Abstract
Avocado (Persea americana Mill.) is a widely cultivated fruit known for its nutritional benefits, with the seed representing a significant portion of the fruit that is often discarded as waste. In the Dominican Republic, the cultivar Semil 34 represents 58% of the national production. This study aimed to explore the potential of Semil 34 avocado seed (AS) as a source of bioactive compounds with applications in the food industry. We conducted the chemical characterization of the seed extract, focusing on its total phenolic content, total flavonoids, and antioxidant capacity. High-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (HPLC-QTOF-MS) was employed to identify key phytochemicals, including phenolic acids and flavonoids, that were responsible for the antioxidant properties of the extract. The hydroalcoholic extract of the Semil 34 seeds exhibited an antioxidant capacity of 1743.3 ± 52.3 µM Trolox/g extract, total phenolic content of 25.86 ± 2.17 mg gallic acid equivalents/g extract, and total flavonoid content of 2.09 ± 0.10 mg quercetin equivalents/g extract. However, the extract’s antioxidant capacity was found to be sensitive to pH changes, suggesting the need for stabilization when used in acidic or basic food matrices. The present work identified 53 compounds in the Semil 34 seed extracts; among these, 23 are being reported for the first time in avocado seeds. This study demonstrates the potential of the avocado seed as a source of bioactive compounds and hence a functional ingredient, supporting its value in sustainable production and its possible contribution to environmental goals by reducing waste in the avocado industry.
1. Introduction
Avocado (Persea americana Mill.) is a ubiquitous fruit with origins in Central America. The avocado fruit is known for its high nutritional value and health benefits [1]. Several varieties of avocado have been reported, including Bacon, Fuerte, Gwen, and Hass, with the latter being the most commercialized [2]. In 2022, the global avocado production was approximately 8.98 million metric tons, with the Dominican Republic being the fourth-largest producer of avocado, with 737,200 metric tons [3,4]. In 2023, 21,875 hectares were dedicated to avocado production in the Dominican Republic. Different cultivars, such as Hass, Popenoe, Simmonds, Pollock, Doctor Dupoi, and Carla, are commercially available in different seasons. Regarding exportation, the most popular variety is Semil 34, which accounts for 58% of the total national avocado production [3,5,6]. Semil 34 is a hybrid between the Guatemalan and the West Indian varieties [7]. Previous studies have reported that, on average, the seed, pulp, and peel constitute 13.93, 79.07, and 7.00%, respectively, of the avocado, as well as an oil and dry matter percentage of approximately 9% and 20% [8]. However, there is limited information available about Semil 34.
Throughout the industrialization process of avocados, the pulp is the only part of the fruit utilized, and its byproducts, such as the avocado seed (AS) and peel, are discarded into the environment, contributing to environmental concerns surrounding the produced waste. The AS represents approximately 13% of the fruit [9], which, according to the numbers previously mentioned, translates into 1.16–1.62 million metric tons of waste globally per year and 317,000–439,000 metric tons of AS annually in the Dominican Republic. In the Dominican Republic, initiatives have been undertaken to utilize the avocado residue to produce oil, and this has demonstrated remarkable effectiveness, utilizing approximately 1.5 million kilograms of discarded avocados between 2020 and 2021 [10]. Nevertheless, other management options are to be considered to drive more environmentally friendly production and meet the Sustainable Development Goals proposed by the United Nations, particularly the objectives of sustainable industrialization and production [11].
Numerous studies have explored the potential applications of AS residues in various industries, such as pharmaceuticals, food and cosmetics, highlighting its potential as a source of many bioactive compounds that can provide antioxidant, anticancer, anti-inflammatory, and antibacterial properties [12,13]. AS also contains significant concentrations of carbohydrates, fiber, protein, lipids, and vitamins, such as A, B1, B2, B3, C, and E [14,15,16]. There are several methods available to recover bioactive compounds, including novel green technologies like ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and Ohmic heating-assisted extraction (OHAE), as well as conventional solid–liquid extractions (SLEs) like maceration, cooking, and infusion. Among these, UAE has been widely applied for the recovery of phytochemicals from plant material [17]. This method uses ultrasound waves to facilitate the rupture of cell walls and the release of compounds at moderate temperatures with little solvent, which enables the high recovery of compounds.
The identification of these health-promoting phytonutrients in the AS extract often requires the utilization of sophisticated techniques such as nuclear magnetic resonance and, more recently, high-performance liquid chromatography–electrospray ionization–mass spectrometry in tandem (HPLC-ESI-MS/MS), which allows the separation and identification of molecules. With these techniques, various phenolic acids, including 3-O-caffeoylquinic acid, 3-p-coumaroylquinic acid, caffeoylquinic acid, and derivates of chlorogenic acid, have been characterized from different AS extracts, as well as terpenes and flavonoids like catechin, epicatechin, procyanidins, luteolin, apigenin, kaempferol, and quercetin [18,19,20,21]. Additionally, sodium, calcium, magnesium, zinc, potassium, and other minerals have been found in AS [22,23].
Driven by the promising composition of avocado waste, many authors have studied the potential industrial applications, particularly for avocado seeds. Previous research has highlighted the broad possibilities to repurpose these byproducts. David et al. observed antimicrobial activity in an ethanolic extract of dry avocado seeds [24]. Similarly, avocado seed extracts were reported to reduce the viability of human cancer cell lines through in vitro trials [15]. Previously, in 2011, Dabas et al. studied the potential use of an avocado seed extract as a natural colorant, finding that the development of the orange color is dependent on a polyphenol oxidase reaction [25]. Later, in 2019, Hatzakis et al. isolated the major colored compound in AS extract using high-performance liquid chromatography and identified it as perseorangin, a yellow-orange solid containing glycosylated benzotropone, with a distinctive orange color when in solution [26]. The ability of avocado to produce an orange dye, associated with the many bioactive compounds present in AS, suggests its substantial potential for applications in food, cosmetics, and pharmaceuticals as an added-value natural colorant, as well as a source of antioxidants. This study aims to characterize the phytochemicals present in Semil 34 seed colored extract for potential applications in the food industry. The study also explores the chemical characterization of avocado cultivar Semil 34, focusing on the total phenolic content and total flavonoids and the antioxidant capacity of its seed. We assess the impact of pH variations on the antioxidant activity and identify key phytochemicals present in the extract. Our findings suggest that incorporating this extract into food matrices could enhance their nutritional value, thanks to its diverse phytochemicals. However, stabilizing the extract for use in acidic or basic matrices is crucial, as its antioxidant capacity is influenced by pH variations.
