Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.)
Abstract
1. Introduction
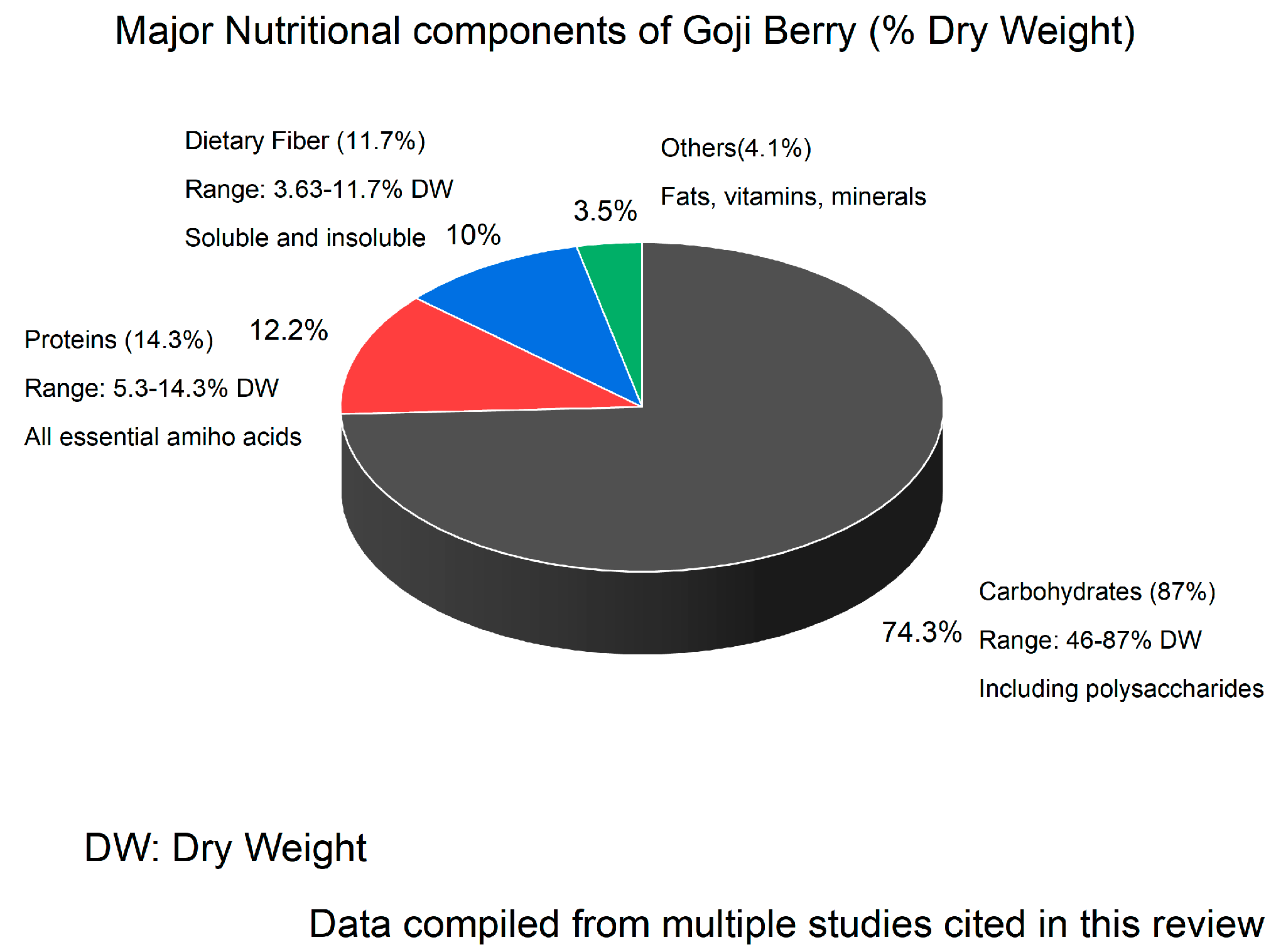
2. Nutritional Composition of Goji Berry
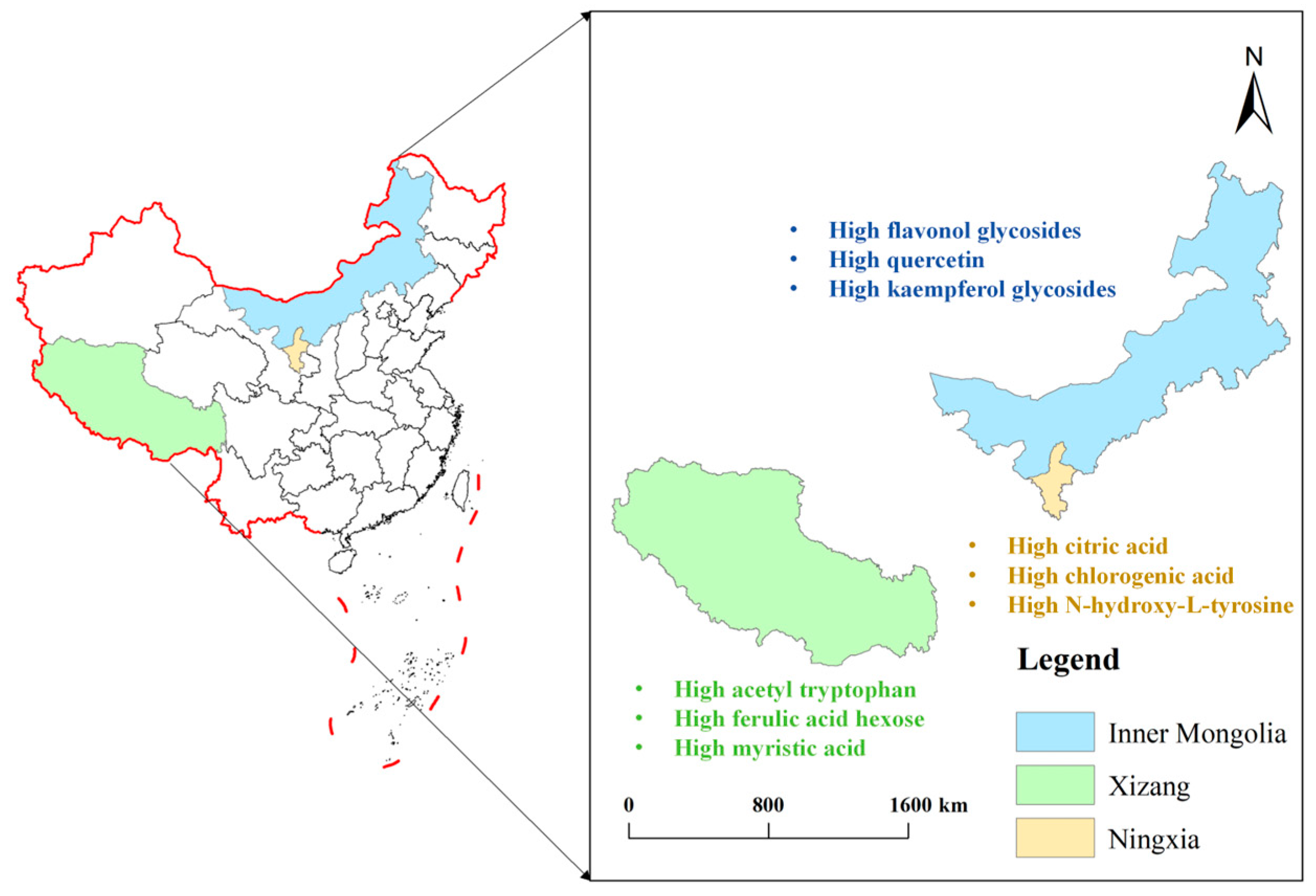
3. Bioactive Compounds in Goji Berry and Advancements in Their Cultivations
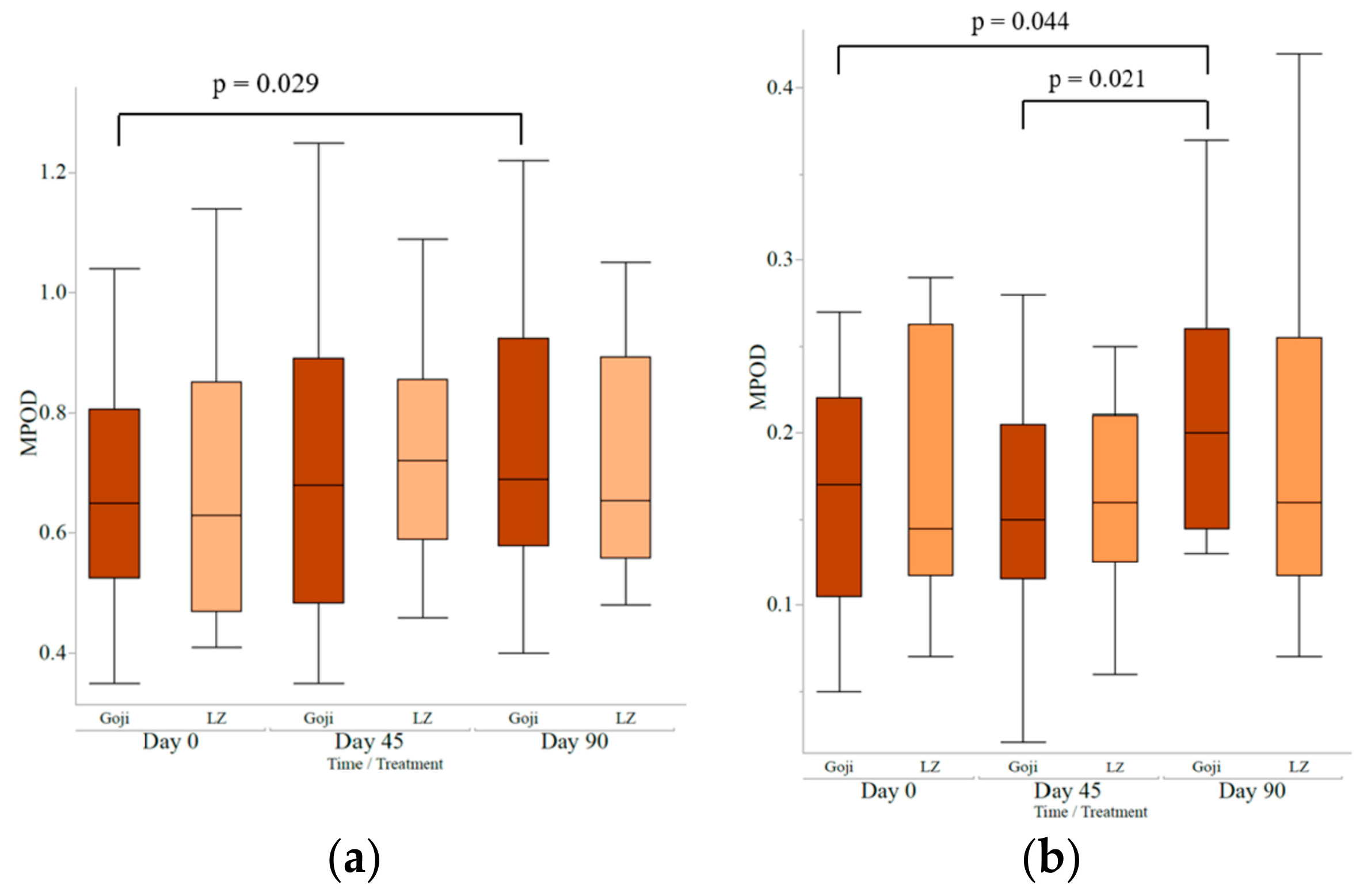
4. Health Benefits and Nutraceutical Properties
5. Future Perspectives and Research Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Tian, F.; Feng, Z.; Fan, X.; Pan, Z.; Zhou, J. Antioxidant Activity and Physicochemical Properties of Chitosan Films Incorporated with Lycium barbarum Fruit Extract for Active Food Packaging. Int. J. Food Sci. Technol. 2015, 50, 458–464. [Google Scholar] [CrossRef]
- Ma, R.-H.; Zhang, X.-X.; Ni, Z.-J.; Thakur, K.; Wang, W.; Yan, Y.-M.; Cao, Y.-L.; Zhang, J.-G.; Rengasamy, K.R.R.; Wei, Z.-J. Lycium barbarum (Goji) as Functional Food: A Review of Its Nutrition, Phytochemical Structure, Biological Features, and Food Industry Prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A. Microstructural and Histochemical Characteristics of Lycium barbarum L. Fruits Used in Folk Herbal Medicine and as Functional Food. Protoplasma 2018, 255, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, X.; Cao, F.; Guo, Q.; Wang, J. Phytochemicals and Bioactivities of Goji (Lycium barbarum L. and Lycium chinense Mill.) Leaves and Their Potential Applications in the Food Industry: A Review. Int. J. Food Sci. Technol. 2022, 57, 1451–1461. [Google Scholar] [CrossRef]
- Carnés, J.; de Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; López-Matas, M.A.; Pagán, J.A.; Navarro, L.A.; García-Abujeta, J.L.; Vicario, S.; Peña, M. Recently Introduced Foods as New Allergenic Sources: Sensitisation to Goji Berries (Lycium Barbarum). Food Chem. 2013, 137, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-H.; Zhang, X.-X.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Research Progress of Lycium Barbarum L. as Functional Food: Phytochemical Composition and Health Benefits. Curr. Opin. Food Sci. 2022, 47, 100871. [Google Scholar] [CrossRef]
- Xin, T.; Yao, H.; Gao, H.; Zhou, X.; Ma, X.; Xu, C.; Chen, J.; Han, J.; Pang, X.; Xu, R.; et al. Super Food Lycium Barbarum (Solanaceae) Traceability via an Internal Transcribed Spacer 2 Barcode. Food Res. Int. 2013, 54, 1699–1704. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quetglas-Llabrés, M.M.; Sureda, A.; Mardones, L.; Villagran, M.; Sönmez Gürer, E.; Živković, J.; Ezzat, S.M.; Zayed, A.; Gümüşok, S.; et al. Supercharging Metabolic Health with Lycium barbarum L.: A Review of the Therapeutic Potential of This Functional Food for Managing Metabolic Syndrome. Food Front. 2024, 5, 420–434. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A Review of Botanical Characteristics, Phytochemistry, Clinical Relevance in Efficacy and Safety of Lycium Barbarum Fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Medica 2009, 76, 7–19. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The Genus Lycium as Food and Medicine: A Botanical, Ethnobotanical and Historical Review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hu, Y.; Yan, Y.; Zhou, W.; Chen, G.; Zeng, X.; Cao, Y. Characterization and Evaluation of Antioxidant and Anti-Inflammatory Activities of Flavonoids from the Fruits of Lycium barbarum. Foods 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S.; Kokkalou, E. Phytochemical Analysis and Antioxidant Activity of Lycium barbarum (Goji) Cultivated in Greece. Pharm. Biol. 2017, 55, 596–602. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Chu, Q.; Fang, Y.; Ye, J. Determination of Taurine in Lycium barbarum L. and Other Foods by Capillary Electrophoresis with Electrochemical Detection. Electroanalysis 2003, 15, 898–902. [Google Scholar] [CrossRef]
- Lin, L.; Luo, C.; Li, C.; Abdel-Samie, M.A.; Cui, H. Eugenol/Silk Fibroin Nanoparticles Embedded Lycium Barbarum Polysaccharide Nanofibers for Active Food Packaging. Food Packag. Shelf Life 2022, 32, 100841. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Guo, B.; Zhang, T.; Wang, L.; Ji, Q.; Xia, H.; Sun, G. Comparative Metabolic Profiling of Lycium Fruits (Lycium barbarum and Lycium chinense) from Different Areas in China and from Nepal. J. Food Qual. 2019, 2019, 4396027. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Skenderidis, P.; Leontopoulos, S.; Lampakis, D. Goji Berry: Health Promoting Properties. Nutraceuticals 2022, 2, 32–48. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium Barbarum Polysaccharides: Extraction, Purification, Structural Characterisation and Evidence about Hypoglycaemic and Hypolipidaemic Effects. A Review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Lycium barbarum Berries (Solanaceae) as Source of Bioactive Compounds for Healthy Purposes: A Review. Int. J. Mol. Sci. 2023, 24, 4777. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.C.; Sánchez-Mata, M.-C.; Pérez-Rodríguez, M.L.; Cámara, M.; López-Colón, J.L.; Bach, F.; Bellettini, M.; Haminiuk, C.W.I. Qualitative and Nutritional Comparison of Goji Berry Fruits Produced in Organic and Conventional Systems. Sci. Hortic. 2019, 257, 108660. [Google Scholar] [CrossRef]
- Kruczek, A.; Krupa-Małkiewicz, M.; Lachowicz, S.; Oszmiański, J.; Ochmian, I. Health-Promoting Capacities of In Vitro and Cultivated Goji (Lycium chinense Mill.) Fruit and Leaves; Polyphenols, Antimicrobial Activity, Macro- and Microelements and Heavy Metals. Molecules 2020, 25, 5314. [Google Scholar] [CrossRef] [PubMed]
- Endes, Z.; Uslu, N.; Özcan, M.M.; Er, F. Physico-Chemical Properties, Fatty Acid Composition and Mineral Contents of Goji Berry (Lycium barbarum L.) Fruit. J. Agroaliment. Process. Technol. 2015, 21, 36–40. [Google Scholar]
