Vibroarthrography as a Noninvasive Screening Method for Early Diagnosis of Knee Osteoarthritis: A Review of Current Research
Abstract
:1. Introduction
2. Methods of Diagnosing Cartilage Lesions
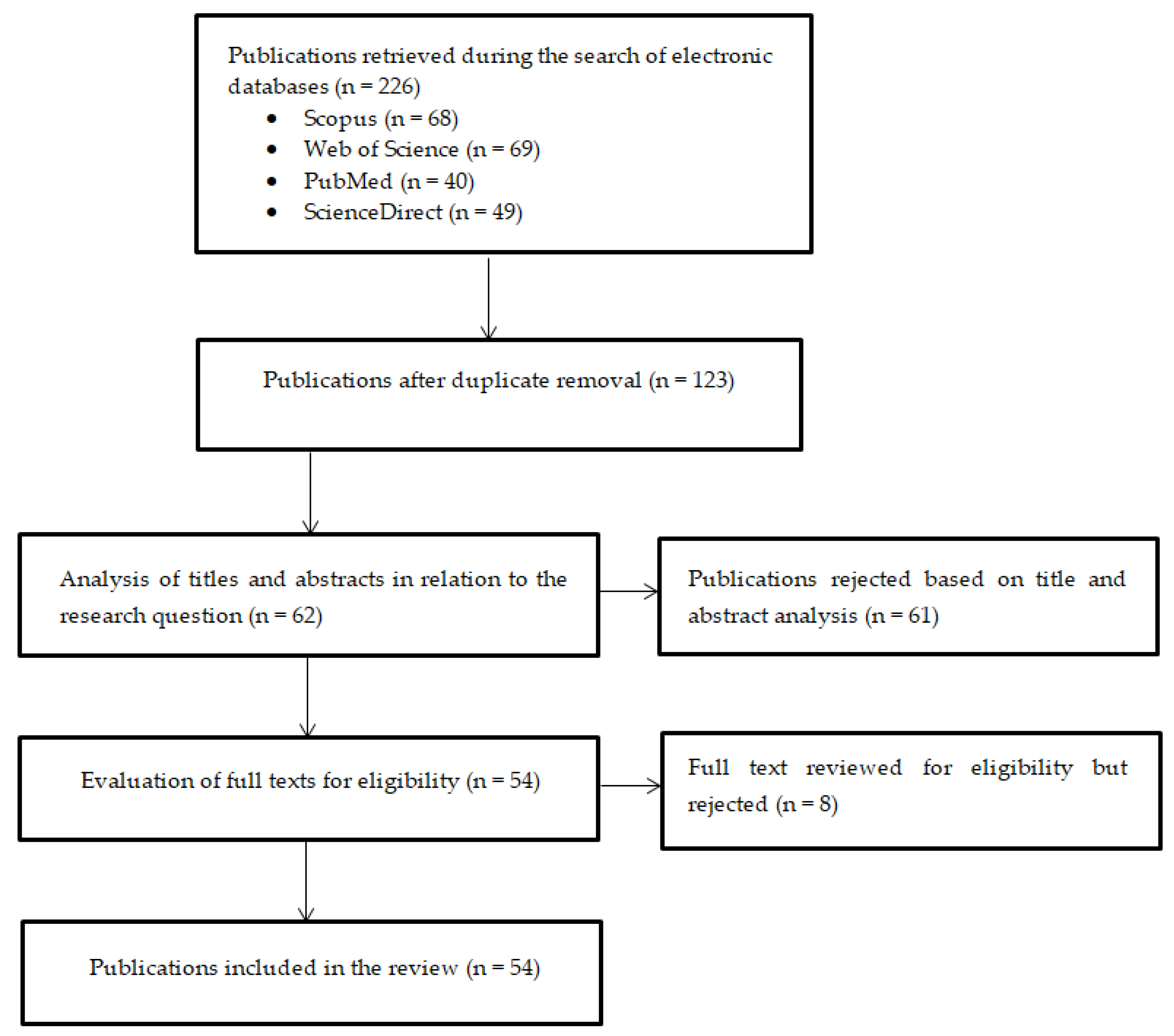
3. Review Methodology
- Publication date older than 2014;
- Lack of availability of full texts;
- Type of publication (review articles);
- Duplicates;
- Lack of empirical data.
4. Vibroarthrography of the Knee Joint
4.1. Data Acquisition
| Authors of Research Study | Population | Sensor Type and Model | Sensor Placement | Knee Movement During the Examination | Data Acquisition |
|---|---|---|---|---|---|
| Wu et al., 2014 [96] | 45 normal, 20 abnormal | Accelerometer (3115A, Dytran Instruments, Chatsworth, CA, USA) | Mid-patella | Flexion/extension | Universal amplifier, LabVIEW software, electro-stethoscope, Matlab R2011b software |
| Sarille et al., 2014 [88] | 8 normal, 9 abnormal | AE sensors | Patella | Sit-stand-sit, swing the leg | Integrated AE system and data translation, preamplifier, interface module, GSL transformer, 24 bits A/D converter, MATLAB software, nominal centre frequency 190 kHz, sampling rate: 1000 Hz |
| Bączkowicz et al., 2014 [97] | 32 normal, 73 abnormal | Accelerometer (4513B-002, Brüel and Kjær, Nærum, Denmark) | Above the apex of the patella | Flexion/extension | Multichannel Nexus conditioning amplifier, MATLAB software, periodicity between 0.7 and 1000 Hz, frequency: 10 kHz, filtered: fourth-order zero-phase Butterworth band-pass digital filter with cutoff frequencies at 50 Hz and 1000 Hz |
| Bączkowicz et al., 2015 [98] | 220 normal | Accelerometer (4513B-002, Brüel and Kjær, Nærum, Denmark) | Above the apex of the patella | Flexion/extension | Electrogoniometer, transducer, low-noise measuring amplified series Nexus, computer equipped with a measuring card, AcquiFlex software, periodicity between 0.7 and 1000 Hz, frequency: 10 kHz, filtered: fourth-order zero-phase Butterworth band-pass digital filter with cutoff frequencies at 50 Hz and 1000 Hz |
| Ota et al., 2016 [94] | 16 normal, 17 abnormal | General stethoscope, nursing scope double (No. 120, Kenzmedico Co., Saitama, Japan) | Medial and lateral epicondyle, patella, tibia | Sit-stand-sit | Operational amplifier, electrodynamic shaker, MATLAB R2013a software, sampling rate: 50 kHz, band-pass filter: 100 Hz–2 kHz |
| Wu et al., 2016 [99] | 55 normal, 18 abnormal | Accelerometers (xyzPlux, PLUX Wireless Biosignals S.A., Lisbon, Portugal) | Mid-patella, proximal patella | Flexion/extension | Signal acquisition hub, sampling rate: 1 kHz, OpenSignals software platform |
| Kręcisz et al., 2018 [100] | 72 abnormal, 33 normal | Accelerometer, (4513B-002, Brüel and Kjær, Nærum, Denmark) | Above the apex of the patella | Flexion/extension | Electrogoniometer, transducer, low-noise measuring amplified series Nexus, computer equipped with a measuring card, AcquiFlex software, periodicity between 0.7 and 1000 Hz, frequency 10 Khz, filtered: fourth-order zero-phase Butterworth band-pass digital filter with cutoff frequencies at 50 Hz and 1000 Hz |
| Khan et al., 2018 [89] | 38 normal, 11 abnormal | Frequency acoustic emission sensor (R6α, Physical Acoustics Corporation, Princeton Jct, NJ, USA) | Femur, tibia | Sit-stand-sit | Dual-channel goniometer (SG150, Biometrics Ltd., Caerphilly, Wales), software: no data |
| Befrui et al., 2018 [11] | 30 normal, 39 abnormal | Accelerometers (352A24, Piezotronics, Inc., Depew, NY, USA) piezoelectric disk (EPZ-27MS44F, Elektrotechnik Karl-Heinz Mauz GmbH, Ostfildern, Germany) | Patella, medial tibial and lateral tibial plateau | Flexion/extension | Potentiometer, four single-ended simultaneous channels with 24-bit resolution, support for IEPE inputs, MATLAB software |
| Choi et al., 2018 [69] | 20 normal, 14 abnormal | piezoelectric sensors, custom-made | Medial and lateral epicondyle of the tibia, patella | Flexion/extension, sit-stand-sit | Sampling rate of 50 kHz, software: no data |
| Andersen et al., 2018 [101] | 11 normal | Accelerometers (LIS344ALH, ST microelectronics, Geneva, Switzerland) | Quadriceps tendon, lateral and medial side of the knee, patella, tibial tuberosity | Flexion/extension | Custom-made device based on the Trentadue wireless multichannel surface electromyography (SEMG) recorder, custom adaptor, accelerometer probes made using micro-machined accelerometers, MATLAB 2016a software, amplification and filtering: recording device, low pass third-order sync filter 250 Hz, high-pass filter 10 Hz, sampling at 1000 Hz |
| Sharma et al., 2018 [102] | 51 normal, 38 abnormal | Accelerometer (3115a, Dytran, Chatsworth, CA, USA) | Mid-patella | Flexion/extension | Signal prefiltration range: 10–10,000 Hz, sampling rate: 2 kHz, software: no data |
| Kiselev et al., 2019 [90] | 29 abnormal | Piezoelectric sensor | Lateral and medial part of the knee, except for the patellar cartilage | Squats | Band-pass filters that pass frequencies in the range of 70–85 kHz, software: no data |
| Bączkowicz et al., 2019 [103] | 62 normal, 38 abnormal | Accelerometer (4513B-002, Brüel and Kjær, Nærum, Denmark) | Above the apex of the patella | Sit-stand-sit, flexion/extension | 10 kHz sampling rate, data filtering: fourth-order zero-phase Butterworth band-pass filter with cutoff frequencies 50–1000 Hz, software: no data |
| Kalo et al., 2020 [84] | 12 normal | Microphone (SPU0414HR5H-SB, Knowles Electronics, Itasca, IL, USA) | Medial tibial plateau, patella | Sit-to-stand, downstairs, flexion/extension | A/D converter, MATLAB R2018b, signal filtering using Butterworth hand-pass digital filter with cutoff frequencies of 100 Hz and 300 Hz |
| Gong et al., 2020 [104] | 26 normal, 25 with OA | Accelerometer (BW21SG2, Fuji Ceramics, Fujinomiya, Japan) | Mid-patella | Sit-stand-sit | Conversion connector, preamplifier, sampling rate 25 kHz, PC oscilloscope, PicoScope 6 Software |
