Study of Interface Adhesion Between Polyurethane and Aggregate Based on Surface Free Energy Theory and Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
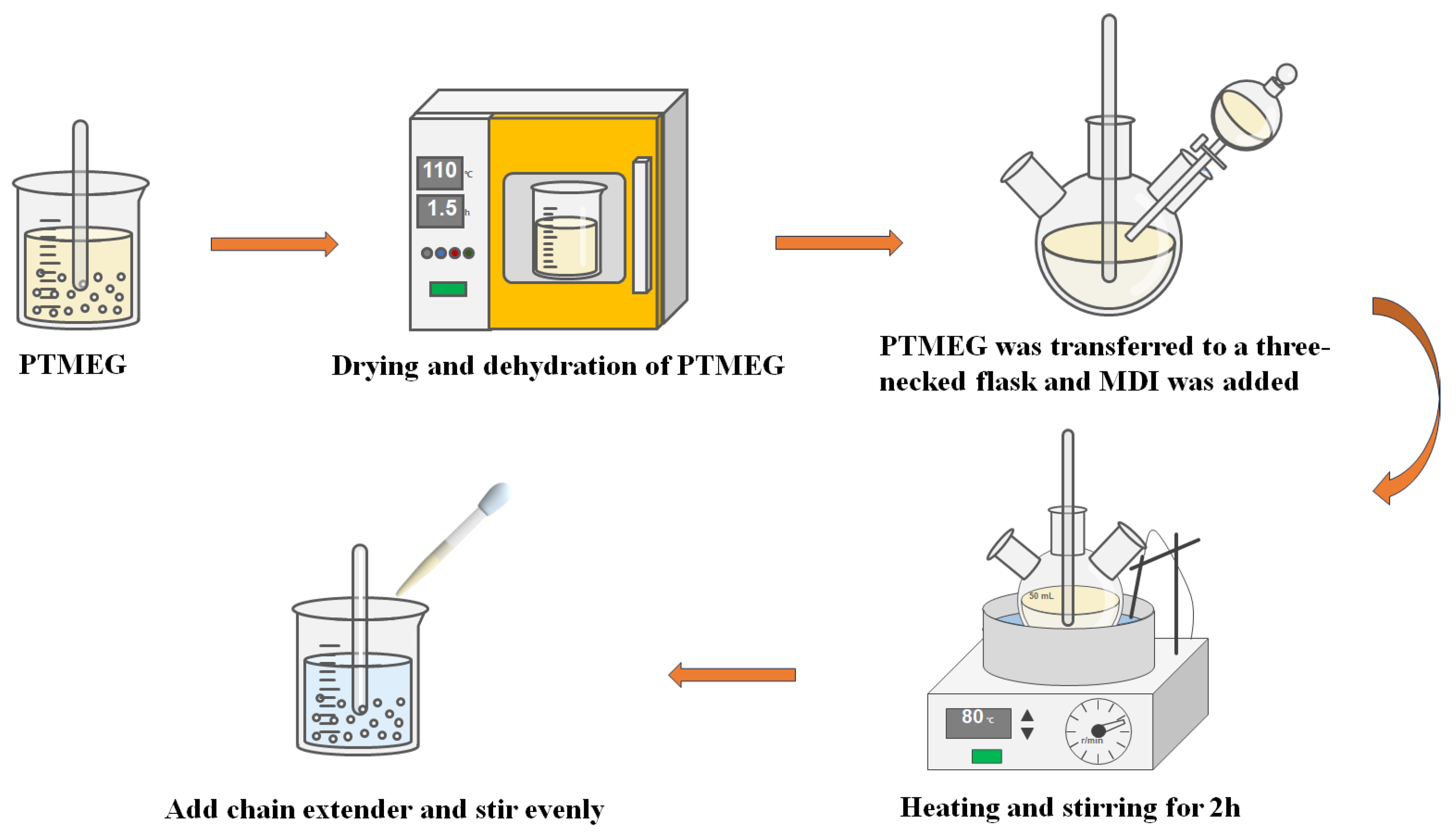
2.2. Synthesis of PU
2.3. Contact Angle Test
2.4. Calculation of Surface Free Energy
2.5. Calculation of Adhesion Work and Spalling Work
