Constituents from Ageratina pichinchensis and Their Inhibitory Effect on Nitric Oxide Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Plant Material
2.2.1. Purification and Identification of Compounds from A. pichinchensis Leaves
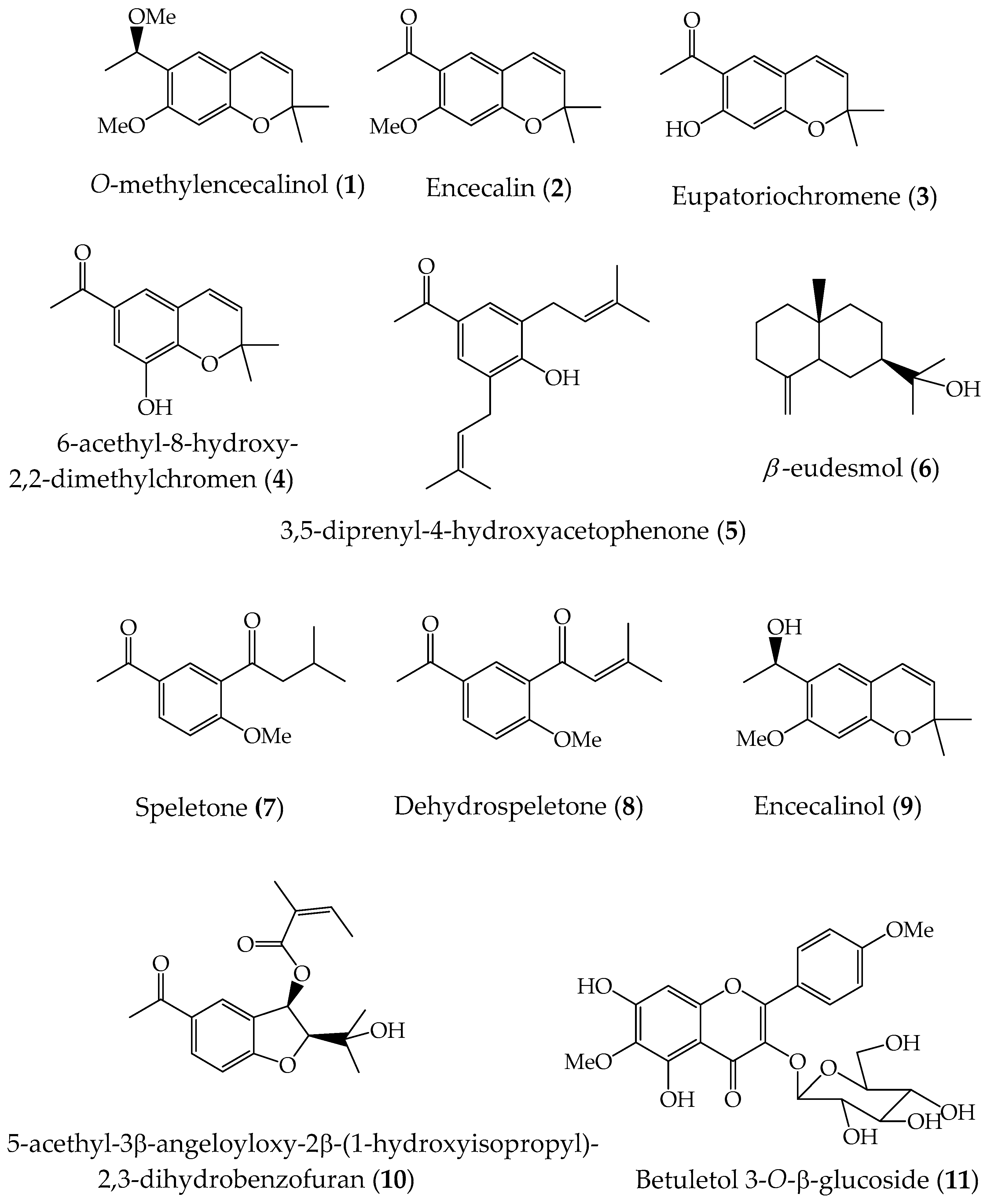
O-Methylencecalinol (1)
Encecalin (2)
Euparoriochromene (3)
6-Acethyl-8-Hydroxy-2,2-Dimethylchromene (4)
3.5-Diprenyl-4-Hydroxyacetophenone (5)
β-Eudesmol (6)
Speletone (7)
Dehydrospeletone (8)
Encecalinol (9)
5-Acetyl-3β--Angeloyloxy-2β-(1-Hydroxyisopropyl)-2,3-Dihydrobenzofurane (10)