| Kalo et al., 2020 [91] | 19 normal | Acoustic sensors (SPU0414HR5H-SB, Knowles Electronics, Itasca, IL, USA) | Medial tibial plateau, patella | Sit-stand-sit | A/D converter with a sampling rate of 16,000 Hz, 8 AI (14-bit, 48 kS/s), 2 AO (150 Hz), 13 DIO USB multifunction I/O device, MATLAB R2018b |
| Madeleine et al., 2020 [105] | 20 normal, 20 abnormal | Accelerometers (LIS344ALH, ST microelectronics, Geneva, Switzerland) | Patella, tibial tuberosity, above the knee, next to the lateral and medial epicondyle of femur | Sit-to-stand, stairs descent, stairs ascent | Custom-made Trentadue wireless multichannel recorder, custom 16-channel accelerometer adaptor, MATLAB 2016a, sensitivity: 600 mV/g, linear transmission: 0–1800 Hz, band-pass filter: 10–500 Hz, sampling rate: 2000 Hz |
| Befrui et al., 2020 [106] | 30 normal, 39 abnormal | Accelerometer (352A24, PCB Piezotronics Inc., Depew, NY, USA) | Patella, medial and lateral tibial plateau | Flexion/extension | Frequency range 1–8000 Hz, sensitivity 10.09 mV/m/s2, Measurement range 50 m/s, resonant frequency 38,300 Hz, constant current excitation 2–20 mA, piezoelectric disk with resonant frequency 4400 Hz, impedance 300 Ω, potentiometer, dynamic signal analyser, software: no data |
| Khan et al., 2021 [31] | 41 normal, 23 abnormal | Piezoelectric sensors (R6α, Physical Acoustics Corporation, Princeton Jct, NJ, USA) | Medial and lateral condyle of the tibia, medial and lateral epicondyle of the femur | Sit-stand-sit | Four preamplifiers, AE main amplifier, AE acquisition device, electrogoniometer, amplification unit, software: no data |
| Ozmen et al., 2021 [107] | 10 normal | Accelerometer (3225F7, Dytran Instruments, Chatsworth, CA, USA) | Mid-patella | Flexion/extension | Sensitivity 100 mV/g in the 50 Hz–10 kHz frequency band, USB-4431 data acquisition unit, power amplifier 2781, electrodynamic mini shaker, impedance head with sensitivity 30 pC/g, charge to constant current live drive converter, MATLAB software |
| Gong et al., 2021 [92] | 36 normal | Piezoelectric sensors (7BB-20-6L0, Murata, Kyoto, Japan) | Tibia | Sit-stand-sit | Accelerometer, tri-axis accelerometer, analogue-to-digital converter, sampling rate: 2000 Hz, software: no data |
| Shidore et al., 2021 [108] | 51 normal, 39 abnormal | Accelerometer (3115A, Dytran Instruments, Chatsworth, CA, USA) | Mid-patella | Flexion/extension | LabVIEW software, sampling rate: 2 kHz, frequency range: 10 Hz–1 kHz |
| Karpiński et al., 2021 [30] | 10 normal, 10 abnormal | Piezoelectric contact microphone (CM-01B, TE Connectivity, Schaffhausen, Switzerland) | Patella | Flexion/extension | Measurement system based on Arduino Mega2560 board: bandwidth spans from 8 Hz to 2.2 kHz, sampling frequency 1400 Hz with 10-bit resolution, digital encoder, 11.1 V lithium-ion battery. Data logging using RealTerm software in ASCII format |
| Nevalainen et al., 2021 [72] | 55 normal, 54 abnormal | Air microphone (Audio-Technica AT899, Stow, OH, USA), IMU Sensors (SparkFun 6 DOF IMU Digital Combo Board-ITG3200/ADXL345 | Medial and lateral sides of the bone, thigh, and shin | Flexion/extension, sit-to-stand, one-leg stand | Sampling frequency 44.1 kHz, soundcard, frequency 100 Hz, software: no data |
| Karpiński 2022 [85] | 25 normal, 25 abnormal | Piezoelectric contact microphone (CM-01B, TE Connectivity, Schaffhausen, Switzerland) | Patella | Sit-stand-sit | Bandwidth from 10 Hz to 2 kHz, software: no data |
| Karpiński et al., 2022 [76] | 33 normal, 34 abnormal | Piezoelectric contact microphone (CM-01B, TE Connectivity, Schaffhausen, Switzerland) | Patella | Flexion/extension, sit-stand-sit | Bandwidth from 10 Hz to 2 kHz, orthosis with vibration transducers, 10-bit Bourns magnetic digital encoder, 8-bit Atmega2560 microcontroller with 10-bit ADC, ADuM4160 USB 2.0 isolator, software: no data |
| Karpiński et al., 2022 [77] | 33 normal, 34 abnormal | Piezoelectric contact microphone (CM-01B, TE Connectivity, Schaffhausen, Switzerland) | Patella | Flexion/extension, sit-stand-sit | Bandwidth from 10 Hz to 2 kHz, orthosis with vibration transducers, 10-bit Bourns magnetic digital encoder, 8-bit Atmega2560 microcontroller with 10-bit ADC, ADuM4160 USB 2.0 isolator, software: no data |
| Jeong et al., 2022 [109] | 16 normal | Accelerometers (3225, Dytran Instruments, Chatsworth, CA, USA) | Patella | Squats | Sensitivity: 100 mV/g, broad bandwidth: up to 10 kHz, low noise floor: 700 ugrms, sampling rate: 25 kHz, device: NI USB-4432, National Instruments Corporation, MATLAB software |
| Vatolik et al., 2022 [70] | 15 normal | Microphone (MR-28406-000, Knowles Electronics, Itasca, IL, USA) | Lateral soft part of the knee below the patellofemoral joint | Sit-stand-sit | Frequency range 100 Hz–4.7 kHz, Laryngograph DSP Unit, Speech Filing System software |
| Khokhlova et al., 2022 [93] | 8 normal | PK151 AE sensor | Right medial tibial condyle area | Cycling | USB AE node monitoring system, acoustic sensor with frequency range of 100–450 kHz, integrated preamplifier, software: no data |
| Kręcisz et al., 2022 [110] | 220 normal | Accelerometer with a multichannel NEXUS conditioning amplifier (413B-002, Brüel and Kjær Sound and Vibration Measurement A/S, Nærum, Denmark) | Above the patella apex | Flexion/extension | Frequency range: 0.7–1000 Hz, sampling rate 10 kHz, signal filtering: fourth-order zero-phase Butterworth band-pass digital filter with cutoff frequencies 50–1000 Hz, software: no data |
| Khoklova et al., 2023 [21] | 51 normal | PK3I—30 kHz AE sensor with low-power, integral preamp, Physical Acoustics; PK15I—150 kHz sensor with low-power, integral preamp, Physical Acoustic | Right medial tibial condyle area | Cycling | MATLAB software |
| Karpiński et al., 2023 [19] | 40 normal, 44 abnormal | Piezoelectric contact microphone (CM-01B, TE Connectivity, Schaffhausen, Switzerland) | Patella | Flexion/extension | Bandwidth from 10 Hz to 2 kHz, Bourns digital encoder, RealTerm software, in ASCII format |