- Sun, Q.; Du, M.; Kang, Y.; Zhu, M.-J. Prebiotic Effects of Goji Berry in Protection against Inflammatory Bowel Disease. Crit. Rev. Food Sci. Nutr. 2023, 63, 5206–5230. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Savolainen, O.; Törrönen, R.; Martinez, J.A.; Poutanen, K.; Hanhineva, K. Metabolic Profiling of Goji Berry Extracts for Discrimination of Geographical Origin by Non-Targeted Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry. Food Res. Int. 2014, 63, 132–138. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, F.; Liu, X.; Zheng, Y.; Fu, L.; Shi, H.; Wang, F.; Xu, Z. First Investigation of Electrochemical Behavior and Detection of 2-O-(β-D-Glucopyranosyl) Ascorbic Acid. Int. J. Electrochem. Sci. 2021, 16, 211115. [Google Scholar] [CrossRef]
- Shi, X.; Fan, B.; Zheng, Y.; Wang, X.; Zhang, Y.; Fu, L. Feasibility Study on the Geographical Indication of Lycium Barbarum Based on Electrochemical Fingerprinting Technique. Int. J. Electrochem. Sci. 2021, 16, 210714. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Amodio, M.L.; Colelli, G. Quality of Goji Berry Fruit (Lycium barbarum L.) Stored at Different Temperatures. Foods 2022, 11, 3700. [Google Scholar] [CrossRef]
- Kosińska-Cagnazzo, A.; Weber, B.; Chablais, R.; Vouillamoz, J.F.; Molnár, B.; Crovadore, J.; Lefort, F.; Andlauer, W. Bioactive Compound Profile and Antioxidant Activity of Fruits from Six Goji Cultivars Cultivated in Switzerland. J. Berry Res. 2017, 7, 43–59. [Google Scholar] [CrossRef]
- Gębalski, J.; Małkowska, M.; Graczyk, F.; Słomka, A.; Piskorska, E.; Gawenda-Kempczyńska, D.; Kondrzycka-Dąda, A.; Bogucka-Kocka, A.; Strzemski, M.; Sowa, I.; et al. Phenolic Compounds and Antioxidant and Anti-Enzymatic Activities of Selected Adaptogenic Plants from South America, Asia, and Africa. Molecules 2023, 28, 6004. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kima, J.-H.; Jeung, E.-S.; Park, I.-S.; Choe, C.-H.; Kwon, T.-H.; Yu, K.-Y.; Jeong, S.-I. Inhibitory Effects of Stilbene Glucoside Isolated from the Root of Polygonum Multiflorum on Tyrosinase Activity and Melanin Biosynthesis. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 342–345. [Google Scholar] [CrossRef]
- Yang, R.; Zhao, C.; Chen, X.; Chan, S.; Wu, J. Chemical Properties and Bioactivities of Goji (Lycium Barbarum) Polysaccharides Extracted by Different Methods. J. Funct. Foods 2015, 17, 903–909. [Google Scholar] [CrossRef]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of Ultrasound Assisted Extraction of of Goji Berry (Lycium barbarum) Fruits and Evaluation of Extracts’ Bioactivity. J. Food Process Eng. 2017, 40, e12522. [Google Scholar] [CrossRef]
- Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R.J.; Ye, X.; Chen, S. Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, L.; Liu, J.-M.; Zhang, J.; Wang, W.; Li, B.-G.; Dong, C.-X.; Bai, C.-C. Lycium Genus Polysaccharide: An Overview of Its Extraction, Structures, Pharmacological Activities and Biological Applications. Separations 2022, 9, 197. [Google Scholar] [CrossRef]
