Analysis of Operated Ankle Fractures in Elderly Patients: Are They All Osteoporotic?
Abstract
:1. Introduction
1.1. Diagnosis of Osteoporosis
- T-score from +2.5 to −1.0 SD (normal);
- T-score between −1.0 and −2.5 SD (osteopenia);
- T-score < −2.5 SD (osteoporosis);
- T-score < −2.5 SD in the presence of at least one fragility fracture (severe osteoporosis).
1.2. Risk Factors
1.3. Fragility Fractures
1.4. Objective of the Study
2. Materials and Methods
- Patients aged ≥60 years;
- Ankle fracture treated surgically in our Hospital “Santa Maria della Misericordia” in Perugia from 1 May 2022 to 31 October 2022;
- DXA performed between 6 months and 1 year from the date of the surgery at the contralateral side relative to the fracture.
- Previous ankle fractures;
- Severe ankle osteoarthritis previous to surgery;
- Irreducible ankle dislocation;
- Patients with complications after surgery.
2.1. Clinical and Anamnestic Evaluation
- The fracture traumatic mechanism description is to divide it into high- or low-energy traumas. Low-energy traumas were defined in agreement with P. Kannus [20,21], who included in the minor sprain type traumas affecting the ankle, i.e., slips, jumps, and falls from a standing position or from a height in any case lower than the patient’s height. The category of high-energy traumas includes those that occurred due to falls from a height greater than one’s own height, car and motorcycle accidents, and others.
- The menstrual history, considering as a risk factor the presence of amenorrhea periods in fertile age exceeding 9 months, early menopause (before age 45), bilateral ovariectomy during fertile age (iatrogenic early menopause).
- Diabetes mellitus, alcohol consumption greater than 3 units/day, smoking habit, corticosteroid oral therapy (5 mg/day or more) at the time of evaluation or in the previous 3 months.
- Protracted period of bed rest, immobilization, or total absence of load on both lower limbs (at least one month) in the immediate period of time preceding the execution of the DXA. This was decided as under these conditions it would not have been possible to discern whether any reduction in BMD was linked to this period of no weight bearing or to an underlying condition preceding the fracture.
2.2. Postoperative Radiographic Evaluation of BMD
2.3. Risk of Future Fractures
2.4. Postoperative Clinical Evaluation
2.5. Statistics
3. Results
3.1. Demographic and Clinical Evaluation
3.2. BMD
3.2.1. BMD and Type of Fracture
3.2.2. BMD and Type of Trauma
3.3. FADI
3.3.1. FADI and BMD
3.3.2. FADI and Type of Fracture
3.3.3. FADI and Type of Trauma
3.4. FRAX®