| Borzucka et al., 2024 [111] | 38 normal | Accelerometer with a multichannel NEXUS conditioning amplifier (413B-002, Brüel and Kjær Sound and Vibration Measurement A/S, Nærum, Denmark) | Patella | Squats | Frequency range: 0.7–1000 Hz, sampling rate 10 kHz, signal filtering: fourth-order zero-phase Butterworth band-pass digital filter with cutoff frequencies 50–1000 Hz, software: no data |
| Machrowska et al., 2024 [86] | 63 normal, 49 abnormal | Piezoelectric contact microphones (CM-01B, TE Connectivity, Schaffhausen, Switzerland) | Condyle of the femur on the lateral and medial sides, patella | Flexion/extension | Arduino Mega2560 R3 module, Bourns encoder, software: no data |
| Machrowska et al., 2024 [87] | 51 normal, 47 abnormal | Piezoelectric contact microphones (CM-01B, TE Connectivity, Schaffhausen, Switzerland) | Patella | Flexion/extension | Arduino Mega2560 R3 module, Bourns encoder, software: no data |
4.2. VAG Signal Preprocessing and Filtration
4.3. Feature Extraction and Classification
4.3.1. Feature Extraction and Selection
4.3.2. Classification
5. Discussion, Limitations, and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Cempel, C. Diagnostyka Wibroakustyczna Maszyn; PWN: Warszawa, Poland, 1989; ISBN 978-83-01-09696-0. [Google Scholar]
- Fletcher, N.H. Acoustic Systems in Biology; Oxford University Press: Oxford, UK, 1992; ISBN 978-0-19-506940-2. [Google Scholar]
- Kernohan, W.G.; Beverland, D.E.; McCoy, G.F.; Hamilton, A.; Watson, P.; Mollan, R. Vibration Arthrometry. Acta Orthop. Scand. 1990, 61, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, W.E. Auscultation of the Knee Joint. Boston Med. Surg. J. 1902, 146, 63–66. [Google Scholar] [CrossRef]
- Yiallourides, C.; Naylor, P.A. Time-Frequency Analysis and Parameterisation of Knee Sounds for Non-Invasive Detection of Osteoarthritis. IEEE Trans. Biomed. Eng. 2021, 68, 1250–1261. [Google Scholar] [CrossRef]
- Andersen, R.E.; Arendt-Nielsen, L.; Madeleine, P. A Review of Engineering Aspects of Vibroarthography of the Knee Joint. Crit. Rev. Phys. Rehabil. Med. 2016, 28, 13–32. [Google Scholar] [CrossRef]
- Lafortune, M.A.; Cavanagh, P.R.; Sommer, H.J.; Kalenak, A. Three-Dimensional Kinematics of the Human Knee during Walking. J. Biomech. 1992, 25, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Shark, L.-K.; Chen, H.; Goodacre, J. Knee Acoustic Emission: A Potential Biomarker for Quantitative Assessment of Joint Ageing and Degeneration. Med. Eng. Phys. 2011, 33, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Cibere, J.; Sayre, E.C.; Guermazi, A.; Nicolaou, S.; Kopec, J.A.; Esdaile, J.M.; Thorne, A.; Singer, J.; Wong, H. Natural History of Cartilage Damage and Osteoarthritis Progression on Magnetic Resonance Imaging in a Population-Based Cohort with Knee Pain. Osteoarthr. Cartil. 2011, 19, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.H.; Spindler, K.P. Risk Factors for Radiographic Joint Space Narrowing and Patient Reported Outcomes of Post-Traumatic Osteoarthritis after ACL Reconstruction: Data from the MOON Cohort: PTOA after ACL reconstruction in MOON. J. Orthop. Res. 2017, 35, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Befrui, N.; Elsner, J.; Flesser, A.; Huvanandana, J.; Jarrousse, O.; Le, T.N.; Müller, M.; Schulze, W.H.W.; Taing, S.; Weidert, S. Vibroarthrography for Early Detection of Knee Osteoarthritis Using Normalized Frequency Features. Med. Biol. Eng. Comput. 2018, 56, 1499–1514. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Conconi, M.; Halilaj, E.; Parenti Castelli, V.; Crisco, J.J. Is Early Osteoarthritis Associated with Differences in Joint Congruence? J. Biomech. 2014, 47, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current Evidence on Risk Factors for Knee Osteoarthritis in Older Adults: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef]
- Williams, J.; Pierre-Louis, K. Osteoarthritis of the Knee. Physician Assist. Clin. 2024, 9, 59–69. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of selected classification methods based on machine learning as a diagnostic tool for knee joint cartilage damage based on generated vibroacoustic processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef]
- Khokhlova, L.; Komaris, D.-S.; O’Flynn, B.; Tedesco, S. Acoustic Emissions and Age-Related Changes of the Knee. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; IEEE: Sydney, Australia, 2023; pp. 1–4. [Google Scholar]
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and Osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI Guidelines for the Non-Surgical Management of Knee Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Osteoarthritis: New Insights. Part 1: The Disease and Its Risk Factors. Ann. Intern. Med. 2000, 133, 635. [Google Scholar] [CrossRef]
- Brahim, A.; Jennane, R.; Riad, R.; Janvier, T.; Khedher, L.; Toumi, H.; Lespessailles, E. A Decision Support Tool for Early Detection of Knee OsteoArthritis Using X-Ray Imaging and Machine Learning: Data from the OsteoArthritis Initiative. Comput. Med. Imaging Graph. 2019, 73, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, J.; Cheon, S. A Deep Neural Network-Based Method for Early Detection of Osteoarthritis Using Statistical Data. IJERPH 2019, 16, 1281. [Google Scholar] [CrossRef]
- Ota, S.; Sasaki, E.; Sasaki, S.; Chiba, D.; Kimura, Y.; Yamamoto, Y.; Kumagai, M.; Ando, M.; Tsuda, E.; Ishibashi, Y. Relationship between Abnormalities Detected by Magnetic Resonance Imaging and Knee Symptoms in Early Knee Osteoarthritis. Sci. Rep. 2021, 11, 15179. [Google Scholar] [CrossRef] [PubMed]
- Gądek, A.; Liszka, H.; Zając, M. The Effect of Pre-Operative High Doses of Methylprednisolone on Pain Management and Convalescence after Total Hip Replacement in Elderly: A Double-Blind Randomized Study. Int. Orthop. (SICOT) 2021, 45, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Favero, M.; Ramonda, R.; Goldring, M.B.; Goldring, S.R.; Punzi, L. Early Knee Osteoarthritis. RMD Open 2015, 1, e000062. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Estimation of Differences in Selected Indices of Vibroacoustic Signals between Healthy and Osteoarthritic Patellofemoral Joints as a Potential Non-Invasive Diagnostic Tool. J. Phys. Conf. Ser. 2021, 2130, 012009. [Google Scholar] [CrossRef]