- Cardoso, M.A.P.; Haminiuk, C.W.I.; Pedro, A.C.; de Andrade Arruda Fernandes Fernandes, I.; Yamato, M.A.C.; Maciel, G.M.; Prado, I.N.D. Biological Effects of Goji Berry and the Association with New Industrial Applications: A Review. Food Rev. Int. 2023, 39, 2990–3007. [Google Scholar] [CrossRef]
- Wu, D.-T.; Guo, H.; Lin, S.; Lam, S.-C.; Zhao, L.; Lin, D.-R.; Qin, W. Review of the Structural Characterization, Quality Evaluation, and Industrial Application of Lycium Barbarum Polysaccharides. Trends Food Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Feng, L.; Xiao, X.; Liu, J.; Wang, J.; Zhang, N.; Bing, T.; Liu, X.; Zhang, Z.; Shangguan, D. Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules 2020, 25, 1351. [Google Scholar] [CrossRef]
- Shi, X.; Man, J.; Ye, W.; Zhu, J.; Fu, L.; Zheng, Y.; Yin, Y.; Niu, Y.; Wang, X. Lycium Species and Variety Recognition Technology Based on Electrochemical Sensing of Leaf Signals. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13054. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell Wall Polysaccharides of Chinese Wolfberry (Lycium barbarum): Part 2. Characterisation of Arabinogalactan-Proteins. Carbohydr. Polym. 2011, 84, 1075–1083. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Chen, B.H. Chromatographic Determination of Polysaccharides in Lycium barbarum Linnaeus. Food Chem. 2009, 116, 595–603. [Google Scholar] [CrossRef]
- Gong, G.; Fan, J.; Sun, Y.; Wu, Y.; Liu, Y.; Sun, W.; Zhang, Y.; Wang, Z. Isolation, Structural Characterization, and Antioxidativity of Polysaccharide LBLP5-A from Lycium barbarum Leaves. Process Biochem. 2016, 51, 314–324. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, X.; Yao, W.; Niu, Y.; Gao, X. Structure Characterization and Hypoglycemic Activity of a Polysaccharide Isolated from the Fruit of Lycium barbarum L. Carbohydr. Polym. 2010, 80, 1161–1167. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Y.; Wang, W.; Liu, N.; Zhang, H.; Zhu, Z.; Liu, A. Polysaccharides from Lycium barbarum Leaves: Isolation, Characterization and Splenocyte Proliferation Activity. Int. J. Biol. Macromol. 2012, 51, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, X.; Wang, F.; Zhang, Q.; Zhang, Z. Characterization of Lycium barbarum Polysaccharide and Its Effect on Human Hepatoma Cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and Hypoglycemic Effect of a Polysaccharide Extracted from the Fruit of Lycium barbarum L. Carbohydr. Polym. 2013, 98, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, X.-J.; Han, Z.-H.; Yu, H.-Y.; Lin, Y.; Zhang, W.-G.; Sun, F.-H.; Wang, T.-J. Extraction, Purification of Lycium barbarum Polysaccharides and Bioactivity of Purified Fraction. Carbohydr. Polym. 2011, 86, 136–141. [Google Scholar] [CrossRef]