2.2.2. Purification and Identification of Compounds from A. pichinchensis Flowers
Betuletol 3-O-β-Glucoside (11)
2.3. Anti-Inflammatory Assays
2.3.1. TPA-Induced Mouse Ear Edema
2.3.2. In Vitro Anti-Inflammatory Activities
Macrophage Culture in 75 cm2 Flasks
Cell Viability of RAW 264.7 Macrophages
Treatment of Macrophages with Compounds and LPS
Determination of NO in RAW 264.7 Macrophages
Determination of the IL-6 Concentration in RAW 264.7 Macrophages
RAW-Blue Cell Culture
RAW-Blue Macrophage Treatment
2.4. Statistical Analysis
3. Results and Discussion
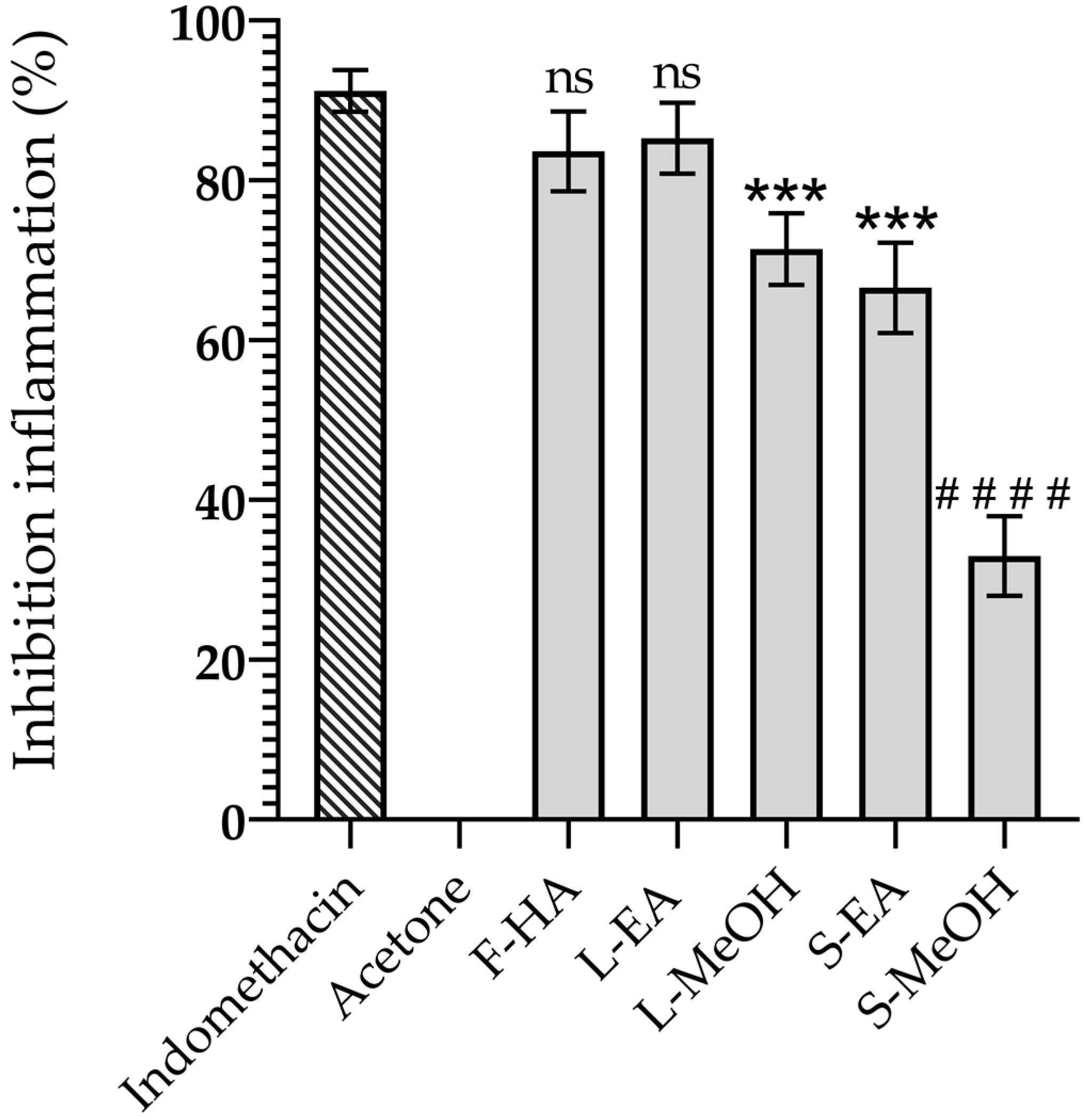