- Khan, T.I.; Hassan, M.M.; Kurihara, M.; Ide, S. Research on Diagnosis of Knee Osteoarthritis Using Acoustic Emission Technique. Acoust. Sci. Tech. 2021, 42, 241–251. [Google Scholar] [CrossRef]
- Peat, G. Clinical Classification Criteria for Knee Osteoarthritis: Performance in the General Population and Primary Care. Ann. Rheum. Dis. 2006, 65, 1363–1367. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, M.; Chambat, P. (Eds.) Osteoartritis of the Knee; Approche pratique en orthopédie—traumatologie; Springer: Paris, France; Berlin, Germany, 2008; ISBN 978-2-287-74174-6. [Google Scholar]
- Hayashi, D.; Roemer, F.W.; Guermazi, A. Imaging of Osteoarthritis by Conventional Radiography, MR Imaging, PET–Computed Tomography, and PET–MR Imaging. PET Clin. 2019, 14, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Latourte, A. Osteoarthritis: Value of imaging and biomarkers. Rev. Prat. 2019, 69, 507–509. [Google Scholar]
- Ahn, J.M.; El-Khoury, G.Y. Computed Tomography of Knee Injuries. Imaging Decis. 2006, 10, 14–23. [Google Scholar] [CrossRef]
- Chan, W.P.; Lang, P.; Stevens, M.P.; Sack, K.; Majumdar, S.; Stoller, D.W.; Basch, C.; Genant, H.K. Osteoarthritis of the Knee: Comparison of Radiography, CT, and MR Imaging to Assess Extent and Severity. Am. J. Roentgenol. 1991, 157, 799–806. [Google Scholar] [CrossRef]
- Palczewski, P. Imaging Diagnosis of Osteoarthritis. Pol. J. Sports Med. 2021, 37, 103–115. [Google Scholar] [CrossRef]
- Mazrani, W.; McHugh, K.; Marsden, P.J. The Radiation Burden of Radiological Investigations. Arch. Dis. Child. 2007, 92, 1127–1131. [Google Scholar] [CrossRef]
- Möller, I.; Bong, D.; Naredo, E.; Filippucci, E.; Carrasco, I.; Moragues, C.; Iagnocco, A. Ultrasound in the Study and Monitoring of Osteoarthritis. Osteoarthr. Cartil. 2008, 16, S4–S7. [Google Scholar] [CrossRef]
- Wu, Y. Knee Joint Vibroarthrographic Signal Processing and Analysis; SpringerBriefs in Bioengineering; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-44283-8. [Google Scholar]
- Mathiessen, A.; Cimmino, M.A.; Hammer, H.B.; Haugen, I.K.; Iagnocco, A.; Conaghan, P.G. Imaging of Osteoarthritis (OA): What Is New? Best Pract. Res. Clin. Rheumatol. 2016, 30, 653–669. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.G. The Development and Clinical Applications of Musculoskeletal Ultrasound. Skelet. Radiol. 2011, 40, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Nogalski, A.; Jurkiewicz, A.; Karpiński, R.; Maciejewski, R.; Jonak, J. Comparison of Diagnostic Accuracy of Physical Examination and MRI in the Most Common Knee Injuries. Appl. Sci. 2019, 9, 4102. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Jojczuk, M.; Nogalska, A.; Jonak, J. Knee MRI Underestimates the Grade of Cartilage Lesions. Appl. Sci. 2021, 11, 1552. [Google Scholar] [CrossRef]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.J.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the Knee in Osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Singer, P.; Zeller, P.; Mandl, I.; Haller, J.; Trattnig, S. Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) for the Evaluation of Autologous Chondrocyte Transplantation: Determination of Interobserver Variability and Correlation to Clinical Outcome after 2 Years. Eur. J. Radiol. 2006, 57, 16–23. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, Z. Gadolinium-based Contrast Agents for Magnetic Resonance Cancer Imaging. WIREs Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Runge, V.M. Critical Questions Regarding Gadolinium Deposition in the Brain and Body After Injections of the Gadolinium-Based Contrast Agents, Safety, and Clinical Recommendations in Consideration of the EMA’s Pharmacovigilance and Risk Assessment Committee Recommendation for Suspension of the Marketing Authorizations for 4 Linear Agents. Investig. Radiol. 2017, 52, 317–323. [Google Scholar] [CrossRef]
- Fraum, T.J.; Ludwig, D.R.; Bashir, M.R.; Fowler, K.J. Gadolinium-based Contrast Agents: A Comprehensive Risk Assessment. Magn. Reson. Imaging 2017, 46, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, C.L.; Mallio, C.A.; Vaccarino, F.; Grasso, R.F.; Zobel, B.B. Imaging of Knee Osteoarthritis: A Review of Multimodal Diagnostic Approach. Quant. Imaging Med. Surg. 2023, 13, 7582–7595. [Google Scholar] [CrossRef]
- Chalian, M.; Pooyan, A.; Alipour, E.; Roemer, F.W.; Guermazi, A. What Is New in Osteoarthritis Imaging? Radiol. Clin. North Am. 2024, 62, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Oo, W.M.; Linklater, J.M. What Is the Role of Imaging in the Clinical Diagnosis of Osteoarthritis and Disease Management? Rheumatology 2018, 57, iv51–iv60. [Google Scholar] [CrossRef] [PubMed]
- Tanamas, S.K.; Jones, G. Imaging of Knee Osteoarthritis. Clin. Pract. 2010, 7, 635. [Google Scholar] [CrossRef]
- Fukuda, T.; Yonenaga, T.; Miyasaka, T.; Kimura, T.; Jinzaki, M.; Ojiri, H. CT in Osteoarthritis: Its Clinical Role and Recent Advances. Skelet. Radiol. 2023, 52, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Turmezei, T.D.; Lomas, D.J.; Hopper, M.A.; Poole, K.E.S. Severity Mapping of the Proximal Femur: A New Method for Assessing Hip Osteoarthritis with Computed Tomography. Osteoarthr. Cartil. 2014, 22, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Gielis, W.P.; Weinans, H.; Nap, F.J.; Roemer, F.W.; Foppen, W. Scoring Osteoarthritis Reliably in Large Joints and the Spine Using Whole-Body CT: OsteoArthritis Computed Tomography-Score (OACT-Score). JPM 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, V.; Sorriento, A.; Cafarelli, A.; Donati, D.; Papalexis, N.; Russo, A.; Lisignoli, G.; Ricotti, L.; Spinnato, P. Ultrasound Imaging in Knee Osteoarthritis: Current Role, Recent Advancements, and Future Perspectives. JCM 2024, 13, 4930. [Google Scholar] [CrossRef]
