Guided Tissue Regeneration Membranes: Review of Innovations and Applications in Immunocompromised Patients
Abstract
:1. Introduction
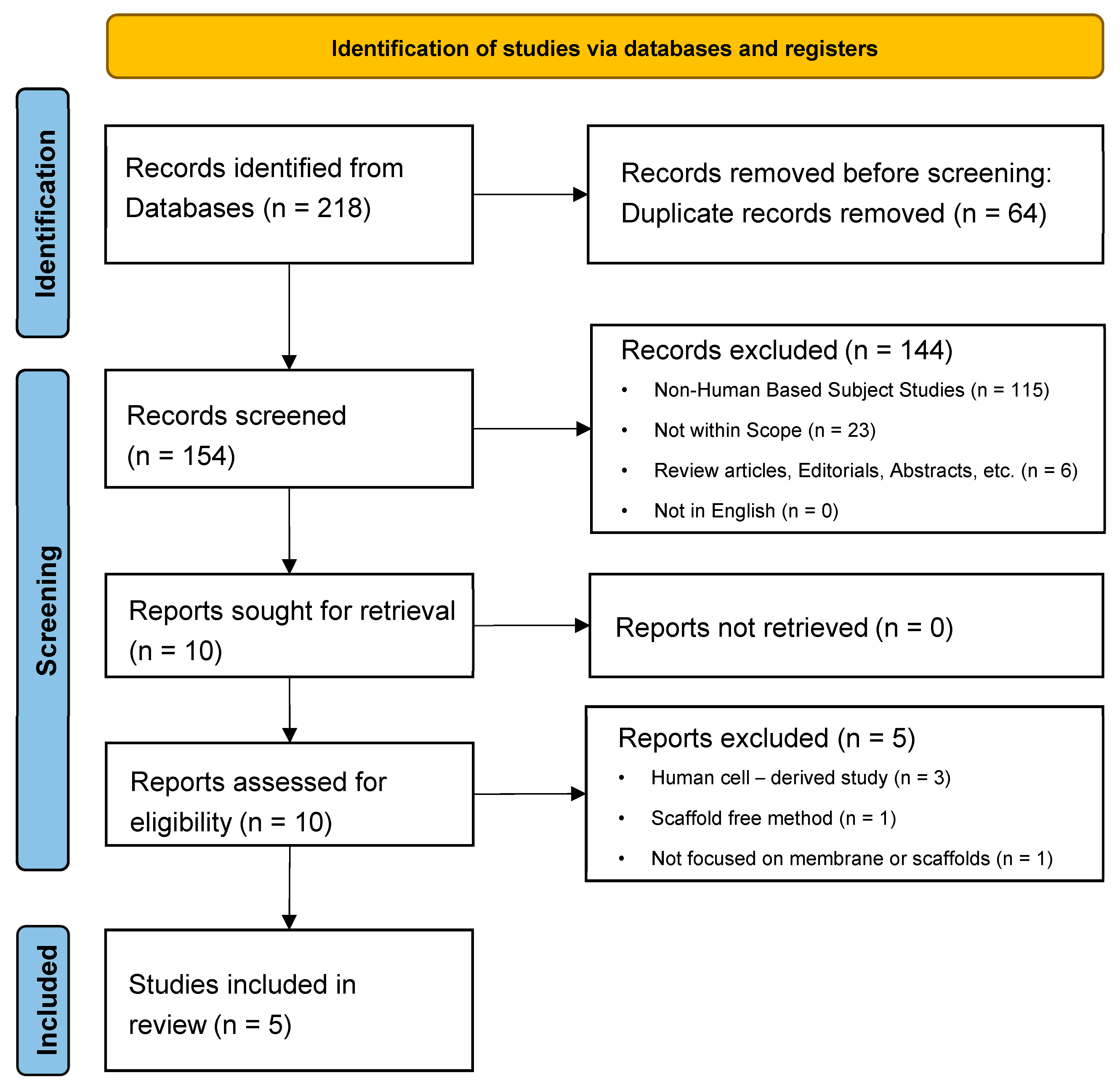
2. Methods
3. Results
4. Discussion
4.1. Integration of Stem Cells with GTR Membranes
4.2. Architectural and Material Innovations in GTR Membranes
4.3. Scaffold-Free Tissue Engineering
4.4. Preclinical Models and Translational Potential
4.5. Gaps and Challenges
4.6. Clinical Implications
4.6.1. Personalized Approaches to Regeneration
4.6.2. Enhanced Material Design for Clinical Applications
4.6.3. Scaffold-Free Constructs for Immunocompromised Patients
4.6.4. Preclinical Findings and Translational Potential
4.6.5. Addressing Challenges and Building on Current Innovations
4.7. Limitations of This Review
4.8. Future Integration into Standard Care
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fort Collins. Key Benefits of Guided Tissue Regeneration. Available online: https://www.fortcollinsperio.com/benefits-of-guided-tissue-regeneration (accessed on 5 December 2024).
