Gamma-Polyglutamic Acid Reduces Heavy Metal Uptake and Stabilize Microbial Biosafety in Edible Mushroom Cultures
Abstract
1. Introduction
2. Materials and Methods
- ○
- Step 1: Cultivation of in vitro mushroom mycelia with or without metals.
- ○
- Step 2: Biosynthesis and purification of γ-PGA.
- ○
- Cultivation with metals and increasing γ-PGA concentrations (1, 2, 5 mL/250 mL medium).
- ○
- Sorption and desorption efficiency of metals in biomass.
- ○
- Bioavailability assessment via artificial digestive juices (gastric and intestinal phases).
- ○
- Kirby–Bauer disk diffusion assay with Salmonella enteritidis and E. coli strains on mushroom cultures ± γ-PGA.
2.1. Mushrooms Biomass
2.2. Poly-Gamma Glutamic Acid (γ-PGA)
2.3. Reagents
2.4. Analysis Effectiveness of Metal Adsorption and Desorption of Toxic Metals in the Biomass Obtained in Stage 1 from In Vitro Cultures with Added Poly-Gamma Glutamic Acid (γ-PGA) (Stage 2)
2.5. Assessment of Metal Extraction by Simulated Digestive Juices from Mushroom Biomass Obtained Under Cultivation Conditions with and Without γ-PGA (Stage 3)
2.6. Testing Material Properties (Biomass and γ-PGA)
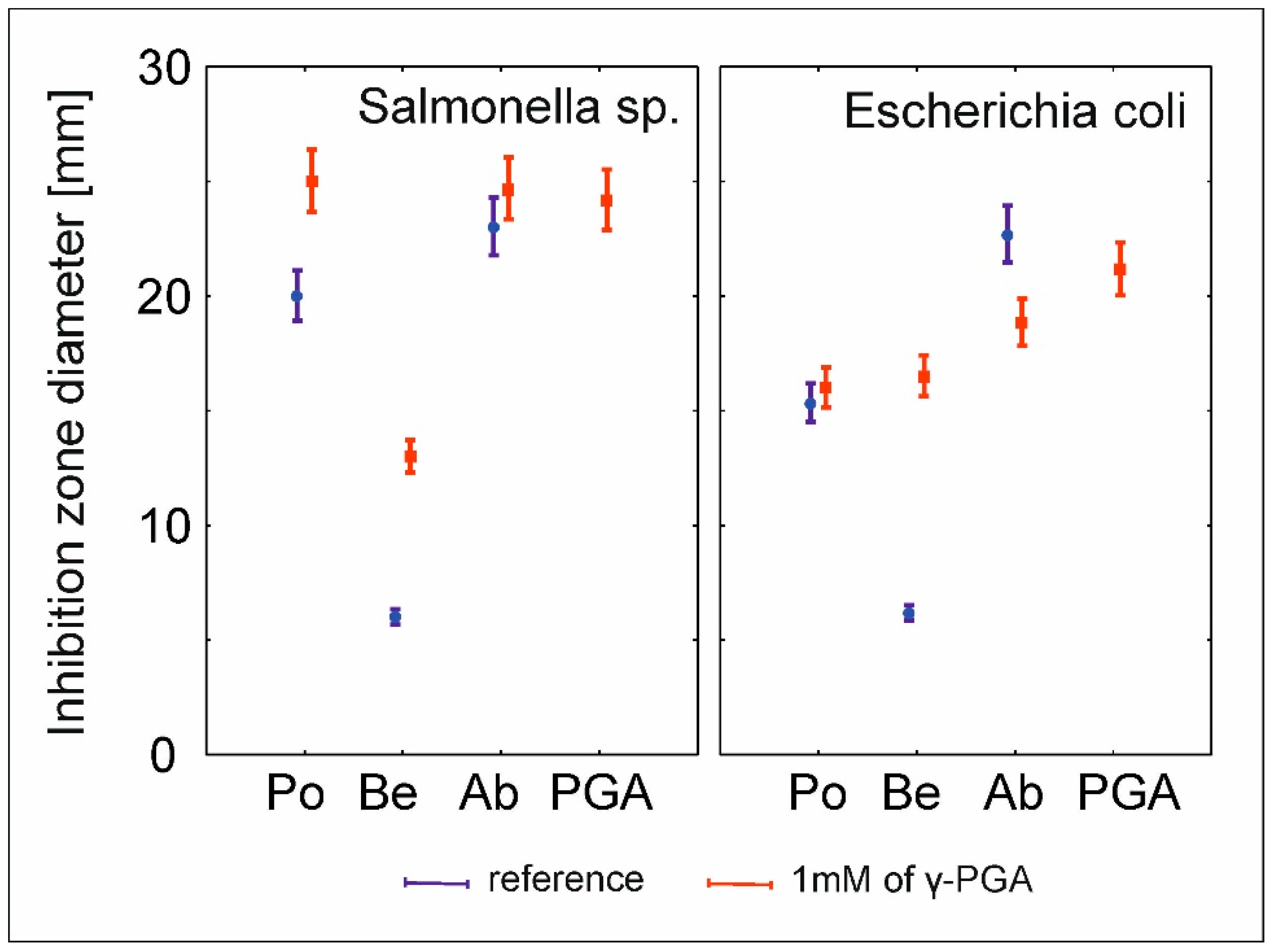
2.7. Antimicrobial Susceptibility Testing
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization Materials (Biomass and γ-PGA)
3.2. Structure Examination of Mycelium Biomass
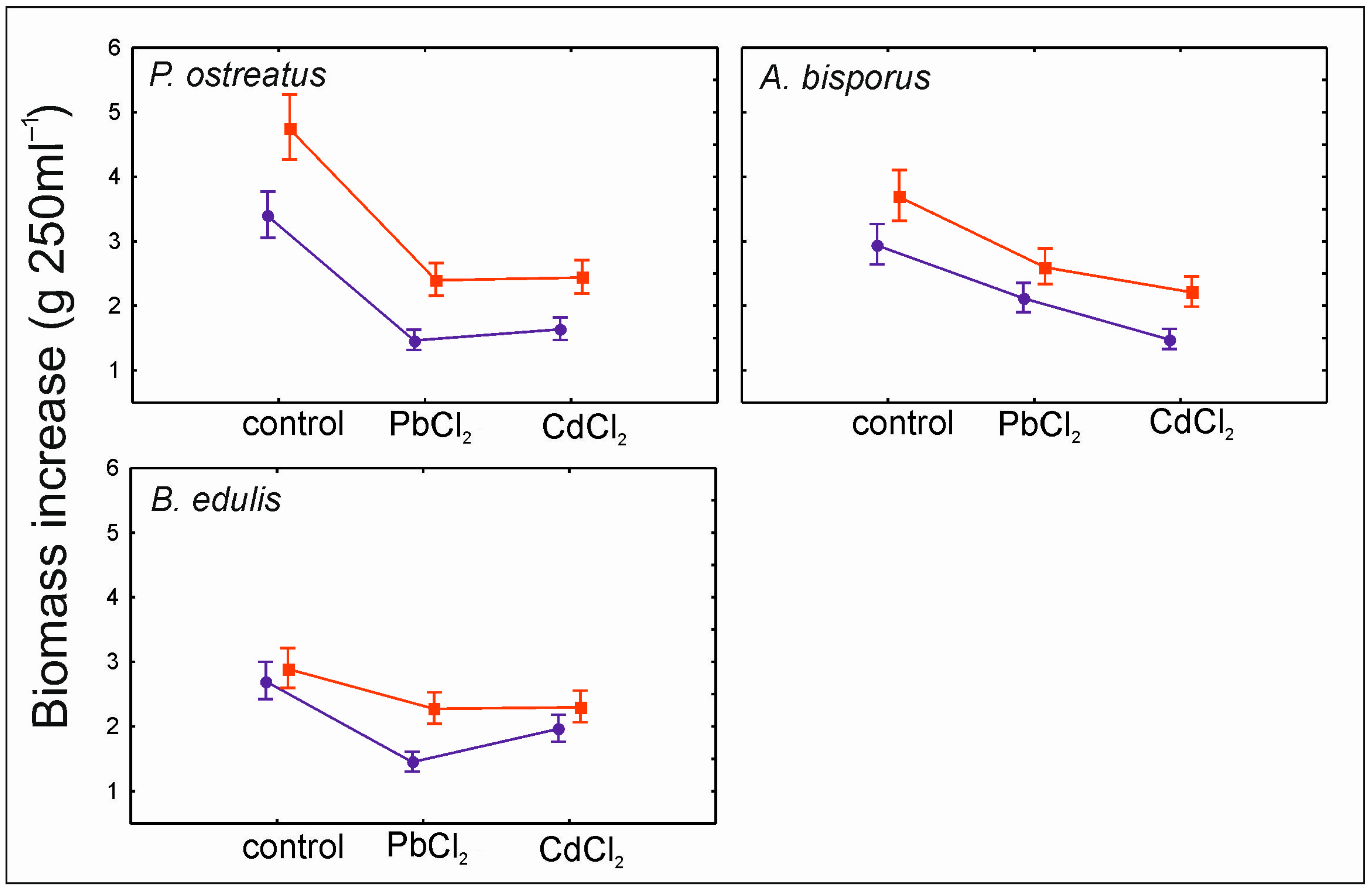
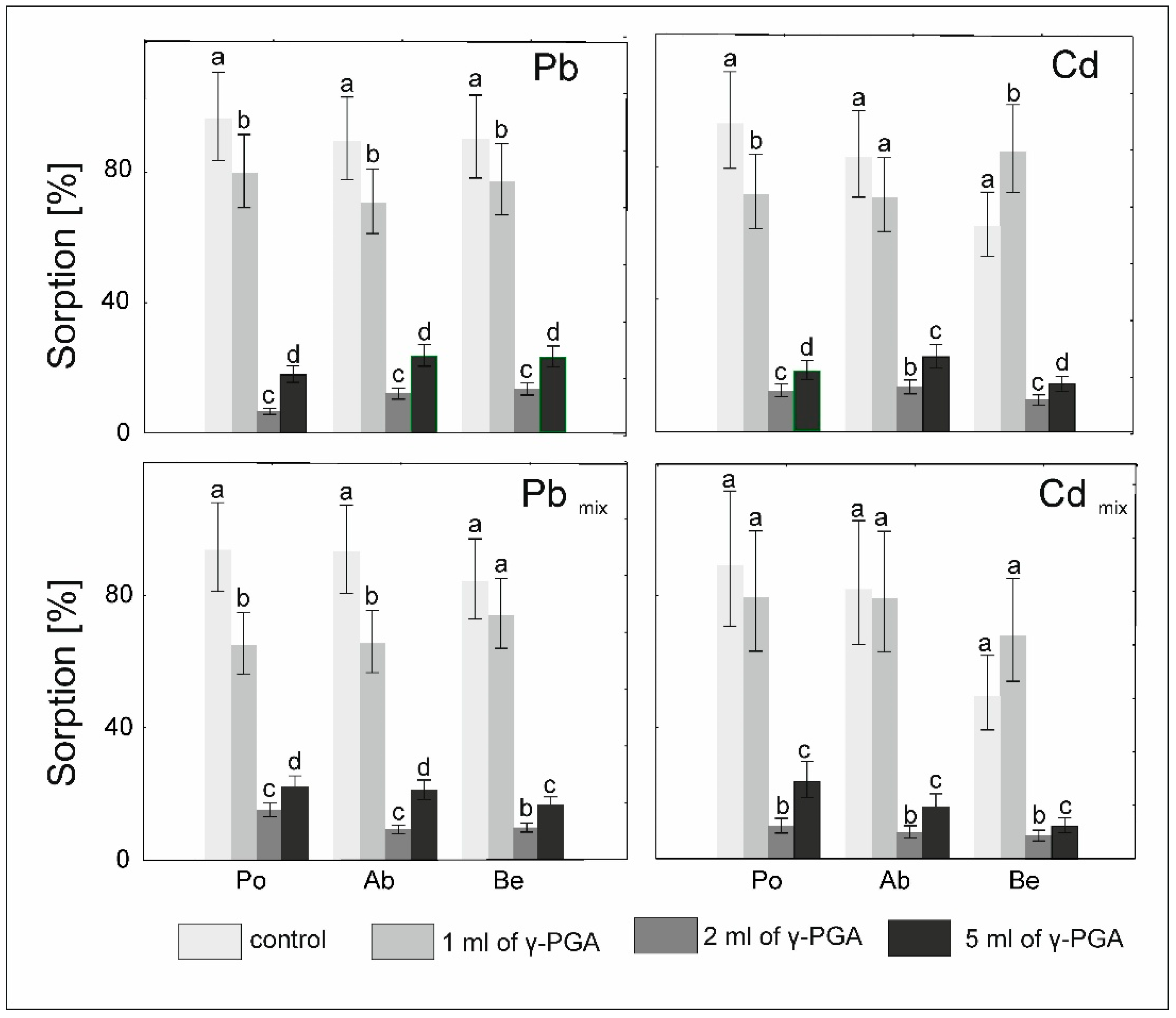
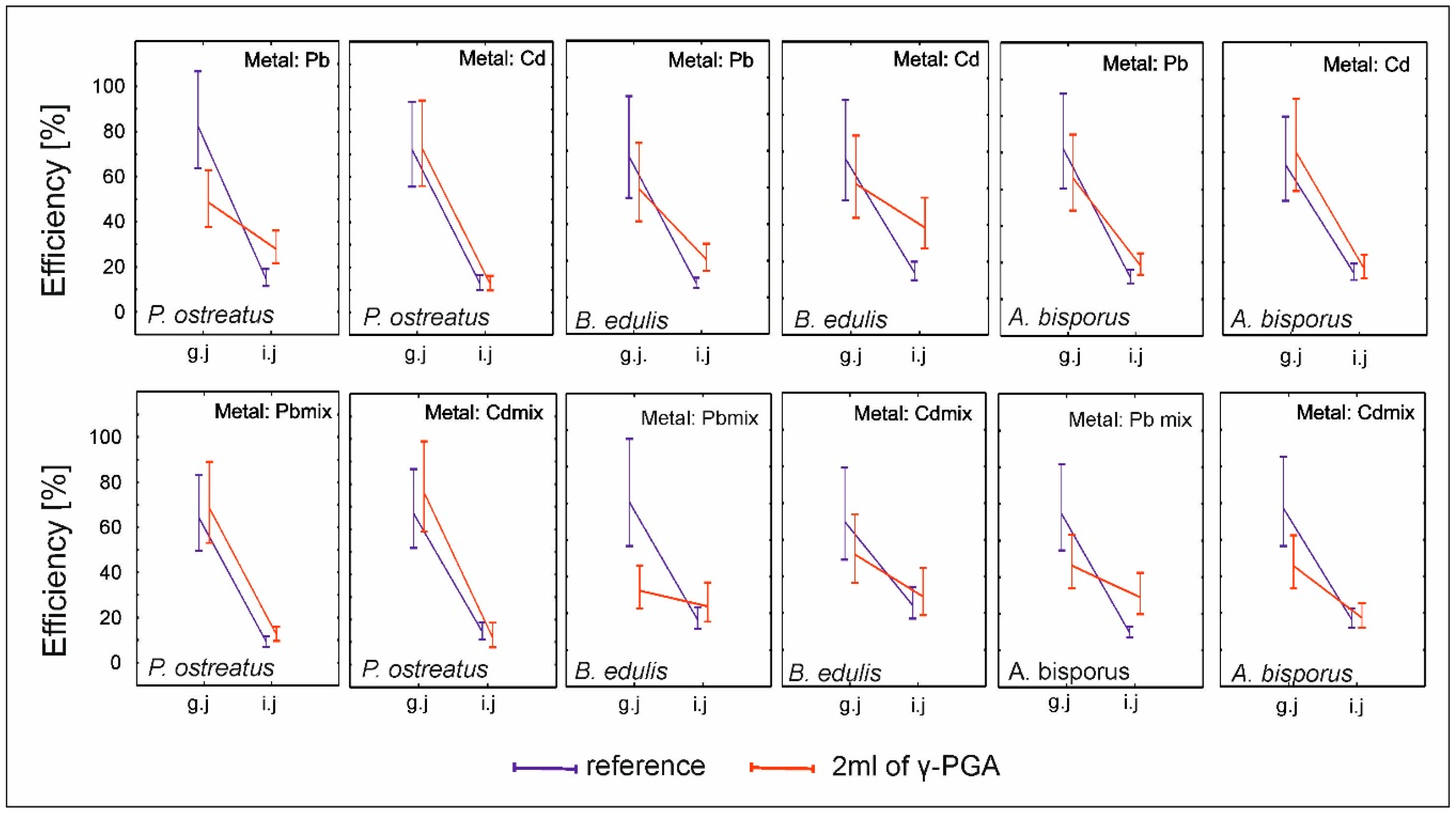
3.3. Sorption and Desorption by Mycelia In Vitro Cultures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [PubMed]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2012, 103, 180–189. [Google Scholar] [CrossRef]
- Sesli, E.; Tuzen, M. Levels of trace elements in the fruiting bodies of macrofungi growing in the East Black Sea region of Turkey. Food Chem. 1999, 65, 453–460. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Mleczek, M.; Niedzielski, P. The effect of heavy metals on the growth and accumulation of bioelements and heavy metals in edible mushrooms. ESPR 2020, 27, 35503–35513. [Google Scholar]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. Environ. Sci. Technol. 2009, 43, 4664–4670. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and me: An interview. Hydrometallurgy 2007, 84, 3–7. [Google Scholar]
- Zhang, Y. Application of polyglutamic acid in heavy metal removal from aqueous solutions: A review. Chemosphere 2015, 134, 273–282. [Google Scholar]
- Shih, I.L.; Van, Y.T. The production and characterization of poly-γ-glutamic acid from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Singhal, R.S. Poly (γ-glutamic acid)—An emerging biopolymer of commercial interest. Bioresour. Technol. 2011, 102, 5551–5561. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2014, 49, 750–759. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, E.A. Remediation and detection techniques for heavy metals in the environment. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 205–222. [Google Scholar]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef] [PubMed]
- Bontà, V.; Calvio, C. Poly-γ-Glutamic Acid and Its Application in Bioremediation: A Critical Review. In Modern Approaches in Waste Bioremediation: Environmental Microbiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 211–225. [Google Scholar]
- Ogunleye, A.O. Bacterial Poly-gamma-glutamic Acid (γ-PGA): A Promising Biosorbent of Heavy Metals. Ph.D. Thesis, University of Wolverhampton, Wolverhampton, UK, 2015. [Google Scholar]
- Pang, X.; Lei, P.; Feng, X.; Xu, Z.; Xu, H.; Liu, K. Poly-γ-glutamic acid, a bio-chelator, alleviates the toxicity of Cd and Pb in the soil and promotes the establishment of healthy Cucumis sativus L. seedling. Environ. Sci. Pollut. Res. 2018, 25, 19975–19988. [Google Scholar] [CrossRef]
- Sharma, M.; Tellili, N.; Kacem, I.; Rouissi, T. Microbial biopolymers: From production to environmental applications—A review. Appl. Sci. 2024, 14, 5081. [Google Scholar] [CrossRef]