- Chunhui, Q.; Linjuan, H.; Yongxiang, Z.; Xiunan, Z.; Gengyuan, T.; Xiangbin, R.; Beifen, S. Chemical Structure and Immunoactivity of the Glycoconjugates and Their Glycan Chains from the Fruit of Lycium barbarum L. Chin. J. Pharmacol. Toxicol. 2001, 15, 185–190. [Google Scholar]
- Peng, X.; Tian, G. Structural Characterization of the Glycan Part of Glycoconjugate LbGp2 from Lycium barbarum L. Carbohydr. Res. 2001, 331, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Linjuan, H.; Gengyuan, T.; Chunhui, Q.; Yongxiang, Z. Structure Elucidation and Immunoactivity Studies of Glycan of Glycoconjugate LbGp4 Isolated from the Fruit of Lycium barbarum L. Chem. J. Chin. Univ. 2001, 22, 407–411. [Google Scholar]
- Huang, L.; Lin, Y.; Tian, G.; Ji, G. Isolation, Purification and Physico-Chemical Properties of Immunoactive Constituents from the Fruit of Lycium barbarum L. Yao Xue Xue Bao= Acta Pharm. Sin. 1998, 33, 512–516. [Google Scholar]
- Ren, L.; Li, J.; Xiao, Y.; Zhang, Y.; Fan, J.; Zhang, B.; Wang, L.; Shen, X. Polysaccharide from Lycium barbarum L. Leaves Enhances Absorption of Endogenous Calcium, and Elevates Cecal Calcium Transport Protein Levels and Serum Cytokine Levels in Rats. J. Funct. Foods 2017, 33, 227–234. [Google Scholar] [CrossRef]
- Peng, Q.; Lv, X.; Xu, Q.; Li, Y.; Huang, L.; Du, Y. Isolation and Structural Characterization of the Polysaccharide LRGP1 from Lycium ruthenicum. Carbohydr. Polym. 2012, 90, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure Characterization, Chemical and Enzymatic Degradation, and Chain Conformation of an Acidic Polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-Soluble Polysaccharide from Dried Lycium barbarum Fruits: Isolation, Structural Features and Antioxidant Activity. Carbohydr. Polym. 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Ma, R.; Sun, X.; Yang, C.; Fan, Y. Integrated Transcriptome and Metabolome Provide Insight into Flavonoid Variation in Goji Berries (Lycium Barbarum L.) from Different Areas in China. Plant Physiol. Biochem. 2023, 199, 107722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Shi, Y.-P. Comprehensive Analysis of Phenolic Compounds in Four Varieties of Goji Berries at Different Ripening Stages by UPLC–MS/MS. J. Food Compos. Anal. 2022, 106, 104279. [Google Scholar] [CrossRef]
- Liu, W.; Xia, M.; Bai, J.; Yang, L.; Wang, Z.; Wang, R.; Shi, Y. Chemical Characterization and 5α-Reductase Inhibitory Activity of Phenolic Compounds in Goji Berries. J. Pharm. Biomed. Anal. 2021, 201, 114119. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Ghisoni, S.; Baccolo, G.; Blasi, F.; Montesano, D.; Trevisan, M.; Lucini, L. UHPLC-ESI-QTOF-MS Profile of Polyphenols in Goji Berries (Lycium Barbarum L.) and Its Dynamics during in Vitro Gastrointestinal Digestion and Fermentation. J. Funct. Foods 2018, 40, 564–572. [Google Scholar] [CrossRef]