- Wang, D.; Zhou, X.; Cao, H.; Zhang, H.; Wang, D.; Guo, J.; Wang, J. Barrier membranes for periodontal guided bone regeneration: A potential therapeutic strategy. Front. Mater. 2023, 10, 1220420. [Google Scholar] [CrossRef]
- Liu, W.-S.; Liu, Y.; Gao, J.; Zheng, H.; Lu, Z.-M.; Li, M. Biomembrane-Based Nanostructure- and Microstructure-Loaded Hydrogels for Promoting Chronic Wound Healing. Int. J. Nanomed. 2023, 18, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Chen, X.; Shahabi, S.; Nasiri, N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater. Au 2023, 3, 394–417. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chen, J.-Y.; Suo, W.-H.; Shao, W.-R.; Huang, C.-Y.; Li, M.-T.; Li, Y.-Y.; Li, Y.-H.; Liang, E.-L.; Chen, Y.-H.; et al. Unlocking the Future of Periodontal Regeneration: An Interdisciplinary Approach to Tissue Engineering and Advanced Therapeutics. Biomedicines 2024, 12, 1090. [Google Scholar] [CrossRef]
- Pandit, N.; Malik, R.; Philips, D. Tissue engineering: A new vista in periodontal regeneration. J. Indian Soc. Periodontol. 2011, 15, 328–337. [Google Scholar] [CrossRef]
- Jiamset, I.; Hanprasertpong, J. Impact of diabetes mellitus on oncological outcomes after radical hysterectomy for early stage cervical cancer. J. Gynecol. Oncol. 2016, 27, e28. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Mahasarakham, C.P.N.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef]
- Yadav, J.P.; Verma, A.; Pathak, P.; Dwivedi, A.R.; Singh, A.K.; Kumar, P.; Khalilullah, H.; Jaremko, M.; Emwas, A.-H.; Patel, D.K. Phytoconstituents as modulators of NF-κB signalling: Investigating therapeutic potential for diabetic wound healing. Biomed. Pharmacother. 2024, 177, 117058. [Google Scholar] [CrossRef]
- Wolf, S.J.; Melvin, W.J.; Gallagher, K. Macrophage-mediated inflammation in diabetic wound repair. Semin. Cell Dev. Biol. 2021, 119, 111–118. [Google Scholar] [CrossRef]
- Deptuła, M.; Zieliński, J.; Wardowska, A.; Pikuła, M. Wound healing complications in oncological patients: Perspectives for cellular therapy. Postep. Dermatol. Alergol. 2019, 36, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Słonimska, P.; Sachadyn, P.; Zieliński, J.; Skrzypski, M.; Pikuła, M. Chemotherapy-Mediated Complications of Wound Healing: An Understudied Side Effect. Adv. Wound Care 2024, 13, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Bajpai, D.; Rajasekar, A. Recent advances in GTR scaffolds. Bioinformation 2022, 18, 1181–1185. [Google Scholar] [CrossRef]
- Aryal, R.; Chen, X.p.; Fang, C.; Hu, Y.c. Bone Morphogenetic Protein-2 and Vascular Endothelial Growth Factor in Bone Tissue Regeneration: New Insight and Perspectives. Orthop. Surg. 2014, 6, 171–178. [Google Scholar] [CrossRef]
- Hassan, S.U.; Bilal, B.; Nazir, M.S.; Naqvi, S.A.R.; Ali, Z.; Nadeem, S.; Muhammad, N.; Palvasha, B.A.; Mohyuddin, A. Recent progress in materials development and biological properties of GTR membranes for periodontal regeneration. Chem. Biol. Drug Des. 2021, 98, 1007–1024. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Quteish, D.; Dolby, A.E. The use of irradiated-crosslinked human collagen membrane in guided tissue regeneration. J. Clin. Periodontol. 1992, 19, 476–484. [Google Scholar] [CrossRef]
- Solomon, S.-M.; Sufaru, I.-G.; Teslaru, S.; Ghiciuc, C.; Stafie, C. Finding the Perfect Membrane: Current Knowledge on Barrier Membranes in Regenerative Procedures: A Descriptive Review. Appl. Sci. 2022, 12, 1042. [Google Scholar] [CrossRef]
- Choi, J.F.; Chang, P. Oral Surgery, Extraction of Unerupted Teeth; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- International Diabetes Federation. Facts & Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 5 December 2024).