- Schill, S.; Stessl, B.; Meier, N.; Tichy, A.; Wagner, M.; Ludewig, M. Microbiological Safety and Sensory Quality of Cultivated Mushrooms (Pleurotus eryngii, Pleurotus ostreatus and Lentinula edodes) at Retail Level and Post-Retail Storage. Foods 2021, 10, 816. [Google Scholar] [CrossRef]
- Doran, G.; Sheridan, F.; DeLappe, N.; O’ Hare, C.; Anderson, W.; Corbett-Feeney, G.; Cormican, M. Salmonella enterica serovar Kedougou contamination of commercially grown mushrooms. Diagn. Microbiol. Infect. Dis. 2005, 51, 73–76. [Google Scholar] [CrossRef]
- Jiang, H.; Miraglia, D.; Ranucci, D.; Donnini, D.; Roila, R.; Branciari, R.; Li, C. High microbial loads found in minimally-processed sliced mushrooms from Italian market. Ital. J. Food Saf. 2018, 7, 7000. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Le Port, G.; Gormley, R. Post-harvest treatment with citric acid or hydrogen peroxide to extend the shelf life of fresh sliced mushrooms. Lebensm Wiss Technol. 2000, 33, 285–289. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Del-Toro-Sánchez, L.; Alvarez-Parilla, E.; González-Aguilar, G.A. High relative humidity inpackage of fresh-cut fruits and vegetables: Advantage or disadvantage considering microbiological problems and antimicrobial delivering systems? J. Food. Sci. 2008, 73, 41–47. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh minimally-processed fruit and vegetable, and sprouts from retail estrablishment. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef]
- Öncül, N.; Çiftçi, M. Microbiological quality of some fresh wild edible mushrooms. Food Health 2023, 9, 323–330. [Google Scholar] [CrossRef]
- Chikthimmah, N.; Beelman, R.B. Microbial spoilage of fresh mushrooms. In Microbiology of Fruits and Vegetables; Sapers, G.M., Gorny, J.R., Yousef, A.E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 135–158. ISBN 0-8493-2261-8. [Google Scholar]
