Abstract
Arm swing is an inherent aspect of human locomotion that enhances lower limb (LL) muscle activation, which may explain its benefits for stroke rehabilitation over fixed-arm approaches. This study analyzed how restricting arm movement affects LL muscle coordination by comparing treadmill walking with arms (WWA) and walking with no arms (WNA) through muscle synergy analysis. Surface electromyography was recorded from eight LL muscles in ten healthy participants. Significant differences were found in muscle activity envelopes at 50% of the gait cycle (GC) for the Gluteus Medius, 0% and 100% GC for the Vastus Lateralis and Semitendinosus, and 25% GC for the Semitendinosus, Gastrocnemius Medialis, and Soleus. The Rectus Femoris and Vastus Lateralis showed higher variability and activation in WNA compared to WWA. Synergy analysis revealed four muscle synergies, with a median global variance of 95%. While spatial components were similar, temporal differences emerged at 0% GC for Synergy 1, 5% and 90% GC for Synergy 2, and 95% GC for Synergy 3 (p < 0.05). Our results suggest that arm swing influences LL muscle activity and coordination during walking. Future studies will aim at understanding the effects of arm swing in stroke rehabilitation, which could help design more effective gait rehabilitation protocols including arm swing.
1. Introduction
Locomotion, defined as a purposeful and goal-directed behavior, is a fundamental aspect of human mobility involving cyclic and rhythmic movements of the limbs. When a person is walking, there are rhythmic automatic processes involving coordination between the central nervous system and the musculoskeletal system that enable smooth and efficient movements [1]. This coordination is orchestrated by locomotor networks known as central pattern generators [2].
While the biomechanics and functions of the lower limbs (LLs) during gait have been widely studied [3,4], there is a growing interest in understanding the role of the rhythmic swinging movement of upper limbs (ULs) [5,6]. This natural swinging movement of the ULs is referred to as arm swing and has been suggested to be an integral component of human bipedal locomotion [7,8]. For instance, when a disturbance is introduced during gait, such as tripping, the arm swing is prolonged and even accelerated, which has a beneficial effect on balance recovery and helps to avoid falling [9]. Likewise, arm swing contributes to energetic efficiency [10,11,12] and has been linked to decreased ground reaction moments [8]. Additionally, recent research suggests that the kinematics of arm swing may differ between genders during locomotor tasks like running [13]. Furthermore, arm swing during gait is not a passive pendular action, as it requires active muscle contractions in the ULs [14,15,16]. A study that investigated coupling between UL and LL muscles during gait revealed that arm swing during gait is an active contributor to efficient activation of LL muscles [16]. This contributes to the evidence supporting a connection between the neural pathways controlling arm movement and those controlling LL muscles [1,6,17,18]. Altogether, these findings support the importance of arm swing in human walking.
Regarding its impact in the neurorehabilitation field, adding arm swing to robotic therapy improved functional outcomes compared to conventional training with arm restraint in stroke survivors [19]. Some possible explanations are that arm swing allows maintenance of trunk and body stability with a reduced center of mass lateral displacement [10], or that active arm swing influences LL muscle activation and modulates corticospinal excitability [19]. Yet, these hypotheses have not been be tested. Although some groups have tried to design robotic devices that allow natural arm swing [20,21,22], current treadmill rehabilitation assisted by commercial robots is mostly performed with constrained arm movements due to safety reasons and the lateral encumbrance of the robot [23].
In the past decades, several studies have suggested that the central nervous system modulates neural commands by activating groups of muscles rather than individual muscles to execute a given task [24,25,26,27,28,29,30]. Under this hypothesis, each group of co-activated muscles acts synergistically. Analysis of muscle synergies, which can be used to characterize muscle coordination [31], also reduces the high-dimensional space of multiple muscle activation recordings and degrees of freedom [25,30] to a low-dimensional space of a few muscle synergies. Muscle synergies have been studied in a wide range of rhythmic tasks, such as walking [32], running [33], and cycling [24,34,35,36], by measuring surface electromyography (sEMG). The analysis of muscle synergies can be used, for example, to compare muscle coordination between different tasks [24,33,37,38]. Regarding human locomotion, some groups have studied the effect of walking speed, body weight support, or walking in the water on LL muscle synergies [38,39,40]. Boccia et al. [37] compared UL and LL muscle synergies during treadmill walking and Nordic treadmill walking, an activity that requires precise UL movements while walking. However, though several groups have investigated the origin and purpose of arm swing during walking in terms of metabolic cost, kinematics, sEMG, or spatiotemporal parameters [10,11,41,42], to the best of our knowledge, none have quantified the effect of arm swing on LL muscle synergies during walking.
In this study, our goal was to investigate how arm swing influences LL muscle activity and muscle coordination in healthy subjects. Considering that WWA and WNA are similar tasks, we hypothesized that muscle synergies would show minimal differences between these conditions, as the neural control of human locomotion may be strongly encoded at the spinal level [1]. However, individual EMG activation patterns could vary due to the presence or absence of arm swing. These differences might highlight the modulatory role of arm swing in LL limb muscle coordination, potentially influencing the timing and amplitude of muscle activation during walking. This can be considered a first step towards understanding if the lack of arm swing during current robot-based walking rehabilitation approaches might hinder LL muscle coordination, and thus affect the efficiency of rehabilitation sessions.
2. Materials and Methods
Ten healthy young participants (seven men and three women, mean age: 24.3 ± 2.26 years old) volunteered for this study. All participants were physically active in daily life, though none were engaged in any formal athletic training. They were informed about the procedures involved in the study. The protocol was approved by an institutional Ethics Committee (the Ethical Committee for Clinical Research of the Hospital Universitario Severo Ochoa de Leganés) under the code CYCLING-AL in October 2022 and was conducted in accordance with the Declaration of Helsinki at the Center for Clinical Neuroscience of Hospital Los Madroños. Prior to data collection, informed consent was obtained from the participants.
First, participants walked on a sensorized Y-Mill treadmill (Motek Medical, B.V., Houten, The Netherlands) for 2 min, so they could familiarize themselves with the treadmill and select a speed. During this initial trial, experimenters determined the desired walking cadence by manually fitting a metronome to the participants’ natural cadence. Then, participants walked for an additional minute on the treadmill to familiarize themselves with the selected metronome. The sensorized treadmill is part of the Bestable testbed [43] of the EUROBENCH project (https://cordis.europa.eu/project/id/779963/es (accessed on 1 October 2022)), and allows: (1) measurement of ground reaction force; (2) recording of foot strike and foot off events; and (3) reading of an external analog signal for synchronization by means of a digital acquisition board (NI USB-6210, National Instruments Corp, Austin, Texas, USA).
Next, surface electrodes (Ambu WhiteSensor WSP30, Ambu A/S, Ballerup, Denmark) were placed on each participant’s dominant leg to measure the muscle activity of eight muscles––the Gluteus Medius (GLM), Rectus Femoris (RF), Vastus Lateralis (VL), Biceps femoris (BF), Semitendinosus (ST), Gastrocnemius Medialis (GaM), Soleus (SOL), and Tibialis Anterior (TA)––following SENIAM guidelines [44]. Additionally, one reference electrode was placed on the surface of the lateral malleolus of the same leg. sEMG was recorded with a sampling frequency of 2000 Hz (Sessantaquattro+, OTBioelettronica SRL, Torino, Italy).