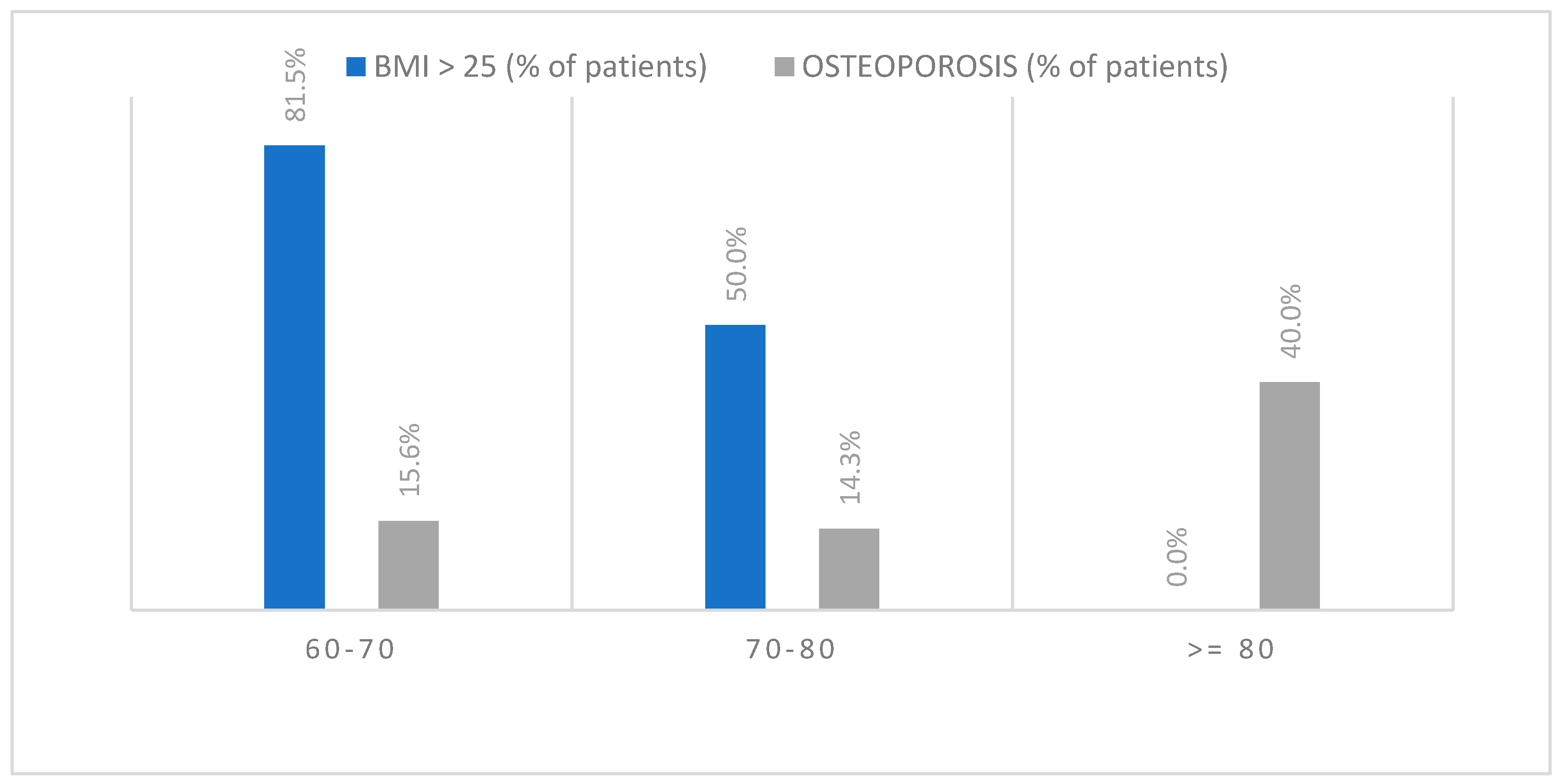
3.5. BMI
3.5.1. BMI and BMD
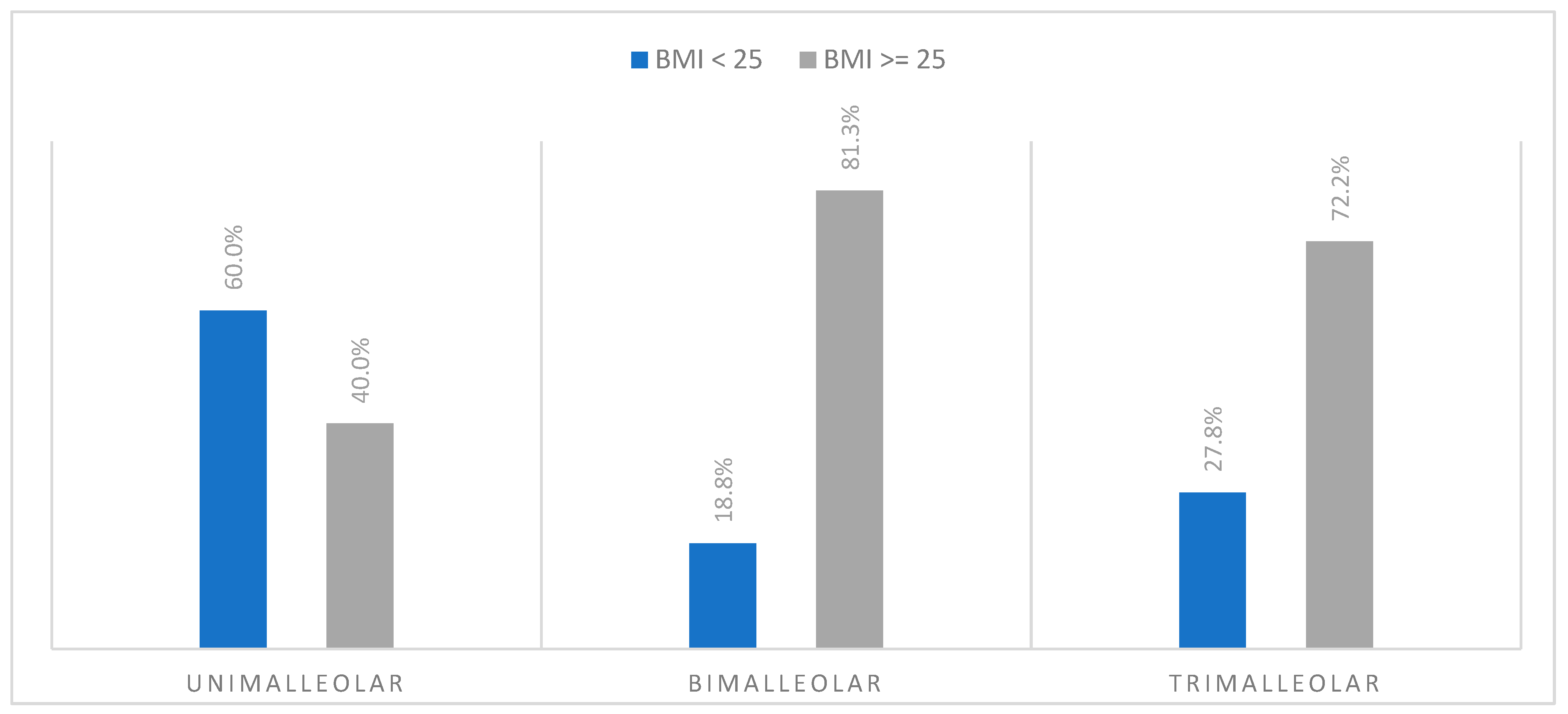
3.5.2. BMI and Type of Fracture
3.5.3. BMI and Type of Trauma
3.5.4. BMI and FADI
4. Discussion
4.1. Ankle Fractures and Osteoporosis
4.2. FADI and Ankle Fracture
4.3. BMI and Ankle Fractures
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wa, P. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Therdyothin, A.; Phiphopthatsanee, N.; Wajanavisit, W.; Woratanarat, P.; Laohajaroensombat, S.; Tawonsawatruk, T. Is ankle fracture related to low bone mineral density and subsequent fracture? A systematic review. Osteoporos. Sarcopenia 2020, 6, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Rinonapoli, G.; Ruggiero, C.; Meccariello, L.; Bisaccia, M.; Ceccarini, P.; Caraffa, A. Osteoporosis in men: A review of an underestimated bone condition. Int. J. Mol. Sci. 2021, 22, 2105. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C.; et al. Clinical Guidelines for the Prevention and Treatment of Osteoporosis: Summary Statements and Recommendations from the Italian Society for Orthopaedics and Traumatology; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 18. [Google Scholar]
- Lu, Y.; Genant, H.K.; Shepherd, J.; Zhao, S.; Mathur, A.; Fuerst, T.P.; Cummings, S.R. Classification of osteoporosis based on bone mineral densities. J. bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 901–910. [Google Scholar] [CrossRef]
- Morris, J.; Karkenny, A.J.; Toro, J.B. The management of osteoporosis after fragility fracture: The orthopaedic perspective. JBJS Rev. 2017, 5, e4. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Oden, A.; Johnell, O.; Johansson, H.; De Laet, C.; Brown, J.; Burckhardt, P.; Cooper, C.; Christiansen, C.; Cummings, S.; et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos. Int. 2007, 18, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Hans, D.; Cooper, C.; Baim, S.; Bilezikian, J.P.; Binkley, N.; Cauley, J.A.; Compston, J.E.; Dawson-Hughes, B.; El-Hajj Fuleihan, G.; et al. Interpretation and use of FRAX in clinical practice. Osteoporos. Int. 2011, 22, 2395–2411. [Google Scholar] [CrossRef]
- Rinonapoli, G.; Pace, V.; Ruggiero, C.; Ceccarini, P.; Bisaccia, M.; Meccariello, L.; Caraffa, A. Obesity and Bone: A Complex Relationship. Int. J. Mol. Sci. 2021, 22, 13662. [Google Scholar] [CrossRef]