3.1. Anti-Inflammatory Activity of the Extract from the Aerial Parts of A. pichinchensis
3.2. Chemical Composition of Anti-Inflammatory Extracts
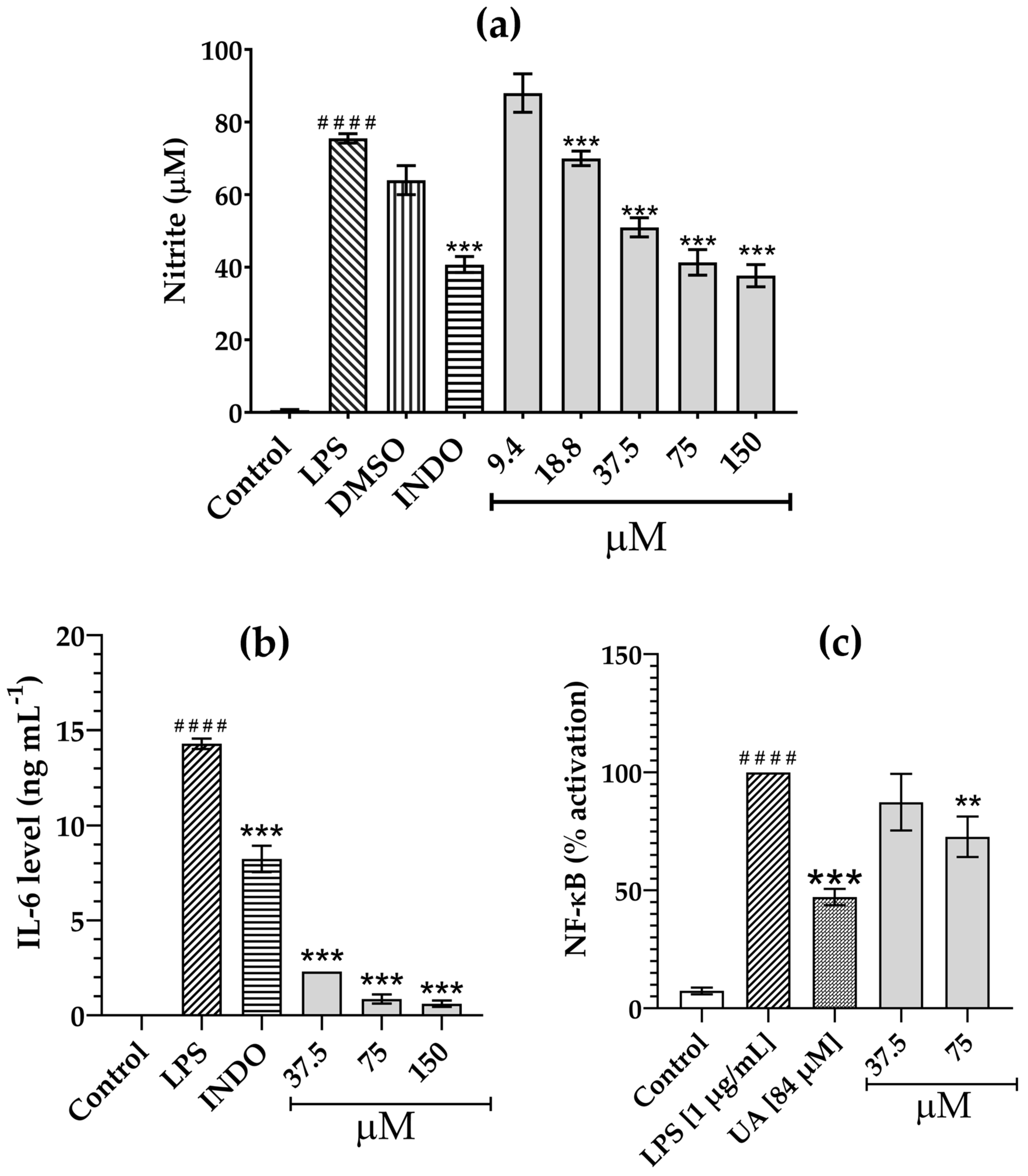
3.3. Inhibition of LPS-Induced NO Production by Compounds 1–11
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McVaugh, R. Compositae. Flora Novo-Galiciana. In A Descriptive Account of the Vascular Plants of Western Mexico; The University of Michigan Press: Ann Arbor, MI, USA, 1984; Volume 12. [Google Scholar]
- GBFI. Ageratina Pichinchensis. Available online: https://www.gbif.org/es/species/5400249 (accessed on 1 December 2023).