- Nelson, A.E. Ultrasound in Osteoarthritis. In Musculoskeletal Ultrasound in Rheumatology Review; Kohler, M.J., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 405–424. ISBN 978-3-030-73554-8. [Google Scholar]
- Ehmig, J.; Engel, G.; Lotz, J.; Lehmann, W.; Taheri, S.; Schilling, A.F.; Seif Amir Hosseini, A.; Panahi, B. MR-Imaging in Osteoarthritis: Current Standard of Practice and Future Outlook. Diagnostics 2023, 13, 2586. [Google Scholar] [CrossRef]
- Mallio, C.A.; Bernetti, C.; Agostini, F.; Mangone, M.; Paoloni, M.; Santilli, G.; Martina, F.M.; Quattrocchi, C.C.; Zobel, B.B.; Bernetti, A. Advanced MR Imaging for Knee Osteoarthritis: A Review on Local and Brain Effects. Diagnostics 2022, 13, 54. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Jonak, J.; Maciejewski, R. Evaluation of Diagnostic Accuracy of Physical Examination and MRI for Ligament and Meniscus Injuries. J. Phys. Conf. Ser. 2021, 1736, 012027. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J. Evaluation of the Diagnostic Accuracy of MRI in Detection of Knee Cartilage Lesions Using Receiver Operating Characteristic Curves. J. Phys. Conf. Ser. 2021, 1736, 012028. [Google Scholar] [CrossRef]
- De Tocqueville, S.; Marjin, M.; Ruzek, M. A Review of the Vibration Arthrography Technique Applied to the Knee Diagnostics. Appl. Sci. 2021, 11, 7337. [Google Scholar] [CrossRef]
- McCoy, G.; McCrea, J.; Beverland, D.; Kernohan, W.; Mollan, R. Vibration Arthrography as a Diagnostic Aid in Diseases of the Knee. A Preliminary Report. J. Bone Jt. Surgery. Br. Vol. 1987, 69, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Jonak, J.; Karpinski, R.; Machrowska, A.; Krakowski, P.; Maciejewski, M. A Preliminary Study on the Use of EEMD-RQA Algorithms in the Detection of Degenerative Changes in Knee Joints. IOP Conf. Ser. Mater. Sci. Eng. 2019, 710, 012037. [Google Scholar] [CrossRef]
- Karpiński, R.; Machrowska, A.; Maciejewski, M. Application of acoustic signal processing methods in detecting differences between open and closed kinematic chain movement for the knee joint. Appl. Comput. Sci. 2019, 15, 36–48. [Google Scholar] [CrossRef]
- Choi, D.; Ahn, S.; Ryu, J.; Nagao, M.; Kim, Y. Knee Acoustic Emission Characteristics of the Healthy and the Patients with Osteoarthritis Using Piezoelectric Sensor. Sens. Mater. 2018, 30, 1629. [Google Scholar] [CrossRef]
- Vatolik, I.; Everington, M.; Hunter, G.; Swann, N.; Augousti, A.T. Development of a Multi-Modal Sensor Network to Detect and Monitor Knee Joint Condition. Meas. Sens. 2022, 24, 100483. [Google Scholar] [CrossRef]
- Schlüter, D.K.; Spain, L.; Quan, W.; Southworth, H.; Platt, N.; Mercer, J.; Shark, L.-K.; Waterton, J.C.; Bowes, M.; Diggle, P.J.; et al. Use of Acoustic Emission to Identify Novel Candidate Biomarkers for Knee Osteoarthritis (OA). PLoS ONE 2019, 14, e0223711. [Google Scholar] [CrossRef]
- Nevalainen, M.T.; Veikkola, O.; Thevenot, J.; Tiulpin, A.; Hirvasniemi, J.; Niinimäki, J.; Saarakkala, S.S. Acoustic Emissions and Kinematic Instability of the Osteoarthritic Knee Joint: Comparison with Radiographic Findings. Sci. Rep. 2021, 11, 19558. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Analysis of Differences in Vibroacoustic Signals between Healthy and Osteoarthritic Knees Using EMD Algorithm and Statistical Analysis. J. Phys. Conf. Ser. 2021, 2130, 012010. [Google Scholar] [CrossRef]
- Karpiński, R.; Machrowska, A.; Maciejewski, M.; Jonak, J.; Krakowski, P. Concept and validation of a system for recording vibroacoustic signals of the knee joint. IAPGOS 2024, 14, 17–21. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Krakowski, P.; Jonak, J. Diagnostic factors for opened and closed kinematic chain of vibroarthrography signals. Appl. Comput. Sci. 2019, 15, 34–44. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part I: Femoral-Tibial Joint. Sensors 2022, 22, 2176. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part II: Patellofemoral Joint. Sensors 2022, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, D.; Calvo, R.; Vaisman, A.; Carrasco, M.A.; Moraga, C.; Delgado, I. Knee Chondral Lesions: Incidence and Correlation Between Arthroscopic and Magnetic Resonance Findings. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 312–315. [Google Scholar] [CrossRef]
- Bredella, M.A.; Tirman, P.F.; Peterfy, C.G.; Zarlingo, M.; Feller, J.F.; Bost, F.W.; Belzer, J.P.; Wischer, T.K.; Genant, H.K. Accuracy of T2-Weighted Fast Spin-Echo MR Imaging with Fat Saturation in Detecting Cartilage Defects in the Knee: Comparison with Arthroscopy in 130 Patients. Am. J. Roentgenol. 1999, 172, 1073–1080. [Google Scholar] [CrossRef]
- Edelsten, L.; Jeffrey, J.E.; Burgin, L.V.; Aspden, R.M. Viscoelastic Deformation of Articular Cartilage during Impact Loading. Soft Matter 2010, 6, 5206. [Google Scholar] [CrossRef]
- Temple, D.K.; Cederlund, A.A.; Lawless, B.M.; Aspden, R.M.; Espino, D.M. Viscoelastic Properties of Human and Bovine Articular Cartilage: A Comparison of Frequency-Dependent Trends. BMC Musculoskelet. Disord. 2016, 17, 419. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, C.; Liu, S.; Ma, S.; Zhang, A.; You, Z.; Chen, L.; Zhao, H. Interleukin-6 Induces Extracellular Matrix Degradation and Angiogenesis in Osteoarthritis Models of Temporomandibular Joint via Estrogen-Related Receptor γ. SSRN J. 2019. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone Remodelling in Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kalo, K.; Niederer, D.; Sus, R.; Sohrabi, K.; Banzer, W.; Groß, V.; Vogt, L. The Detection of Knee Joint Sounds at Defined Loads by Means of Vibroarthrography. Clin. Biomech. 2020, 74, 1–7. [Google Scholar] [CrossRef]