- International Agency for Research on Cancer. Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020. Available online: https://www.iarc.who.int/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/ (accessed on 5 December 2024).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Yamaguchi, S.; Sato, Y.; Harada, K. Sphere-Derived Multipotent Progenitor Cells Obtained From Human Oral Mucosa Are Enriched in Neural Crest Cells. Stem Cells Transl. Med. 2015, 5, 117–128. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Georgopoulou, A.; Grivas, I.; Bekiari, C.; Prymak, O.; Loza, Κ.; Epple, M.; Papadopoulos, G.C.; Koidis, P.; Chatzinikolaidou, Μ. Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent. Mater. 2019, 35, 310–327. [Google Scholar] [CrossRef]
- Barthel, E.R.; Levin, D.E.; Speer, A.L.; Sala, F.G.; Torashima, Y.; Hou, X.; Grikscheit, T.C. Human tissue-engineered colon forms from postnatal progenitor cells: An in vivo murine model. Regen. Med. 2012, 7, 807–818. [Google Scholar] [CrossRef]
- Basu, A.; Rothermund, K.; Ahmed, M.N.; Syed-Picard, F.N. Self-Assembly of an Organized Cementum-Periodontal Ligament-Like Complex Using Scaffold-Free Tissue Engineering. Front. Physiol. 2019, 10, 422. [Google Scholar] [CrossRef]
- Graziano, A.; d’Aquino, R.; Angelis, M.G.C.-D.; Laino, G.; Piattelli, A.; Pacifici, M.; De Rosa, A.; Papaccio, G. Concave pit-containing scaffold surfaces improve stem cell-derived osteoblast performance and lead to significant bone tissue formation. PLoS ONE 2007, 2, e496. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Ma, Y.; Tan, S.; Ren, B.; Liu, S.; Dai, H.; Xu, Z. Application of dental pulp stem cells in oral maxillofacial tissue engineering. Int. J. Med. Sci. 2022, 19, 310–320. [Google Scholar] [CrossRef]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Alqahtani, A.M. Guided Tissue and Bone Regeneration Membranes: A Review of Biomaterials and Techniques for Periodontal Treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef]
- Gao, P.; Kajiya, M.; Motoike, S.; Ikeya, M.; Yang, J. Application of mesenchymal stem/stromal cells in periodontal regeneration: Opportunities and challenges. Jpn. Dent. Sci. Rev. 2024, 60, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Angjelova, A.; Jovanova, E.; Polizzi, A.; Annunziata, M.; Laganà, L.; Santonocito, S.; Isola, G. Insights and Advancements in Periodontal Tissue Engineering and Bone Regeneration. Medicina 2024, 60, 773. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Velikic, G.; Maric, D.M.; Maric, D.L.; Supic, G.; Puletic, M.; Dulic, O.; Vojvodic, D. Harnessing the Stem Cell Niche in Regenerative Medicine: Innovative Avenue to Combat Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 993. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Chu, D.-T.; Nguyen, T.T.; Tien, N.L.B.; Tran, D.-K.; Jeong, J.-H.; Anh, P.G.; Thanh, V.V.; Truong, D.T.; Dinh, T.C. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells 2020, 9, 563. [Google Scholar] [CrossRef]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Eslaminejad, M.B.; Seitz, H. Advancements in hydrogel design for articular cartilage regeneration: A comprehensive review. Bioact. Mater. 2024, 43, 1–31. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A.A. Asymmetric resorbable-based dental barrier membrane for periodontal guided tissue regeneration and guided bone regeneration: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2157–2182. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Liu, T.; Liu, H.; Huang, L. Application of BMP in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef]
- Pramanik, S.; Aggarwal, A.; Kadi, A.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Koul, K.; Deepak, A.; Bellucci, S. Chitosan alchemy: Transforming tissue engineering and wound healing. RSC Adv. 2024, 14, 19219–19256. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Zamani, M.; Huang, N.F. Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering. J. Cardiovasc. Dev. Dis. 2021, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. NPJ Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Murphy, K.G.; Gunsolley, J.C. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann. Periodontol. 2003, 8, 266–302. [Google Scholar] [CrossRef]