- Villaseñor, R.; Espinosa, G.F.J. Catálogo de Malezas de México; Universidad Nacional Autónoma de México: México City, México, 1998; pp. 1–448. [Google Scholar]
- INAH. Jardín Etnobotánico y Museo de la Medicina Tradicional. 2023. Available online: https://lugares.inah.gob.mx/es/museos-inah/colecciones/piezas/12915-12915-axihuitl.html?lugar_id=389 (accessed on 1 December 2023).
- Argueta, A.; Cano, L.; Rodarte, M. Atlas de la Medicina Tradicional Mexicana, Tomo 1–3; Instituto Nacional Indigenista: Mexico City, Mexico, 1994; p. 1786. [Google Scholar]
- Ríos, M.Y.; Aguilar-Guadarrama, B.; Navarro, V. Two new benzofurans from Eupatorium aschenbornianum and their antimicrobial activity. Planta Med. 2003, 69, 967–970. [Google Scholar] [PubMed]
- Navarro-García, V.M.; Gonzalez, A.; Fuentes, M.; Aviles, M.; Ríos, M.Y.; Zepeda, G.; Rojas, M.G. Antifungal activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol. 2003, 87, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; Sánchez-Gómez, P.; Rodriguez-Silverio, J.; Castillo-Henkel, C.; Cervantes-Cuevas, H.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschembornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia 2010, 81, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mendoza, M.; Rodriguez-Silverio, J.; Rivero-Cruz, J.F.; Rocha-González, H.; Pineda-Farías, J.; Arrieta, J. Antinociceptive effect and gastroprotective mechanisms of 3,5-diprenyl-4-hydroxyacetophenone from Ageratina pichinchensis. Fitoterapia 2013, 87, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Ríos, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; Jiménez, E.; Tortoriello, J. Effect on the wound healing process and in vitro cell proliferation by the medical Mexican plant Ageratina pichinchensis. Planta Med. 2011, 77, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barajas, L.; Rojas-Vera, J.; Morales-Méndez, A.; Rojas-Fermín, L.; Lucena, M.; Buitrago, A. Chemical composition and evaluations of antibacterial activity of oils of Ageratina jahnii and Ageratina pichinchensis collected in Mérida, Venezuela. Bol. Latinioam. Caribe Plant. Med. Aromat. 2013, 12, 92–98. [Google Scholar]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar-Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622–627. [Google Scholar] [CrossRef] [PubMed]
- White, M.M.D. Mediators of inflammation and the inflammatory process. J. Allergy Clin. Immunol. 1999, 103, S978–S981. [Google Scholar] [CrossRef]
- Duleba, M.D.A.J.; Dokras, M.D.A. Is PCOS an inflammatory process? Fert. Steril. 2012, 97, 7–12. [Google Scholar] [CrossRef]
- Adbulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the resolutions of the inflammatory response. Trends Immunol. 2019, 1545, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Veríssimo, F.J.; Oliveira, C.R.N.; Nunes, J.C.A.; Araújo, M.F.T.A.; Veríssimo, A.J.W.; Galvao, A.J.M. The role of the mediators of inflammation in cancer development. Pathol. Onco. Res. 2015, 21, 527–534. [Google Scholar]
- Salgado, A.; Bóveda, J.L.; Monasterio, J.; Segura, R.M.; Mourelle, M.; Gómez-Jiménez, J.; Peracaula, R. Inflammatory mediators and their influence on haemostasis. Haemostasis 1994, 24, 132–138. [Google Scholar] [CrossRef]