- Su, C.; Tseng, C.; Wu, S.; Shih, B.; Chen, Y.; Fang, H. Poly-Gamma-Glutamic Acid Functions as an Effective Lubricant with Antimicrobial Activity in Multipurpose Contact Lens Care Solutions. Polymers 2019, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Ajayeoba, T.; Dula, S.; Ijabadeniyi, O. Properties of Poly-γ-Glutamic Acid Producing- Bacillus Species Isolated from Ogi Liquor and Lemon-Ogi Liquor. Front. Microbiol. 2019, 16, 771. [Google Scholar] [CrossRef]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected edible medicinal mushrooms from Pleurotus genus as an answer for human civilization diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Guo, Y.; Ding, S.; Chen, G.; Liang, Z.; Zeng, W. An integrated strategy for recovery and purification of poly-γ-glutamic acid from fermentation broth and its techno-economic analysis. Sep. Purif. Technol. 2021, 278, 119575. [Google Scholar] [CrossRef]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiol. 2015, 161, 1–17. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Wang, S. Statistical optimization of fermentation conditions for γ-polyglutamic acid production by Bacillus subtilis NX-2. J. Microbiol. Biotechnol. 2019, 29, 565–573. [Google Scholar]
- Arvidson, K.; Johasson, E.G. Galvanic current between dental alloys in vitro. Scand. J. Dent. Res. 1985, 93, 467–473. [Google Scholar] [CrossRef]
- Neumann, M.; Goderska, K.; Grajek, K.; Grajek, W. Modele przewodu pokarmowego in vitro do badań nad biodostępnością składników odżywczych. Żywność Nauka Technol. Jakość 2006, 13, 30–45. [Google Scholar]
- Opoka, W.; Muszyńska, B.; Rojowski, J.; Rumian, J. Gastroel–2014. Poland Patent Application P 417238, 18 May 2016. [Google Scholar]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized linear models. J. R. Stat. Soc. Ser. A 1972, 135, 370–384. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 1989. [Google Scholar]
- Byers, S. Biostatistics with R: An Introduction to Statistics Through Biological Data; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models, 3rd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Javaid, A.; Bajwa, R.; Shafique, U.; Anwar, J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenergy 2011, 35, 1675–1682. [Google Scholar] [CrossRef]