- Chai, T.; Zhang, W.-H.; Jiao, H.; Qiang, Y. Hydroxycinnamic Acid Amide Dimers from Goji Berry and Their Potential Anti-AD Activity. Chem. Biodivers. 2021, 18, e2100436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, F.; Wang, J.; Yang, Q.; Wang, P.; Zhao, H.; Wang, J.; Wang, C.; Xu, X. Salicylic Acid Inhibits the Postharvest Decay of Goji Berry (Lycium Barbarum L.) by Modulating the Antioxidant System and Phenylpropanoid Metabolites. Postharvest Biol. Technol. 2021, 178, 111558. [Google Scholar] [CrossRef]
- Rodrigues Sá, R.; da Cruz Caldas, J.; de Andrade Santana, D.; Vieira Lopes, M.; dos Santos, W.N.L.; Graças Andrade Korn, M.; de Freitas Santos Júnior, A. Multielementar/Centesimal Composition and Determination of Bioactive Phenolics in Dried Fruits and Capsules Containing Goji Berries (Lycium Barbarum L.). Food Chem. 2019, 273, 15–23. [Google Scholar] [CrossRef]
- Fratianni, A.; Niro, S.; Alam, M.D.R.; Cinquanta, L.; Di Matteo, M.; Adiletta, G.; Panfili, G. Effect of a Physical Pre-Treatment and Drying on Carotenoids of Goji Berries (Lycium Barbarum L.). LWT 2018, 92, 318–323. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and Carotenoid Profile of New Goji Cultivars and Their Anti-Hyperglycemic, Anti-Aging and Antioxidant Properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
- Akbarizare, M. Black Goji Berry Extract as a Natural Photosensitizer for Photodynamic Inactivation of Microbial Strains: A Promising Approach. J. Med. Microbiol. Infect. Dis. 2023, 11, 222–225. [Google Scholar] [CrossRef]
- Ciceoi, R.; Luchian, V.; Tabacu, A.F.; Gutue, M.; Stavrescu-Bedivan, M.M. Goji Berry Gall Mite Expansion in Europe, with Emphasis on Southeastern Part of Romania | Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Food Science and Technology. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2021, 78, 93–99. [Google Scholar]
- Ren, H.; Bai, H. Study on the Development Status, Dilemmas and Countermeasures of Wolfberry Industry in Ningxia. Gansu Agric. Sci. Tech. 2022, 53, 10–14. [Google Scholar]
- Ju, J.-I.; Paik, S.-W.; Yun, T.-S.; Park, Y.-C.; Lee, B.-H.; Son, S.W. Variety, Cutting Date and Physiological Functionality for Production of Leaves in Goji Berry (Lycium chinense Mill.). Korean J. Plant Resour. 2020, 33, 436–445. [Google Scholar]
- Li, M.; Zheng, G.; Zhu, J.; Feng, Y. Research Progress on Resource Utilization of Chinese Wolfberry Branch Waste. Heilongjiang Agric. Sci. 2021, 327, 134–138. [Google Scholar]
- Zhao, J.; Ge, L.-Y.; Xiong, W.; Leong, F.; Huang, L.-Q.; Li, S.-P. Advanced Development in Phytochemicals Analysis of Medicine and Food Dual Purposes Plants Used in China (2011–2014). J. Chromatogr. A 2016, 1428, 39–54. [Google Scholar] [CrossRef] [PubMed]
- GH/T 1237-2019; Chinese Wolfberry Pulp. All China Federation of Supply and Marketing Cooperatives: Beijing China, 2019.
- T/NXFSA 002S-2020; Wolfberry Orginal Pulp. Ningxia Food Safety Association: Yinchuan, China, 2020.