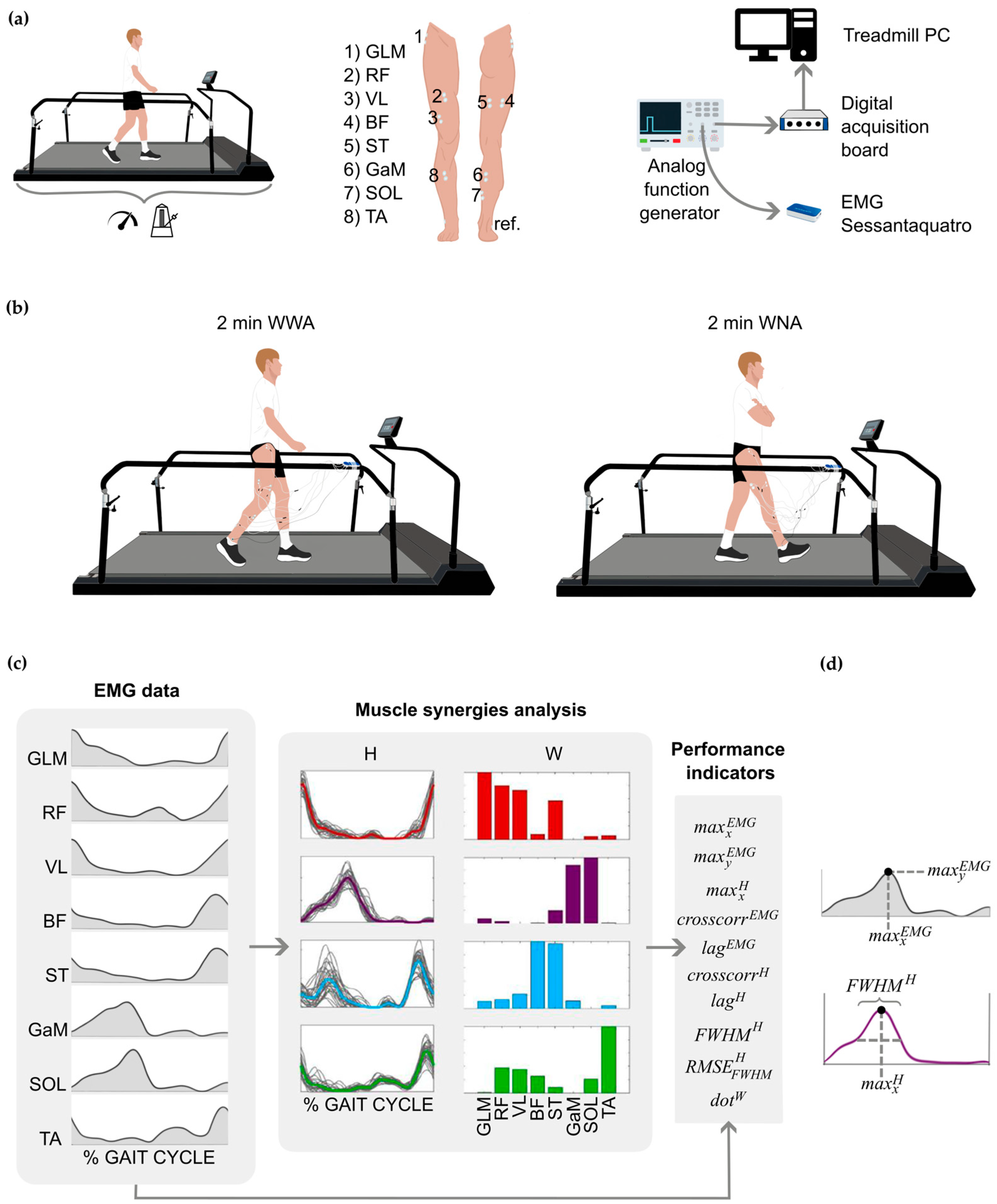
Then, participants performed 2 min of treadmill walking with arm swing (walking with arms, WWA) and 2 min of treadmill walking without arm swing (walking with no arms, WNA). In both tasks, participants were asked to synchronize their walking cadence with the metronome. We used a metronome to control walking cadence and ensure that any differences in muscle activation and coordination were not due to variations in gait speed, allowing for more accurate comparison between walking with and without arm swing. During WWA, subjects were asked to swing the arms naturally, which resulted in a 1:1 arm-leg coordination that was visually verified [45]. The two recording systems (the sensorized treadmill and the EMG recorder) were synchronized by means of an analog signal pulse of 100 ms and an amplitude of around 3V delivered in parallel by an analog function generator (HDG2102B, Hantek, Asturias, Spain). The experimental setup and protocol are shown in Figure 1a and Figure 1b, respectively.
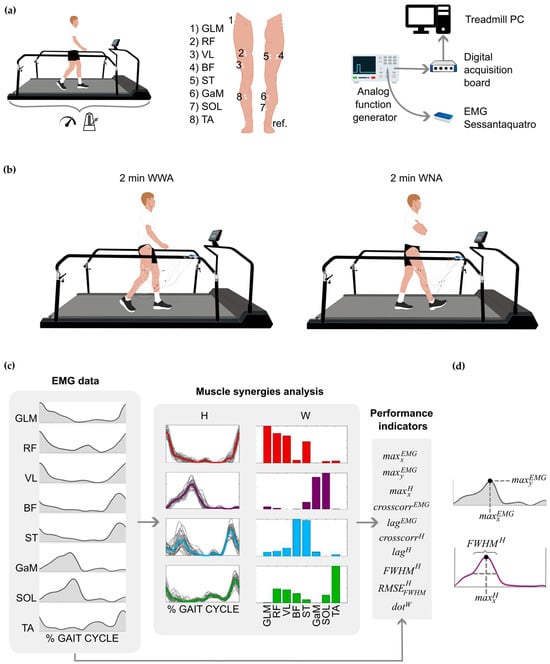
Figure 1.
(a) Setup of the experimental protocol. From left to right: participants walked for 2 min on a sensorized treadmill to record comfortable speed and cadence; electrode placement on each participant’s dominant leg; synchronization between devices by means of an analog signal delivered in parallel to the treadmill computer and the Sessataquattro+ EMG recorder. (b) Experimental protocol: participants walked for 2 min on a sensorized treadmill under two conditions: walking with arms (WWA) and walking with no arms (WNA). (c) Example of the electromyographic (EMG) analysis and extraction of muscle synergies. From left to right: EMG envelopes of the eight measured muscles; four synergies extracted (red Syn 1, purple Syn 2, blue Syn 3, green Syn 4); and performance indicators extracted from the EMG data and muscle synergies. (d) Schematic representation of , , and (), all explained in the Supplementary Material (Table S1). GLM, Gluteus Medius; RF, Rectus Femoris; VL, Vastus Lateralis; BP, Biceps Femoris; ST, Semitendinosus; GaM, Gastrocnemius Medialis; SOL, Soleus; TA, Tibialis Anterior; ref., reference electrode; W and H, matrices that represent the spatial time-independent and the time-dependent coefficients of a synergy, respectively.
The EMG signals were analyzed in Matlab R2021a using SynergyLab [24] and custom Matlab scripts. Signals were first high-pass filtered with a third-order Butterworth filter with a cutoff frequency of 20 Hz, then demeaned, rectified, and low-pass filtered with a third-order Butterworth filter with a cutoff frequency of 5 Hz to obtain the EMG envelopes (). The EMG envelopes were then segmented based on the events recorded by the sensorized treadmill. Segmentation was carried out for 30 cycles, normally the central cycles [46]. Each selected cycle was interpolated to 101 samples (from 0% to 100%), de-offset, and normalized to the mean value of the peaks of the selected 30 cycles. Finally, synergies were calculated from the selected cycles using the non-negative matrix factorization algorithm (NNMF) [47], as this method has been shown to effectively estimate LL joint movements by leveraging muscle patterns [48]. For each task and subject, four synergies were extracted [24,38,39], each including the spatial time-independent and the time-dependent coefficients of activation (matrices W and H, respectively), which are used to reconstruct with an error e, based on the following equation:
The size of the EMG0 and EMGR matrices is 8 × 101, with each row corresponding to a muscle and each column to a gait cycle percentage. The size of the H matrix is 4 × 101, with each row corresponding to a synergy and each column to a gait cycle percentage. The size of the W matrix is 8 × 4, with each row corresponding to a muscle and each column to a synergy. The and the reconstructed EMG () were used to calculate the variability accounted for (VAF) metric, which quantifies the accuracy of the EMG reconstruction [36]. The VAF was computed for each muscle individually (VAFmuscle) and for all of them together (VAFglobal). Minimum values of 90% for VAFglobal [49] and 75% for VAFmuscle [50] were selected to accept the reconstruction. A diagram of the EMG signals and synergy extraction is shown in Figure 1c.
Muscle activation and coordination were compared between WWA and WNA using 10 performance indicators from the EMG0 and spatial time-independent (W) and time-dependent (H) coefficients of activation. The performance indicators considered were: the position of the EMG peak () as a function of gait cycle (GC) [51], the difference (in %) between the value of the EMG peak in µV () during WWA and WNA (Δ), as in [33], the position of the synergy peak () in % of GC [33,51], the cross-correlation between the EMG activity of WWA and WNA () [24,37,40], the lag of () [37], the cross-correlation between time-dependent H coefficients of WWA and WNA () [37,40], the lag of () [37,40], the full width at half maximum of the time-dependent H coefficients () [37,52], the root mean square of () [37,52], and the normalized dot product between W of WWA and WNA () [24,33,41,53,54]. The detailed definitions and mathematical computations of these performance indicators are shown in Figure 1c,d and in the Supplementary Material (Table S1).