- Schlag, G.; Redl, H. Mediators of injury and inflammation. World J. Sug. 1996, 20, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.S.; Geller, D.A. Molecular regulation of the human inducible nitric oxidase synthase (iNOS) gene. Shock 2000, 13, 413–424. [Google Scholar]
- Krishna, R.K.M. Molecular mechanisms regulating iNOS expression in various cell types. J. Toxicol. Environ. Health B Crit. Rev. 2000, 3, 27–58. [Google Scholar]
- Chiou, W.-F.; Chen, C.-F.; Lin, J.-J. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br. J. Pharmacol. 2000, 129, 1553–1560. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Poteser, M.; Wakabayashi, I. Serum albumin induces iNOS expression and NO production in RAW 264.7 macrophages. Br. J. Pharmacol. 2004, 143, 143–151. [Google Scholar] [CrossRef]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef]
- Xie, Q.-W.; Nathan, C. The high-output nitric oxide pathway: Role and regulation. J. Leuk. Biol. 1994, 56, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Clark, J.M.; Buzás, E.I.; Gorman, C.L.; Cope, A.P. Nitric oxide, chronic inflammation and autoimmune. Immunol. Let. 2007, 111, 1–5. [Google Scholar] [CrossRef]
- Laroux, F.S.; Pavlick, K.P.; Hines, I.N.; Kawachi, S.; Harada, H.; Bharwani, S.; Hoffman, J.M.; Grisham, M.B. Role of nitric oxide in inflammation. Acta Physiol. Scand. 2001, 173, 113–118. [Google Scholar] [CrossRef]
- Hurtado-Díaz, I.; Sánchez-Carranza, J.N.; Romero-Estrada, A.; González-Maya, L.; González-Christen, J.; Herrera-Ruiz, M.; Alvarez, L. 16-Hydroxy-Lycopersene, a Polyisoprene Alcohol Isolated from Tournefortia hirsutissima, Inhibits Nitric Oxide Production in RAW 264.7 Cells and Induces Apoptosis in Hep3B Cells. Molecules 2019, 24, 2366. [Google Scholar] [CrossRef] [PubMed]
- Romero-Estrada, A.; Maldonado-Magaña, A.; González-Christen, J.; Marquina Bahena, S.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC CAM 2016, 16, 422. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Marquina, B.S.; Romero-Estrada, A.; Bernabé-Antonio, B.; Cruz-Sosa, F.; González-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Alvarez, L. Establishment and phytochemical analysis of a callus cultures from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [Google Scholar] [CrossRef]
- Becerra, J.; Silva, M.; Delle-Monache, G.; Delle-Monache, F.; Botta, M. Two new chromenes from Eupatorium glechonophyllum Less. Rev. Lat. Quím. 1983, 14, 92–94. [Google Scholar]
- Steinbeck, C.; Spitzer, V.; Starosta, M.; Poser, G. Identification of Two Chromenes from Calea serrata by Semiautomatic Structure Elucidation. J. Nat. Prod. 1997, 60, 627–628. [Google Scholar] [CrossRef]
- Shamsuddin, K.M.; Musharraf, M.A.; Zobairi, M.O.; Ali, N. Demethylacetovanillochromene from Tithonia diversifolia (Hemes1.) A. Gray. Indian J. Chem. Sect. B Org. Med. Chem. 2001, 8, 751–752. [Google Scholar]
- Zhai, H.L.; Zhao, G.J.; Yang, G.J.; Sun, H.; Yi, B.; Sun, L.N.; Chen, W.S.; Zheng, S.Q. A new chromene glycoside from Tithonia diversifolia. Chem. Nat. Compd. 2010, 46, 198–200. [Google Scholar] [CrossRef]
- Bjeldanes, L.; Geissman, T. Euparinoid constituents of Encelia californica. Phytochemistry 1969, 8, 1293–1296. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Franke, H. Naturally occurring coumarin derivatives. IX. Constituents of the genus Gerbera. Chem. Ber. 1973, 106, 382–387. [Google Scholar] [CrossRef]