- Karpiński, R. Knee joint osteoarthritis diagnosis based on selected acoustic signal discriminants using machine learning. ACS 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P.; Syta, A. Application of Recurrence Quantification Analysis in the Detection of Osteoarthritis of the Knee with the Use of Vibroarthrography. Adv. Sci. Technol. Res. J. 2024, 18, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P. Application of eemd-dfa algorithms and ann classification for detection of knee osteoarthritis using vibroarthrography. Appl. Comput. Sci. 2024, 20, 90–108. [Google Scholar] [CrossRef]
- Sarillee, M.; Hariharan, M.; Anas, M.N.; Omar, M.I.; Aishah, M.N.; Oung, Q.W. Assessment of Knee Joint Abnormality Using Acoustic Emission Sensors. In Proceedings of the 2014 IEEE International Conference on Control System, Computing and Engineering (ICCSCE 2014), Penang, Malaysia, 28–30 November 2014; IEEE: Penang, Malaysia, 2014; pp. 378–383. [Google Scholar]
- Khan, T.I.; Kusumoto, M.; Nakamura, Y.; Ide, S.; Yoshimura, T. Acoustic Emission Technique as an Adaptive Biomarker in Integrity Analysis of Knee Joint. J. Phys. Conf. Ser. 2018, 1075, 012020. [Google Scholar] [CrossRef]
- Kiselev, J.; Ziegler, B.; Schwalbe, H.J.; Franke, R.P.; Wolf, U. Detection of Osteoarthritis Using Acoustic Emission Analysis. Med. Eng. Phys. 2019, 65, 57–60. [Google Scholar] [CrossRef]
- Kalo, K.; Niederer, D.; Sus, R.; Sohrabi, K.; Groß, V.; Vogt, L. Reliability of Vibroarthrography to Assess Knee Joint Sounds in Motion. Sensors 2020, 20, 1998. [Google Scholar] [CrossRef]
- Gong, R.; Ohtsu, H.; Hase, K.; Ota, S. Vibroarthrographic Signals for the Low-Cost and Computationally Efficient Classification of Aging and Healthy Knees. Biomed. Signal Process. Control 2021, 70, 103003. [Google Scholar] [CrossRef]
- Khokhlova, L.; Komaris, D.-S.; Tedesco, S.; O’Flynn, B. Test-Retest Reliability of Acoustic Emission Sensing of the Knee during Physical Tasks. Sensors 2022, 22, 9027. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Ando, A.; Tozawa, Y.; Nakamura, T.; Okamoto, S.; Sakai, T.; Hase, K. Preliminary Study of Optimal Measurement Location on Vibroarthrography for Classification of Patients with Knee Osteoarthritis. J. Phys. Ther. Sci. 2016, 28, 2904–2908. [Google Scholar] [CrossRef]
- Prill, R.; Walter, M.; Królikowska, A.; Becker, R. A Systematic Review of Diagnostic Accuracy and Clinical Applications of Wearable Movement Sensors for Knee Joint Rehabilitation. Sensors 2021, 21, 8221. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, S.; Zheng, F.; Cai, S.; Lu, M.; Wu, M. Removal of Artifacts in Knee Joint Vibroarthrographic Signals Using Ensemble Empirical Mode Decomposition and Detrended Fluctuation Analysis. Physiol. Meas. 2014, 35, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Bączkowicz, D.; Majorczyk, E. Joint Motion Quality in Vibroacoustic Signal Analysis for Patients with Patellofemoral Joint Disorders. BMC Musculoskelet. Disord. 2014, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Bączkowicz, D.; Majorczyk, E.; Kręcisz, K. Age-Related Impairment of Quality of Joint Motion in Vibroarthrographic Signal Analysis. BioMed Res. Int. 2015, 2015, 591707. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, P.; Luo, X.; Huang, H.; Liao, L.; Yao, Y.; Wu, M.; Rangayyan, R.M. Quantification of Knee Vibroarthrographic Signal Irregularity Associated with Patellofemoral Joint Cartilage Pathology Based on Entropy and Envelope Amplitude Measures. Comput. Methods Programs Biomed. 2016, 130, 1–12. [Google Scholar] [CrossRef]
- Kręcisz, K.; Bączkowicz, D. Analysis and Multiclass Classification of Pathological Knee Joints Using Vibroarthrographic Signals. Comput. Methods Programs Biomed. 2018, 154, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.E.; Arendt-Nielsen, L.; Madeleine, P. Knee Joint Vibroarthrography of Asymptomatic Subjects during Loaded Flexion-Extension Movements. Med. Biol. Eng. Comput. 2018, 56, 2301–2312. [Google Scholar] [CrossRef]
- Sharma, M.; Acharya, U.R. Analysis of Knee-Joint Vibroarthographic Signals Using Bandwidth-Duration Localized Three-Channel Filter Bank. Comput. Electr. Eng. 2018, 72, 191–202. [Google Scholar] [CrossRef]
- Bączkowicz, D.; Kręcisz, K.; Borysiuk, Z. Analysis of Patellofemoral Arthrokinematic Motion Quality in Open and Closed Kinetic Chains Using Vibroarthrography. BMC Musculoskelet. Disord. 2019, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Hase, K.; Goto, H.; Yoshioka, K.; Ota, S. Knee Osteoarthritis Detection Based on the Combination of Empirical Mode Decomposition and Wavelet Analysis. JBSE 2020, 15, 20-00017. [Google Scholar] [CrossRef]
- Madeleine, P.; Andersen, R.E.; Larsen, J.B.; Arendt-Nielsen, L.; Samani, A. Wireless Multichannel Vibroarthrographic Recordings for the Assessment of Knee Osteoarthritis during Three Activities of Daily Living. Clin. Biomech. 2020, 72, 16–23. [Google Scholar] [CrossRef]
- Befrui, N.; Elsner, J.; Flesser, A.; Huvanandana, J.; Jarrousse, O.; Le, T.N.; Müller, M.; Schulze, W.H.W.; Taing, S.; Weidert, S. Detection and Grading of Knee Joint Cartilage Defect Using Multi-Class Classification in Vibroarthrography. CAOS 2018, 2, 6–11. [Google Scholar]
- Ozmen, G.C.; Safaei, M.; Lan, L.; Inan, O.T. A Novel Accelerometer Mounting Method for Sensing Performance Improvement in Acoustic Measurements from the Knee. J. Vib. Acoust. 2021, 143, 031006. [Google Scholar] [CrossRef] [PubMed]
- Shidore, M.M.; Athreya, S.S.; Deshpande, S.; Jalnekar, R. Screening of Knee-Joint Vibroarthrographic Signals Using Time and Spectral Domain Features. Biomed. Signal Process. Control 2021, 68, 102808. [Google Scholar] [CrossRef]
- Jeong, H.K.; An, S.; Herrin, K.; Scherpereel, K.; Young, A.; Inan, O.T. Quantifying Asymmetry Between Medial and Lateral Compartment Knee Loading Forces Using Acoustic Emissions. IEEE Trans. Biomed. Eng. 2022, 69, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, K.; Bączkowicz, D.; Kawala-Sterniuk, A. Using Nonlinear Vibroartrographic Parameters for Age-Related Changes Assessment in Knee Arthrokinematics. Sensors 2022, 22, 5549. [Google Scholar] [CrossRef] [PubMed]