Statistical analysis was carried out to verify that cadences were not significantly different between tasks and in order to study the difference in EMG activity and muscle synergies between tasks. Cadence was computed in Matlab R2021a from heel strike events, whereas cadence distributions between WWA and WNA were compared in SPSS (IBM SPSS 29) using the Wilcoxon signed-rank test, after verifying that the data were not normally distributed by means of the Shapiro–Wilk test. Then, statistical differences among the performance indicators , , and for WWA and WNA were analyzed by means of the Wilcoxon signed-rank test. Finally, statistical differences between WWA and WNA for EMG envelopes and time-dependent coefficients H were calculated at each percent of the gait cycle by means of the Wilcoxon signed-rank test, following the methodology of previous research [38,51], and a non-parametric two-tailed paired t-test using the Statistical Parametric Mapping (SPM). All SPM analyses were performed with a spm1d open code (www.spm1d.org) in its non-parametric version (SnPM). Statistical significance was considered when the p-value was <0.05. However, to account for a possible higher false positive rate, we elected to combine the statistical results with SPM analysis. To justify the sample size, a post hoc power analysis was performed in G*Power for a Wilcoxon signed-rank test. Using a conservative estimate of the effect size, based on previous findings by Boccia et al. [37], a minimum of seven participants was required to achieve a statistical power of 0.85 at α = 0.05. Statistical significance was set at p < 0.05, combining the results of the Wilcoxon test with SPM analyses to reduce the risk of false positives.
3. Results
3.1. Speed and Cadence
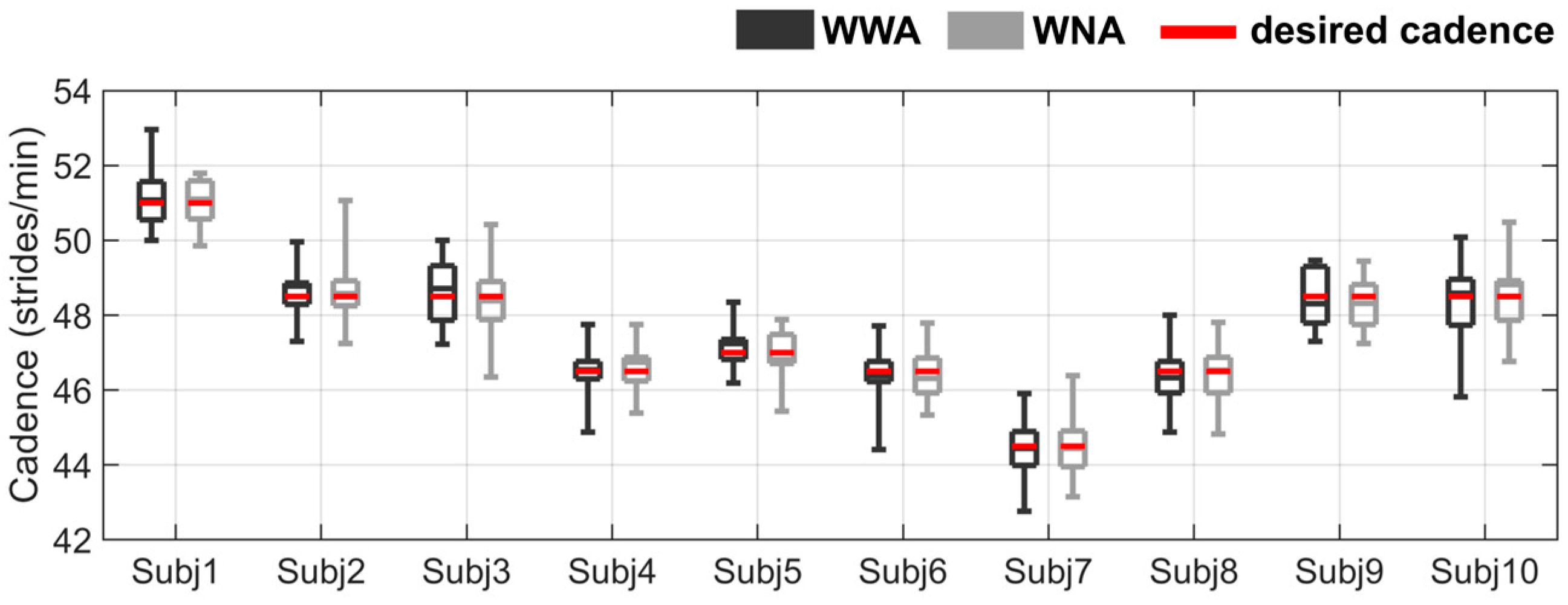
The mean self-selected speed of all subjects was 1.0 ± 0.1 m/s, and the mean cadence was 47.6 ± 1.7 strides/min. Two subjects (Subject 4 and Subject 5) repeated the WWA condition to ensure that the walking cadence was similar to the metronome cadence. After repeating the WWA trial, no statistical differences were found between WWA and WNA for any subject. The cadence distribution for the 30 selected steps of all subjects is shown in Figure 2. More details about the speed and cadence of all subjects can be found in the Supplementary Material (Table S2 and Table S3, respectively).
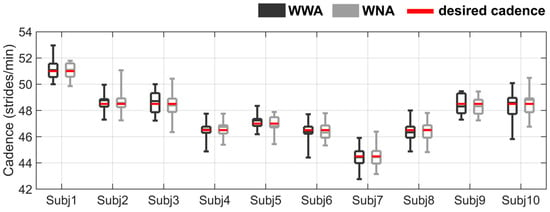
Figure 2.
Cadence distribution for all subjects during walking with arms (WWA in black) and walking with no arms (WNA in grey). The cadence specified by the metronome (desired cadence) is shown as a red horizontal line. See the Supplementary Material (Table S3) for more detailed data on the cadence statistics results.
3.2. EMG Envelopes and Performance Indicators
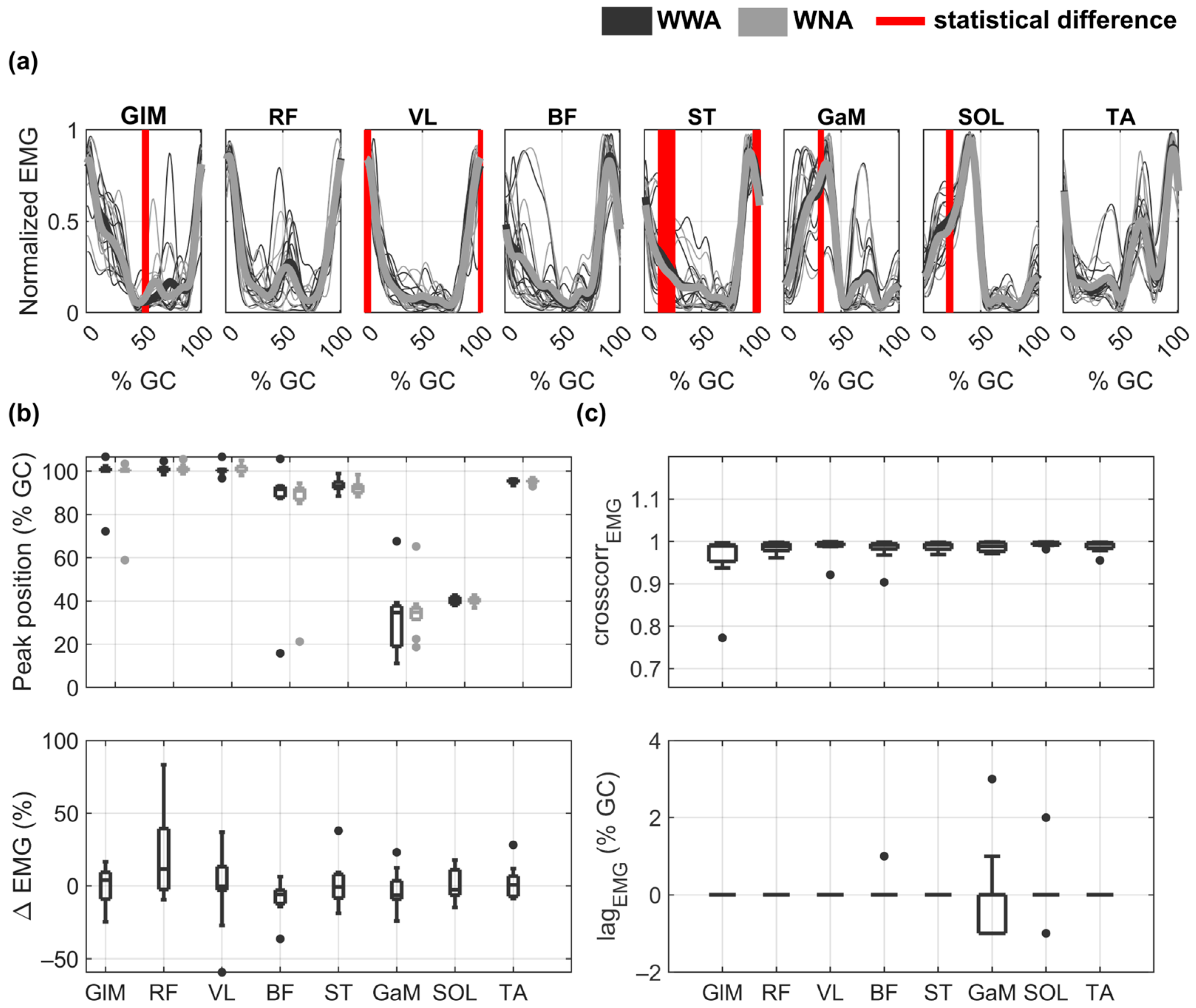
The EMG envelopes and performance indicators obtained for all subjects during WWA and WNA are shown in Figure 3. Although the shapes of the EMG envelopes looked similar between WWA and WNA (see Figure 3a), statistically significant differences were found between the two tasks at 50% of GC for GLM (minimum p-value = 0.037, Cohen’s d = 0.62), at around 0% of GC (or 100% of GC) for VL (minimum p-value = 0.037, Cohen’s d = 0.26) and ST (minimum p-value = 0.027, Cohen’s d = 0.55), and at 25% of GC for ST (minimum p-value = 0.002, Cohen’s d = 0.27), GaM (minimum p-value = 0.037, Cohen’s d = 0.41), and SOL (minimum p-value = 0.037, Cohen’s d = 0.26). Peak muscle activity was obtained at around 0% of GC (or 100% of GC) for GLM, RF, and VL, at around 90% of GC for BF, ST, and TA, and at around 40% of GC for GaM and SOL (Figure 3b). Additional details on the timing of the EMG maximum amplitude as a function of the GC can be found in Table S4 of the Supplementary Material. The distribution of the EMG differences between WWA and WNA (Δ) are shown in Figure 3b. Median Δ values are