- Bohlmann, F.; Rao, N. New hydroxyacetophenone derivatives from Espeletia schultzii. Chem. Ber. 1973, 106, 3035–3038. [Google Scholar] [CrossRef]
- Dupre, S.; Bohlmann, F.; Knox, E. Prenylated p-hydroxyacetophenone derivatives from the giant Senecio johnstonii. Biochem. Syst. Ecol. 1990, 18, 149–150. [Google Scholar] [CrossRef]
- Achenbach, H.; Waibel, R.; Addae-Mensah, I. Constituents of West African medicinal plants. Part 17. Sesquiterpenes from Carissa edulis. Phytochemistry 1985, 24, 2325–2328. [Google Scholar] [CrossRef]
- Schwartz, M.A. Syntheses of (+)-α- and (+)-β-eudesmol and their diastereomers by intramolecular nitrone-olefin cycloaddition. J. Org. Chem. 1985, 50, 1359–1365. [Google Scholar] [CrossRef]
- Castañeda, P.; Gómez, L.; Mata, R.; Lotina-Hennsen, B.; Anaya, A.L.; Bye, R. Phytogrowth-Inhibitory and Antifungal Constituents of Helianthella quinquenervis. J. Nat. Prod. 1996, 59, 323–326. [Google Scholar] [CrossRef]
- Merfort, I.; Wendisch, D. Flavonoid glycosides from Arnica montana and Arnica chamissonis. Planta Med. 1987, 53, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Merfort, I.; Wendisch, D. New flavonoid glycosides from Arnicae flos DAB 91. Planta Med. 1992, 58, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rebolledo, G.; Garduño-Siciliano, L.; García-Rodríguez, R.; Pérez-González, M.; Chávez, M.I.; Bah, M.; Siordia-Reyes, G.; Chamorro-Cevallos, G.; Jiménez-Arellano, M.A. Antiinflammatory and toxicological evaluation of Moussonia deppeana (Schldl. & Cham) Hanst and verbascoside as a main active metabolite. J. Ethnopharmacol. 2016, 187, 269–280. [Google Scholar]
- Owona, B.A.; Njayou, N.F.; Laufer, S.; Moundipa, P.F.; Schluesener, H.J. A fraction of stem bark extract of Entada africana suppresses lipopolysaccharide-induced inflammation in RAW 264.7 cells. J. Ethnopharmacol. 2013, 149, 162–168. [Google Scholar] [CrossRef]
- An, X.; Gil, S.L.; Kang, H.; Heo, H.J.; Cho, Y.S.; Ok, D.M. Antioxidant and Anti-Inflammatory Effects of Various Cultivars of Kiwi Berry (Actinidia arguta) on Lipopolysaccharide-Stimulated RAW 264.7 Cells. J. Microbiol. Biotechnol. 2016, 26, 1367–1374. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Won, T.-J.; Hwang, B.-Y.; Kim, H.-R.; Hwang, K.-W.; Sul, D.; Park, S.-Y. The new diterpene isodojaponin D inhibited LPS-induced microglial activation through NF-kappaB and MAPK signaling pathways. Eur. J. Pharmacol. 2010, 642, 10–18. [Google Scholar] [CrossRef]
- Hadi, G.; Ghanbari, R.; Delezar, A.; Nejad, E.S.; Yousef, M.M.; Bamdad, M.S.; Hamedayazdan, S.; Nazemiyeh, H. Caccinia macrathera Brand var. macranthera: Phytochemical analysis, phytotoxicity and antimicrobial investigations of essential oils with concomitant in silico molecular docking based on OPLS force-field. Toxicon 2023, 234, 107291. [Google Scholar]
- Merrill, G.B. Eupatoriochromene and encecalin, plant growth regulators from yellow starthistle (Centaurea solstitialis L.). J. Chem. Ecol. 1989, 15, 2073–2087. [Google Scholar] [CrossRef]