2. Materials and Methods
2.1. Chemical and Reagents
A DPPH antioxidant assay kit, including DDPH reagent, Trolox standard, and assay buffer, was purchased from Dojindo Laboratories (Rockville, MD, USA). Folin–Ciocalteu reagent, gallic acid, quercetin, calcium carbonate anhydrous, and aluminum chloride were purchased from Sigma Aldrich (St. Louis, MO, USA). All solvents (ethanol and methanol) were obtained from Fisher Scientific (Waltham, MA, USA).
2.2. Avocado Samples and Processing
Persea americana Mill. variety Semil 34 samples were collected in the municipality of Tenares (Hermanas Mirabal, Dominican Republic). The avocados were collected through random sampling and transported to the Universidad Nacional Pedro Henríquez Ureña in Santo Domingo for processing. The seeds were manually separated and allowed to dry for 48 h at 55 °C, and the membrane that covered the seed was removed. The seeds were cut into small pieces, crushed into a fine powder, and stored at −20 °C in an amber bottle until further analysis.
2.3. Extraction of Phytochemicals Through Cooking
Five grams of the previously pulverized avocado seed was mixed with 50 mL of solvent (water or water/ethanol mixture) and placed on a hotplate and gently boiled for 5 min. Then, the mixture was removed from the heat source and left to cool down to room temperature. The solution was centrifuged (1500 rpm, 10 min) and vacuum-filtered (0.2 microns, Millipore; Burlington, MA, USA) to remove suspended particles. Samples were run in triplicate.
2.4. Extraction of Phytochemicals Through Infusion
Five grams of the pulverized avocado seed was mixed with 50 mL of solvent (water or water/ethanol mixture) and placed on a hotplate until a temperature of 65 °C was reached. The mixture was then removed from the heat source and left to cool down to room temperature. The solution was centrifuged (1500 rpm, 10 min) and vacuum-filtered to remove suspended particles.
2.5. Extraction of Phytochemicals Through Ultrasound-Assisted Extraction
Five grams of the pulverized avocado seed was mixed with 50 mL of 50% ethanol aqueous solution and placed in the ultrasonic homogenizer cabin (U.S. Solid; Cleveland, OH, USA). The mixture was sonicated for 10 min with the following probe settings: 2 s on time, 3 s off time, maximum temperature 65 °C, power 240 W, and frequency 20 KHz. The solution was then centrifuged (1500 rpm, 10 min) and vacuum-filtered (0.2 microns, Millipore; Burlington, MA, USA) to remove suspended particles.
2.6. Apparent Color Determination
The avocado seed extract was transferred to an empty comparison tube and placed in the space between the Gardner Liquid Standards L230 (Columbia, MD, USA), as recommended by the manufacturer. When the color of the extract is darker than the standard, a (+) is added to the number.
2.7. Antioxidant Activity Assays
The instructions from Dojindo’s DPPH Antioxidant Assay Kit were followed accordingly [27]. A preliminary assay to determine the IC50 was performed. The sample, Trolox standards, and blanks were prepared by mixing the sample solution, solvent, ethanol, assay buffer, and DPPH working solution, following the manufacturer’s protocol. The mixtures were incubated for 30 min, and the absorbance was measured on a microplate reader (ELISA READER ADX-110, Poway, CA, USA) at 492 nm. The antioxidant activity of the seed extract was determined from the calibration plot (Y= −3.0841 × 10−4x + 0.2573, R2 = 0.9608) and expressed as the Trolox-equivalent antioxidant capacity (TEAC)/g seed. All determinations were carried out in triplicate.
2.8. Total Phenolic Compounds
The total phenolic compounds (TPC) were measured following the method proposed by Singleton and Lamuela-Reventós, with a few modifications [28]. One milliliter of avocado seed extract was mixed inside a 100 mL volumetric flask. The solution was treated with 5 mL of Folin–Ciocalteu reagent, followed by 15 mL of sodium carbonate 1.89 M. The mixture was then swirled, filled to volume with distilled water, and left to react for 2 h before recording the absorbance at 760 nm (E-2100UV UV–Vis Spectrometer, Peak Instruments, Inchinnan, UK). Gallic acid (GA) was used as a standard and the TPC was determined using the calibration curve (Y = 0.9225x − 0.0227, R2 = 0.9994). The results were expressed as mg of gallic acid equivalents (GAE)/g seed. All determinations were carried out in triplicate.
2.9. Total Flavonoids
The flavonoid content was determined following the method described by Chandra et al., with minor modifications [29]. Methanolic standard solutions of quercetin were created (9–220 μg /mL). Separately, 1 mL of the avocado extract and each of the standards were mixed with 1 mL of aluminum chloride 2% on cuvettes incubated for 60 min. The absorbance of the mixtures was measured against a blank at 420 nm. The concentration of total flavonoid content in the AS extract was calculated using the calibration plot (Y = 0.0089x + 0.0268, R2 = 0.9885) and expressed as mg quercetin equivalents (QE)/g seed. All determinations were carried out in triplicate.