- Borzucka, D.; Kręcisz, K.; Bączkowicz, D. Influence of External Load during Back Squats on Knee Joint Arthrokinematics Analyzed by Vibroarthrography. Research Square 2024. [Google Scholar] [CrossRef]
- Lee, T.-F.; Lin, W.-C.; Wu, L.-F.; Wang, H.-Y. Analysis of Vibroarthrographic Signals for Knee Osteoarthritis Diagnosis. In Proceedings of the 2012 Sixth International Conference on Genetic and Evolutionary Computing, Kitakyushu, Japan, 25–28 August 2012; IEEE: Kitakyushu, Japan, 2012; pp. 223–228. [Google Scholar]
- Kim, K.S.; Seo, J.H.; Kang, J.U.; Song, C.G. An Enhanced Algorithm for Knee Joint Sound Classification Using Feature Extraction Based on Time-Frequency Analysis. Comput. Methods Programs Biomed. 2009, 94, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cai, S.; Yang, S.; Zheng, F.; Xiang, N. Classification of Knee Joint Vibration Signals Using Bivariate Feature Distribution Estimation and Maximal Posterior Probability Decision Criterion. Entropy 2013, 15, 1375–1387. [Google Scholar] [CrossRef]
- Rangayyan, R.M.; Wu, Y. Analysis of Vibroarthrographic Signals with Features Related to Signal Variability and Radial-Basis Functions. Ann. Biomed. Eng. 2009, 37, 156–163. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, S.; Xu, F.; Shi, L.; Krishnan, S. Chondromalacia Patellae Detection by Analysis of Intrinsic Mode Functions in Knee Joint Vibration Signals. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Beijing, China, 31–26 May 2012; Long, M., Ed.; IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2013; Volume 39, pp. 493–496. ISBN 978-3-642-29304-7. [Google Scholar]
- Cai, S.; Wu, Y.; Xiang, N.; Zhong, Z.; He, J.; Shi, L.; Xu, F. Detrending Knee Joint Vibration Signals with a Cascade Moving Average Filter. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: San Diego, CA, USA, 2012; pp. 4357–4360. [Google Scholar]
- Wang, Y. (Ed.) Transdisciplinary Advancements in Cognitive Mechanisms and Human Information Processing; IGI Global: Hershey, PA, USA, 2011; ISBN 978-1-60960-553-7. [Google Scholar]
- Yang, S.; Cai, S.; Zheng, F.; Wu, Y.; Liu, K.; Wu, M.; Zou, Q.; Chen, J. Representation of Fluctuation Features in Pathological Knee Joint Vibroarthrographic Signals Using Kernel Density Modeling Method. Med. Eng. Phys. 2014, 36, 1305–1311. [Google Scholar] [CrossRef]
- Bachi, L.; Billeci, L.; Varanini, M. QRS Detection Based on Medical Knowledge and Cascades of Moving Average Filters. Appl. Sci. 2021, 11, 6995. [Google Scholar] [CrossRef]
- Ma, C.; Yang, J.; Wang, Q.; Liu, H.; Xu, H.; Ding, T.; Yang, J. A Method of Feature Fusion and Dimension Reduction for Knee Joint Pathology Screening and Separability Evaluation Criteria. Comput. Methods Programs Biomed. 2022, 224, 106992. [Google Scholar] [CrossRef]
- Azami, H.; Mohammadi, K.; Bozorgtabar, B. An Improved Signal Segmentation Using Moving Average and Savitzky-Golay Filter. JSIP 2012, 03, 39–44. [Google Scholar] [CrossRef]
- Rangayyan, R.M.; Oloumi, F.; Wu, Y.; Cai, S. Fractal Analysis of Knee-Joint Vibroarthrographic Signals via Power Spectral Analysis. Biomed. Signal Process. Control 2013, 8, 23–29. [Google Scholar] [CrossRef]
- Cai, S.; Yang, S.; Zheng, F.; Lu, M.; Wu, Y.; Krishnan, S. Knee Joint Vibration Signal Analysis with Matching Pursuit Decomposition and Dynamic Weighted Classifier Fusion. Comput. Math. Methods Med. 2013, 2013, 904267. [Google Scholar] [CrossRef] [PubMed]
- Nalband, S.; Sundar, A.; Prince, A.A.; Agarwal, A. Feature Selection and Classification Methodology for the Detection of Knee-Joint Disorders. Comput. Methods Programs Biomed. 2016, 127, 94–104. [Google Scholar] [CrossRef]
- Caesarendra, W.; Tjahjowidodo, T. A Review of Feature Extraction Methods in Vibration-Based Condition Monitoring and Its Application for Degradation Trend Estimation of Low-Speed Slew Bearing. Machines 2017, 5, 21. [Google Scholar] [CrossRef]
- Yiakopoulos, C.T.; Gryllias, K.C.; Antoniadis, I.A. Rolling Element Bearing Fault Detection in Industrial Environments Based on a K-Means Clustering Approach. Expert. Syst. Appl. 2011, 38, 2888–2911. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. [Google Scholar] [CrossRef]
- Szabelski, J. Effect of Incorrect Mix Ratio on Strength of Two Component Adhesive Butt-Joints Tested at Elevated Temperature. MATEC Web Conf. 2018, 244, 01019. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef]
- Özhan, O. Short-Time-Fourier Transform. In Basic Transforms for Electrical Engineering; Springer International Publishing: Cham, Switzerland, 2022; pp. 441–464. ISBN 978-3-030-98845-6. [Google Scholar]
- Rhif, M.; Ben Abbes, A.; Farah, I.R.; Martínez, B.; Sang, Y. Wavelet Transform Application for/in Non-Stationary Time-Series Analysis: A Review. Appl. Sci. 2019, 9, 1345. [Google Scholar] [CrossRef]
- Percival, D.B.; Walden, A.T. Wavelet Methods for Time Series Analysis; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-511-84104-0. [Google Scholar]
- Ocak, H. A Medical Decision Support System Based on Support Vector Machines and the Genetic Algorithm for the Evaluation of Fetal Well-Being. J. Med. Syst. 2013, 37, 9913. [Google Scholar] [CrossRef]
- Oh, S.L.; Adam, M.; Tan, J.H.; Hagiwara, Y.; Sudarshan, V.K.; Koh, J.E.W.; Chua, K.C.; Chua, K.P.; Tan, R.S.; Ng, E.Y.K. Automated identification of coronary artery disease from short-term 12 lead electrocardiogram signals by using wavelet packet decomposition and common spatial pattern techniques. J. Mech. Med. Biol. 2017, 17, 1740007. [Google Scholar] [CrossRef]
- Ho, T.K. Random Decision Forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; IEEE Computer Society Press: Montreal, QC, Canada, 1995; Volume 1, pp. 278–282. [Google Scholar]
- Wu, X.; Kumar, V.; Ross Quinlan, J.; Ghosh, J.; Yang, Q.; Motoda, H.; McLachlan, G.J.; Ng, A.; Liu, B.; Yu, P.S.; et al. Top 10 Algorithms in Data Mining. Knowl. Inf. Syst. 2008, 14, 1–37. [Google Scholar] [CrossRef]