around zero for all muscles, due to the inter-subject variability. Subjects responded differently to walking without arms for RF (Δ ∈ (−10,83)), VL (Δ ∈ (−59,37)), ST (Δ ∈ (−19,38)), and GaM (Δ ∈ (−24,23)). Detailed data on the EMG amplitude of each subject can be found in Table S5 of the Supplementary Material. Some subjects increased RF and VL activity (17, 39, 21, and 83% for RF and 24, 27, 13, and 9% for VL Subjects 2, 3, 5, and 8, respectively) during WNA compared to WWA. Others decreased the activity of the quadriceps muscles (−7% and −10% for RF and −59 and −27% for VL for Subject 4 and 7, respectively) under WNA conditions compared to WWA condition. Subject 10 increased BF activity by 36% but decreased ST activity by 38% during WNA. Subjects 1, 6, and 9 showed less relevant changes (Δ < 15%). Moreover, there was a very high cross-correlation between WWA and WNA in all muscles, with a median value of 0.99 in all muscles and a maximum lag of 3% of GC for GaM (Figure 3c). No statistically significant differences were observed in the SPM analysis.
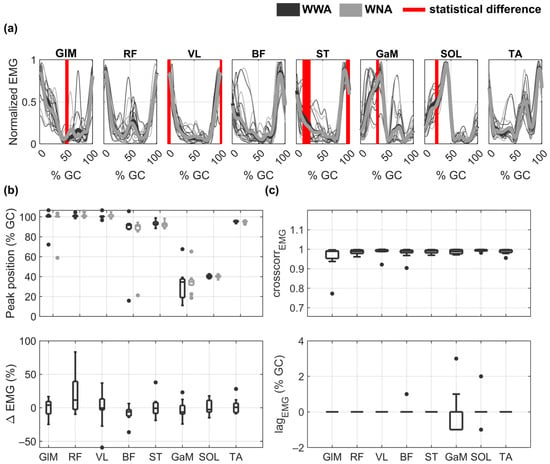
Figure 3.
EMG envelopes and performance indicators during walking with arms (WWA in black) and walking with no arms (WNA in grey). (a) Normalized mean EMG envelopes for each subject (thin lines) and mean of all subjects (bold lines). Vertical red lines show statistically significant differences between WWA and WNA. (b) Distribution of the peak position () as a function of gait cycle (GC) (upper plot) and distribution of the EMG differences between WWA and WNA (Δ) (lower plot). (c) Distribution of the cross-correlation () between WWA and WNA (upper plot) and its lag as a function of GC (lower plot) for each muscle. Black boxplots correspond to WWA and grey boxplots to WNA.
3.3. Muscle Synergy Components
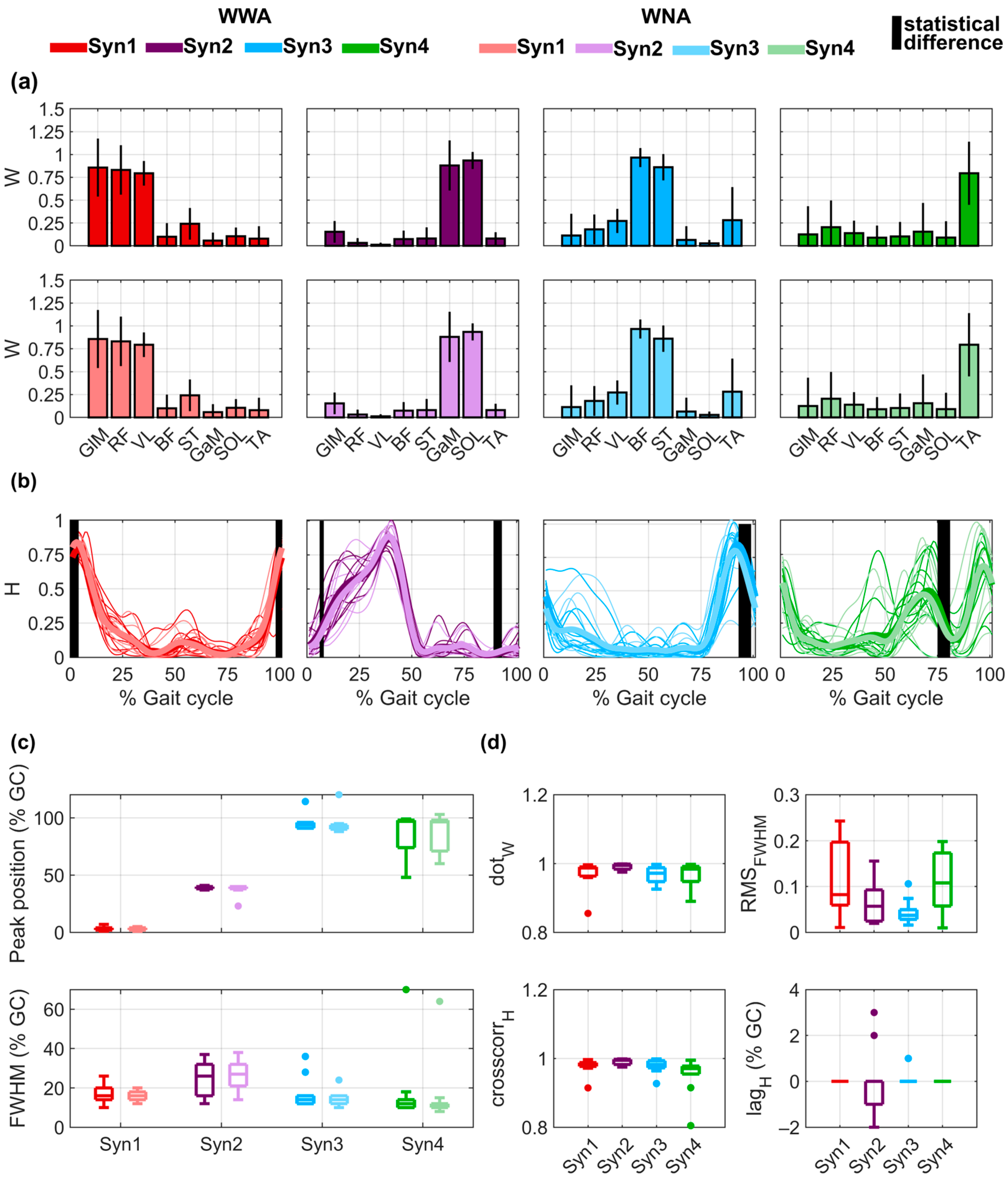
For all subjects, four synergies (shown in Figure 4a,b) were computed, with a median VAFglobal of 95% (minimum value of 92%) for WWA and WNA and a median VAFmuscle of 95% for WWA (minimum value of 84%) and 95% for WNA (minimum value of 83%). Detailed VAF values for each subject, muscle, and task are provided in Table S7 of the Supplementary Material. For both WWA and WNA, Synergy 1 consisted mainly of the activity of GLM, RF, and VL, with a median maximum value occurring at 3.5% of GC for WWA and 3% of GC for WNA (Figure 4c). Synergy 2 involved GaM and SOL, with a median maximum value at 39% of GC for WWA and WNA. Synergy 3 involved mainly BF and ST, with a median maximum value of 94% of GC for WWA and 92.5% for WNA. Synergy 4 was mainly explained by bimodal activity of TA, with the main peak at 97% of GC for WWA and 96.5% for WNA. Detailed data on the spatial time-independent coefficients of activation of all muscles in each synergy can be found in the Supplementary Material for WWA (Table S8) and WNA (Table S9). Statistically significant differences were observed for the time-dependent H of all synergies in specific phases of the gait cycle: around 0% of GC for Synergy 1 (minimum p-value = 0.019 and Cohen’s d = 0.64), around 5% and 90% of GC for Synergy 2 (respectively, minimum p-value = 0.048 and Cohen’s d = 0.31 and minimum p-value = 0.027 and Cohen’s d = 0.55), around 95% of GC for Synergy 3 (minimum p-value = 0.027 and Cohen’s d = 0.78), and around 75% of GC for Synergy 4 (minimum p-value = 0.01 and Cohen’s d = 0.44). No statistically significant differences were observed in the SPM analysis. More detailed data on the time-dependent coefficients of activation for all subjects performing both tasks can be found in Table S10 of the Supplementary Material. FWHM was around 15% of GC for Synergies 1, 3, and 4 and around 25% of GC for Synergy 2 (see Figure 4b, lower plot). The FWHM values of the time-dependent coefficients of activation for all subjects performing both tasks can be found in Table S11 of the Supplementary Material.