2.10. Identification and Characterization of Bioactive Compounds by HPLC-QTOF-MS/MS
The bioactive molecules were analyzed using a quadrupole time-of-flight liquid chromatograph mass spectrometer (LCMS-9050, Shimadzu, Canby, OR, USA). Analyte separation was performed with a Restek Raptor C18 column (2.1 mm ID × 100mm, 1.8 µm particle size). The mobile phases for both positive and negative modes consisted of 0.1% formic acid aqueous solution (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The gradient began at 5% B, was held for 0.6 min, and then increased to 95% B over 27 min. Following the gradient was a 4 min wash at 95% and a 12 min equilibration period. The flow rate was set to 0.3 mL/min, and the column was maintained at 60 °C. Ionization was achieved in positive and negative modes using an electrospray ion source, with nitrogen serving as the drying and nebulizing gas and argon as the collision gas. Data analysis was performed using MZmine [30] and Sirius [31]. The raw files were converted into mzMz files using the POSTRUN Shimadzu software for processing in MZmine (4.3.0). The noise level for mass detection was 3 × 103 for MS1 and 2 × 102 for MS2. The ion chromatogram was extracted using the ADAP module builder with a minimum absolute height of 6 × 103 and 5 ppm m/z tolerance (scan-to-scan). The generated features were resolved using the local minimum feature resolver with a chromatogram threshold of 80%, minimum absolute height of 1 × 103, and peak duration range of 0.1 to 1.12 min/mobility. Finally, the detected features were annotated against multiple spectral libraries, such as NIST and MSbank, with precursor tolerance of 0.01 m/z or 10.0 ppm, a minimum of 5 matched signals, and a cosine similarity score of 0.6 using NIST11 weights. The features extracted from MZmine were further processed in Sirius 5.8.3 for molecular formula assignments and structure predictions for compounds.
2.11. Statistical Analysis
Statistical analysis and plotting were performed using Origin (Origin (Pro), version 2022). Significance was assessed using a two-way two-sample t-test (p < 0.05). The standard deviation was calculated from the triplicates and plotted accordingly.
3. Results and Discussion
3.1. Total Phenolic Compounds, Flavonoids, and Antioxidant Capacity
For the present study, a hydroalcoholic solvent was chosen based on the possibility of industrial applications in food and cosmetic products. Various extraction methods were previously tested (see Supplementary Information) and corroborated with the literature. Different extraction times were tested; however, past 10 min of sonication, the temperature of the mixture would rise beyond our 65 °C cutoff. Utami et al. (2021) previously concluded that ultrasound-assisted extraction (UAE) produced the best avocado dye recoveries at shorter times [32]. On the other hand, Xu et al. (2015) previously observed that greater ethanol concentrations yielded better antioxidant activity in flower extracts [33]. Lastly, Alara et al. (2021) observed that phenolic compounds were easier to extract using semi-polar organic solvents, which agrees with our findings [34].
The Gardner index of the AS extract was 15+, resulting in an orange-colored extract with potential applications in the food industry (see Supplementary Information). Other parameters tested included the recovery yield, pH, and absorbance, with 8.11%, 5.97, and 2.89 (λmax = 450), respectively. The protection of a biological system against oxidative stress and its harmful effects relies on the antioxidant capacity [19]. Therefore, to assess the potential benefits of the Semil 34 AS extract, we studied the antioxidant capacity (TEAC) as well as the total phenolic content (TPC) and total flavonoid content (TFC). Table 1 compares the results obtained in this study with those of previous studies found in the literature for other varieties of avocado. The TEAC, TPC, and TFC obtained for the Semil 34 AS extract were 1743.33 µM/g seed, 25.86 mg GAE/g extract, and 2.0911 mg QE/g extract, respectively. In a previous study, Araujo et al. reported TEAC of 1065.00 µM/g seed (units were converted) for a Hass cultivar AS extract [18]. Interestingly, another study by Rosero et al. reported a TEAC value of 18,400 µM/g seed and a TPC value of 1303.0 mg GAE/g extract, which differ from our results (see Table 1). The chemical composition of avocado can vary from cultivar to cultivar, depending on several factors, such as the variety, processing method, region, climate, and precipitation [18,35]; this explains the variability found within the values for the TEAC, TPC, and TFC from different authors, even when the same variety of avocado is evaluated. This also highlights the importance of preliminary studies of the avocado residues prior to processing for the targeted industry.

Table 1.
Total phenolic compounds, total flavonoids, and antioxidant capacity of Semil 34 seed extract. Different letters represent different expressions of the concentration: a = (µM/g extract); b = (mg GAE/g extract); c = (mg QE/g extract); d = (mmol Trolox/g dried extract); e = (mg GAE/g dried extract); f = (mMol Trolox eq./100 g of extract); g = mMol GA/100 g of extract; h = mMol Cat. eq./100 g of extract; i = μg TE/g dried extract; j = mg TE/g extract.
3.2. Effect of pH on Antioxidant Activity
The effect of the pH on the antioxidant capacity of the AS extracts was tested at pH values of 3 and 9. As shown in Figure 1, a significant drop in the TEAC was observed in both acidic and basic conditions when compared to the unmodified extract. This agrees with previous studies that observed a pH-dependent effect on the antioxidant activity of flavonoids [36]. These results are key when exploring future applications of AS extracts. For example, to benefit from its additional antioxidant properties in the food industry, the colored extract must be incorporated in a matrix with a relatively neutral pH, which excludes processed dairy products such as yogurt. The stabilization of the phytochemical compounds found in the extract through encapsulation and emulsion technologies should be considered to ensure the bioactivity when incorporated into cosmetics or food products [16].
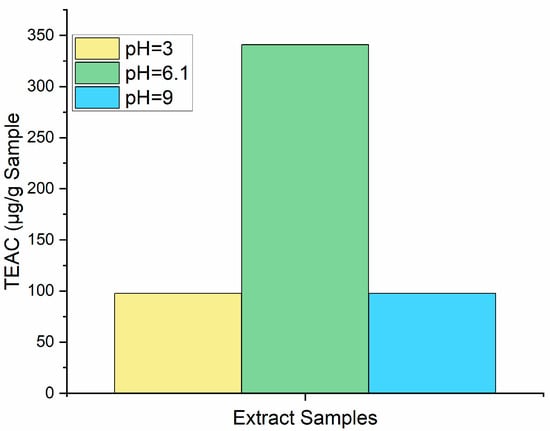
Figure 1.
pH effects on antioxidant activity of Semil 34 seed extract. pH 6.1 is observed in the processed extracts of Semil 34 avocado seeds.