- Geng, J.; Zhao, L.; Zhang, H. Formation Mechanism of Isoprene Compounds Degraded from Carotenoids during Fermentation of Goji Wine. Food Qual. Saf. 2021, 5, fyaa033. [Google Scholar] [CrossRef]
- Magalhães, V.; Silva, A.R.; Silva, B.; Zhang, X.; Dias, A.C.P. Comparative Studies on the Anti-Neuroinflammatory and Antioxidant Activities of Black and Red Goji Berries. J. Funct. Foods 2022, 92, 105038. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative Studies on Phenolic Profiles, Antioxidant Capacities and Carotenoid Contents of Red Goji Berry (Lycium barbarum) and Black Goji Berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical Characterization, Antioxidant and Antimicrobial Properties of Goji Berries Cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (Goji) Juice Improves in Vivo Antioxidant Biomarkers in Serum of Healthy Adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, F.; Coşkun, H.; Soytürk, H.; Bozat, B. Anxiolytic, Antioxidant, and Neuroprotective Effects of Goji Berry Polysaccharides in Ovariectomized Rats: Experimental Evidence from Behavioral, Biochemical, and Immunohistochemical Analyses. Turk. J. Biol. 2020, 44, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Jia, H.; Li, X.; Bai, Z.; Liu, Z.; Sun, L.; Zhu, Z.; Bucheli, P.; Ballevre, O.; Wang, J. A Milk-Based Wolfberry Preparation Prevents Prenatal Stress-Induced Cognitive Impairment of Offspring Rats, and Inhibits Oxidative Damage and Mitochondrial Dysfunction in Vitro. Neurochem. Res. 2010, 35, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Chung, W.Y.; Wang, J.; Richelle, M.; Bucheli, P. Enhanced Bioavailability of Zeaxanthin in a Milk-Based Formulation of Wolfberry (Gou Qi Zi; Fructus barbarum L.). Br. J. Nutr. 2006, 96, 154–160. [Google Scholar] [CrossRef]
- Toyoda-Ono, Y.; Maeda, M.; Nakao, M.; Yoshimura, M.; Sugiura-Tomimori, N.; Fukami, H. 2-O-(β-D-Glucopyranosyl) Ascorbic Acid, a Novel Ascorbic Acid Analogue Isolated from Lycium Fruit. J. Agric. Food Chem. 2004, 52, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Quiles, J.L.; Mezzetti, B.; Xiao, J.; Giampieri, F.; Battino, M. The Efficacy of Berries against Lipopolysaccharide-Induced Inflammation: A Review. Trends Food Sci. Technol. 2021, 117, 74–91. [Google Scholar] [CrossRef]
- Ávila, C.N.; Trindade, F.M.R.; Penteado, J.O.; Janke, F.; Schneider, J.P.; Uecker, J.N.; Rincón, J.A.A.; de Barros, C.C.; Andreazza, R.; Pieniz, S. Anti-Inflammatory Effect of a Goji Berry Extract (Lycium barbarum) in Rats Subjected to Inflammation by Lipopolysaccharides (LPS). Braz. Arch. Biol. Technol. 2020, 63, e20180612. [Google Scholar] [CrossRef]
- de Souza Zanchet, M.Z.; Nardi, G.M.; de Oliveira Souza Bratti, L.; Filippin-Monteiro, F.B.; Locatelli, C. Lycium barbarum Reduces Abdominal Fat and Improves Lipid Profile and Antioxidant Status in Patients with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2017, 2017, 9763210. [Google Scholar] [CrossRef] [PubMed]
- Kwaśnik, P.; Lemieszek, M.K.; Rzeski, W. Impact of Phytochemicals and Plant Extracts on Viability and Proliferation of NK Cell Line NK-92–a Closer Look at Immunomodulatory Properties of Goji Berries Extract in Human Colon Cancer Cells. Ann. Agric. Environ. Med. 2021, 28, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yang, G.; Zhang, S.; Ross, C.F.; Zhu, M.-J. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-Deficient Mice. Mol. Nutr. Food Res. 2018, 62, 1800535. [Google Scholar] [CrossRef] [PubMed]
- Soytürk, H.; Bozat, B.G.; Karakas, F.P.; Coskun, H.; Firat, T. Neuroprotective Effects of Goji Berry (Lycium barbarum L.) Polysaccharides on Depression-like Behavior in Ovariectomized Rats: Behavioral and Biochemical Evidence. Croat. Med. J. 2023, 64, 231–242. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, L.; Mo, M.; Kong, X.; Chai, Z.; Deng, L.; Zhang, J.; Cao, K.; Wei, C.; Xu, L.; et al. Research Advances on Antioxidation, Neuroprotection, and Molecular Mechanisms of Lycium barbarum Polysaccharides. Brain Sci. Adv. 2021, 7, 207–219. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Q.; Li, S.; Pu, L.; Yu, L.; Liu, Y.; Lai, X. Effects of Lycium barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review. Molecules 2022, 27, 5628. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef]
- Miranda, M.R.; Vestuto, V.; Amodio, G.; Manfra, M.; Pepe, G.; Campiglia, P. Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential. Life 2024, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Czerwonka, A.; Okła, K.; Rzeski, W. Anticancer Effect of Ethanol Lycium barbarum (Goji Berry) Extract on Human Breast Cancer T47D Cell Line. Nat. Prod. Res. 2016, 30, 1993–1996. [Google Scholar] [CrossRef]