- Zaidi, A.; Oves, M.; Ahmad, E.; Khan, M.S. Importance of free-living fungi in heavy metal remediation. In Biomanagement of Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2011; pp. 479–494. [Google Scholar]
- Zhang, D.; Frankowska, A.; Jarzyńska, G.; Kojta, A.K.; Drewnowska, M.; Wydmańska, D.; Falandysz, J. Metals of King Bolete (Boletus edulis) Bull.: Fr. collected at the same site over two years. Afr. J. Agric. Res. 2010, 5, 3050–3055. [Google Scholar]
- Falandysz, J. Nutritional and other trace elements and their associations in raw King Bolete mushrooms, Boletus edulis. Int. J. Environ. Res. Public Health 2021, 19, 417. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Frankowska, A.; Jarzyńska, G.; Dryżałowska, A.; Kojta, A.K.; Zhang, D. Survey on composition and bioconcentration potential of 12 metallic elements in King Bolete (Boletus edulis) mushroom that emerged at 11 spatially distant sites. J. Environ. Sci. Health. 2011, 46, 231–246. [Google Scholar] [CrossRef]
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) glutamic acid: A unique microbial biopolymer with diverse commercial applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef]
- Najar, I.N.; Das, S. Poly-glutamic acid (PGA)-Structure, synthesis, genomic organization and its application: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 2258. [Google Scholar]
- Zhou, K.; Yin, D.; Liu, C.; Sun, R. Investigating the role of poly-γ-glutamic acid in Pennisetum giganteum phytoextraction of mercury-contaminated soil. Sci. Total Environ. 2024, 944, 173707. [Google Scholar] [CrossRef]
- Muszyńska, B.; Rojowski, J.; Łazarz, M.; Kała, K.; Dobosz, K.; Opoka, W. The accumulation and release of Cd and Pb from edible mushrooms and their biomass. Pol. J. Environ. Stud. 2018, 27, 223–230. [Google Scholar] [CrossRef]