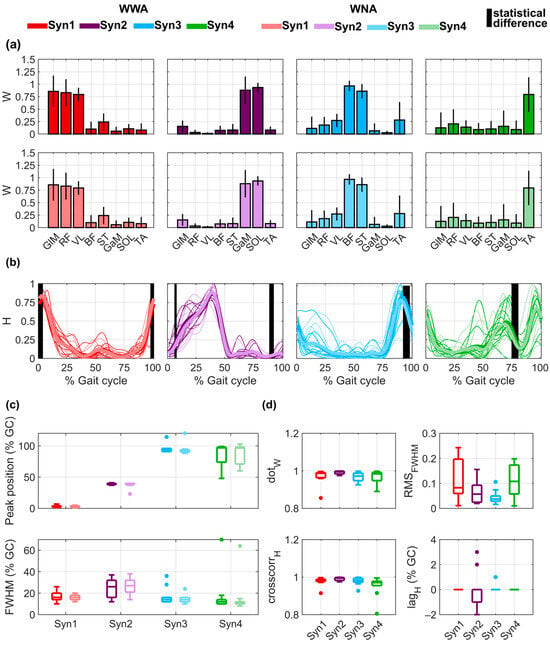
Figure 4.
Muscle synergy components extracted for walking with arms (WWA) and walking with no arms (WNA), considering four synergies (Syn 1, Syn 2, Syn 3, Syn 4). (a) Mean ± std of the spatial time-invariant coefficients (W) during WWA (upper plots) and WNA (lower plots). (b) Time-dependent coefficients (H) for all subjects (thin lines) and mean of all subjects (bold lines). Vertical black lines show the statistically significant differences between WWA and WNA. (c) Distribution of the peak position () as a function of the gait cycle (GC) (upper plot) and full width at half maximum (FWHM) (lower plot). (d) Distribution of the dot product (), root mean square error (), cross-correlation (), and between WWA and WNA, for each H.
As shown in Figure 4d, spatial time-independent coefficients (W) were very similar between WWA and WNA, with median values higher than 0.97 for all synergies. The only value lower than 0.90 occurred for Subject 4 in Synergy 1 () and Synergy 4 (. Regarding the time-dependent coefficients (H), median cross-correlation was higher than 0.97 for all synergies. The only cross-correlation lower than 0.90 occurred for Subject 4 in Synergy 4 (. Additionally, Synergy 2 presented lags between −2% and 3% of GC. Detailed statistical results of the muscle synergy components for all synergies and subjects can be found in Table S12 of the Supplementary Material. Finally, median was lower than 0.12 for all synergies. The highest variability was observed for Synergy 1 and Synergy 4, with an IQR of 0.16 and 0.12, respectively. More detailed data on the of both tasks for all synergies and all subjects can be found in Table S13 of the Supplementary Material.
4. Discussion
This study analyzed the influence of arm swing on lower limb (LL) muscle activity and coordination during comfortable treadmill walking. Humans exhibit interlimb coordination between upper limbs (ULs) and LLs during walking, with arm swing being an integral part of this coordination [1,6]. Previous research examined the effects of walking speed, walking surface, or body-weight support on muscle activity and coordination [39,40,55] but, to the best of our knowledge, this is the first time that muscle synergies have been compared between walking with arms (WWA) and walking with no arms (WNA). The ultimate objective of this research was to verify to what extent the absence of arm swing during robot-based treadmill gait therapy may affect muscle activation, which might have an impact on the design of novel devices that allow normal arm swing.
First, we measured the EMG activity of 8 LL muscles from ten young, healthy subjects during treadmill WWA and WNA at comfortable speeds, following a similar protocol to those carried out in previous studies [18,24,38,52,56]. Both the EMG envelopes and the timing of peak occurrence () were similar to those presented in the literature: the activation peak occurred before 20% of the GC for GLM, RF, and VL; at around 90% of the GC for BF and ST; at around 40% of the GC for GaM and SOL; and at the beginning of the GC for TA (at around 0%). As most of the previous studies presented normalized EMG data, the EMG amplitudes obtained in this study cannot be directly compared. Finally, to the best of our knowledge, only a few studies have analyzed EMG activity during WWA and WNA [18,57]. Stephenson et al. [18] showed that the largest statistical differences in EMGs were found in ST and SOL at the beginning of the GC (between 0% and 30%) and for ST at the end of the GC (between 80% and 100%). These results are consistent with our observations. However, we also observed significant differences in the GLM at 50% of GC and sporadic statistical differences for GaM and VL. In our study, we used SPM analysis to examine individual differences in muscle activation patterns [31] between WWA and WNA, but no significant differences were observed. It is important to emphasize that, while SPM compares the mean of the linear envelopes of the EMG signals for each subject and task, the analysis of muscle synergies takes into account the stride-by-stride variability within each subject and task. Moreover, although differences in GLM, VL, ST, GaM, and SOL, as well as in the H components, were identified using the Wilcoxon signed-rank test following the methodology of previous studies [38,51], no significant differences were found in the recruitment timing of each synergy throughout the gait cycle (Hs) when analyzed with SPM using a non-parametric paired t-test. These findings highlight the complementary nature of the two approaches and underscore the need for appropriate statistical methods to minimize false-positive rates. In terms of EMG amplitude, the increased variability and activation of RF and VL during WNA, particularly at the beginning of the stance phase, may reflect compensatory adaptations to the altered stability demands caused by the absence of arm movement [8]. These muscles play a key role in motor control of the load response phase and ensure balance during initial stance [58]. This effect varies between individuals, underscoring inter-subject variability and the limitations of our small sample size. This trend is also reflected in Synergy 1, where greater and earlier quadriceps muscles activation during WNA was observed at 0%, 5%, and 90% of the GC. Interestingly, these findings align with those of Boccia et al. [37], who reported a decrease in RF activity during Nordic walking, a task requiring increased arm movement and effort. This suggests that changes in arm activity, whether increased or absent, can significantly influence LL muscle activation and coordination. Previous studies also found that the activity of the GLM increased when removing arm swing [57], which was not observed either in our study or in another similar study [59]. In addition, the literature has shown that the activity of latissimus dorsi decreases when removing arm swing [57] and increases when adding weight to the arms [59]. Therefore, it might be interesting to also analyze the activity of low-back muscles in future studies comparing WWA and WNA.
To compare WWA and WNA, we calculated the cross-correlation between EMG patterns and found a minimum between-subject cross-correlation of 0.99. Cross-correlation was also very high (0.96) for the LL muscles evaluated by Boccia et al., who compared WWA and Nordic walking [37], whereas higher variability was observed in the UL muscles. Nordic walking is a task that actively involves the ULs by integrating walking poles. In our study, we did not ask the subjects to specifically swing the arms in a controlled manner, nor did we control arm swing amplitude or ask them to add weights to the wrists, which are all conditions that have been shown to have an impact on LL muscle activity, intermuscular coherence, walking speed, and other spatiotemporal parameters [15,45,59,60,61]. However, considering only natural arm swing such as that that involved in this study, some studies have concluded that WNA only leads to shorter and more frequent steps [18,47], as most did not find any statistical differences in spatiotemporal parameters and kinematics under both uncontrolled and controlled cadences [10,61]. Though measuring additional parameters such as metabolic consumption, spatiotemporal parameters, or kinematics might be interesting in order to obtain a global picture of the effects of arm swing in walking efficiency, stability, and symmetry, we believe that the added complexity does not compensate for the expected results. Therefore, in future studies we plan to keep a comfortable walking speed with a controlled cadence, but we will increase the number of measured muscles, e.g., by including UL muscles, to quantify muscle effort during arm swing.