- Gezici, S. Goji Berry Fruit Extracts Induce Cytotoxicity and Apoptotic Cell Death in Breast Cancer Cells. J. Res. Pharm. 2023, 27, 1751–1759. [Google Scholar] [CrossRef]
- Chien, K.-J.; Horng, C.-T.; Huang, Y.-S.; Hsieh, Y.-H.; Wang, C.-J.; Yang, J.-S.; Lu, C.-C.; Chen, F.-A. Effects of Lycium barbarum (Goji Berry) on Dry Eye Disease in Rats. Mol. Med. Rep. 2018, 17, 809–818. [Google Scholar] [CrossRef]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Yiu, G.; Hackman, R.M. Goji Berry Intake Increases Macular Pigment Optical Density in Healthy Adults: A Randomized Pilot Trial. Nutrients 2021, 13, 4409. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, N.; Lin, L.; Sun, E.-D.; Li, J.-D.; Li, P.-K. Macular Pigment and Serum Zeaxanthin Levels with Goji Berry Supplement in Early Age-Related Macular Degeneration. Int. J. Ophthalmol. 2018, 11, 970–975. [Google Scholar] [CrossRef]
- Liu, R.; Tam, T.W.; Mao, J.; Salem, A.; Arnason, J.T.; Krantis, A.; Foster, B.C. In Vitro Activity of Lycium barbarum (Goji) against Major Human Phase I Metabolism Enzymes. J. Complement. Integr. Med. 2016, 13, 257–265. [Google Scholar] [CrossRef]
- Rivera, C.A.; Ferro, C.L.; Bursua, A.J.; Gerber, B.S. Probable Interaction between Lycium barbarum (Goji) and Warfarin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, e50–e53. [Google Scholar] [CrossRef] [PubMed]
- Larramendi, C.; García-Abujeta, J.; Vicario, S.; García-Endrino, A.; López-Matas, M.; García-Sedeño, M.; Carnés, J. 4 Goji Berries (Lycium barbarum): Risk of Allergic Reactions in Individuals with Food Allergy. J. Investig. Allergol. Clin. Immunol. 2012, 22, 345. [Google Scholar]
- Leung, H.; Hung, A.; Hui, A.; Chan, T. Warfarin Overdose Due to the Possible Effects of Lycium barbarum L. Food Chem. Toxicol. 2008, 46, 1860–1862. [Google Scholar] [CrossRef]
- Guzmán, C.E.; Guzman-Moreno, C.G.; Assad-Morell, J.L.; Carrizales-Sepulveda, E.F. Flecainide Toxicity Associated with the Use of Goji Berries: A Case Report. Eur. Heart J.-Case Rep. 2021, 5, ytab204. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Martinez, Q.; Sáenz, M.J.; Arias, F.A.; Acosta, M.S.J. Lycium barbarum: A New Hepatotoxic “Natural” Agent? Dig. Liver Dis. 2011, 43, 749. [Google Scholar] [CrossRef]
| Compound | Analytical Methods | Reference |
|---|---|---|
| AGP | TSKgel G3000PWXL and GMPWXL columns with RID detection, using 0.1 M NaNO3 as mobile phase | [42] |
| LBP | Cosmosil 5 Diol-300-II column with ELSD detection, using the formic acid solution as mobile phase | [43] |
| LBLP5-A | TSKgel G4000SW column with RID detection, using 0.1 M Na2SO4 as mobile phase | [44] |
| LBP-1 | Shodex KS-805 column with RID detection, using distilled water as mobile phase | [45] |
| LBP-II | TSKgel G4000PWXL column with RID detection, using 0.1 M NaCl as mobile phase | [46] |
| LBP-p8 | Shodex SB-804 HQ column with RID detection, using water as mobile phase | [47] |
| LBP-s-1 | Shodex Sugar KS-805 column with RID detection, using deionized water as mobile phase | [48] |
| LBPF5 | TSKgel G3000PWXL column with RID detection, using 0.7% Na2SO4 as mobile phase | [49] |
| LbGp1 | Sepharose 4B column with phenol-sulfuric acid detection, using 0.1 M KCl as mobile phase | [50] |
| LbGp1-OL | Sepharose 4B column with phenol-sulfuric acid detection, using 0.1 M KCl as mobile phase | [50] |
| LbGp2 | Sepharose 4B column with phenol-sulfuric acid detection, using 0.1 M KCl as mobile phase | [51] |
| LbGp3 | Sepharose 4B column with phenol-sulfuric acid detection, using 0.1 M KCl as mobile phase | [52] |
| LbGp4 | Sepharose 4B column with phenol-sulfuric acid detection, using 0.1 M KCl as mobile phase | [53] |
| LP | Shodex OHpak SB-806M column with RID-MALLS detection, using 0.1 M NaNO3 as mobile phase | [54] |
| LRGP1 | TSKgel G3000SW column with RID detection, using 0.1 M Na2SO4 as mobile phase | [55] |
| p-LBP | TSKgel G4000PWXL column with RID-MALLS detection, using 50 mM Na2SO4 as mobile phase | [56] |
| PLBP | Superdex column with RID detection, using 0.7% Na2SO4 as mobile phase | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Wang, X.; Zheng, Y.; Fu, L. Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.). Appl. Sci. 2025, 15, 262. https://doi.org/10.3390/app15010262
Shi X, Wang X, Zheng Y, Fu L. Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.). Applied Sciences. 2025; 15(1):262. https://doi.org/10.3390/app15010262
Chicago/Turabian StyleShi, Xin, Xiaojing Wang, Yuhong Zheng, and Li Fu. 2025. "Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.)" Applied Sciences 15, no. 1: 262. https://doi.org/10.3390/app15010262
APA StyleShi, X., Wang, X., Zheng, Y., & Fu, L. (2025). Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.). Applied Sciences, 15(1), 262. https://doi.org/10.3390/app15010262