3.3. Proposed Identification of Chemicals in Avocado (P. americana Mill.) Semil 34 Seed
The chemical characterization of Semil 34 was conducted using negative and positive electrospray ionization (ESI) modes via UPLC-MS (Figure S5). A total of 53 putative compounds were identified, based on their predicted chemical formulas and high mass accuracy (Table 2). The chemical classification tools Classyfire and NPClassifier were also utilized to group the previously identified compounds [37,38,39], allowing the classification of the metabolites into carboxylic acids and derivatives, cinnamic acid and derivatives, fatty acyls, flavonoids, furanoid ligans, glycerolipids, glycerophospholipids, organic sulfuric acids and derivatives/arylsulfates/phenylsulfates, organooxygen compounds, and prenol lipids.
Three molecules were tentatively identified among the carboxylic acids and derivates. For example, compound 2 showed a precursor ion at m/z [M-H]− 191.0188, assigned to citric acid. Previous studies have identified citric acid in avocado seeds with precursor ions at m/z 191.021 and 191.0539, which aligns with our findings [19,40]. Within cinnamic acid and derivatives, a coumaroylquinic acid isomer with m/z [M-H]− 337.0922 was found. This compound was previously reported as 5-O-p-coumaroylquinic acid and cis-3-p-coumarylquinic acid at m/z 337.04 and 337, respectively [41,42]. Caffeic acid [M+H-H2O]+ with m/z 163.0387 was also identified, a compound that is widely studied in avocado [20,41,43,44]. An example within the fatty acyl compounds is 2-linoleoylglycerol [M+H-H2O]+ with m/z 337.2732, which has also been previously reported with a fragment ion at 337 [45]. Examples of organooxygen compounds include perseitol, observed at m/z 211.0815 [M-H]−, previously identified by Rojas-García et al. at m/z 211.0805, as well as other authors [19,45,46]. Quinic acid with m/z 191.0553 [M-H]− and neochlorogenic acid at m/z 353.0878 [M-H]− were also identified and corroborated by the literature [19,45,47]. Regarding flavonoids, compounds 29 and 30 were found, with proposed compounds Procyanidin B1 and B2, with m/z 579.1490 and 579.1493 [M+H]+, respectively, agreeing with previous studies focused on the Hass and Fuerte varieties [48]. Molecules specifically found in avocado, such as compounds 15, 16, 17, and 20, were presented as avocadenol A with m/z 231.2107 [M+H]+, 4-AcO-avocadyne with m/z 309.2424 [M+H-H2O]+, avocadene acetate with m/z 293.2472 [M+H-2H2O]+, and avocadyne with m/z 249.2210 [M+H-2H2O]+, which have also been previously reported [45,49,50].

Table 2.
Compounds identified from Semil 34 seed extract using HPLC-QTOF-MS/MS.
Table 2.
Compounds identified from Semil 34 seed extract using HPLC-QTOF-MS/MS.
| Peak No. | RT | Proposed Compound Identification | Molecular Formula | Ionization | m/z Observed | m/z Theoretical | Error (ppm) | References |
|---|---|---|---|---|---|---|---|---|
| Carboxylic acids and derivatives | ||||||||
| 1 | 0.98 | gamma-Aminobutyric acid | C4H9NO2 | [M+H]+ | 104.0703 | 104.0706 | −2.8 | [51] |
| 2 | 1.14 | Citric acid | C6H8O7 | [M-H]− | 191.0188 | 191.0197 | −4.7 | [40,47] |
| 3 | 1.36 | Pantothenic acid | C9H17NO5 | [M+H]+ | 220.1178 | 220.1179 | −0.4 | [51] |
| Cinnamic acid and derivatives | ||||||||
| 4 | 1.64 | Neochlorogenic acid | C16H18O9 | [M-H]− | 353.0873 | 353.0878 | −1.4 | [47] |
| 5 | 2.85 | Caffeic acid | C9H8O4 | [M+H-H2O]+ | 163.0387 | 163.0389 | −1.2 | [41] |
| 6 | 2.85 | Caffeoylquinic acid | C16H18O9 | [M+H]+ | 355.1022 | 355.1023 | −0.3 | [20,41,48] |
| 7 | 3.53 | 3-O-p-Coumaroylquinic acid | C16H18O8 | [M-H]− | 337.0922 | 337.0929 | −2 | [18,42] |
| Fatty acyls | ||||||||
| 8 | 15.92 | Methyl tetradecanoate | C15H30O2 | [M+H]+ | 243.2316 | 243.2318 | −0.6 | [51] |
| 9 | 17.60 | 9,10-Methylenehexadecanoic acid | C17H32O2 | [M+H-H2O]+ | 251.2367 | 251.2369 | −0.7 | |
| 10 | 27.45 | 2-Linoleoylglycerol | C21H38O4 | [M+H-H2O]+ | 337.2732 | 337.2737 | −1.4 | [52] |
| 11 | 28.32 | 1-Palmitoyl-2-linoleoyl-rac-glycerol | C37H68O5 | [M+H]+ | 593.5131 | 593.5138 | −1.17 | [52] |
| 12 | 20.39 | Monopalmitolein | C19H36O4 | [M+H-H2O]+ | 311.2569 | 311.2580 | −3.5 | |
| 13 | 6.51 | 3-Hydroxy-3-methylglutaric acid | C6H10O5 | [M+H]+ | 163.0599 | 163.0601 | −1.2 | [53] |
| 14 | 21.99 | Linoelaidic acid | C18H32O2 | [M+H-H2O]+ | 263.2367 | 263.2369 | −0.7 | |
| 15 | 18.36 | Avocadenol A | C17H32O3 | [M+H-3H2O]+ | 231.2105 | 231.2107 | −0.8 | [54,55] |
| 16 | 18.52 | 4-AcO-avocadyne | C19H34O4 | [M+H-H2O]+ | 309.2423 | 309.2424 | −0.3 | [54,55] |
| 17 | 19.24 | Avocadene acetate | C19H36O4 | [M+H-2H2O]+ | 293.2472 | 293.2475 | −1.02 | [54,55,56] |
| 18 | 13.58 | 16-Heptadecyn-4-one, 1,2-dihydroxy | C17H30O3 | [M+H-H2O]+ | 265.2160 | 265.2162 | −0.7 | |
| 19 | 18.03 | 9E,11E-Octadecadienoic acid | C18H32O2 | [M+H-H2O]+ | 263.2367 | 263.2369 | −0.7 | [57] |