- Krakowska, A.; Reczyński, W.; Krakowski, T.; Szewczyk, K.; Opoka, W.; Muszyńska, B. A new biotechnology method of bioelements’ accumulation monitoring in in vitro culture of Agaricus bisporus. Molecules 2020, 26, 5165. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Krakowska, A.; Sułkowska-Ziaja, K.; Suchanek, M.; Zięba, P.; Opoka, W.; Muszyńska, B. Pleurotus spp. Mycelia Enriched in Magnesium and Zinc Salts as a Potential Functional Food. Molecules 2020, 26, 162. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Liu, Y. Advances in microbial production of poly-γ-glutamic acid: Strategies and applications. Microorganisms 2023, 11, 917. [Google Scholar]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible mushrooms. Trends Food Sci. Technol. 2012, 23, 430–442. [Google Scholar]
- Wasser, S.P. Medicinal mushroom science: History, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms 2014, 16, 1–16. [Google Scholar] [CrossRef]
- Tiong, S.P.; Liew, W.P.; Loo, Y.Y. Food safety concerns of edible mushrooms: A review. Food Sci. Nutr. 2020, 8, 2303–2316. [Google Scholar]
- Li, W. Biocontrol potential of γ-polyglutamic acid against foodborne pathogens in fresh produce. Food Control 2023, 142, 109163. [Google Scholar]
- Lambert, P.A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 2005, 98, S16–S21. [Google Scholar]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 137–145. [Google Scholar] [CrossRef]
- Sharma, S. Microbial contamination of edible mushrooms: A review. Food Microbiol. 2014, 88, 101621. [Google Scholar]
- FDA Food Safety Modernization Act (FSMA); U.S. Food & Drug Administration: Silver Spring, MD, USA, 2011.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on heavy metals in food. EFSA J. 2009, 7, 1570. [Google Scholar]
- Kahl, S. Consumer trends towards natural food preservatives and implications for microbiological safety. J. Food Prot. 2020, 83, 1457–1469. [Google Scholar]
- OECD Guidelines for the Testing of Chemicals. 2023. Available online: https://www.oecd.org/en/topics/sub-issues/testing-of-chemicals/test-guidelines.html (accessed on 25 July 2025).