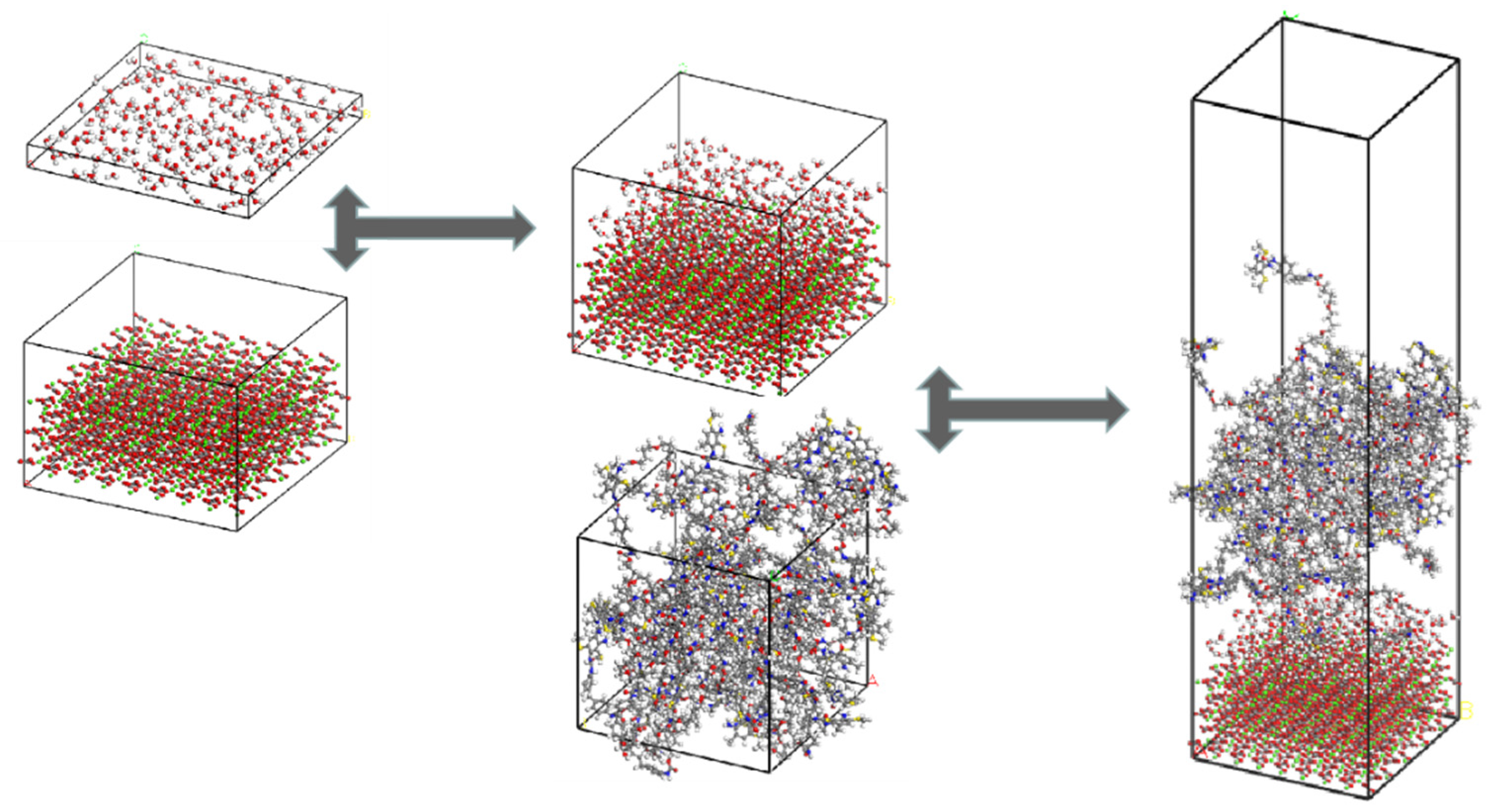
2.6. Construction and Validation of Molecular Models
2.6.1. Molecular Model of PU
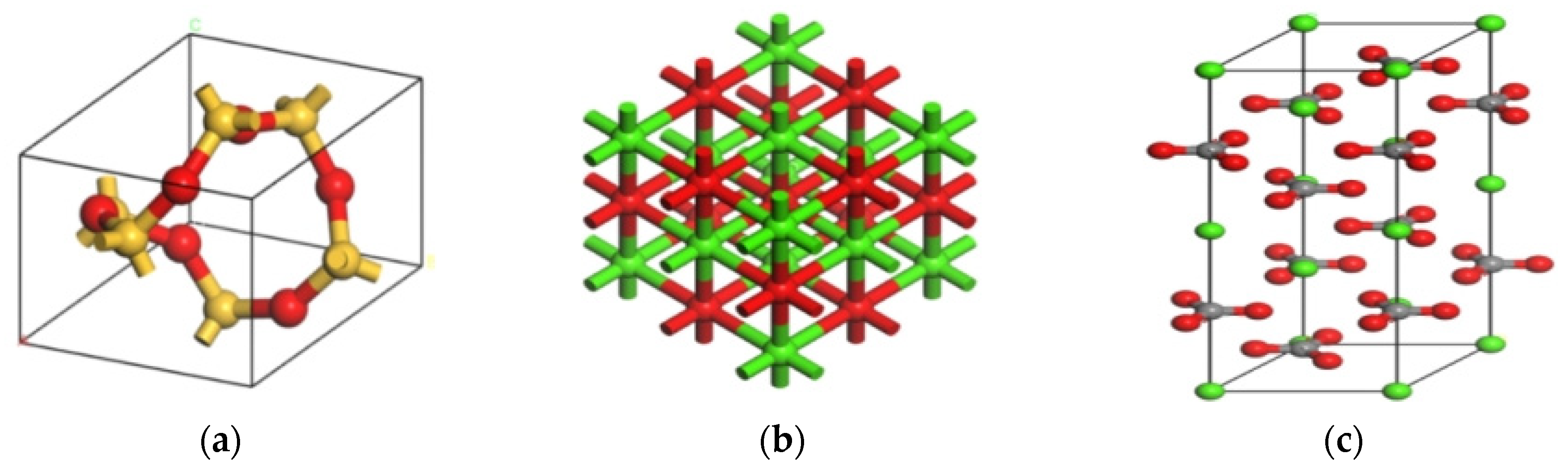
2.6.2. Molecular Model of Aggregate
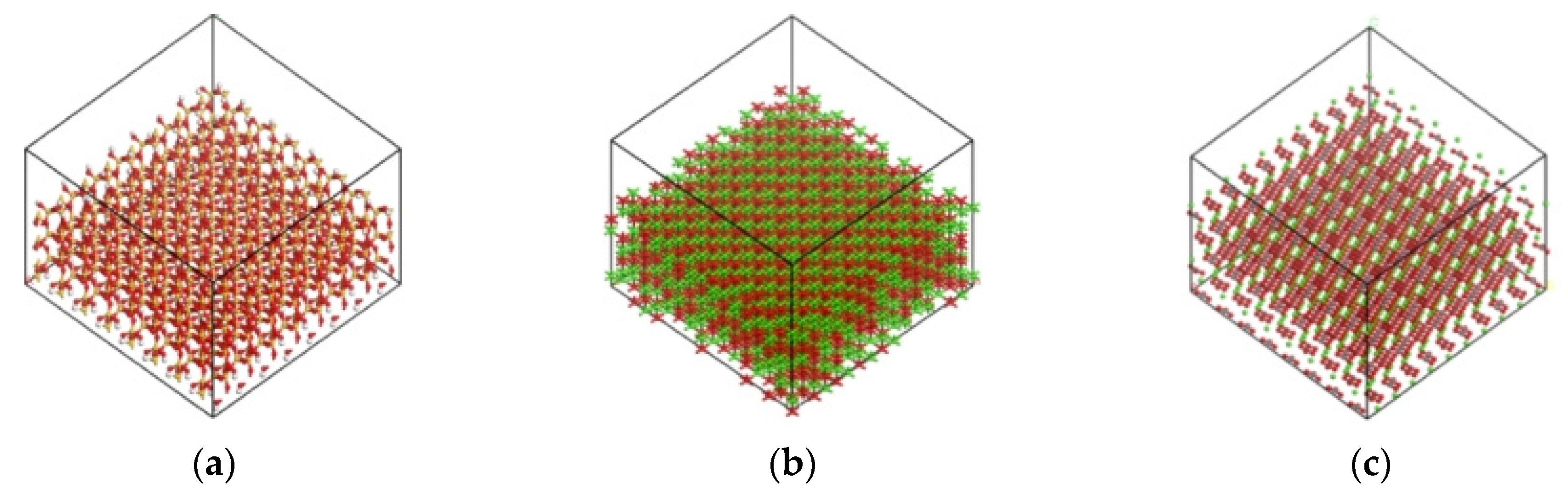
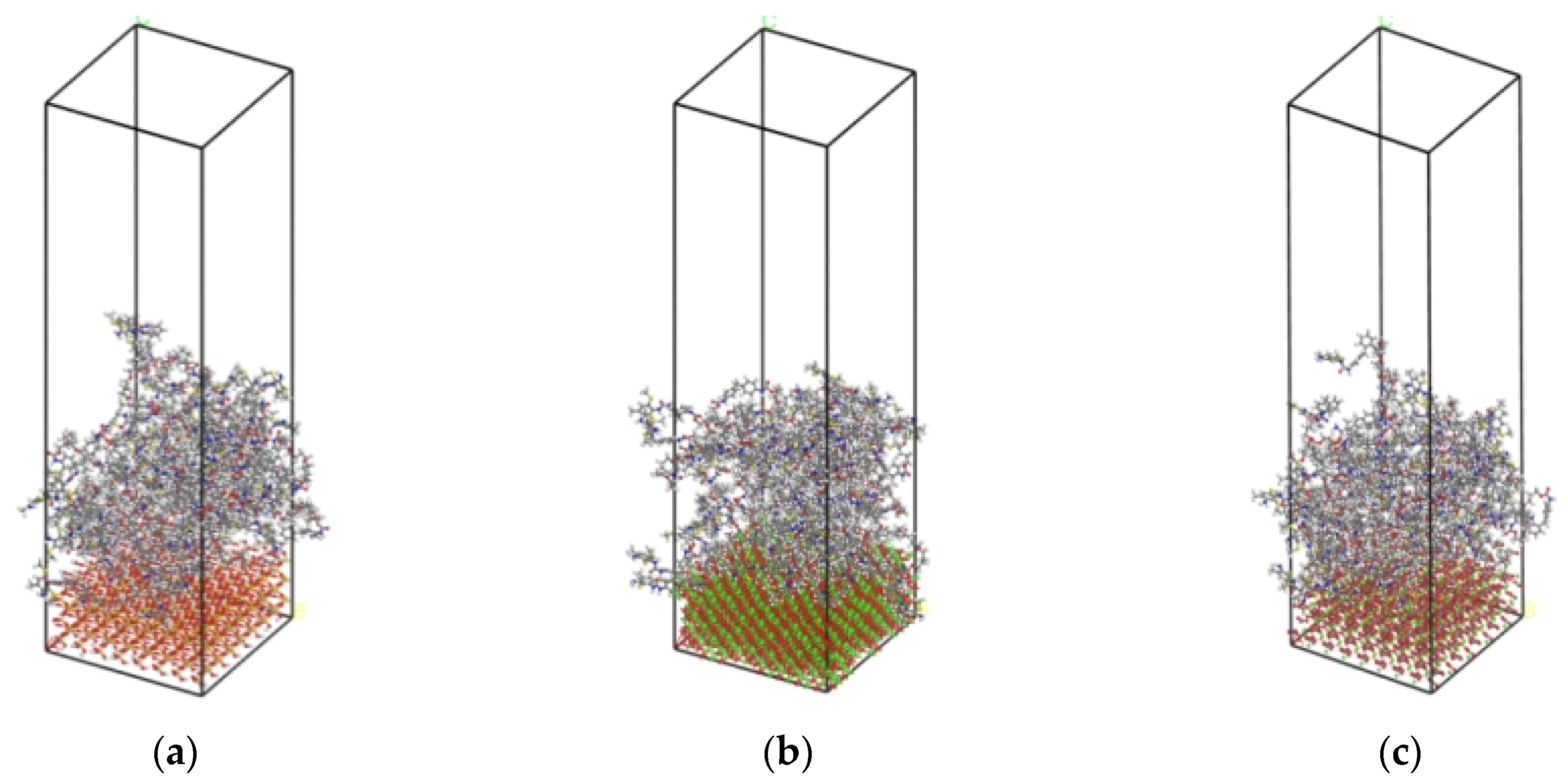
2.6.3. Interface Model of PU–Aggregate