| 20 | 18.62 | Avocadyne | C17H32O3 | [M+H-2H2O]+ | 249.2210 | 249.2213 | −1.2 | [58] |
| 21 | 18.63 | 9,10-Methylenehexadecanoic acid | C17H32O2 | [M+H-2H2O]+ | 233.2262 | 233.2272 | −4.2 | |
| 22 | 9.96 | Nonadecanedioic acid | C19H36O4 | [M+H-H2O]+ | 311.2575 | 311.2581 | −1.9 | |
| 23 | 23.66 | Oleoyl ethylamide | C20H39NO | [M+H]+ | 310.3101 | 310.3103 | −0.6 | |
| 24 | 25.47 | cis-13-Docosenoic acid | C22H42O2 | [M+H-H2O]+ | 321.3149 | 321.3152 | −0.9 | [59] |
| 25 | 10.62 | (9Z)-5,8,11-Trihydroxyoctadec-9-enoic acid | C18H34O5 | [M-H]− | 329.2329 | 329.2333 | −1.2 | |
| 26 | 14.84 | (12Z)-9,10,11-Trihydroxyoctadec-12-enoic acid | C18H34O5 | [M-H-H2O]− | 311.2221 | 311.2228 | −2.2 | |
| 27 | 25.47 | Erucamide | C22H43NO | [M+H]+ | 338.3435 | 338.3417 | 5.3 | |
| 28 | 23.0805 | 1,2,4-nonadecanetriol | C19H40O3 | [M+H]+ | 299.2945 | 299.2945 | 0.0 | |
| Flavonoids | ||||||||
| 29 | 2.41 | Procyanidin B2 | C30H26O12 | [M+H]+ | 579.1493 | 579.1497 | −0.6 | [48] |
| 30 | 3.82 | Procyanidin B1 | C30H26O12 | [M+H]+ | 579.1490 | 579.1497 | −1.2 | [48] |
| 31 | 4.04 | Procyanidin A1 | C30H24O12 | [M+H]+ | 577.1338 | 577.1341 | −0.5 | [58] |
| 32 | 3.54 | Pavetannin B6 | C45H36O18 | [M+H]+ | 865.1968 | 865.1974 | 1.3 | |
| Furanoid ligans | ||||||||
| 33 | 8.16 | Syringaresinol | C22H26O8 | [M+H-H2O]+ | 401.1592 | 401.1595 | 0.3 | [20] |
| Glycerolipids | ||||||||
| 34 | 18.57 | Lichesterinic acid | C19H32O4 | [M-H]− | 325.2377 | 325.238 | −0.9 | |
| Glycerophospholipids | ||||||||
| 35 | 18.11 | 1-Oleoyl-sn-glycero-3-phosphocholine | C26H52NO7P | [M+CHO2]− | 566.3457 | 566.3452 | 0.8 | [45] |
| 36 | 15.84 | D-myo-Inositol, 1-[2-hydroxy-3-[(1-oxo-9,12-octadecadienyl)oxy]propyl hydrogen phosphate], [S-(Z,Z)]- | C25H49O12P | [M-H]− | 595.2885 | 595.2889 | 0.4 | |
| 37 | 15.35 | 1-Myristoyl-sn-glycero-3-phosphocholine (LPC 14:0) | C22H46NO7P | [M+H]+ | 468.3079 | 468.3085 | −1.2 | |
| 38 | 16.01 | 1-palmitoleoyl-glycerophosphocholine (LPC 16:1) | C24H48NO7P | [M+H]+ | 494.3237 | 494.3241 | −0.8 | |
| 39 | 17.08 | 1-(10Z-heptadecenoyl)-2-hydroxy-sn-glycero-3-phosphocholine (17:1 Lyso PC) | C25H50NO7P | [M+H]+ | 508.3393 | 508.3397 | −0.7 | |
| 40 | 17.11 | 1-Palmitoyl-sn-glycero-3-phosphocholine (LPC 16:0) | C24H50NO7P | [M+H]+ | 496.3394 | 496.3398 | −0.8 | [60] |
| 41 | 17.40 | 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (LPE 16:0) | C21H44NO7P | [M+H]+ | 454.2923 | 454.2928 | −1.1 | |
| 42 | 19.73 | 1-Stearoyl-sn-glycero-3-phosphocholine (LPC 18:0) | C26H54NO7P | [M+H]+ | 524.3715 | 524.3710 | 0.9 | [60] |
| Organic sulfuric acids and derivatives/arylsulfates/phenylsulfates | ||||||||
| 43 | 3.04 | 3-[3-(Sulfooxy)phenyl]propanoic acid | C9H10O6S | [M-H]− | 245.0117 | 245.0125 | −3.2 | [61] |
| Organooxygen compounds | ||||||||
| 44 | 0.75 | Perseitol | C7H16O7 | [M-H]− | 211.0815 | 211.0823 | −3.7 | [62] |
| 45 | 26.67 | Soyacerebroside I | C40H75NO9 | [M-H]− | 712.5361 | 712.5369 | −1.1 | |
| 46 | 5.12 | Phenetyl rutenoside | C20H30O10 | [M+NH4]+ | 448.2170 | 448.2188 | −4.0 | |
| 47 | 0.94 | Quinic acid | C7H12O6 | [M-H]− | 191.0553 | 191.0561 | 0.8 | [41] |
| 48 | 0.99 | Volemitol | C7H16O7 | [M-H]− | 211.0814 | 211.082 | 0.6 | [63] |
| 49 | 1.22 | D-Picose | C6H12O6 | [M-H2O-H]− | 161.0446 | 161.0455 | −5.5 | |
| 50 | 6.16 | Isomaltulose | C12H22O11 | [M+H-3H2O]+ | 289.0917 | 289.0928 | −3.8 | |
| 51 | 6.16 | Sucrose | C12H22O11 | [M+H-2H2O]+ | 307.1022 | 307.1034 | 3.9 | [64] |
| 52 | 2.95 | (1r,3R,4s,5S)-4-(((2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl)oxy)-1,3,5-trihydroxycyclohexanecarboxylic acid | C16H18O9 | [M-H]− | 353.0873 | 353.0878 | −1.4 | |
| Prenol lipids | ||||||||
| 53 | 7.52 | Abscisic acid | C15H20O4 | [M+H-H2O]+ | 247.1326 | 247.1329 | 0.3 | [65] |
3.4. Proposed Identification of Novel Chemicals in Avocado (P. americana Mill.) Semil 34 Seed
We also present 23 tentative compounds that, to our knowledge, have not been previously reported in the literature. These compounds belong to various classes, including 10 fatty acyl compounds, five glycerophospholipids, six organooxygen compounds, one glycolipid, and one flavonoid. Among the fatty acyl compounds, pavetannin B1 was provisionally identified with a precursor ion at m/z 865.1968 [M+H]+, displaying major fragment ions at m/z 695, 575, 533, and 287. Another example is linoelaidic acid, detected at m/z 263.2367 [M+H-H2O]+ with a mass error of −0.7 ppm. Its fragmentation spectrum exhibited major product ions at m/z 245, 109, and 95. An example of an organooxygen compound is (1r,3R,4s,5S)-4-(((2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl)oxy)-1,3,5 trihydroxycyclohexanecarboxylic acid, with m/z of 353.0878 [M-H]− and major ions such as 191, 179, and 134. Other proposed compounds include isomaltulose, with a precursor ion at m/z 289.0917 [M+H-3H2O]+, D-picose at m/z 161.0446 [M-H2O-H]−, and LPE 16:0 with m/z 454.2923 [M-H]+, among others.