Muscle synergies were extracted using NNMF, which is the most commonly used factorization method for analysis of muscle synergies [62] and, as has been recently demonstrated by Zhang et al. [48], highlights the potential of this method in accurately estimating LL joint movements. We considered four synergies, consistent with previous studies [3,24,38,63,64], and obtained VAFglobal and VAFmuscle values above 90% and 75%. These thresholds are used in the literature to consider EMG reconstruction acceptable [24]. Our analysis revealed that spatial time-independent coefficients W were comparable to those found in previous studies [3,24,38,63]. Some differences were observed in the synergies in which TA is involved: we identified a single synergy that accounted for most of the variability of TA, which is similar to previous research [38,63]. In contrast, Barroso et al. reported that hamstring muscles (BF and ST) work together with TA at slow cadences [24], while Clark et al. found a synergistic behavior between RF and TA, mainly due to the higher activation of RF and TA during late stance (at around 70%) [3]. These differences might be due to the different walking speeds and cadences used in each protocol. As observed by Barroso et al. [24], the main changes in spatial time-independent W coefficients depending on speed are related to TA, which changes from working with the hamstring muscles in late swing to working with the GLM during the initial stance when increasing speed. During this phase, different motor patterns contribute to the controlled transfer of body weight to the support limb. These patterns include: the eccentric action of TA to control the descent of the foot to the ground; the eccentric contraction of RF, which moderates knee flexion and facilitates the absorption of energy from the trunk; and pelvic obliquity controlled by the hip abductor muscles, such as GLM, to prevent the elevation of the center of gravity induced by the body mass over the hip [58]. Therefore, this phase, corresponding to Synergy 1, may exhibit individual variations due to its complexity. As to the spatial time-independent coefficients W of Synergy 2, 3, and 4, they were composed of GaM and SOL; BF and ST; and TA, respectively, which is consistent with their functional contributions. In Synergy 2, GaM and SOL are activated concentrically during midstance and late stance, supporting body weight and aiding propulsion. During WNA, their increased activation throughout stance reflects compensatory demands for stability due to the absence of arm swing. In addition, in Synergy 3, BF and ST, key for knee flexion during swing, also decelerate the leg before heel strike and stabilize the ankle during early stance. During WNA, their earlier and lower activation at ~75% of the gait cycle suggests an anticipatory adjustment to shorten swing and maintain balance. Finally, in Synergy 4, TA activates progressively in the second half of the gait cycle to ensure dorsiflexion during swing. During WWA, an earlier peak at ~75% of the cycle likely compensates for reduced stability in treadmill walking with limited arm swing. Time-dependent coefficients H were also comparable to literature [3,24,38,63], as the peaks of the four synergies occurred at the beginning of the GC for Synergy 1, at around 40% of GC for Synergy 2, and at the end of the GC for Synergy 3. Differences were observed in Synergy 4, consistent with the differences observed in the activity of TA. Barroso et al. [24] demonstrated that decreased cadence delays the peak of the synergy related to TA (Synergy 4, in our case). This is consistent with the results observed in the literature: Yokoyama et al. found that the peak occurred at around 70% of GC when participants walked at 0.5 m/s [38]; we observed, as did Rodriguez et al. and Barroso et al., that the peak occurred at 90% of GC when walking at 1.0 m/s [63] or with slow cadence (42 strides/min) [24], whereas when walking at 1.25 m/s [3] or with faster cadences [24], the peak occurred at the beginning of the GC. These results suggest that the timing of TA activation in Synergy 4 is highly dependent on gait speed and cadence. Specifically, at slower speeds or cadences, the prolonged contact time between the foot and the ground may necessitate greater and later TA activation to prevent abrupt foot–ground collisions and maintain ankle stability during the initial stance phase. We also calculated the FWHM, which indicates the percentage of GC at which a synergy can be considered active. Our results are consistent with the observations of Boccia et al. [37] for the WWA task, as all synergies were active around 15% of GC, with a longer duration for the synergy related to GaM and SOL (Synergy 2, in our case). Though the data cannot be directly compared to the literature, Boccia et al. also did not find meaningful changes between WWA and Nordic walking [37]. Moreover, we observed a high correlation between synergies during WWA and WNA, both in the spatial time-independent ( > 0.97) and time-dependent coefficients ( > 0.99, < 0.12). Therefore, considering all performance indicators, our results demonstrate that WWA and WNA present very similar muscle coordination, even closer than WWA and Nordic walking [37] or cycling [24].
In the current work, the sample size was relatively small, consisting of ten young, healthy subjects. While this sample size is consistent with previous studies analyzing muscle synergies in healthy subjects during various locomotor tasks [24,28,33,35,37,38,50], future work should enlarge the sample size to enhance the robustness of the findings and increase the statistical power of the results. The generalizability of our findings is limited by the homogeneity of the sample, which consisted of young, healthy subjects within a narrow age range (24.3 ± 2.26 years). While this minimized inter-subject variability and is in line with similar studies using mixed-sex samples to investigate muscle synergies during treadmill walking [3,28,37,56,65], it does not capture the variability present in broader populations. Although our sample included both sexes, as other synergy analyses across different tasks [24,28,36,37], it would be interesting, with a larger cohort, to investigate potential sex-related differences in muscle synergies, as has been studied in terms of kinematics during running [13]. Additionally, while this study focused on muscle activation and coordination, incorporating kinematic measurements could provide a more comprehensive understanding of how the speed and frequency of arm swing affect muscle activation and coordination, offering a more complete perspective on the biomechanics of locomotion. Furthermore, exploring these effects under different conditions such as overground walking or varying speeds could provide insights into the role of arm swing in more realistic locomotor scenarios. This approach would enhance our understanding of neuromuscular adaptations to varying mechanical demands and offer valuable guidance for rehabilitation applications. Further studies could also investigate the metabolic costs, providing deeper insights into the physiological mechanisms underlying these changes. Although we controlled gait cadence in both tasks, we cannot determine participants’ natural cadence without arm swing, which should be addressed in future studies. Furthermore, investigating how arm swing influences LL muscle synergies under varying conditions, such as on different terrains or at different speeds, would enhance our understanding of its biomechanical implications.
Despite these limitations, this study was designed as an initial step toward understanding the effects of arm swing on muscle coordination under controlled conditions. Future research should extend these findings to clinical populations, such as stroke survivors, alongside age-matched healthy controls. This approach would help evaluate the relevance of these findings in clinical contexts and assess their potential applications in rehabilitation.
5. Conclusions
In conclusion, this study compared EMG activation and muscle synergies between WWA and WNA, with some differences being observed in the activation timings for both EMG and time-dependent synergy coefficients (H). Whether or not these changes affect the efficiency of robot-based gait rehabilitation after stroke, as suggested by previous research [19], is still unclear. Future studies may focus not only on the activity of LL muscles but also on UL and low-back muscles; on analyzing EMG activity and muscle synergies during treadmill walking at different speeds during rehabilitation; on studying the muscular effects of walking in a rehabilitation robot [66]; and on data recording with stroke survivors. Collecting these data would ensure a more comprehensive understanding of muscle coordination during treadmill walking and may shed light on the importance of incorporating arm swing during robotic rehabilitation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15010192/s1. Table S1: Performance indicator (PI) definitions, with the mathematical explanation of computation for each metric extracted, with its respective unit; Table S2: Self-selected speed of all subjects; Table S3: Cadence statistics results: report of the Shapiro–Wilk analysis of 30 cycles to verify the distribution of the analyzed data of the cadence in each task, and a Wilcoxon signed-rank test analysis to determine if there are differences in the cadences between walking with arms (WWA) and walking with no arms (WNA). Statistical significance was set at p < 0.05; Table S4: Electromyography (EMG) results: time of occurrence of the EMG maximum amplitude as a function of the gait cycle (GC) in each muscle of all the subjects performing both tasks, walking with arms (WWA) and walking with no arms (WNA); Table S5: Electromyography (EMG) results: maximum EMG instantaneous amplitude in each muscle of all the subjects performing both tasks, walking with arms (WWA) and walking with no arms (WNA); Table S6: Estimation of the linear relationship between the electromyographic (EMG) time series of walking with arms (WWA) and walking with no arms (WNA) of all muscles; Table S7: Synergy results: variability accounted for (VAF) values for each muscle (VAFmuscle) and the overall VAF of all of the muscles(VAFglobal). Minimum values of 0.75 for VAFmuscle and 0.9 for VAFglobal were used to consider the quality of reconstruction of each muscle and of all muscles overall good; Table S8: Muscle synergy components: spatial time-independent coefficients of activation (Ws) of all muscles in each synergy during walking with arms (WWA); Table S9: Muscle synergy components: spatial time-independent coefficients of activation (Ws) of all muscles during walking with arms (WWA); Table S10: Muscle synergy components: maximum value of time-dependent coefficients of activation (Hs) as a function of the gait cycle (GC) of all the subjects performing both tasks, walking with arms (WWA) and walking with no arms (WNA); Table S11: Muscle synergy components: full width at half maximum (FWHM) values of time-dependent coefficients of activation (Hs) as a function of the gait cycle (GC) of all the subjects performing both tasks, walking with arms (WWA) and walking with no arms (WNA); Table S12: Estimation of the relationship between the synergy components of walking with arms (WWA) and walking with no arms (WNA) for all synergies and all subjects considering the dot product between the time-independent spatial coefficients W, the cross-correlation, and the lag between the time dependent coefficients H. Statistical results of the muscle synergy components; Table S13: Root mean square error between the full-width at half-maximum (FWHM) of both tasks (walking with arms (WWA) and walking with no arms (WNA)) for all synergies and all subjects.