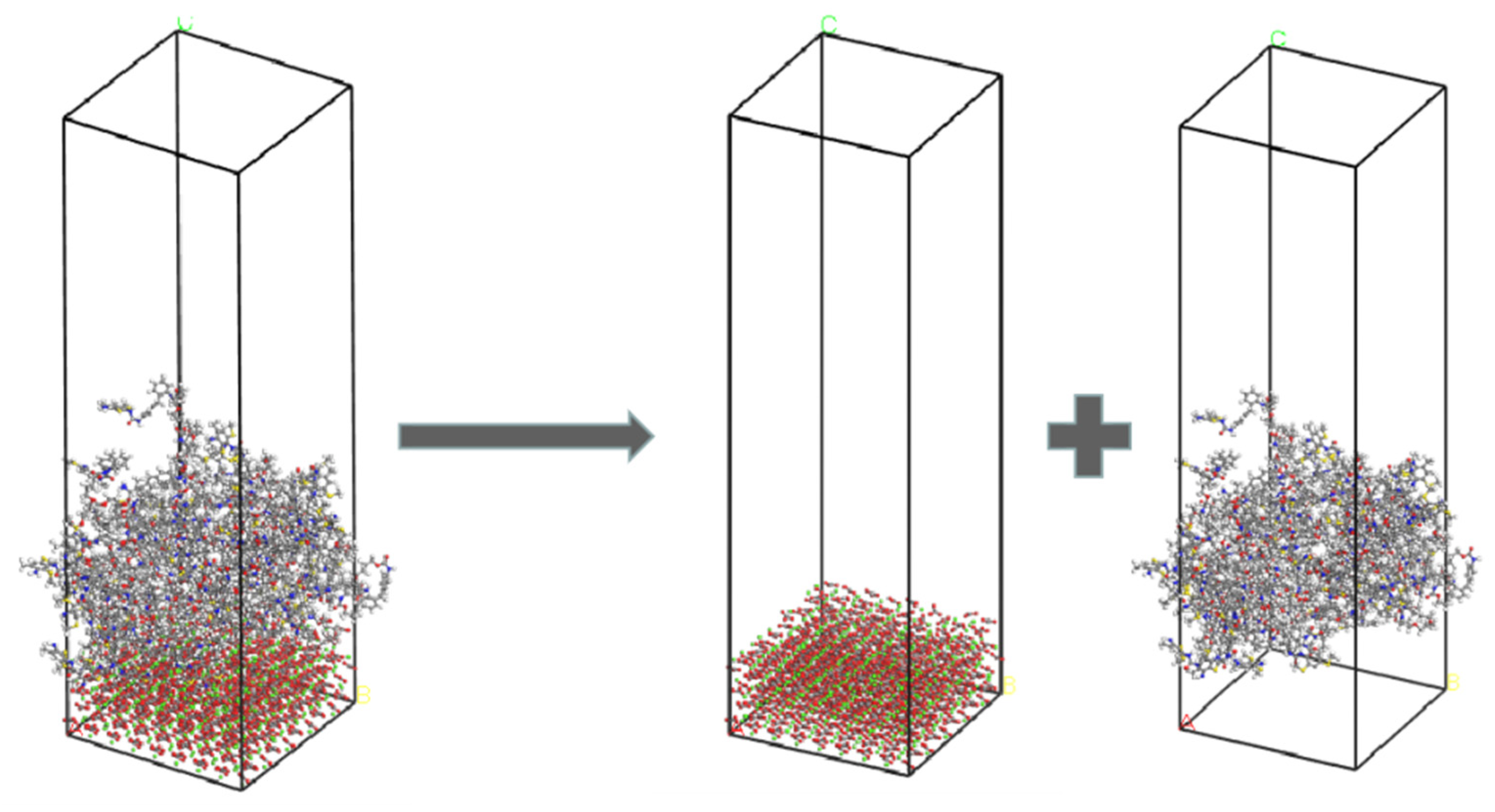
2.6.4. Calculation of Interfacial Interaction Energy
2.7. Model Validation
2.7.1. MSD
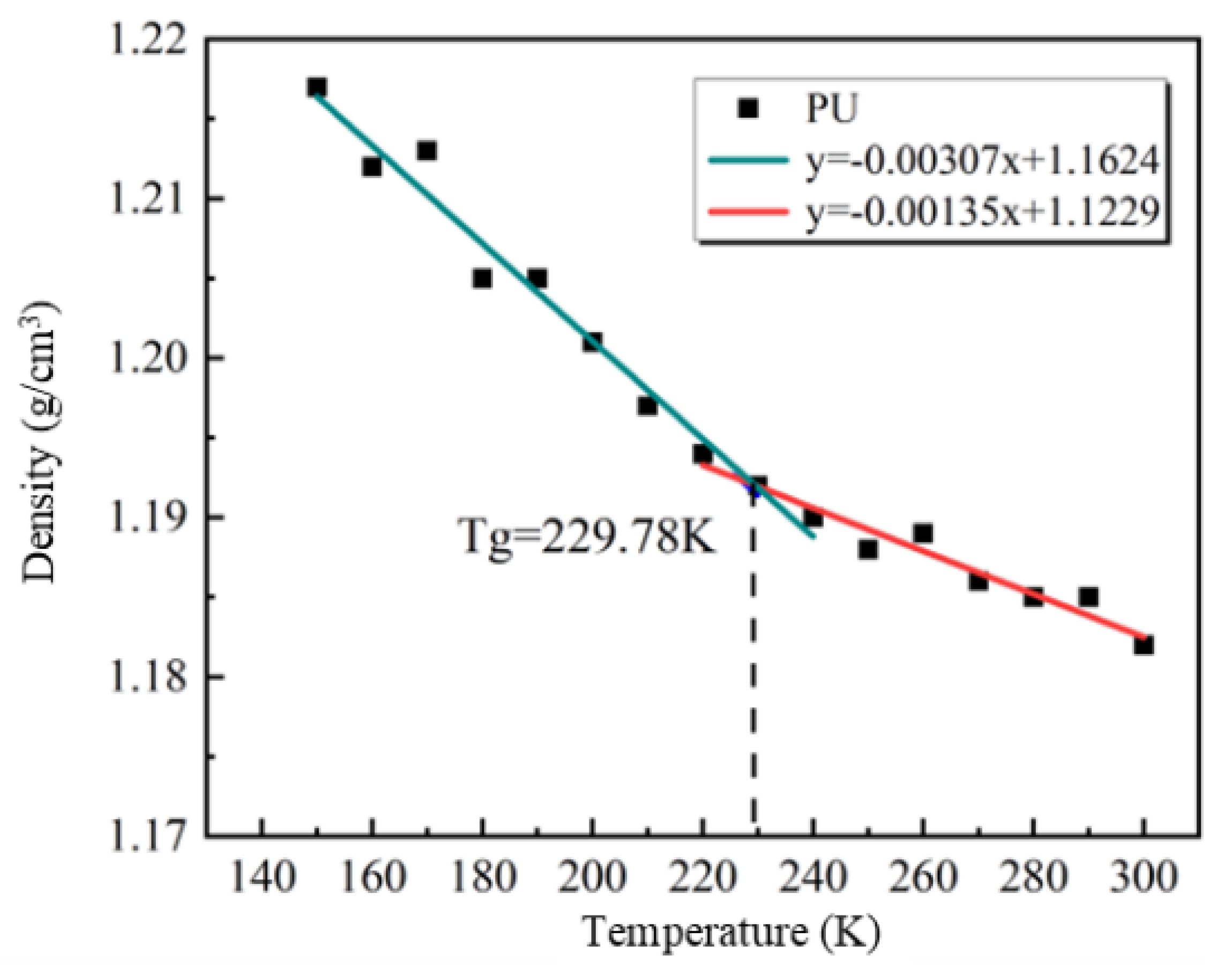
2.7.2. Glass Transition Temperature (Tg)
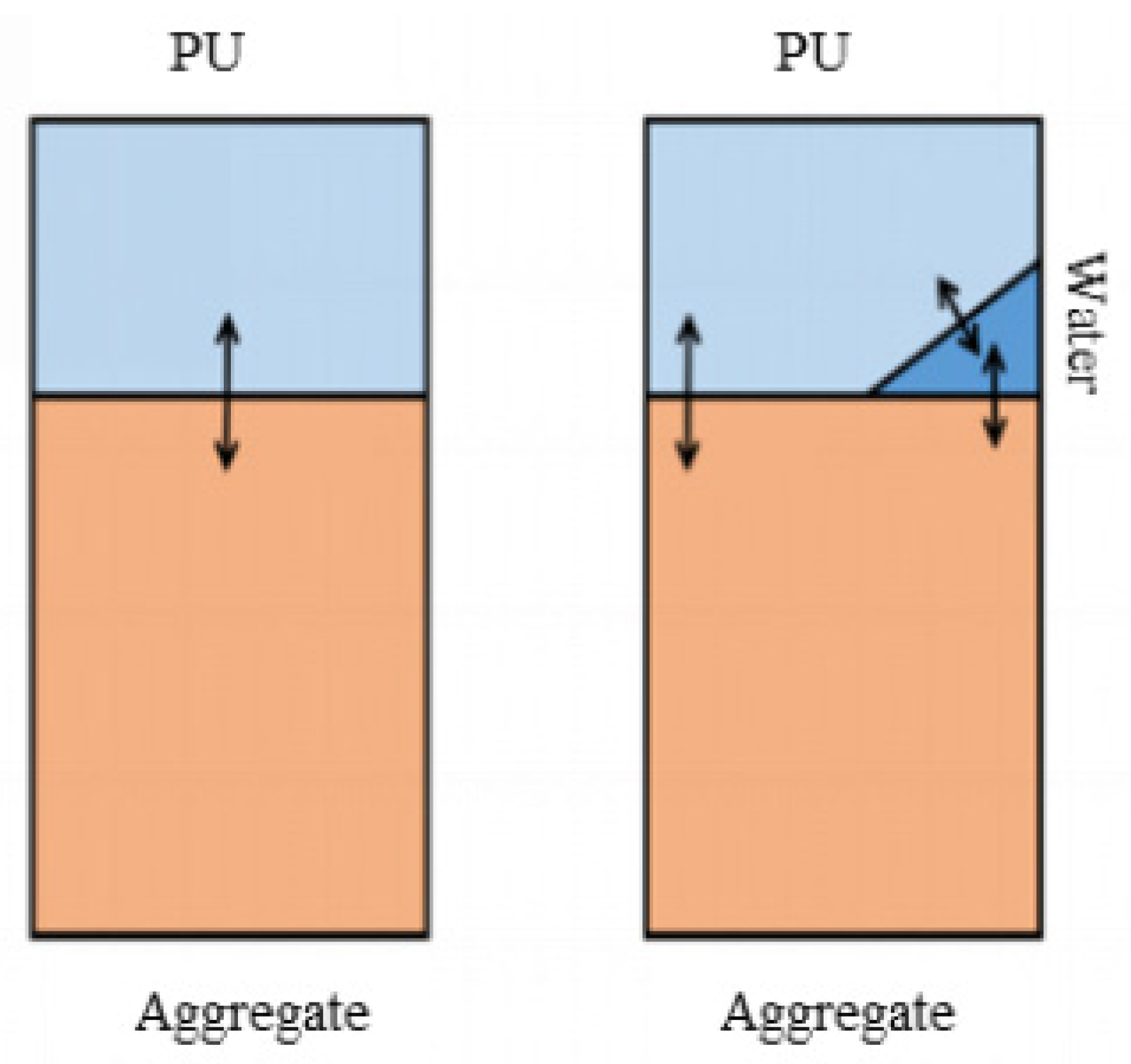
2.8. Water Boiling Method and Water Immersion Method
3. Results and Discussions