- Brito-Machado, D.; Ramos, Y.J.; Antunes, A.C.D.; Azevedo, G.Q.; Franklin, E.G.; Lima, M.D. Volatile chemical variation of essential oils and their correlation with insects, phenology, ontogeny and microclimate: Piper mollicomum Kunth, a case of study. Plants 2022, 11, 3535. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-S.; Gao, B.; Dong, Z.-D.; Qiao, Z.-A.; Yan, M.; Han, W.-W.; Li, W.-H.; Han, L. Chemical compounds, antioxidant activities, and inhibitory activities against Xanthine oxidase of the essential oils from the three varieties of sunflower (Helianthus annuus L.) Receptacles. Front. Nutr. 2021, 8, 737157. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Chhetri, B.K.; Setzer, W.N. Chemical composition and phytotoxicity of the essential oil of Encelia farinosa growing in the Sonoran desert. Am. J. Essent. Oil. Nat. Prod. 2013, 1, 18–22. [Google Scholar]
- Zeeshan, M.; Rizvi, S.M.D.; Khan, M.S.; Kumar, A. Isolation, partial purification and evaluation of bioactive compounds from leaves of Ageratum houstonianum. EXCLI J. 2012, 11, 78–88. [Google Scholar]
- Yuan, Y.; Liub, Y.J.; Huanga, L.Q.; Cuia, G.H.; Fua, G.F. Soil acidity elevates some phytohormone and β-eudesmol contents in roots of Atractylodes lancea. Russ. J. Plant Physiol. 2009, 56, 147–151. [Google Scholar] [CrossRef]
- Yua, F.; Haradab, H.; Yamasakia, K.; Okamotoa, S.; Hirasec, S.; Tanakac, Y.; Norihiko, N.M.; Utsumia, R. Isolation and functional characterization of a β-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet. FEBS Lett. 2008, 582, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Britto, A.C.S.; de Oliveira, A.C.A.; Henriques, R.M.; Cardoso, G.M.B.; Bomfim, D.S.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Pinheiro, M.L.B.; Costa, E.V.; et al. In vitro and In Vivo antitumor effects of the Essential Oil from the leaves of Guatteria friesiana. Planta Med. 2012, 78, 409–414. [Google Scholar] [CrossRef]
- Xue You, C.; Yang, K.; Fang, C.W.; Zhang, W.J.; Wang, Y.; Jiao, J.H.; Fan, L.; Shan, S.D.; Feng, Z.G.; Wei, Z.D. Cytotoxic Compounds Isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.L.; Li, Y.C.; Tsuneki, H.; Xiao, J.F.; Xia, M.; Wang, M.W.; Kimura, I. β-Eudesmol suppresses tumor growth through inhibition of tumor neovascularization and tumor cell proliferation. J. Asian Nat. Prod. Res. 2008, 10, 159–167. [Google Scholar] [CrossRef]
- Seo, M.J.; Kim, S.J.; Kang, T.H.; Rim, H.K.; Jeong, H.J.; Um, J.Y.; Hong, S.H.; Kim, H.M. The regulatory mechanism of β-eudesmol is through the suppression of caspase-1 activation in mast cell–mediated inflammatory response. Immunopharmacol. Immunotoxicol. 2011, 33, 178–185. [Google Scholar] [CrossRef]
- Nam, S.Y.; Kim, H.Y.; Kim, H.M.; Jeong, H.J. Βeta-eudesmol reduces stem cell factor-induced mast cell migration. Int. Immunopharmacol. 2017, 48, 1–7. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2019, 40, 158–189. [Google Scholar] [CrossRef]
- Hong, H.-J.; Loh, S.-H.; Yen, M.-H. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br. J. Pharmacol. 2000, 131, 631–637. [Google Scholar] [CrossRef]