Author Contributions
Conceptualization, C.B.S.-M., R.U. and J.T.; methodology, C.B.S.-M., R.U. and J.T.; software, F.O.B. and C.B.S.-M.; validation, T.R.-M., C.B.S.-M. and A.C.; formal analysis, T.R.-M., C.B.S.-M. and A.C.; investigation, T.R.-M. and A.C.; data curation, T.R.-M., C.B.S.-M. and A.C.; writing—original draft preparation, T.R.-M. and C.B.S.-M.; writing—review and editing, A.C., R.U., F.O.B., J.L.P. and J.T.; visualization, T.R.-M. and C.B.S.-M.; supervision, J.L.P. and J.T.; project administration, J.T.; funding acquisition, J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Center for Clinical Neuroscience of Hospital Los Madroños. F.O.B. received the support of a fellowship from “la Caixa” Foundation (ID 100010434, fellowship code LCF/BQ/PR23/11980042) and also from Grant RYC2022-036551-I, funded by MICIU/AEI/10.13039/501100011033 and by “ESF+ Investing in your future”.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (Ethical Committee for Clinical Research) of Hospital Universitario Severo Ochoa de Leganés, Madrid, Spain, on 26 October 2022 under the protocol code CYCLING-AL.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding authors and after approval of institutional authorities (Center for Clinical Neuroscience-HLM). SynergyLab is the software used by Barroso et al. (2014) [24]; however, it is not formally published nor available on a public website. For further information, we recommend contacting its creator and co-author of this manuscript, Filipe Oliveira Barroso (email: filipe.barroso@cajal.csic.es).
Acknowledgments
We express our sincere gratitude to Natacha León, head of the Advanced Neurorehabilitation Unit, for her leadership in this research, and we thank the study participants for their time and patience.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef]
- Zehr, E.P.; Carroll, T.J.; Chua, R.; Collins, D.F.; Frigon, A.; Haridas, C.; Hundza, S.R.; Thompson, A.K. Possible contributions of CPG activity to the control of rhythmic human arm movement. Can. J. Physiol. Pharmacol. 2004, 82, 556–568. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Figueiredo, J.; Fonseca, P.; Vilas-Boas, J.P.; Santos, C.P. Lower limb kinematic, kinetic, and EMG data from young healthy humans during walking at controlled speeds. Sci. Data 2021, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Leng, Y.; Lei, D.; Yu, Q.; Li, K.; Ding, M.; Lo, W.L.A. A protocol to analyze the global literature on the clinical benefit of interlimb-coordinated intervention in gait recovery and the associated neurophysiological changes in patients with stroke. Front. Neurol. 2022, 13, 959917. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Barss, T.S.; Dragert, K.; Frigon, A.; Vasudevan, E.V.; Haridas, C.; Hundza, S.; Kaupp, C.; Klarner, T.; Klimstra, M.; et al. Neuromechanical interactions between the limbs during human locomotion: An evolutionary perspective with translation to rehabilitation. Exp. Brain Res. 2016, 234, 3059–3081. [Google Scholar] [CrossRef] [PubMed]
- Massaad, F.; Levin, O.; Meyns, P.; Drijkoningen, D.; Swinnen, S.P.; Duysens, J. Arm sway holds sway: Locomotor-like modulation of leg reflexes when arms swing in alternation. Neuroscience 2014, 258, 34–46. [Google Scholar] [CrossRef]
- Meyns, P.; Bruijn, S.M.; Duysens, J. The how and why of arm swing during human walking. Gait Posture 2013, 38, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Pijnappels, M.; Kingma, I.; Wezenberg, D.; Reurink, G.; van Dieën, J.H. Armed against falls: The contribution of arm movements to balance recovery after tripping. Exp. Brain Res. 2010, 201, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.D.; Fehlman, L.A.; Farley, C.T. Effects of aging and arm swing on the metabolic cost of stability in human walking. J. Biomech. 2008, 41, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Umberger, B.R. Effects of suppressing arm swing on kinematics, kinetics, and energetics of human walking. J. Biomech. 2008, 41, 2575–2580. [Google Scholar] [CrossRef]
- Yizhar, Z.; Boulos, S.; Inbar, O.; Carmeli, E. The effect of restricted arm swing on energy expenditure in healthy men. Int. J. Rehabil. Res. 2009, 32, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Schleichardt, A.; Warschun, F.; Walter, N.; Fleckenstein, D.; Berkel, F.; Ueberschär, O. Relationship between Longitudinal Upper Body Rotation and Energy Cost of Running in Junior Elite Long-Distance Runners. Sports 2023, 11, 204. [Google Scholar] [CrossRef]
- Ballesteros, M.L.F.; Buchthal, F.; Rosenfalck, P. The pattern of muscular activity during the arm swing of natural walking. Acta Physiol. Scand. 1965, 63, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, M.; Jonkers, I.; van Dieen, J.H.; Bruijn, S.M. Arm swing in human walking: What is their drive? Gait Posture 2014, 40, 321–326. [Google Scholar] [CrossRef]
- Weersink, J.B.; de Jong, B.M.; Halliday, D.M.; Maurits, N.M. Intermuscular coherence analysis in older adults reveals that gait-related arm swing drives lower limb muscles via subcortical and cortical pathways. J. Physiol. 2021, 599, 2283–2298. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Huang, H.J.; Kao, P.C. Moving the arms to activate the legs. Exerc. Sport Sci. Rev. 2006, 34, 113–120. [Google Scholar] [CrossRef]
- Stephenson, J.L.; De Serres, S.J.; Lamontagne, A. The effect of arm movements on the lower limb during gait after a stroke. Gait Posture 2010, 31, 109–115. [Google Scholar] [CrossRef]
- Kang, T.W.; Oh, D.W.; Lee, J.H.; Cynn, H.S. Effects of integrating rhythmic arm swing into robot-assisted walking in patients with subacute stroke: A randomized controlled pilot study. Int. J. Rehabil. Res. 2018, 41, 57–62. [Google Scholar] [CrossRef]
- Aoyagi, D.; Ichinose, W.E.; Harkema, S.J.; Reinkensmeyer, D.J.; Bobrow, J.E. A robot and control algorithm that can synchronously assist in naturalistic motion during body-weight-supported gait training following neurologic injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 387–400. [Google Scholar] [CrossRef]
- Bishop, L.; Omofuma, I.; Stein, J.; Agrawal, S.; Quinn, L. Treadmill-Based Locomotor Training With Robotic Pelvic Assist and Visual Feedback: A Feasibility Study. J. Neurol. Phys. Ther. 2020, 44, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Hunt, K.J. Mechanical Design and Control System Development of a Rehabilitation Robotic System for Walking With Arm Swing. Front. Rehabil. Sci. 2021, 2, 720182. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Baeyens, J.P.; Knaepen, K.; Michielsend, M.; Clijsene, R.; Beckwéea, D.; Kerckhofs, E. Robot-assisted walking with the Lokomat: The influence of different levels of guidance force on thorax and pelvis kinematics. Clin. Biomech. 2015, 30, 254–259. [Google Scholar] [CrossRef]
- Barroso, F.O.; Torricelli, D.; Moreno, J.C.; Taylor, J.; Gomez-Soriano, J.; Bravo-Esteban, E.; Piazza, S.; Santos, C.; Pons, J.L. Shared muscle synergies in human walking and cycling. J. Neurophysiol. 2014, 112, 1984–1998. [Google Scholar] [CrossRef]
- Bernstein, N. The Co-Ordination and Regulation of Movements; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- d’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- Dominici, N.; Ivanenko, Y.P.; Cappellini, G.; d’Avella, A.; Mondì, V.; Cicchese, M.; Fabiano, A.; Silei, T.; Di Paolo, A.; Giannini, C.; et al. Locomotor primitives in newborn babies and their development. Science 2011, 334, 997–999. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Cappellini, G.; Dominici, N.; Poppele, R.E.; Lacquaniti, F. Modular control of limb movements during human locomotion. J. Neurosci. 2007, 27, 11149–11161. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of Muscle Synergy Outcomes in Clinics, Robotics, and Sports: A Systematic Review. Appl. Bionics Biomech. 2018, 2018, 3934698. [Google Scholar] [CrossRef]