3.1. Contact Angle
3.1.1. Contact Angles of PU
3.1.2. Contact Angle of Aggregates
3.2. Surface Free Energy
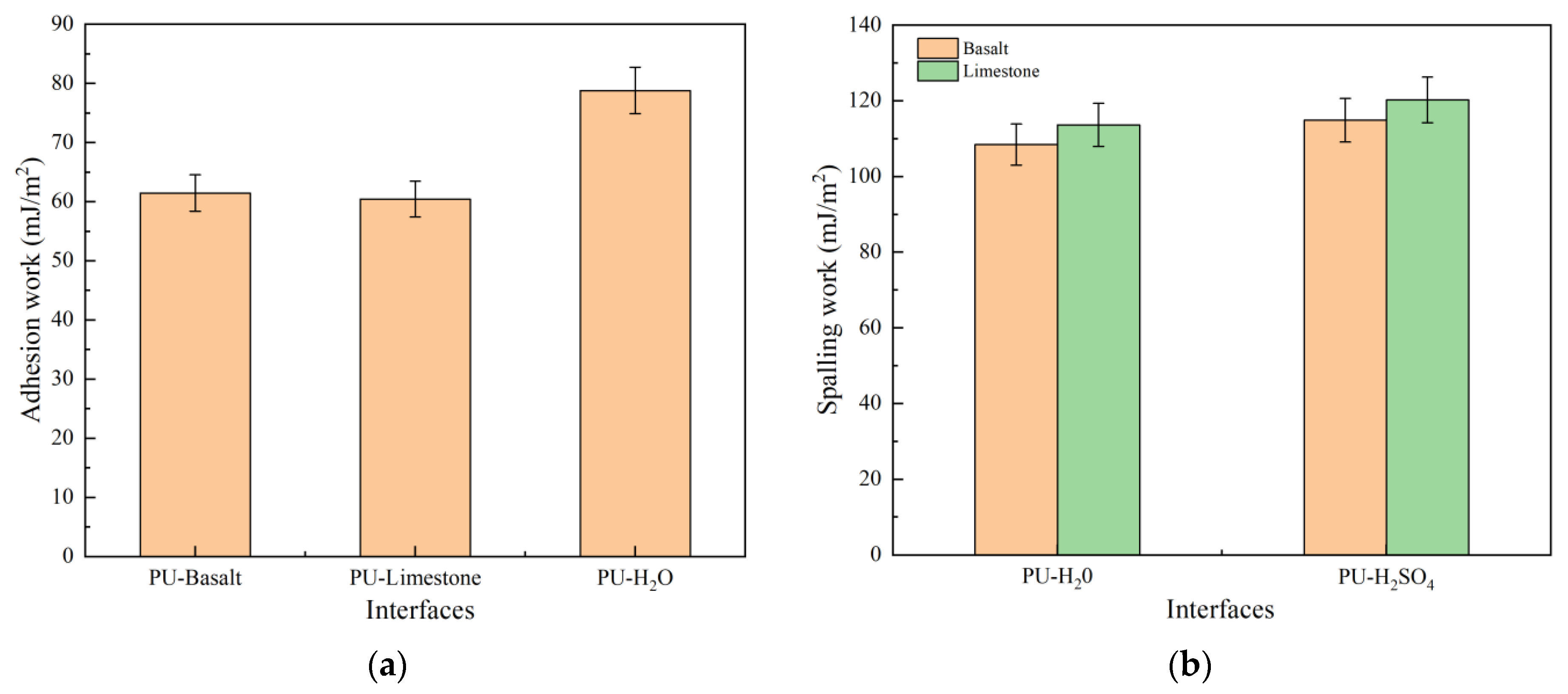
3.3. Adhesion Work and Spalling Work
3.4. Results of Molecular Dynamics Simulation
3.4.1. Interaction Energy
3.4.2. Adhesion Work and Spalling Work
3.5. Results of the Water Boiling Method and Water Immersion Method
4. Conclusions
- (1)
- The surface free energy of PU showed a polarity similar to that of the aggregate, indicating good adhesion between PU and aggregate. However, the surface free energy of HPU was lower than that of PU, suggesting that adhesion between PU and aggregate decreases under acidic conditions.