- Fassio, A.; Idolazzi, L.; Rossini, M.; Gatti, D.; Adami, G.; Giollo, A.; Viapiana, O. The obesity paradox and osteoporosis. Eat. Weight Disord. 2018, 23, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Felsenberg, D.; Silman, A.J.; Lunt, M.; Armbrecht, G.; Ismail, A.A.; Finn, J.D.; Cockerill, W.C.; Banzer, D.; Benevolenskaya, L.I.; Bhalla, A.; et al. Incidence of vertebral fracture in europe: Results from the european prospective osteoporosis study (EPOS). J. Bone Miner. Res. 2002, 17, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Campion, G.; Melton, L.J. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Waterloo, S.; Ahmed, L.A.; Center, J.R.; Eisman, J.A.; Morseth, B.; Nguyen, N.D.; Nguyen, T.; Sogaard, A.J.; Emaus, N. Prevalence of vertebral fractures in women and men in the population-based Tromsø study. BMC Musculoskelet. Disord. 2012, 13, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Elaine, M.; Cooper, C. The Epidemiology of Osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hasselman, C.T.; Vogt, M.T.; Stone, K.L.; Cauley, J.A.; Conti, S.F. Foot and ankle fractures in elderly white women. Incidence and risk factors. J. Bone Jt. Surg. Am. 2003, 85, 820–824. [Google Scholar] [CrossRef] [PubMed]
- So, E.; Juels, C.; Scott, R.T.; Sietsema, D.L. A Comparison of Ankle Fractures Relative to Other Fragility Fractures: A Review and Analysis of the American Orthopaedic Association’s Own the Bone Database. Foot Ankle Int. 2023, 44, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Zampi, E.; Rinonapoli, G.; Baroni, M.; Serra, R.; Zengarini, E.; Baglioni, G.; Duranti, G.; Ercolani, S.; Conti, F.; et al. Fracture prevention service to bridge the osteoporosis care gap. Clin. Interv. Aging 2015, 10, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Palvanen, M.; Niemi, S.; Parkkari, J.; Jrvinen, M. Increasing number and incidence of low-trauma ankle fractures in elderly people: Finnish statistics during 1970-2000 and projections for the future. Bone 2002, 31, 430–433. [Google Scholar] [CrossRef]
- Kannus, P.; Parkkari, J.; Niemi, S.; Palvanen, M. Epidemiology of osteoporotic ankle fractures in elderly persons in Finland. Ann. Intern. Med. 1996, 125, 975–978. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis seminar 2019. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Gregson, C.L.; Armstrong, D.J.; Bowden, J.; Cooper, C.; Edwards, J.; Gittoes, N.J.L.; Harvey, N.; Kanis, J.; Leyland, S.; Low, R.; et al. UK Clinical Guideline for the Prevention and Treatment of Osteoporosis. Arch. Osteoporos. 