- Hamdy, T.M. Dental Biomaterial Scaffolds in Tooth Tissue Engineering: A Review. Curr. Oral. Health Rep. 2023, 10, 14–21. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Lin, J.; Zhang, S. Challenges to Improve Bone Healing Under Diabetic Conditions. Front. Endocrinol. 2022, 13, 861878. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Liu, J.; Wei, L.; Wu, G. BMP-functionalised coatings to promote osteogenesis for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 10150–10168. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, S.; Shi, B.; Wang, Y.; Chen, Y.; Wang, X.; Lee, E.-S.; Jiang, H.-B. Advances in Modification Methods Based on Biodegradable Membranes in Guided Bone/Tissue Regeneration: A Review. Polymers 2022, 14, 871. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.C.; Kraynak, C.A.; Huang, W.; Suggs, L.J. Cell-Inspired Biomaterials for Modulating Inflammation. Tissue Eng. Part. B Rev. 2022, 28, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun Biomimetic Nanofibrous Scaffolds: A Promising Prospect for Bone Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- DuRaine, G.D.; Brown, W.E.; Hu, J.C.; Athanasiou, K.A. Emergence of Scaffold-free Approaches for Tissue Engineering Musculoskeletal Cartilages. Ann. Biomed. Eng. 2014, 43, 543–554. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Forum on Regenerative Medicine. Emerging Technologies and Innovation in Manufacturing Regenerative Medicine Therapies: Proceedings of a Workshop—In Brief; National Academies Press (US): Washington, DC, USA, 2024. [Google Scholar]
- Yang, Z.; Wu, C.; Shi, H.; Luo, X.; Sun, H.; Wang, Q.; Zhang, D. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front. Bioeng. Biotechnol. 2022, 10, 921576. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Database (Date of Search) | Search Strategies | Number of Articles Found |
|---|---|---|
| PubMed (7 November 2023) | ((“Membranes, Artificial”[Mesh]) OR (“Tissue Scaffolds”[Mesh]) OR (“absorbable membrane”[tiab]) OR (“absorbable membranes”[tiab]) OR (“Semi-absorbable membrane”[tiab]) OR (“Semi-absorbable membranes”[tiab]) OR (“non-absorbable membranes”[tiab]) OR (“non-absorbable membrane”[tiab]) OR (“Barrier Membrane”[tiab]) OR (“Barrier Membranes”[tiab]) OR (“synthetic membrane”[tiab]) OR (“synthetic membranes”[tiab])) AND ((“Guided Tissue Regeneration”[Mesh]) OR (“Bone Growth”[tiab]) OR (“Tissue Engineering”[Mesh]) OR (“Guided Tissue Regeneration, Periodontal”[Mesh]) OR (“Bone Regeneration”[Mesh]) OR (“Nerve Regeneration”[Mesh])) AND ((“Immunocompromised Host”[Mesh]) OR (“immunodeficiency”[tiab]) OR (“immunocompromised”[tiab]) OR (“immunosuppressed”[tiab])) NOT ((“Review”[Publication Type]) OR (“Meta-analysis”[Publication Type]) OR (“Systematic review”[Publication Type]) OR (“Case reports”[Publication Type]) OR (“Editorial”[Publication Type]) OR (“News”[Publication Type]) OR (“Newspaper Article”[Publication Type])) | 66 |
| Cochrane Library—Clinical Trials (7 November 2023) | (((Artificial Membranes) OR (Artificial Membrane) OR (Tissue Scaffolds) OR (Tissue Scaffold) OR (absorbable membrane) OR (absorbable membranes) OR (Semi-absorbable membrane) OR (Semi-absorbable membranes) OR (non-absorbable membranes) OR (non-absorbable membrane) OR (Barrier Membrane) OR (Barrier Membranes) OR (synthetic membrane) OR (synthetic membranes)) AND ((Guided Tissue Regeneration) OR (Bone Regeneration) OR (Bone Regenerations) OR (Periodontal Guided Tissue Regeneration) OR (Guided Periodontal Tissue Regeneration) OR (Tissue Engineering) OR (Nerve Regeneration) OR (Nerve Tissue Regeneration) OR (Nervous Tissue Regeneration) OR (Neural Tissue Regeneration)) AND ((Immunocompromised Host) OR (Immunosuppressed Host) OR (Immunocompromised Patient) OR (Immunocompromised Hosts) OR (Immunosuppressed Hosts) OR (immunodeficiency) OR (immunocompromised) OR (immunosuppressed))):ti,ab,kw | 2 |