- (2)
- Interaction energies between PU, H2O, and H2SO4 with the three minerals were calculated through MD simulation. In terms of aggregate type, the adhesion of PU with basalt was found to be superior to that with limestone, and limestone exhibited stronger hydrophilicity.
- (3)
- The adhesion work and spalling work obtained by surface free energy theory and molecular dynamics calculations have the same trend, and the results of the adhesion work and spalling work fully indicate that the adhesion properties of PU with basalt are better than with limestone. While the adhesion properties of PU with limestone and basalt decreased under acidic conditions, the adhesion properties of PU with basalt were better than those of limestone, indicating that the combination of PU–basalt is more suitable for acid rain-prone areas. In addition, the water boiling method and water immersion method verified the rationality of the PU–basalt combination.
- (4)
- The surface free energy measurement based on the contact angle test and MD simulation provides a multiscale perspective on the adhesion properties between PU and aggregate, enhancing the feasibility of applying permeable PU mixtures. The study of factors affecting PU–aggregate adhesion properties contributes to the further optimization of PU mixture formulations. Moreover, the calculation results of the surface free energy method and MD simulation showed good correlation and were consistent with macroscopic performance tests, which can be extended to the study of the adhesion properties of other materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Li, T.; Peng, H.Y. A new structure of permeable pavement for mitigating urban heat island. Sci. Total Environ. 2018, 634, 1119–1125. [Google Scholar] [CrossRef]
- Chen, J.Q.; Chu, R.X.; Wang, H.; Zhang, L.C.; Chen, X.D.; Du, Y.F. Alleviating urban heat island effect using high-conductivity permeable concrete pavement. J. Clean Prod. 2019, 237, 117722. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, X. Preparation and Component Optimization of Resin-Based Permeable Brick. Materials 2020, 13, 2701. [Google Scholar] [CrossRef]
- Zhou, J.J.; Pang, Y.L.; Du, W.; Huang, T.Y.; Wang, H.; Zhou, M.L.; Liu, J.H. Review of the development and research of permeable pavements. Hydrol. Process. 2024, 38, e15179. [Google Scholar] [CrossRef]
- Cai, J.; Song, C.; Zhou, B.C.; Tian, Y.F.; Li, R.; Zhang, J.P.; Pei, J.Z. Investigation on high-viscosity asphalt binder for permeable asphalt concrete with waste materials. J. Clean Prod. 2019, 228, 40–51. [Google Scholar] [CrossRef]
- An, Y.; Du, L.Z.; Dai, W.T.; Zhang, K.; Huang, M.X.; Guo, W. Siltation behavior characterization and evaluation of permeable asphalt mixture based on computed tomography method. Constr. Build. Mater. 2024, 411, 134360. [Google Scholar] [CrossRef]
- Lian, C.; Zhuge, Y. Optimum mix design of enhanced permeable concrete—An experimental investigation. Constr. Build. Mater. 2010, 24, 2664–2671. [Google Scholar] [CrossRef]
- Kia, A.; Delens, J.M.; Wong, H.S.; Cheeseman, C.R. Structural and hydrological design of permeable concrete pavements. Case Stud. Constr. Mater. 2021, 15, e00564. [Google Scholar] [CrossRef]
- Yang, Z.H.; Wang, L.B.; Xu, B.; Li, S.L.; Cao, D.W.; Yuan, B.; Dong, R. Study of the Durability of a Fully Permeable Asphalt Pavement Structure Based on the Accelerated Pavement Test Method under Saturated Conditions. J. Transp. Eng. Part B Pavements 2022, 148, 04021081. [Google Scholar] [CrossRef]
- Kia, A.; Wong, H.S.; Cheeseman, C.R. High-strength clogging resistant permeable pavement. Int. J. Pavement Eng. 2021, 22, 271–282. [Google Scholar] [CrossRef]
- Kia, A.; Wong, H.S.; Cheeseman, C.R. Freeze-Thaw Durability of Conventional and Novel Permeable Pavement Replacement. J. Transp. Eng. Part B Pavements 2022, 148, 132767. [Google Scholar] [CrossRef]
- Kia, A. Freeze-thaw durability of air-entrained high-strength clogging resistant permeable pavements. Constr. Build. Mater. 2023, 400, 04022051. [Google Scholar] [CrossRef]
- Gao, Y.; Romero, P.; Zhang, H.L.; Huang, M.; Lai, F. Unsaturated polyester resin concrete: A review. Constr. Build. Mater. 2019, 228, e49008. [Google Scholar] [CrossRef]
- Zhao, B.W.; Du, Y.B.; Ren, L.Y.; Wang, J.Y. Preparation and performance of epoxy resin permeable bricks for sponge city construction. J. Appl. Polym. Sci. 2020, 137, 116709. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Sun, Q.S.; Ding, H.J.; Jia, D.Z.; Li, C.W. Effect of binder-aggregate ratio on the mechanical and functional properties of porous polyurethane cement mixture. Int. J. Pavement Eng. 2022, 23, 5101–5117. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Liu, H.; Ban, X.Y.; Liu, X.S.; Guo, Y.X.; Sun, J.; Liu, Y.Q.; Zhang, S.Y.; Lei, J.H. Thermosetting resin modified asphalt: A comprehensive review. J. Traffic Transp. Eng.-Engl. Ed. 2023, 10, 1001–1036. [Google Scholar] [CrossRef]
- Cong, L.; Yang, F.; Guo, G.H.; Ren, M.D.; Shi, J.C.; Tan, L. The use of polyurethane for asphalt pavement engineering applications: A state-of-the-art review. Constr. Build. Mater. 2019, 225, 1012–1025. [Google Scholar] [CrossRef]
- Li, X.R.; Li, J.; Wang, J.Y.; Yuan, J.; Jiang, F.; Yu, X.Y.; Xiao, F.P. Recent applications and developments of Polyurethane materials in pavement engineering. Constr. Build. Mater. 2021, 304, 124639. [Google Scholar] [CrossRef]
- Liu, X.S.; Zhang, Z.P.; Luo, Y.; Sun, J.; Tian, P.J.; Liu, H.; Mao, Z.Y.; Kan, S.Y.; Yu, X.H. Curing characteristics and performance of a bio-based polyurethane engineered sealant with high bonding strength: Effect of R value and chain extender content. Constr. Build. Mater. 2024, 432, 136684. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Sun, J.; Jia, M.; Qi, B.; Zhang, H.L.; Lv, W.J.; Mao, Z.Y.; Chang, P.T.; Peng, J.; Liu, Y.C. Study on a thermosetting polyurethane modified asphalt suitable for bridge deck pavements: Formula and properties. Constr. Build. Mater. 2020, 241, 118122. [Google Scholar] [CrossRef]