2022, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Gogate, N.; Satpute, K.; Hall, T. The effectiveness of mobilization with movement on pain, balance and function following acute and sub acute inversion ankle sprain—A randomized, placebo controlled trial. Phys. Ther. Sport 2021, 48, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.J.; Ahearn, B.M.; Schwartz, A.M.; Tenenbaum, S.; Bariteau, J.T. Ankle fractures in the elderly: Risks and management challenges. Orthop. Res. Rev. 2017, 9, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Koval, K.J.; Lurie, J.; Zhou, W.; Sparks, M.B.; Cantu, R.V.; Sporer, S.M.; Weinstein, J. Ankle fractures in the elderly: What you get depends on where you live and who you see. J. Orthop. Trauma 2005, 19, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Li, X.; Franklin, P.; Wixted, J.J. Ankle fractures in the elderly: Initial and long-term outcomes. Foot Ankle Int. 2008, 29, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Toole, W.P.; Elliott, M.; Hankins, D.; Rosenbaum, C.; Harris, A.; Perkins, C. Are low-energy open ankle fractures in the elderly the new geriatric hip fracture? J. Foot Ankle Surg. 2015, 54, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.J.; Hsu, R.Y.; Hayda, R.; Lee, Y.; Bariteau, J.T. Evaluation of one-year mortality after geriatric ankle fractures in patients admitted to nursing homes. Injury 2015, 46, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.Y.; Lee, Y.; Hayda, R.; DiGiovanni, C.W.; Mor, V.; Bariteau, J.T. Morbidity and mortality associated with geriatric ankle fractures. J. Bone Jt. Surg. Am. Vol. 2014, 97, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Valtola, A.; Honkanen, R.; Kröger, H.; Tuppurainen, M.; Saarikoski, S.; Alhava, E. Lifestyle and other factors predict ankle fractures in perimenopausal women: A population-based prospective cohort study. Bone 2002, 30, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Biver, E.; Durosier, C.; Chevalley, T.; Herrmann, F.R.; Ferrari, S.; Rizzoli, R. Prior ankle fractures in postmenopausal women are associated with low areal bone mineral density and bone microstructure alterations. Osteoporos. Int. 2015, 26, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.; Kröger, H.; Tuppurainen, M.; Alhava, E.; Saarikoski, S. Fractures and low axial bone density in perimenopausal women. J. Clin. Epidemiol. 1995, 48, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Liu, X.S.; Nickolas, T.L.; Cohen, A.; Thomas, V.; McMahon, D.J.; Zhang, C.; Cosman, F.; Nieves, J.; Greisberg, J.; et al. Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. J. Clin. Endocrinol. Metab. 2011, 96, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-O.; Kim, J.-H.; Yoo, B.-C.; Yoo, J.-H. Is osteoporosis a risk factor for ankle fracture?: Comparison of bone mineral density between ankle fracture and control groups. Osteoporos. Sarcopenia 2017, 3, 192–194. [Google Scholar] [CrossRef] [PubMed]