- Möller, B.; Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases. Springer Semin. Immun. 2006, 27, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Cicuttini, F.; Li, J.; Jones, G. Target IL-6 in the treatment of inflammatory and autoimmune diseases. Exper Opin. Investig. Drugs 2009, 18, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.G.S.; Souza, G.R.; Silva, J.C.; Saraiva, S.R.G.D.; Junior, R.G.O.; Quintans, J.S.S.; Barreto, R.S.S.; Bonjardim, L.R.; Cavalcanti, S.C.H.; Junior, L.J.Q. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 2013, 808460. [Google Scholar] [CrossRef] [PubMed]
- Mankhong, S.; Iawsipo, P.; Srisook, E.; Srisook, K. 4-methoxycinnamyl p-coumarate isolated from Etlingera pavieana rhizomes inhibits inflammatory response via suppression of NF-κβ, Akt and AP-1 signaling in LPS-stimulated RAW 264.7 macrophages. Phytomedicine 2019, 54, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Han, F.; Luan, S.S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.X. Triterpenoids from Ganoderma lucidum and Their Potential Anti-inflammatory Effect. J. Agric. Food Chem. 2019, 67, 5147–5158. [Google Scholar] [CrossRef]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-κβ. Biol. Chem. 1997, 378, 951–961. [Google Scholar] [CrossRef]
| Compounds | Cell Viability (%) a | NO Inhibition 75 µM (%) | NO Inhibition (IC50, µM) |
|---|---|---|---|
| 1 | 109.9 ± 7.16 | 0.95 ± 1.35 | >75 |
| 2 | 112.4 ± 24.08 | 16.75 ± 5.36 | >75 |
| 3 | 115.6 ± 1.58 | 11.98 ± 7.85 | >75 |
| 4 | 99.33 ± 12.39 | 22.63 ± 10.38 | >75 |
| 5 | 100.20 ± 2.95 | 29.77 ± 18.27 | >75 |
| 6 | 61.14 ± 6.31 | ----- | ----- |
| 7 | 104.7 ± 1.82 | 5.90 ± 8.35 | >75 |
| 8 | 103.9 ± 3.83 | 36.73 ± 16.93 | >75 |
| 9 | 110.9 ± 8.3 | 29.77 ± 9.37 | >75 |
| 10 | 121.2 ± 10.20 | 5.98 ± 5.22 | >75 |
| 11 | 101.3 ± 1.62 | 75.08 ± 3.07 | 20.55 ± 0.27 |
| DMSO b | ----- | ----- | ----- |
| Indomethacin c (84 µM) | ----- | 65.93 ± 6.03 | 54.69 ± 10.34 |
| Etoposide d (68 µM) | 42.02 ± 4.23 | ----- | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ramos, M.; Guerrero-Alonso, A.; Romero-Estrada, A.; González-Christen, J.; Alvarez, L.; Acevedo-Fernández, J.J.; Román-Guerrero, A.; Cruz-Sosa, F.; Marquina-Bahena, S. Constituents from Ageratina pichinchensis and Their Inhibitory Effect on Nitric Oxide Production. Appl. Sci. 2024, 14, 3942. https://doi.org/10.3390/app14093942
Sánchez-Ramos M, Guerrero-Alonso A, Romero-Estrada A, González-Christen J, Alvarez L, Acevedo-Fernández JJ, Román-Guerrero A, Cruz-Sosa F, Marquina-Bahena S. Constituents from Ageratina pichinchensis and Their Inhibitory Effect on Nitric Oxide Production. Applied Sciences. 2024; 14(9):3942. https://doi.org/10.3390/app14093942
Chicago/Turabian StyleSánchez-Ramos, Mariana, Araceli Guerrero-Alonso, Antonio Romero-Estrada, Judith González-Christen, Laura Alvarez, Juan José Acevedo-Fernández, Angélica Román-Guerrero, Francisco Cruz-Sosa, and Silvia Marquina-Bahena. 2024. "Constituents from Ageratina pichinchensis and Their Inhibitory Effect on Nitric Oxide Production" Applied Sciences 14, no. 9: 3942. https://doi.org/10.3390/app14093942
APA StyleSánchez-Ramos, M., Guerrero-Alonso, A., Romero-Estrada, A., González-Christen, J., Alvarez, L., Acevedo-Fernández, J. J., Román-Guerrero, A., Cruz-Sosa, F., & Marquina-Bahena, S. (2024). Constituents from Ageratina pichinchensis and Their Inhibitory Effect on Nitric Oxide Production. Applied Sciences, 14(9), 3942. https://doi.org/10.3390/app14093942