- Huang, G.; Yang, T.H.; He, Z.Y.; Yu, L.; Xiao, H.X. Polyurethane as a modifier for road asphalt: A literature review. Constr. Build. Mater. 2022, 356, 129058. [Google Scholar] [CrossRef]
- Sun, M.; Zheng, M.L.; Qu, G.Z.; Yuan, K.; Bi, Y.F.; Wang, J. Performance of polyurethane modified asphalt and its mixtures. Constr. Build. Mater. 2018, 191, 386–397. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Yu, X.H.; Wang, Z.F.; Zhang, S.Y.; Wang, Y.X.; Liu, H.; Kan, S.Y.; Ji, J.J.; Shi, E. MDI-PTMG-based TPU modified asphalt: Preparation, rheological properties and molecular dynamics simulation. Fuel 2025, 379, 133007. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Huang, T.; Sun, J.; Wang, Z.F.; Wang, L.; Li, X.; Liu, H.; Zhang, D.L. Laboratory Study and Molecular Dynamics Simulation of High- and Low-Temperature Properties of Polyurethane-Modified Asphalt. J. Mater. Civ. Eng. 2023, 35, 04023257. [Google Scholar] [CrossRef]
- Zhong, K.; Li, S.L.; Sun, M.Z. Research Status Review of Water Stability for Polyurethane Mixtures. J. Highw. Transp. Res. Dev. Engl. Ed. 2022, 16, 1–6. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.Z.; Duan, M.X.; Ji, J.; Xu, S.F. Compaction Characteristics of Single-Component Polyurethane Mixtures. J. Mater. Civ. Eng. 2021, 33, 04021221. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, L.; Lv, X.P.; Chou, Z.J.; Li, W.J.; Ji, J.; Xu, S.F. Improvement of Water Stability of Macroporous Polyurethane Mixture. J. Mater. Civ. Eng. 2022, 34, 04022285. [Google Scholar] [CrossRef]
- Xu, J.Y.; Ma, B.; Mao, W.J.; Si, W.; Wang, X.Q. Review of interfacial adhesion between asphalt and aggregate based on molecular dynamics. Constr. Build. Mater. 2023, 362, 129642. [Google Scholar] [CrossRef]
- Elphingstone, G.M. Adhesion and Cohesion in Asphalt-Aggregate Systems; Texas A&M University: College Station, TX, USA, 1997. [Google Scholar]
- Moraes, R.; Velasquez, R.; Bahia, H. Using bond strength and surface energy to estimate moisture resistance of asphalt-aggregate systems. Constr. Build. Mater. 2017, 130, 156–170. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Guo, M. Using surface free energy method to study the cohesion and adhesion of asphalt mastic. Constr. Build. Mater. 2013, 47, 254–260. [Google Scholar] [CrossRef]
- Guo, P.; Feng, Y.X.; Wei, W.F.; He, L.H.; Tang, B.M. Adhesion of Warm-Mix Recycled Asphalt Aggregate Mixtures Based on Surface Free Energy Theory. J. Mater. Civ. Eng. 2019, 31, 04019209. [Google Scholar] [CrossRef]
- Li, C.Y.; Strachan, A. Molecular Scale Simulations on Thermoset Polymers: A Review. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 103–122. [Google Scholar] [CrossRef]
- Qu, X.; Wang, D.W.; Wang, L.B.; Huang, Y.C.; Hou, Y.; Oeser, M. The State-of-the-Art Review on Molecular Dynamics Simulation of Asphalt Binder. Adv. Civ. Eng. 2018, 2018, 4546191. [Google Scholar] [CrossRef]
- Yu, M.Y.; Zhang, H.T.; Wang, J.J.; Sun, J.F.; Liu, D.G.; Sun, Y.H.; Chen, J.Y. Multi-scale analysis of virgin/aged/recycled asphalt self-healing performance based on molecular simulation and micro-macro tests. Constr. Build. Mater. 2024, 431, 1136535. [Google Scholar] [CrossRef]
- Lu, P.Z.; Ma, Y.H.; Ye, K.; Huang, S.M. Analysis of high-temperature performance of polymer-modified asphalts through molecular dynamics simulations and experiments. Constr. Build. Mater. 2022, 350, 128903. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wang, C.H.; Yang, Y. The Progress of the Interfacial Diffusion between Virgin and Aged Asphalt Based on Molecular Dynamics Simulation: A Review. Processes 2023, 11, 3024. [Google Scholar] [CrossRef]
- Wu, S.Y.; Liu, Q.; Yang, J.; Yang, R.C.; Zhu, J.P. Study of adhesion between crack sealant and pavement combining surface free energy measurement with molecular dynamics simulation. Constr. Build. Mater. 2020, 240, 117900. [Google Scholar] [CrossRef]
- Huang, X.F.; Luo, J.H.; Xie, C.; Cheng, D.D.; Tang, P.F.; Li, G.; Chen, J. Study of Interfacial Adhesion of Pavement Sealants Based on Molecular Dynamics. Mater. Tehnol. 2023, 57, 41–48. [Google Scholar] [CrossRef]
- Wu, M.; Xu, G.J.; Luan, Y.C.; Zhu, Y.J.; Ma, T.; Zhang, W.G. Molecular dynamics simulation on cohesion and adhesion properties of the emulsified cold recycled mixtures. Constr. Build. Mater. 2022, 333, 127403. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Wu, R.; Li, A.; Neumann, A.W. Contact angle measurements and interpretation: Wetting behavior and solid surface tensions for poly (alkyl methacrylate) polymers. J. Adhes. Sci. Technol. 2000, 14, 719–743. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. B 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. J. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zhu, S.X.; Kong, L.Y.; Peng, Y.; Chen, Y.; Zhao, T.H.; Jian, O.Y.; Zhao, P.H.; Sheng, X.; Li, Z. Mechanisms of interface electrostatic potential induced asphalt-aggregate adhesion. Constr. Build. Mater. 2024, 438, 137255. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, C.J.; Ouyang, J. Rejuvenation effect of waste cooking oil on the adhesion characteristics of aged asphalt to aggregates. Constr. Build. Mater. 2022, 327, 126907. [Google Scholar] [CrossRef]
- Gao, L.; Ji, X.; Tan, Y.W.; Wang, Z.Q.; Zhang, Y.; Kong, H.M. Molecular dynamics simulation of interfacial adhesion behavior between waterborne epoxy resin emulsified asphalt and aggregate. Compos. Interfaces 2023, 30, 749–770. [Google Scholar] [CrossRef]
- Suyoung, Y.; Seunghwa, Y.; Maenghyo, C. Multi-scale modeling of cross-linked epoxy nanocomposites. Polymer 2009, 50, 945–952. [Google Scholar]
- Fox, T.G.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- JTG E20-2011; Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. China Communications Press: Beijing, China, 2011.