- AIFA. Nota 79. 2015; pp. 4–12. Available online: https://www.aifa.gov.it/documents/20142/1728074/nota-79.pdf (accessed on 29 March 2024).
- Bergh, C.; Wennergren, D.; Möller, M.; Brisby, H. Fracture incidence in adults in relation to age and gender: A study of 27,169 fractures in the Swedish Fracture Register in a well-defined catchment area. PLoS ONE 2020, 15, e0244291. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Baroni, M.; Talesa, G.R.; Cirimbilli, A.; Prenni, V.; Bubba, V.; Parretti, L.; Bogini, R.; Duranti, G.; Caraffa, A.; et al. The interdisciplinary fracture liaison service improves health-related outcomes and survival of older adults after hip fracture surgical repair. Arch. Osteoporos. 2022, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Kuru, P.; Akyüz, G.; Cerşit, H.P.; Çelenlioğlu, A.E.; Cumhur, A.; Biricik, Ş.; Kozan, S.; Gökşen, A.; Özdemir, M.; Lüleci, E. Fracture history in osteoporosis: Risk factors and its effect on quality of life. Balk. Med. J. 2014, 31, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Niemi, S.; Parkkari, J.; Sievänen, H. Declining incidence of fall-induced ankle fractures in elderly adults: Finnish statistics between 1970 and 2014. Arch. Orthop. Trauma Surg. 2016, 136, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Chung, C.Y.; Kwon, S.S.; Won, S.H.; Lee, S.Y.; Chung, M.K.; Park, M.S. Ankle fractures have features of an osteoporotic fracture. Osteoporos. Int. 2013, 24, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Seeley, D.G.; Browner, W.S.; Nevitt, M.C.; Genant, H.K.; Scott, J.C.; Cummings, S.R. Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 1991, 115, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.K.; Hasserius, R.; Obrant, K.J. The ankle fracture as an index of future fracture risk. A 25–40 year follow-up of 1063 cases. Acta Orthop. Scand. 1993, 64, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Gary, L.C.; Arora, T.; Becker, D.J.; Curtis, J.R.; Kilgore, M.L.; Morrisey, M.A.; Saag, K.G.; Matthews, R.; Yun, H.; et al. Clinical and demographic factors associated with fractures among older Americans. Osteoporos. Int. 2011, 22, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Charde, S.H.; Joshi, A.; Raut, J. A Comprehensive Review on Postmenopausal Osteoporosis in Women. Cureus 2023, 15, e48582. [Google Scholar] [CrossRef] [PubMed]