| Ovid (7 November 2023) | ((Artificial Membranes) OR (Artificial Membrane) OR (Tissue Scaffolds) OR (Tissue Scaffold) OR (absorbable membrane) OR (absorbable membranes) OR (Semi-absorbable membrane) OR (Semi-absorbable membranes) OR (non-absorbable membranes) OR (non-absorbable membrane) OR (Barrier Membrane) OR (Barrier Membranes) OR (synthetic membrane) OR (synthetic membranes)).ti,ab. AND ((Guided Tissue Regeneration) OR (Bone Regeneration) OR (Bone Regenerations) OR (Periodontal Guided Tissue Regeneration) OR (Guided Periodontal Tissue Regeneration) OR (Tissue Engineering) OR (Nerve Regeneration) OR (Nerve Tissue Regeneration) OR (Nervous Tissue Regeneration) OR (Neural Tissue Regeneration)).ti,ab. AND ((Immunocompromised Host) OR (Immunosuppressed Host) OR (Immunocompromised Patient) OR (Immunocompromised Hosts) OR (Immunosuppressed Hosts) OR (immunodeficiency) OR (immunocompromised) OR (immunosuppressed)).ti,ab. | 1 |
| Scopus (7 November 2023) | TITLE-ABS (((artificial AND membranes) OR (artificial AND membrane) OR (tissue AND scaffolds) OR (tissue AND scaffold) OR (absorbable AND membrane) OR (absorbable AND membranes) OR (semi-absorbable AND membrane) OR (semi-absorbable AND membranes) OR (non-absorbable AND membranes) OR (non-absorbable AND membrane) OR (barrier AND membrane) OR (barrier AND membranes) OR (synthetic AND membrane) OR (synthetic AND membranes)) AND ((guided AND tissue AND regeneration) OR (bone AND regeneration) OR (bone AND regenerations) OR (periodontal AND guided AND tissue AND regeneration) OR (guided AND periodontal AND tissue AND regeneration) OR (tissue AND engineering) OR (nerve AND regeneration) OR (nerve AND tissue AND regeneration) OR (nervous AND tissue AND regeneration) OR (neural AND tissue AND regeneration)) AND ((immunocompromised AND host) OR (immunosuppressed AND host) OR (immunocompromised AND patient) OR (immunocompromised AND hosts) OR (immunosuppressed AND hosts) OR (immunodeficiency) OR (immunocompromised) OR (immunosuppressed))) AND (EXCLUDE (DOCTYPE , “re”) OR EXCLUDE (DOCTYPE , “ch”) OR EXCLUDE (DOCTYPE , “cp”)) AND (LIMIT-TO (LANGUAGE , “English”)) | 79 |
| Web of Science (7 November 2023) | (TI = (((Artificial Membranes) OR (Artificial Membrane) OR (Tissue Scaffolds) OR (Tissue Scaffold) OR (absorbable membrane) OR (absorbable membranes) OR (Semi-absorbable membrane) OR (Semi-absorbable membranes) OR (non-absorbable membranes) OR (non-absorbable membrane) OR (Barrier Membrane) OR (Barrier Membranes) OR (synthetic membrane) OR (synthetic membranes)) AND ((Guided Tissue Regeneration) OR (Bone Regeneration) OR (Bone Regenerations) OR (Periodontal Guided Tissue Regeneration) OR (Guided Periodontal Tissue Regeneration) OR (Tissue Engineering) OR (Nerve Regeneration) OR (Nerve Tissue Regeneration) OR (Nervous Tissue Regeneration) OR (Neural Tissue Regeneration)) AND ((Immunocompromised Host) OR (Immunosuppressed Host) OR (Immunocompromised Patient) OR (Immunocompromised Hosts) OR (Immunosuppressed Hosts) OR (immunodeficiency) OR (immunocompromised) OR (immunosuppressed)))) OR AB = (((Artificial Membranes) OR (Artificial Membrane) OR (Tissue Scaffolds) OR (Tissue Scaffold) OR (absorbable membrane) OR (absorbable membranes) OR (Semi-absorbable membrane) OR (Semi-absorbable membranes) OR (non-absorbable membranes) OR (non-absorbable membrane) OR (Barrier Membrane) OR (Barrier Membranes) OR (synthetic membrane) OR (synthetic membranes)) AND ((Guided Tissue Regeneration) OR (Bone Regeneration) OR (Bone Regenerations) OR (Periodontal Guided Tissue Regeneration) OR (Guided Periodontal Tissue Regeneration) OR (Tissue Engineering) OR (Nerve Regeneration) OR (Nerve Tissue Regeneration) OR (Nervous Tissue Regeneration) OR (Neural Tissue Regeneration)) AND ((Immunocompromised Host) OR (Immunosuppressed Host) OR (Immunocompromised Patient) OR (Immunocompromised Hosts) OR (Immunosuppressed Hosts) OR (immunodeficiency) OR (immunocompromised) OR (immunosuppressed))) | 70 |