- Codex Alimentarius Commission. Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for Its Application; Codex Alimentarius Commission: Rome, Italy, 2003. [Google Scholar]
- Yang, L. Biopolymers as food additives in microbial control. Food Control 2021, 124, 107896. [Google Scholar]
- FSANZ Standards. Food Safety Regulations, Australia New Zealand Food Standards Code; FSANZ Standards: Majura Park, Australia, 2023. [Google Scholar]
- FAO. Sustainable food systems and microbial safety: Regulatory perspectives. FAO Food Saf. Rev. 2022, 18, 1–27. [Google Scholar]
- EMA. Guideline on the Safety Assessment of Natural Polymers in Food; European Medicines Agency: Amsterdam, The Netherlands, 2024. [Google Scholar]

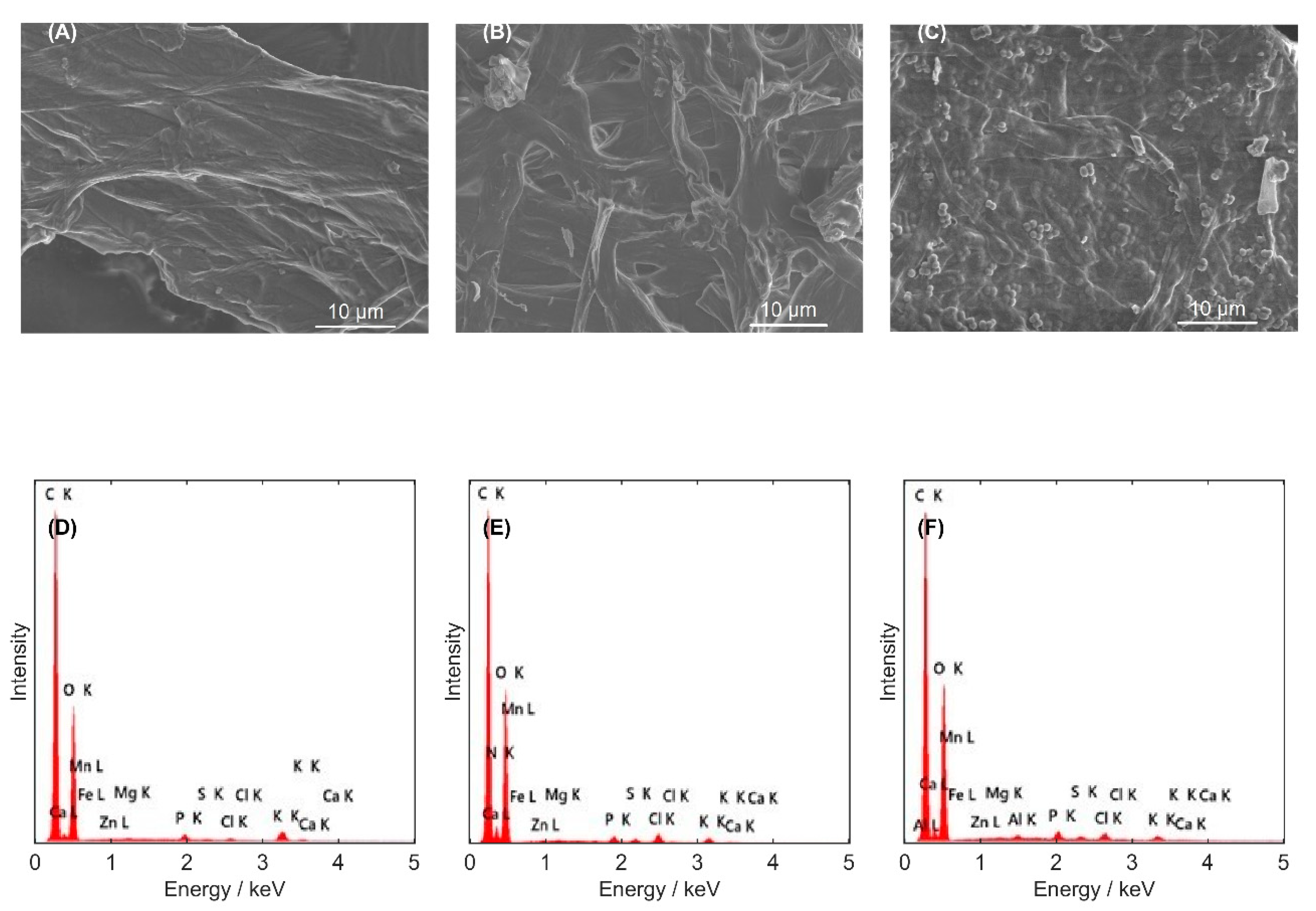

| Type of Biomaterials/Parameters | Ab | Be | Po | γ-PGA |
|---|---|---|---|---|
| Vmicro, cm3/g | 0.0020 | 0.0001 | 0.0028 | 0.031 |
| Smicro, m2/g | 3.5 | 0.4 | 4.6 | 8.1 |
| Apore, nm | 8 | 6 | 13 | 15 |
| df | Walds Stat. | p | |
|---|---|---|---|
| Lead | |||
| Intercept | 1 | 29,449.37 | 0.000 |
| Mushroom species | 2 | 19.34 | 0.000 |
| PGA conc | 3 | 1925.94 | 0.000 |
| Mushroom species*PGA conc | 6 | 46.2 | 0.000 |
| Cadmium | |||
| Intercept | 1 | 23,071.05 | 0.000 |
| Mushroom species | 3 | 1359.29 | 0.000 |
| PGA conc | 2 | 17.55 | 0.000 |
| Mushroom species*PGA conc | 6 | 24.27 | 0.000 |
| Lead mix | |||
| Intercept | 1 | 27,834.16 | 0.000 |
| Mushroom species | 3 | 1703.95 | 0.000 |
| PGA conc | 2 | 13.42 | 0.001 |
| Mushroom species*PGA conc | 6 | 26.77 | 0.000 |
| Cadmium mix | |||
| Intercept | 1 | 9910.183 | 0.000 |
| Mushroom species | 3 | 816.978 | 0.000 |