- Aguado, P.; Garcés, M.V.; Casaús, M.L.G.; del Campo, M.T.; Richi, P.; Coya, J.; Torrijos, A.; Gijón, J.; Mola, E.M.; Martínez, M.E. Alta prevalencia de deficiencia de vitamina D en mujeres posmenopáusicas de una consulta reumatológica en Madrid. Evaluación de dos pautas de prescripción de vitamina D. Med. Clínica 2000, 114, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Hodsman, A.B.; Hanley, D.A.; Josse, R. Do bisphosphonates reduce the risk of osteoporotic fractures? An evaluation of the evidence to date. CMAJ 2002, 166, 1426–1430. [Google Scholar] [PubMed]
- Ziegler, P.; Bahrs, C.; Konrads, C.; Hemmann, P.; Ahrend, M.D. Ankle fractures of the geriatric patient: A narrative review. EFORT Open Rev. 2023, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.R.; Hunter, J.; Baumhauer, J.F. Osteoporotic ankle fractures. Orthop. Clin. N. Am. 2013, 44, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Warner, S.J.; Garner, M.R.; Fabricant, P.D.; Lorich, D.G. Bone density correlates with clinical outcomes after ankle fracture fixation. Arch. Orthop. Trauma Surg. 2018, 138, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.J.; Egol, K.A. The management of ankle fractures in the elderly. Injury 2007, 38, 2–9. [Google Scholar] [CrossRef]
- Hoogervorst, P.; Bergen, C.; Van den Bekerom, M. Management of Osteoporotic and Neuropathic Ankle Fractures in the Elderly. Curr. Geriatr. Rep. 2017, 6, 9–14. [Google Scholar] [CrossRef]
- Ong, T.; Sahota, O.; Tan, W.; Marshall, L. A United Kingdom perspective on the relationship between body mass index (BMI) and bone health: A cross sectional analysis of data from the Nottingham Fracture Liaison Service. Bone 2014, 59, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Watts, N.B.; Chapurlat, R.; Cooper, C.; Boonen, S.; Greenspan, S.; Pfeilschifter, J.; Silverman, S.; Díez-Pérez, A.; Lindsay, R.; et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Premaor, M.O.; Fina Avilés, F.; Hermosilla, E.; Martinez-Laguna, D.; Carbonell-Abella, C.; Nogués, X.; Compston, J.E.; Díez-Pérez, A. The association between fracture and obesity is site-dependent: A population-based study in postmenopausal women. J. bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 294–300. [Google Scholar] [CrossRef] [PubMed]
- King, C.M.; Hamilton, G.A.; Cobb, M.; Carpenter, D.; Ford, L.A. Association between ankle fractures and obesity. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2012, 51, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Cairns, B.J.; Green, J.; Reeves, G.K.; Beral, V.; Armstrong, M.E.G. The Effects of Age, Adiposity, and Physical Activity on the Risk of Seven Site-Specific Fractures in Postmenopausal Women. J. Bone Miner. Res. 2016, 31, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.V.; Paccaud, J.; Dubois-Ferrière, V.; Barea, C.; Hannouche, D.; Veljkovic, A.; Lübbeke, A. The effect of BMI on long-term outcomes after operatively treated ankle fractures: A study with up to 16 years of follow-up. BMC Musculoskelet. Disord. 2022, 23, 317. [Google Scholar] [CrossRef] [PubMed]