| Author (Year) | Country | Study Aims | Findings |
|---|---|---|---|
| Abe et al. (2015) [27] | Japan | To isolate NCSCs from oral mucosa using the neurosphere technique and to establish effective in vivo bone tissue regeneration methods. | OMSFCs have similar properties to NCSCs (self-renewing capabilities and multipotency); OMSFCs were able to generate ectopic bone tissues even in a region not naturally containing hard tissue; regenerated acellular-type hard tissues from the OMSFCs form cementum-like structures, and the use of the neurosphere culture technique allows for a more simplified method for isolating and culturing OMSFCs. |
| Bakopoulou et al. (2019) [28] | Greece | To investigate the potential of combining biomimetic chitosan/gelatin (CS/Gel) scaffolds with dental pulp stem cells (DPSCs) for orofacial bone reconstruction. | This study demonstrated the successful production of a nanocrystalline, mineralized matrix over time in both in vitro and in vivo settings, with CS/Gel-0.1 scaffolds showing more effective upregulation of osteo/odontogenic genes and enhanced bone formation when pre-treated with recombinant human BMP-2. |
| Barthel et al. (2012) [29] | United States | To develop tissue-engineered colons (TECs) from postnatal human organoid units and evaluate their potential for colon tissue regeneration by implanting them into immunocompromised mice. | A TEC was successfully generated from postnatal human organoid units implanted onto biodegradable scaffolds in immunocompromised mice, demonstrating differentiation into mature colon epithelium with supporting mesenchymal components, indicating a potential transition towards human therapy. |
| Basu et al. (2019) [30] | United States | To develop scaffold-free tissue constructs using periodontal ligament cells (PDLCs) that self-assemble into an organized multi-tissue structure mimicking the complex composition of periodontal tissues, specifically comprising a mineralized cementum-like core surrounded by a periodontal ligament (PDL)-like tissue. | Scaffold-free tissue constructs engineered from PDLCs demonstrated an organized multi-tissue structure comprising a mineralized cementum-like core and a PDL-like tissue, which maintained its structural and biochemical characteristics both in vitro and in vivo. |
| Graziano et al. (2007) [31] | Italy | To understand whether a microcavity-rich scaffold had distinct bone-forming capabilities compared to a smooth one. | Cells on the microcavity-rich scaffold released larger amounts of BMP-2 and VEGF into the culture medium and expressed higher alkaline phosphatase activity. The microcavity-rich scaffold enhanced cell adhesion: the cells created initimate contact with secondary microcavities and were polarized. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, M.; Sanders, K.; Deshpande, A.; Trivedi, R.; Schwartz, C.; Mohajeri, A. Guided Tissue Regeneration Membranes: Review of Innovations and Applications in Immunocompromised Patients. Appl. Sci. 2025, 15, 1145. https://doi.org/10.3390/app15031145
Hung M, Sanders K, Deshpande A, Trivedi R, Schwartz C, Mohajeri A. Guided Tissue Regeneration Membranes: Review of Innovations and Applications in Immunocompromised Patients. Applied Sciences. 2025; 15(3):1145. https://doi.org/10.3390/app15031145
Chicago/Turabian StyleHung, Man, Katherine Sanders, Aditya Deshpande, Roshni Trivedi, Connor Schwartz, and Amir Mohajeri. 2025. "Guided Tissue Regeneration Membranes: Review of Innovations and Applications in Immunocompromised Patients" Applied Sciences 15, no. 3: 1145. https://doi.org/10.3390/app15031145
APA StyleHung, M., Sanders, K., Deshpande, A., Trivedi, R., Schwartz, C., & Mohajeri, A. (2025). Guided Tissue Regeneration Membranes: Review of Innovations and Applications in Immunocompromised Patients. Applied Sciences, 15(3), 1145. https://doi.org/10.3390/app15031145