4. Conclusions
The current research targeted the sustainability of the agroindustry through the valorization of the residues produced during its operations. Understanding the phytochemical composition of avocado seeds is key when considering their further applications in food, cosmetics, and/or pharmaceuticals. Our results indicate that seed extracts from the cultivar Semil 34 offer promising health benefits due to their antioxidant capacity and the various chemicals present. These compounds have been associated with antidiabetic, anti-inflammatory, antioxidant, and antimicrobial properties that could benefit several industries, particularly the food sector. Further research is needed to completely characterize the cultivar Semil 34 and fully comprehend all of the possible benefits within this variety. Moreover, prior to its incorporation in food matrices, more research is required to determine the cytotoxicity and negative health impacts and to assess the appropriate technology needed to stabilize the extract and prevent the loss of antioxidants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15020922/s1, Figure S1: Extract recovery comparison between Ultrasound Assisted Extraction (UAE), Cooking, and Infusion; Figure S2: Linear Fit for Total Phenolic Compounds expressed in mg of Gallic Acid Equivalents per mL; Figure S3: Linear Fit for Total Flavonoids Content expressed in µg of Quercetin per mL; Figure S4: Linear Fit for Antioxidant Activity expressed in µM of Trolox Equivalent Antioxidant Capacity; Figure S5: Positive and Negative mode Chromatogram for avocado seed extract.
Author Contributions
Conceptualization, A.F.-J. and R.S.-R.; methodology, L.C., S.V., M.T.A. and R.S.-R.; software, R.S.-R. and M.T.A.; validation, M.T.A. and R.S.-R.; formal analysis, A.F.-J., M.T.A. and R.S.-R.; investigation, L.C., S.V., A.F.-J., M.T.A. and R.S.-R.; resources, M.T.A. and R.S.-R.; data curation, M.T.A. and R.S.-R.; writing—original draft preparation, R.P.-R., A.F.-J., M.A., L.C., S.V. and R.S.-R.; writing—review and editing, A.F.-J., M.T.A. and R.S.-R.; visualization, A.F.-J., M.T.A. and R.S.-R.; supervision, R.S.-R.; project administration, R.S-R.; funding acquisition, R.S-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondo Nacional de Innovación y Desarrollo Científico y Tecnológico, grant number 2022-2D3-075, and the APC was funded by the Universidad Nacional Pedro Henríquez Ureña.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We wish to thank Sergio Sánchez for his time and consideration in critiquing this review. We would also like to thank Berleni Lebrón from Herbario Dr. Henri Alain Liogier (UNPHU) for her time and guidance during the botanical aspects of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Statista. Avocado Production Worldwide 2022. Available online: https://www.statista.com/statistics/577455/world-avocado-production/ (accessed on 9 September 2024).
- World Population Review. Avocado Production by Country 2024. Available online: https://worldpopulationreview.com/country-rankings/avocado-production-by-country (accessed on 9 September 2024).
- Bisonó, S.; Hernández, J. Guía Tecnológica Sobre el Cultivo del Aguacate; Cluster del Aguacate Dominicano Santo Domingo: Dominican Republic, 2008. [Google Scholar]
- IICA. The Avocado Crop: Great Potential for the Dominican Republic. Available online: https://iica.int/es/prensa/noticias/avocado-crop-great-potential-dominican-republic (accessed on 16 October 2024).
- Specialty Produce. Semil 34 Avocados. Available online: https://specialtyproduce.com/produce/Semil_34_Avocados_17119.php (accessed on 10 November 2024).
- Cuevas, M. Non-Destructive Methods and Optimum Harvesting Time of “Semil 34” Avocado (Persea americana Mill.) in the Dominican Republic. Available online: https://www.avocadosource.com/wac6/en/Extenso/4a-164.pdf (accessed on 24 October 2024).
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Onavocados. On Avocados. Available online: https://onavocados.com/ (accessed on 12 November 2024).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development (A/RES/70/1); UN General Assembly: New York, NY, USA, 2015; Available online: https://sdgs.un.org/2030agenda (accessed on 11 November 2024).