| PGA conc | 2 | 34.887 | 0.000 |
| Mushroom species*PGA conc | 6 | 11.912 | 0.044 |
| Effect | df | Wald’s Stat. | p |
|---|---|---|---|
| Intercept | 1 | 46,139.6 | 0 |
| Pathogen | 1 | 0.8 | 0.371141 |
| Fungi | 3 | 2453.7 | 0 |
| γ-PGA | 1 | 4.27 | 0.038761 |
| Pathogen*Fungi | 3 | 168.83 | 0 |
| Pathogen*PGA | 1 | 20.93 | 0.000005 |
| Fungi*PGA | 2 | 629.36 | 0 |
| Pathogen*Fungi*PGA | 2 | 40.29 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowska, A.; Skiba-Kurek, I.; Suchanek, M.; Zontek-Wilkowska, J.; Muszyńska, B.; Skalski, T. Gamma-Polyglutamic Acid Reduces Heavy Metal Uptake and Stabilize Microbial Biosafety in Edible Mushroom Cultures. Appl. Sci. 2025, 15, 10311. https://doi.org/10.3390/app151910311
Krakowska A, Skiba-Kurek I, Suchanek M, Zontek-Wilkowska J, Muszyńska B, Skalski T. Gamma-Polyglutamic Acid Reduces Heavy Metal Uptake and Stabilize Microbial Biosafety in Edible Mushroom Cultures. Applied Sciences. 2025; 15(19):10311. https://doi.org/10.3390/app151910311
Chicago/Turabian StyleKrakowska, Agata, Iwona Skiba-Kurek, Małgorzata Suchanek, Joanna Zontek-Wilkowska, Bożena Muszyńska, and Tomasz Skalski. 2025. "Gamma-Polyglutamic Acid Reduces Heavy Metal Uptake and Stabilize Microbial Biosafety in Edible Mushroom Cultures" Applied Sciences 15, no. 19: 10311. https://doi.org/10.3390/app151910311
APA StyleKrakowska, A., Skiba-Kurek, I., Suchanek, M., Zontek-Wilkowska, J., Muszyńska, B., & Skalski, T. (2025). Gamma-Polyglutamic Acid Reduces Heavy Metal Uptake and Stabilize Microbial Biosafety in Edible Mushroom Cultures. Applied Sciences, 15(19), 10311. https://doi.org/10.3390/app151910311