- Liu, K.; Luo, X.; Yang, S.; Cai, S.; Zheng, F.; Wu, Y. Classification of Knee Joint Vibroarthrographic Signals Using K-Nearest Neighbor Algorithm. In Proceedings of the 2014 IEEE 27th Canadian Conference on Electrical and Computer Engineering (CCECE), Toronto, ON, Canada, 4–7 May 2014; IEEE: Toronto, ON, Canada, 2014; pp. 1–4. [Google Scholar]
- Rogala, M. Neural Networks in Crashworthiness Analysis of Thin-Walled Profile with Foam Filling. Adv. Sci. Technol. Res. J. 2020, 14, 93–99. [Google Scholar] [CrossRef]
- Gajewski, J.; Vališ, D. Verification of the Technical Equipment Degradation Method Using a Hybrid Reinforcement Learning Trees–Artificial Neural Network System. Tribol. Int. 2021, 153, 106618. [Google Scholar] [CrossRef]
- Kruse, R.; Mostaghim, S.; Borgelt, C.; Braune, C.; Steinbrecher, M. Radial Basis Function Networks. In Computational Intelligence; Texts in Computer Science; Springer International Publishing: Cham, Switzerland, 2022; pp. 125–148. ISBN 978-3-030-42226-4. [Google Scholar]

| Diagnostic Method | Advantages | Disadvantages | Sources |
|---|---|---|---|
| Radiography | Noninvasive Inexpensive High availability Time | Exposure to ionising radiation, Shows developed changes in the knee joint, unable to diagnose small cartilage lesions in early stages | [52,53,54,55] |
| Computed tomography | Noninvasive High resolution | Time Lack of cartilage visualisation unless enhanced with the administration of intra-articular contrast agent Based on bone changes, it allows for the estimation of cartilage damage Contrast agent exposure in contrast-enhanced CT | [56,57,58] |
| Ultrasound | Dynamic test Noninvasive Low cost High sensitivity for detecting soft tissue and structures Real-time imaging Ability to assess inflammation | The quality of the examination depends on the experience of the sonographer Lack of visibility of all knee parts as ultrasound cannot penetrate through bones | [59,60] |
| Magnetic resonance imaging | Noninvasive High resolution Quantitative measurements of articular cartilage 3D imaging Detection of damage to cartilage, menisci, ligaments | High cost Complex Long acquisition times Requires specialised equipment and personnel | [61,62,63,64] |
| Vibroarthrography | Noninvasive Low cost Provides information about the condition of the moving joint Repeatability | No dedicated equipment No test protocols | [11,65] |
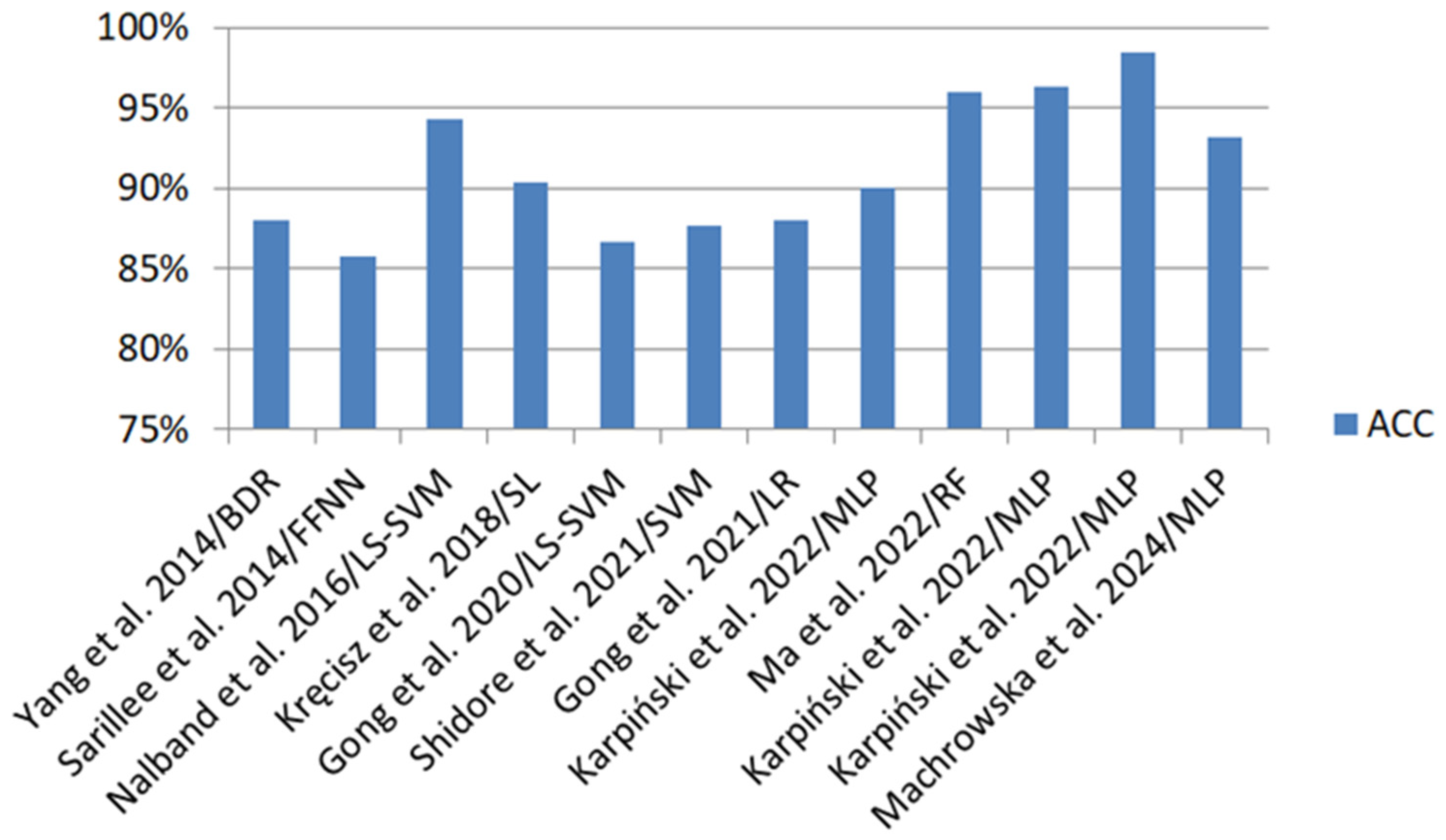
| Authors | Feature Extraction | Classification Methods | Classification Evaluation |
|---|---|---|---|
| Yang et al. [119] | SF (DFA, AEA) | LS-SVM | ACC 82.67%, SEN 0.6429, SPE 0.9362 |
| BDR | ACC 88%, SEN 0.7143, SPE 0.9787 | ||
| Sarillee et al. [88] | SF (K, S) | FFNN | ACC 83.6–85.76% |
| SVM | ACC 83.37–85.74% | ||
| Nalband et al. [125] | TD (RQA, ApEn, SampEn), TFD (Wavelet energy) | LS-SVM | ACC 91.01–94.31%, SEN 0.9.22–0.9807, SPE 0.8333–0.8648 |
| RF | ACC 86.52–91.01%, SEN 0.9411–0.9615, SPE 0.7568–0.8684 | ||
| Kręcisz et al. [100] | TD (VMS, R4), Cf (FF, SHE, TC, DFA, MSE), RQA (RR, DET, LAM, H, TT, LMAX), FDF (FT) | SL | In 2-class classification: ACC 90.4%, SEN 0.934, SPE 0.848 In 5-class classification: ACC 69%, SEN 0.914, SPE 0.69 |
| MLP | In 2-class classification: ACC 88.8%, SEN 0.917, SPE 0.833 In 5-class classification: ACC 69%, SEN 0.912, SPE 0.69 | ||
| RF | In 2-class classification: ACC 87.2%, SEN 0.901, SPE 0.818 In 5-class classification: ACC 62%, SEN 0.899, SPE 0.62 | ||
| SMO | In 2-class classification: ACC 84.5%, SEN 0.893, SPE 0.758 In 5-class classification: ACC 61.5%, SEN 0.89, SPE 0.615 | ||
| Befrui et al. [11] | TD (segmentation), FD (partial sum of the power spectrum) | SVM | SEN 0.80, SPE 0.75 |
| Gong et al. [104] | ST (K, S), TFD (CWT) | LS-SVM | ACC 74.19–86.67% |
| Shidore et al. [108] | TD (SH, IF, MF, CF, S, K), SD (STFT) | SVM | ACC 84.61–87.69%, SEN 0.8571–1.0, SPE 0.7954–0.8461 |
| RF | ACC 81.54–84.61%, SEN 0.7647–0.8846, SPE 0.8158–0.8709 | ||
| NB | ACC 83.07–84.62%, SEN 0.8519–0.8846, SPE 0.8158–0.8205 | ||
| Gong et al. [92] | TD (S, K), TFD (H) | KNN | ACC 87.27%, SEN 0.8846, SPE 0.8821 |
| LR | ACC 88%, SEN 0.875, SPE 0.8519 | ||
| Karpiński [85] | TD (SA, RMS, PV, PPV, CF, IF, SF, VAR, K) | MLP | ACC 86–90%, SEN 0.875–0.917%, SPE 0.846–0.917 |
| RBF | ACC 84–88%, SEN 0.84–0.875, SPE 0.84–0.913 | ||
| Ma et al. [121] | TD (PPV, SA, VAR, MF, K, S, II, FF, VMS, TC) | RF | ACC 93–96%, SEN 0.92–0.96 |
| SVM | ACC 79–95%, SEN 0.65–0.93 | ||
| KNN | ACC 91–95%, SEN 0.90–0.94 | ||
| Karpiński et al. [76] | TD (MV, SA, RMS, PV, PPV, CF, IF, SF, VAR, M6A, M8A) | MLP | ACC 93.7–96.32% |
| RBF | ACC 89.63–91.91% | ||
| Karpiński et al. [77] | TD (MV, SA, RMS, PV, PPV, CF, IF, SF, VAR, K, M6A, M8A) | MLP | ACC 89.71–98.53% |
| RBF | ACC 97.06–98.53% | ||
| Machrowska et al. [87] | VAR, StDev, RMS, FF, CF, FM4, K, PPV, M6A, IF, SM, M, M8A, S, PV | MLP | ACC 91.89–93.24%, SEN 0.889–0.932, SPE 0.932–0.933 |
| RBF | ACC 80.41–81.08%, SEN 0.714–721, SPE 0.84–0.848 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, R.; Prus, A.; Jonak, K.; Krakowski, P. Vibroarthrography as a Noninvasive Screening Method for Early Diagnosis of Knee Osteoarthritis: A Review of Current Research. Appl. Sci. 2025, 15, 279. https://doi.org/10.3390/app15010279
Karpiński R, Prus A, Jonak K, Krakowski P. Vibroarthrography as a Noninvasive Screening Method for Early Diagnosis of Knee Osteoarthritis: A Review of Current Research. Applied Sciences. 2025; 15(1):279. https://doi.org/10.3390/app15010279
Chicago/Turabian StyleKarpiński, Robert, Aleksandra Prus, Kamil Jonak, and Przemysław Krakowski. 2025. "Vibroarthrography as a Noninvasive Screening Method for Early Diagnosis of Knee Osteoarthritis: A Review of Current Research" Applied Sciences 15, no. 1: 279. https://doi.org/10.3390/app15010279
APA StyleKarpiński, R., Prus, A., Jonak, K., & Krakowski, P. (2025). Vibroarthrography as a Noninvasive Screening Method for Early Diagnosis of Knee Osteoarthritis: A Review of Current Research. Applied Sciences, 15(1), 279. https://doi.org/10.3390/app15010279