- Torricelli, D.; Barroso, F.; Coscia, M.; Alessandro, C.; Lunardini, F.; Esteban, E.B.; d’Avella, A. Muscle synergies in clinical practice: Theoretical and practical implications. In Biosystems and Biorobotics; Pons, J., Raya, R., González, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 10, pp. 251–272. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Oliveira Barroso, F.; Torricelli, D.; Pons, J.L.; Politti, F.; Lucareli, P.R.G. Muscle synergies analysis shows altered neural strategies in women with patellofemoral pain during walking. PLoS ONE 2023, 18, e0292464. [Google Scholar] [CrossRef]
- Magrath, W.J.; Qiu, C.S.; Hanwright, P.J.; Tuffaha, S.H.; Khavanin, N. A Systematic Review of Muscle Synergies during a Walking Gait to Define Optimal Donor-Recipient Pairings for Lower Extremity Functional Reconstruction. Plast. Reconstr. Surg. -Glob. Open 2022, 10, e4438. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Gizzi, L.; Ketabi, S.; Farina, D.; Kersting, U.G. Modular Control of Treadmill vs Overground Running. PLoS ONE 2016, 11, e0153307. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.O.; Torricelli, D.; Bravo-Esteban, E.; Taylor, J.; Gómez-Soriano, J.; Santos, C.; Moreno, J.C.; Pons, J.L. Muscle Synergies in Cycling after Incomplete Spinal Cord Injury: Correlation with Clinical Measures of Motor Function and Spasticity. Front. Hum. Neurosci. 2016, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Cartier, T.; Vigouroux, L.; Viehweger, E.; Rao, G. Subject specific muscle synergies and mechanical output during cycling with arms or legs. PeerJ 2022, 10, e13155. [Google Scholar] [CrossRef] [PubMed]
- Hug, F.; Turpin, N.A.; Couturier, A.; Dorel, S. Consistency of muscle synergies during pedaling across different mechanical constraints. J. Neurophysiol. 2011, 106, 91–103. [Google Scholar] [CrossRef]
- Boccia, G.; Zoppirolli, C.; Bortolan, L.; Schena, F.; Pellegrini, B. Shared and task-specific muscle synergies of Nordic walking and conventional walking. Scand. J. Med. Sci. Sports 2018, 28, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Kato, T.; Kaneko, N.; Kobayashi, H.; Hoshino, M.; Kokubun, T.; Nakazawa, K. Basic locomotor muscle synergies used in land walking are finely tuned during underwater walking. Sci. Rep. 2021, 11, 18480. [Google Scholar] [CrossRef]
- Hagedoorn, L.; Zadravec, M.; Olenšek, A.; van Asseldonk, E.; Matjačić, Z. The Existence of Shared Muscle Synergies Underlying Perturbed and Unperturbed Gait Depends on Walking Speed. Appl. Sci. 2022, 12, 2135. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 556 Pt 1, 267–282. [Google Scholar] [CrossRef]
- Coscia, M.; Monaco, V.; Martelloni, C.; Rossi, B.; Chisari, C.; Micera, S. Muscle synergies and spinal maps are sensitive to the asymmetry induced by a unilateral stroke. J. Neuroeng. Rehabil. 2015, 12, 39. [Google Scholar] [CrossRef]
- Stephenson, J.L.; Lamontagne, A.; De Serres, S.J. The coordination of upper and lower limb movements during gait in healthy and stroke individuals. Gait Posture 2009, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Olenšek, A.; Zadravec, M.; Matjačić, Z. Feasibility of Using Visual Cues for Evoking Self-induced Perturbations for Assessing Dynamic Balance During Walking. Biosyst. Biorobotics 2022, 28, 841–844. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H. Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Enschede Roessingh Res. Dev. 2007, 10, 108–112. [Google Scholar]
- Kerkman, J.N.; Bekius, A.; Boonstra, T.W.; Daffertshofer, A.; Dominici, N. Muscle Synergies and Coherence Networks Reflect Different Modes of Coordination During Walking. Front. Physiol. 2020, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Turpin, N.A.; Uriac, S.; Dalleau, G. How to improve the muscle synergy analysis methodology? Eur. J. Appl. Physiol. 2021, 121, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Quan, W.; Gusztav, F.; Baker, J.S.; Gu, Y. Adaptive neuro-fuzzy inference system model driven by the non-negative matrix factorization-extracted muscle synergy patterns to estimate lower limb joint movements. Comput. Methods Programs Biomed. 2023, 242, 107848. [Google Scholar] [CrossRef] [PubMed]
- Hug, F.; Turpin, N.A.; Guével, A.; Dorel, S. Is interindividual variability of EMG patterns in trained cyclists related to different muscle synergies? J. Appl. Physiol. 2010, 108, 1727–1736. [Google Scholar] [CrossRef]
- Hug, F. Can muscle coordination be precisely studied by surface electromyography? J. Electromyogr. Kinesiol. 2011, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lunardini, F.; Casellato, C.; Bertucco, M.; Sanger, T.D.; Pedrocchi, A. Children With and Without Dystonia Share Common Muscle Synergies While Performing Writing Tasks. Ann. Biomed. Eng. 2017, 45, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor patterns in human walking and running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef] [PubMed]
- d’avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA. 2005, 102, 3076–3081. [Google Scholar] [CrossRef]
- Pellegrino, L.; Coscia, M.; Casadio, M. Muscle activities in similar arms performing identical tasks reveal the neural basis of muscle synergies. Exp. Brain Res. 2020, 238, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Semaan, M.B.; Wallard, L.; Ruiz, V.; Gillet, C.; Leteneur, S.; Simoneau-Buessinger, E. Is treadmill walking biomechanically comparable to overground walking? A systematic review. Gait Posture 2022, 92, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kautz, S.A.; Bowden, M.G.; Clark, D.J.; Neptune, R.R. Comparison of motor control deficits during treadmill and overground walking poststroke. Neurorehabil. Neural Repair 2011, 25, 756–765. [Google Scholar] [CrossRef]
- Kim, M.H.; Yoo, W.G. Effect of minimizing arm swing while walking on the trunk and gluteal muscles. J. Phys. Ther. Sci. 2017, 29, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Molina Rueda, F.; Carratalá Tejada, M. La Marcha: Biomecánica, Evaluación y Patología; Editorial Médica Panamericana S.A.: Ciudad de Mexico, Mexico, 2020. [Google Scholar]
- Kim, T.Y.; Yoo, W.G.; An, D.H.; Oh, J.S.; Shin, S.J. The effects of different gait speeds and lower arm weight on the activities of the latissimus dorsi, gluteus medius, and gluteus maximus muscles. J. Phys. Ther. Sci. 2013, 25, 1483–1484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donker, S.F.; Mulder, T.; Nienhuis, B.; Duysens, J. Adaptations in arm movements for added mass to wrist or ankle during walking. Exp. Brain Res. 2002, 146, 26–31. [Google Scholar] [CrossRef]
- Hill, A.; Nantel, J. The effects of arm swing amplitude and lower-limb asymmetry on gait stability. PLoS ONE 2019, 14, e0218644. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Pizzolato, C.; Lloyd, D.G.; Carty, C.P.; Devaprakash, D.; Diamond, L.E. Non-negative matrix factorisation is the most appropriate method for extraction of muscle synergies in walking and running. Sci. Rep. 2020, 10, 8266. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.L.; Roemmich, R.T.; Cam, B.; Fregly, B.J.; Hass, C.J. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 2013, 124, 1390–1397. [Google Scholar] [CrossRef]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture 2013, 38, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, L.; Nielsen, J.F.; Felici, F.; Ivanenko, Y.P.; Farina, D. Impulses of activation but not motor modules are preserved in the locomotion of subacute stroke patients. J. Neurophysiol. 2011, 106, 202–210. [Google Scholar] [CrossRef] [PubMed]
- van Kammen, K.; Boonstra, A.M.; van der Woude, L.H.V.; Reinders-Messelink, H.A.; den Otter, R. Differences in muscle activity and temporal step parameters between Lokomat guided walking and treadmill walking in post-stroke hemiparetic patients and healthy walkers. J. Neuroeng. Rehabil. 2017, 14, 32. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).