- Alkhalaf, M.I.; Alansari, W.S.; Ibrahim, E.A.; Elhalwagy, M.E.A. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ.-Sci. 2019, 31, 1358–1362. [Google Scholar] [CrossRef]
- Dabas, D.; Elias, R.J.; Ziegler, G.R.; Lambert, J.D. In vitro antioxidant and cancer inhibitory activity of a colored avocado seed extract. Int. J. Food Sci. 2019, 2019, 6509421. [Google Scholar] [CrossRef]
- Egbuonu, A.C.C.; Opara, C.I.; Mbah, O.C.A.U.O. Vitamins composition and antioxidant properties in normal and monosodium glutamate-compromised rats’ serum of Persea americana (avocado pear) seed. Open Access J. Chem. 2017, 1, 19–24. [Google Scholar] [CrossRef]
- Siol, M.; Sadowska, A. Chemical composition, physicochemical and bioactive properties of avocado (Persea americana) seed and its potential use in functional food design. Agriculture 2023, 13, 316. [Google Scholar] [CrossRef]
- Féliz-Jiménez, A.; Sanchez-Rosario, R. Bioactive compounds, composition and potential applications of avocado agro-industrial residues: A review. Appl. Sci. 2024, 14, 10070. [Google Scholar] [CrossRef]
- Tan, C.X.; Chin, R.; Tan, S.T.; Tan, S.S. Phytochemicals and antioxidant activity of ultrasound-assisted avocado seed extract. Malays. J. Anal. Sci. 2022, 26, 439–446. [Google Scholar]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process optimization of microwave-assisted extraction of bioactive molecules from avocado seeds. Ind. Crops Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Rojas-García, A.; Villegas-Aguilar, M.D.C.; García-Villegas, A.; Cádiz-Gurrea, M.D.L.L.; Fernández-Ochoa, Á.; Fernández-Moreno, P.; Arráez-Román, D.; Segura-Carretero, A. Characterization and biological analysis of avocado seed and peel extracts for the development of new therapeutic strategies. Biol. Life Sci. Forum 2022, 18, 9. [Google Scholar] [CrossRef]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of phenolic composition of byproducts (seeds and peels) of avocado (Persea americana Mill.) cultivated in Colombia. Molecules 2019, 24, 3209. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Quero, J.; Osada, J.; Martín-Belloso, O.; Rodríguez-Yoldi, M.J. Phenolic-rich extracts from avocado fruit residues as functional food ingredients with antioxidant and antiproliferative properties. Biomolecules 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Arukwe, U.; Amadi, B.A.; Duru, M.K.C.; Agomuo, E.N.; Adindu, E.A.; Odika, P.C.; Lele, K.C.; Egejuru, L.; Anudike, J. Chemical composition of Persea americana leaf, fruit and seed. Int. J. Res. Rev. Appl. Sci. 2012, 11, 346–349. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- David, D.; Alzate, A.F.; Rojano, B.; Copete-Pertuz, L.S.; Echeverry, R. Extraction and characterization of phenolic compounds with antioxidant and antimicrobial activity from avocado seed (Persea americana Mill). Bionatura 2022, 7, 51. [Google Scholar] [CrossRef]
- Dabas, D.; Elias, R.J.; Lambert, J.D.; Ziegler, G.R. A colored avocado seed extract as a potential natural colorant. J. Food Sci. 2011, 76, C1335–C1341. [Google Scholar] [CrossRef] [PubMed]
- Hatzakis, E.; Mazzola, E.P.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Perseorangin: A natural pigment from avocado (Persea americana) seed. Food Chem. 2019, 293, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Ukeda, H. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives—Inter-laboratory evaluation study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Pluskal, T. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Utami, H.; Agustin, V.T.; Novirianti, L.; Darni, Y.; Lesmana, D.; Tagaki, R. The leaching of natural dyes from avocado (Persea americana Mill) seeds using the ultrasonic-assisted extraction method and its application on cellulose fibers. J. Rekayasa Kim. Lingkung. 2021, 16, 100–108. [Google Scholar] [CrossRef]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules 2015, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Sandoval-Contreras, T.; González Chávez, F.; Poonia, A.; Iñiguez-Moreno, M.; Aguirre-Güitrón, L. Avocado waste biorefinery: Towards sustainable development. Recycling 2023, 8, 81. [Google Scholar] [CrossRef]
- Lemańska, K.; Szymusiak, H.; Tyrakowska, B.; Zieliński, R.; Soffers, A.E.; Rietjens, I.M. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Dührkop, K.; Nothias, L.F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Böcker, S. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Wishart, D.S. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.F.; Reher, R.; Kang, K.B.; Cottrell, G.W. NPClassifier: A deep neural network-based structural classification tool for natural products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Contreras, M.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolic profiling of vegetables: Lactuca sativa as an example of its application. J. Chromatogr. A 2013, 1313, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Younis, I.Y.; Khattab, A.R.; Selim, N.M.; Sobeh, M.; Elhawary, S.S.; Bishbishy, M.H.E. Metabolomics-Based Profiling of 4 Avocado Varieties Using HPLC–MS/MS and GC/MS and Evaluation of Their Antidiabetic Activity. Sci. Rep. 2022, 12, 4966. [Google Scholar] [CrossRef] [PubMed]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C. Bioactive Characterization of Persea americana Mill. By-Products: A Rich Source of Inherent Antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef]
- Fan, S.; Qi, Y.; Shi, L.; Giovani, M.; Zaki, N.A.A.; Guo, S.; Suleria, H.A.R. Screening of Phenolic Compounds in Rejected Avocado and Determination of Their Antioxidant Potential. Processes 2022, 10, 1747. [Google Scholar] [CrossRef]
