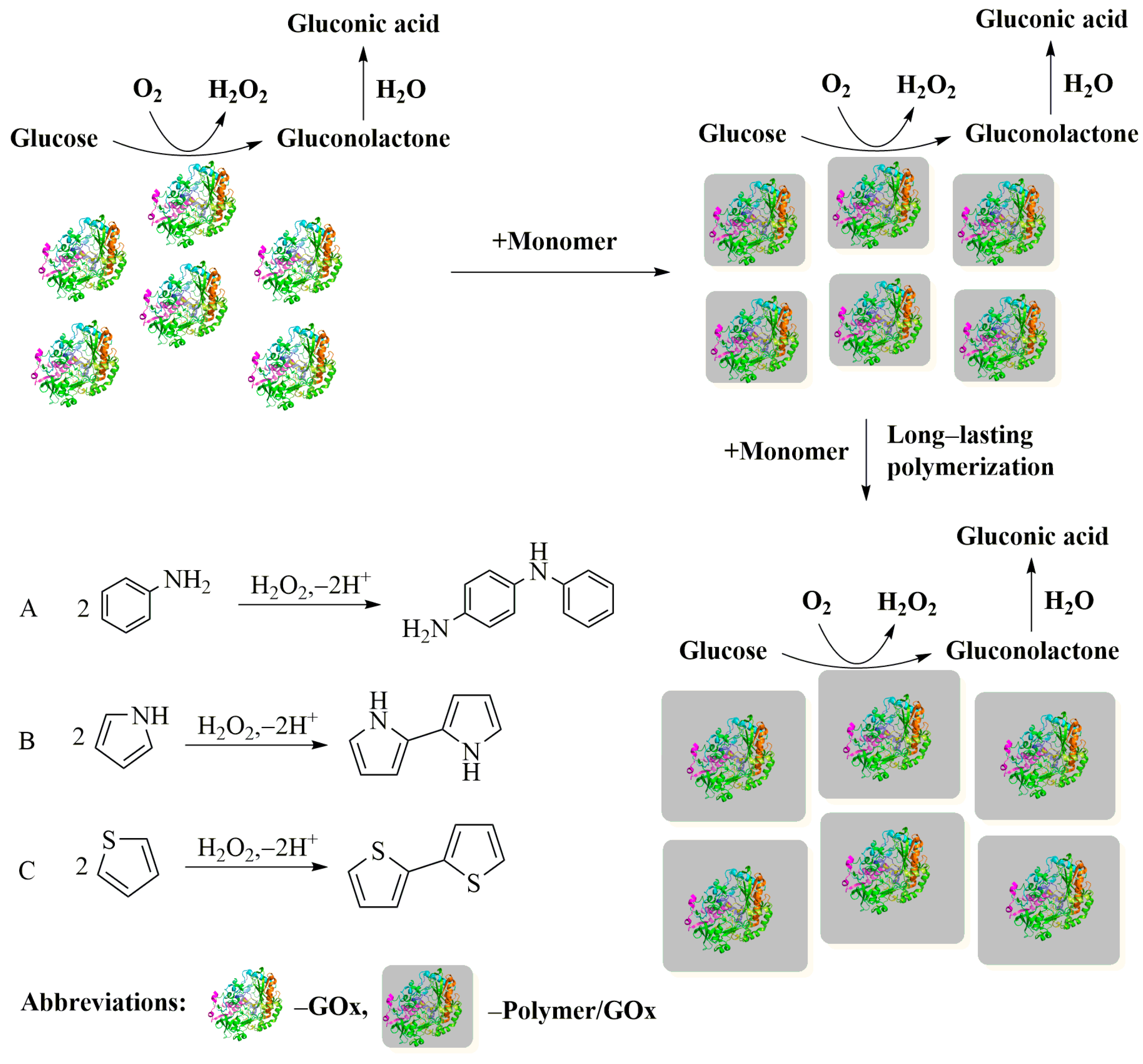
| Enzyme | Metal Ions | Applications | Improved Performance | Ref. |
|---|---|---|---|---|
| ω-Transaminase | Co2+ | Production of chiral amines | Enhanced reusability | [56] |
| lipase | Zn2+ | Regioselective acylation of arbutin | Enhanced reusability | [57] |
| L-arabinose isomerase | Mn2+ | Synthesis of D-tagatose | Enhanced reusability and storage stability | [58] |
| Lactoperoxidase | Cu2+ | Detection of dopamine and epinephrine | Enhanced activity, pH stability, and reusability | [59] |
| Lipase from thermomyces lanuginosus | Ca2+ | Proof of concept | 21.7 times more catalytic activity and thermal stability than a free enzyme | [60] |
| Phosphotriesterase | Co2+ & Mn2+ | Use in nerve agent (GD and VX) degradation | Enhanced stability and reusability | [61] |
| Burkholderia cepacia | Ag+/Fe2+/Cu2+/Au3+ | Proof of concept | Enhanced stability | [62] |
| Aldehyde/ketone reductase and alcoholdehydrogenase | Ca2+ | Synthesis of (S)-1-(2,6-dichloro-3-fluorophenyl) ethyl alcohol | Enhanced thermal stability | [63] |
| Polyketone reductase and glucose dehydrogenase | Ca2+ | Synthesize (R)-(-)-pantolactone | Enhanced stability and reusability | [64] |
| Galactose oxidase and horseradish peroxidase | Mn2+ | Detection of glutamic acid | Enhanced reusability | [65] |
| Horseradish peroxidase and glucose oxidase | Cu2+ | Degradation of acridine and wastewater treatment | Enhanced pH stability | [66] |
| Nucleoside kinase and polyphosphate kinase | Cu2+ | Generation of nucleotides | Enhanced reusability | [67] |
| Glucose oxidase and lipase | Cu2+ | Epoxidation of alkenes | Enhanced reusability | [68] |
| Streptavidin and horseradish peroxidase | Cu2+ | Colorimetric sensor for alpha-fetoprotein (AFP) detection | Enhanced storage stability | [69] |
| Glucose oxidase and horseradish peroxidase | Cu2+ | Counting the number of living bacteria in urine | Enhanced thermostability | [70] |
| MOF | Metal Ions | Enzyme | Applications | Improved Performance | Ref. |
|---|---|---|---|---|---|
| ZIF-8 | Zn2+ | Cytochrome C | Oxidation of Amplex red | Enhanced activity | [103] |
| ZIF-8 | Zn2+ | Horseradish Peroxidase and Glucose Oxidase | Selective glucose detection | Enhanced activity and selectivity | [104] |
| ZIF-8 | Zn2+ | Carbonic Anhydrase | CO2/N2 selectivity composite membranes | Enhanced stability | [105] |