| Age Ranges | Sex | Tot (N°) | Tot (%) |
|---|---|---|---|
| 60–65 | M + F | 18 | 35.3% |
| M | 6 | 33.3% | |
| F | 12 | 66.7% | |
| 65–70 | M + F | 14 | 27.5% |
| M | 5 | 35.7% | |
| F | 9 | 64.3% | |
| 70–75 | M + F | 10 | 19.6% |
| M | 2 | 20.0% | |
| F | 8 | 80.0% | |
| 75–80 | M + F | 4 | 7.8% |
| M | 0 | 0% | |
| F | 4 | 100% | |
| 80–85 | M + F | 5 | 9.8% |
| M | 1 | 20.0% | |
| F | 4 | 80.0% |
| Total Fractures | ||
| Type of Fracture | N | % |
| Unimalleolar | 10 | 19.6 |
| Bimalleolar | 20 | 39.2 |
| Trimalleolar | 21 | 41.2 |
| Non-specificated | 0 | 0.0 |
| Total | 51 | 100.0 |
| Normal BMD (T-Score > −1 DS) | ||
| Type of Fracture | N | % |
| Unimalleolar | 2 | 16.7 |
| Bimalleolar | 7 | 58.3 |
| Trimalleolar | 3 | 25.0 |
| Non-specificated | 0 | 0.0 |
| Total | 12 | 100.0 |
| Osteopenia (−2.5 DS < T-Score < −1 DS) | ||
| Type of Fracture | N | % |
| Unimalleolar | 6 | 20.7 |
| Bimalleolar | 12 | 41.4 |
| Trimalleolar | 11 | 37.9 |
| Non-specificated | 0 | 0.0 |
| Total | 29 | 100.0 |
| Osteoporosis (T-Score < −2.5 DS) | ||
| Type of Fracture | N | % |
| Unimalleolar | 2 | 20.0 |
| Bimalleolar | 1 | 10.0 |
| Trimalleolar | 7 | 70.0 |
| Non-specificated | 0 | 0.0 |
| Total | 10 | 100.0 |
| Normal | Osteopenia | Osteoporosis | ||||
|---|---|---|---|---|---|---|
| Low-energy trauma | 8 | 66.7% | 24 | 82.8% | 10 | 100.0% |
| High-energy trauma | 4 | 33.3% | 5 | 17.2% | 0 | 0.0% |
| TOTAL | 12 | 100.0% | 29 | 100.0% | 10 | 100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinonapoli, G.; Donantoni, M.; Ceccarini, P.; Caraffa, A. Analysis of Operated Ankle Fractures in Elderly Patients: Are They All Osteoporotic? Appl. Sci. 2024, 14, 3787. https://doi.org/10.3390/app14093787
Rinonapoli G, Donantoni M, Ceccarini P, Caraffa A. Analysis of Operated Ankle Fractures in Elderly Patients: Are They All Osteoporotic? Applied Sciences. 2024; 14(9):3787. https://doi.org/10.3390/app14093787
Chicago/Turabian StyleRinonapoli, Giuseppe, Marco Donantoni, Paolo Ceccarini, and Auro Caraffa. 2024. "Analysis of Operated Ankle Fractures in Elderly Patients: Are They All Osteoporotic?" Applied Sciences 14, no. 9: 3787. https://doi.org/10.3390/app14093787
APA StyleRinonapoli, G., Donantoni, M., Ceccarini, P., & Caraffa, A. (2024). Analysis of Operated Ankle Fractures in Elderly Patients: Are They All Osteoporotic? Applied Sciences, 14(9), 3787. https://doi.org/10.3390/app14093787