- Fischer, H.R.; Dillingh, E.C.; Hermse, C.G.M. On the interfacial interaction between bituminous binders and mineral surfaces as present in asphalt mixtures. Appl. Surf. Sci. 2013, 265, 495–499. [Google Scholar] [CrossRef]
- Yang, M.; Huang, T.; Lv, S.T.; Zheng, J.L. Effect and mechanism of acidic aggregate surface silane modification on water stability of asphalt mixture. Road Mater. Pavement Des. 2021, 22, 1654–1666. [Google Scholar] [CrossRef]










| Materials | Molecular Formula | Molecular Weight | Appearance |
|---|---|---|---|
| MDI-50 | C15H10N2O2 | 250.25 | White or light-yellow liquid |
| PTMEG2000 | HO[CH2CH2CH2CH2O]nH | 2000 | White waxy liquid |
| DMTDA | C9H14N2S2 | 214 | Clear yellow to amber liquid |
| Item | Basalt | Limestone |
|---|---|---|
| Apparent density | 2.826 | 2.724 |
| Water absorption (%) | 0.40 | 0.82 |
| Crushed stone value (%) | 11.3 | 15.6 |
| Wear value (%) | 15.9 | 18.7 |
| Polished stone value | 46 | 40 |
| Reagents | Surface Free Energy (mJ/m2) | ||
|---|---|---|---|
| Distilled water | 72.8 | 21.8 | 51.0 |
| Ethylene glycol | 48.3 | 29.3 | 19.0 |
| Formamide | 57.9 | 38.9 | 19.0 |
| Item | Value |
|---|---|
| Tensile strength (MPa) | 13.6 |
| Elongation at break (%) | 672 |
| Shore hardness (HA) | 78 |
| Gel time (min) | 32.2 |
| Glass transition temperature (°C) | −42.33 |
| Mineral Types | Lattice Parameters | |||||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | A (°) | β (°) | γ (°) | |
| SiO2 | 4.913 | 4.913 | 5.405 | 90 | 90 | 120 |
| CaO | 4.810 | 4.810 | 4.810 | 90 | 90 | 90 |
| CaCO3 | 4.990 | 4.990 | 17.061 | 90 | 90 | 120 |
| Materials | Distilled Water | Ethylene Glycol | Formamide | Coefficient of Determination (R2) | |||
|---|---|---|---|---|---|---|---|
| Average/(°) | COV/(%) | Average/(°) | COV/(%) | Average/(°) | COV/(%) | ||
| PU | 87.6 | 0.76 | 61.2 | 0.78 | 77.5 | 0.14 | 0.9649 |
| HPU | 93.1 | 0.32 | 79.9 | 0.41 | 95.3 | 0.49 | 0.9590 |
| Materials | Distilled Water | Ethylene Glycol | Formamide | Coefficient of Determination (R2) | |||
|---|---|---|---|---|---|---|---|
| Average/(°) | COV/(%) | Average/(°) | COV/(%) | Average/(°) | COV/(%) | ||
| Basalt | 75.7 | 0.57 | 40.1 | 0.42 | 59.4 | 0.63 | 0.9602 |
| Limestone | 72.2 | 0.21 | 34.6 | 0.55 | 51.9 | 0.40 | 0.9882 |
| Materials | γ (mJ/m2) | γd (mJ/m2) | γP (mJ/m2) |
|---|---|---|---|
| PU | 23.637 | 14.432 | 9.205 |
| HPU | 16.452 | 4.244 | 12.208 |
| Basalt | 35.647 | 26.605 | 9.042 |
| Limestone | 39.673 | 30.217 | 9.456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ji, J.; Li, X.; Shi, Z.; Wang, J. Study of Interface Adhesion Between Polyurethane and Aggregate Based on Surface Free Energy Theory and Molecular Dynamics Simulation. Appl. Sci. 2025, 15, 606. https://doi.org/10.3390/app15020606
Zhang Z, Ji J, Li X, Shi Z, Wang J. Study of Interface Adhesion Between Polyurethane and Aggregate Based on Surface Free Energy Theory and Molecular Dynamics Simulation. Applied Sciences. 2025; 15(2):606. https://doi.org/10.3390/app15020606
Chicago/Turabian StyleZhang, Zengping, Jiajun Ji, Xue Li, Zhongbao Shi, and Junjie Wang. 2025. "Study of Interface Adhesion Between Polyurethane and Aggregate Based on Surface Free Energy Theory and Molecular Dynamics Simulation" Applied Sciences 15, no. 2: 606. https://doi.org/10.3390/app15020606
APA StyleZhang, Z., Ji, J., Li, X., Shi, Z., & Wang, J. (2025). Study of Interface Adhesion Between Polyurethane and Aggregate Based on Surface Free Energy Theory and Molecular Dynamics Simulation. Applied Sciences, 15(2), 606. https://doi.org/10.3390/app15020606