| ZIF-8 | Zn2+ | Horseradish Peroxidase | Proof of concept | Enhanced thermal stability | [106] |
| ZIF-8 | Zn2+ | Lipase QLM | Kinetic resolution of (R, S)-2-octanol | Enhanced activity and reusability | [107] |
| ZIF-90 | Zn2+ | Catalase | Biocatalysis | Enhanced activity | [108] |
| MIL-53/ NH2-MIL-53 | Al3+ | β-glucosidase/ Laccase | Proof of concept | Enhanced organic solvent stability | [109] |
| MIL-88A | Fe3+ | Glucose Dehydrogenase/ Horseradish Peroxidase /Acetylcholinesterase | Proof of concept | Enhanced reusability | [110] |
| MIL-100 | Fe3+ | Lipase PPL | Synthesis of benzyl cinnamate | Enhanced thermal, pH, and stability | [111] |
| Fe-MOF | Fe3+ | Alcoholdehydrogenase/ Lipase/Glucose Oxidase | Biocatalysis | Enhanced reusability | [112] |
| Polymer | Enzyme | Applications | Improved Performance | Ref. |
|---|---|---|---|---|
| PTh/Ppy/PANI | GOx | Glucose detection biosensor | Enhanced stability | [132] |
| NMPY | ChOx | Cholesterol biosensor | Enhanced charge transfer | [133] |
| PANI | PyOx | Glucose detection biosensor | Enhanced activity and stability | [134] |
| PANI | GOx/Ur | Glucose and urea enzymatic biosensors | Enhanced stability and reusability | [135] |
| Nafion®117 | FDH | Formaldehyde detection biosensor | Enhanced stability and reusability | [136] |
| Chitosan derivatives (CS-Fc) | GOD | Glucose detection biosensor | Enhanced electronic conductivity, electroactive surface area and electrochemical stability | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Liu, P.; Chu, X. Recent Advances in the Strategies of Simultaneous Enzyme Immobilization Accompanied by Nanocarrier Synthesis. Appl. Sci. 2024, 14, 3702. https://doi.org/10.3390/app14093702
Hao X, Liu P, Chu X. Recent Advances in the Strategies of Simultaneous Enzyme Immobilization Accompanied by Nanocarrier Synthesis. Applied Sciences. 2024; 14(9):3702. https://doi.org/10.3390/app14093702
Chicago/Turabian StyleHao, Xinrui, Pengfu Liu, and Xiaohe Chu. 2024. "Recent Advances in the Strategies of Simultaneous Enzyme Immobilization Accompanied by Nanocarrier Synthesis" Applied Sciences 14, no. 9: 3702. https://doi.org/10.3390/app14093702
APA StyleHao, X., Liu, P., & Chu, X. (2024). Recent Advances in the Strategies of Simultaneous Enzyme Immobilization Accompanied by Nanocarrier Synthesis. Applied Sciences, 14(9), 3702. https://doi.org/10.3390/app14093702