- Golukcu, M.; Ozdemir, F. Changes in Phenolic Composition of Avocado Cultivars During Harvesting Time. Chem. Nat. Compd. 2010, 46, 112–115. [Google Scholar] [CrossRef]
- Waly, D.A.; Zeid, A.H.A.; Attia, H.N.; Ahmed, K.A.; El-Kashoury, E.S.A.; El Halawany, A.M.; Mohammed, R.S. Comprehensive Phytochemical Characterization of Persea americana Mill. Fruit via UPLC/HR-ESI–MS/MS and Anti-Arthritic Evaluation Using Adjuvant-Induced Arthritis Model. Inflammopharmacology 2023, 31, 3243–3262. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as Valuable Tools for the Determination of Phenolic and Other Polar Compounds in the Edible Part and By-Products of Avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive Identification of Bioactive Compounds of Avocado Peel by Liquid Chromatography Coupled to Ultra-High-Definition Accurate-Mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; Alencar, S.M.D. Exploration of Avocado By-Products as Natural Sources of Bioactive Compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Kermanshahi, B.; Ghazani, S.M.; Tait, K.; Tcheng, M.; Roma, A.; Callender, S.P.; Smith, R.W.; Tam, W.; Wettig, S.D.; et al. Avocado-Derived Polyols for Use as Novel Co-Surfactants in Low Energy Self-Emulsifying Microemulsions. Sci. Rep. 2020, 10, 5566. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, D.G.; Pacheco, A.; Villarreal-Lara, R.; Ramos-González, M.R.; Ramos-Parra, P.A.; Granados-Principal, S.; Díaz de la Garza, R.I.; García-Rivas, G.; Hernández-Brenes, C. Chemical Profile and Safety Assessment of a Food-Grade Acetogenin-Enriched Antimicrobial Extract from Avocado Seed. Molecules 2019, 24, 2354. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Fernández, E.; Bajoub, A.; Morales, J.C.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Exploratory Analysis of Avocado Extracts by GC-MS: New Insights into the Avocado Fruit Ripening Process. Anal. Methods 2015, 7, 7318–7326. [Google Scholar] [CrossRef]
- Degenhardt, A.G.; Hofmann, T. Bitter-Tasting and Kokumi-Enhancing Molecules in Thermally Processed Avocado (Persea americana Mill.). J. Agric. Food Chem. 2010, 58, 12906–12915. [Google Scholar] [CrossRef] [PubMed]
- Lazare, S.; Yasuor, H.; Yermiyahu, U.; Kuhalskaya, A.; Brotman, Y.; Ben-Gal, A.; Dag, A. It Takes Two: Reciprocal Scion-Rootstock Relationships Enable Salt Tolerance in ‘Hass’ Avocado. Plant Sci. 2021, 312, 111048. [Google Scholar] [CrossRef] [PubMed]
- Tcheng, M.; Ahmed, N.; Spagnuolo, P.A. Structure Defines Bioactivity of Avocado-Derived Acetogenins. Stud. Nat. Prod. Chem. 2023, 78, 1–44. [Google Scholar]
- Louis, M.L.M.; Rani, V.P.; Krishnan, P.; Reegan, A.D.; Balakrishna, K.; Ignacimuthu, S.; Packiam, S.M.; Maheswaran, R.; Shirota, O. Mosquito Larvicidal Activity of Compounds from Unripe Fruit Peel of Avocado (Persea americana Mill.). Appl. Biochem. Biotechnol. 2023, 195, 2636–2647. [Google Scholar] [CrossRef]
- Freitas, M.S.; Pereira, A.H.; Pereira, G.O.; Menezes, I.S.; Lucena, A.R.; Almeida, C.R.; Pereira, E.G.; Santos, L.A.; Tozin, L.R.; Alves, F.M.; et al. Acetogenin-Induced Fibrotic Heart Disease from Avocado (Persea americana, Lauraceae) Poisoning in Horses. Toxicon 2022, 219, 106921. [Google Scholar] [CrossRef] [PubMed]
- Ofunne, C.; Iyekowa, O.; Okolie, H.O. Chemical Analysis and GC-MS Characterization of Ether Fraction from Persea americana Seed. NIPES-J. Sci. Technol. Res. 2019, 1, 2. [Google Scholar]
- del Refugio Ramos-Jerz, M. Phytochemical Analysis of Avocado Seeds (Persea americana Mill., cv Hass); Cuvillier Verlag: Göttingen, Germany, 2007. [Google Scholar]
- Njoku, G.C.; Alisigwe, C.V.; Iloanusi, D.U.; Aririguzo, P.C. Determination of the Total Phenolic Contents of Essential Oil Obtained from Cymbopogon citratus (Lemongrass) and Persea americana Mill (Avocado Pear Seed) and Its Bioactive Component Using GC-MS Analysis. Int. J. Innov. Sci. Res. Technol. 2022, 7, 127–138. [Google Scholar] [CrossRef]
- Pacetti, D.; Boselli, E.; Lucci, P.; Frega, N.G. Simultaneous Analysis of Glycolipids and Phospholipids Molecular Species in Avocado (Persea americana Mill) Fruit. J. Chromatogr. A 2007, 1150, 241–251. [Google Scholar] [CrossRef]
- Lyu, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Phenolic Compounds Profiling and Their Antioxidant Capacity in the Peel, Pulp, and Seed of Australian Grown Avocado. Antioxidants 2023, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sievert, J.; Arpaia, M.L.; Madore, M.A. Postulated Physiological Roles of the Seven-Carbon Sugars, Mannoheptulose, and Perseitol in Avocado. J. Am. Soc. Hortic. Sci. 2002, 127, 108–114. [Google Scholar] [CrossRef]
- Richtmyer, N.K. The Isolation of Volemitol and Other Polyhydric Alcohols from Avocado Seeds. Carbohydr. Res. 1970, 12, 135–138. [Google Scholar] [CrossRef]
- Olmedo, P.; Núñez-Lillo, G.; Ponce, E.; Alvaro, J.E.; Baños, J.; Carrera, E.; González-Fernández, J.J.; Hormaza, J.I.; Campos, D.; Chirinos, R.; et al. Metabolite Profiling and Hormone Analysis of the Synchronized Exocarp-Mesocarp Development during Ripening of cv. ‘Fuerte’ and ‘Hass’ Avocado Fruits. Food Biosci. 2024, 60, 104454. [Google Scholar] [CrossRef]
- del Refugio Ramos, M.; Jerz, G.; Villanueva, S.; López-Dellamary, F.; Waibel, R.; Winterhalter, P. Two Glucosylated Abscisic Acid Derivatives from Avocado Seeds (Persea americana Mill. Lauraceae cv. Hass). Phytochemistry 2004, 65, 955–962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).