Developmental Defects of Enamel and Dental Caries in Pediatric Patients with Chronic Kidney Disease–Mineral Bone Disorders
Abstract
:1. Introduction: Epidemiology of CKD in Pediatric Patients and Main Complications of CKD in Pediatric Patients
2. CKD and Calcium–Phosphorus Metabolism in Pediatric Patients
3. Vitamin D and CKD in Pediatric Patients
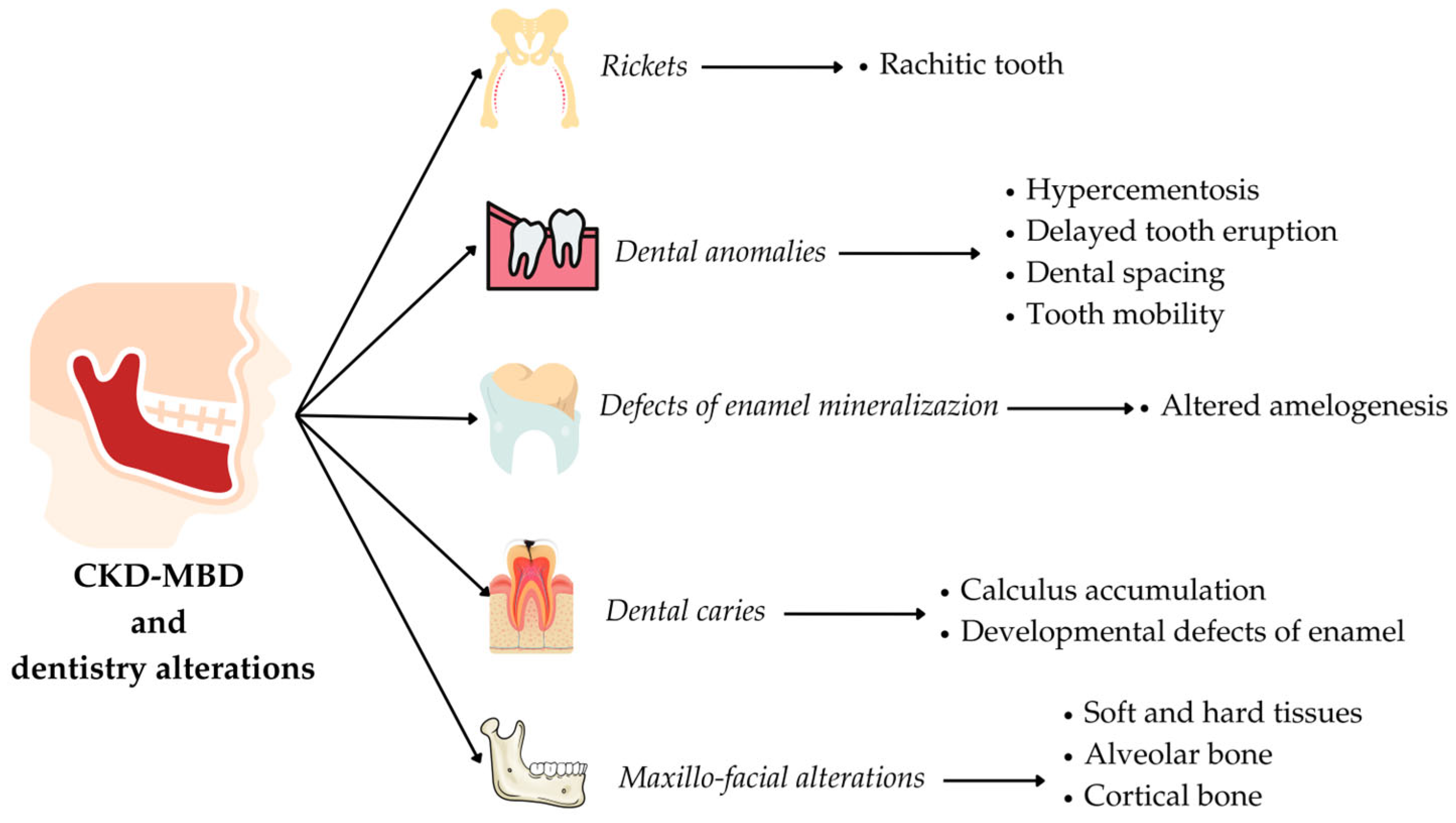
4. Alteration of Calcium–Phosphorus Metabolism and Oral–Maxillofacial Manifestations in Pediatric CKD Patients
4.1. Alteration of Enamel Mineralization
Diagnosis and Treatment of DDEs
4.2. Dental Caries
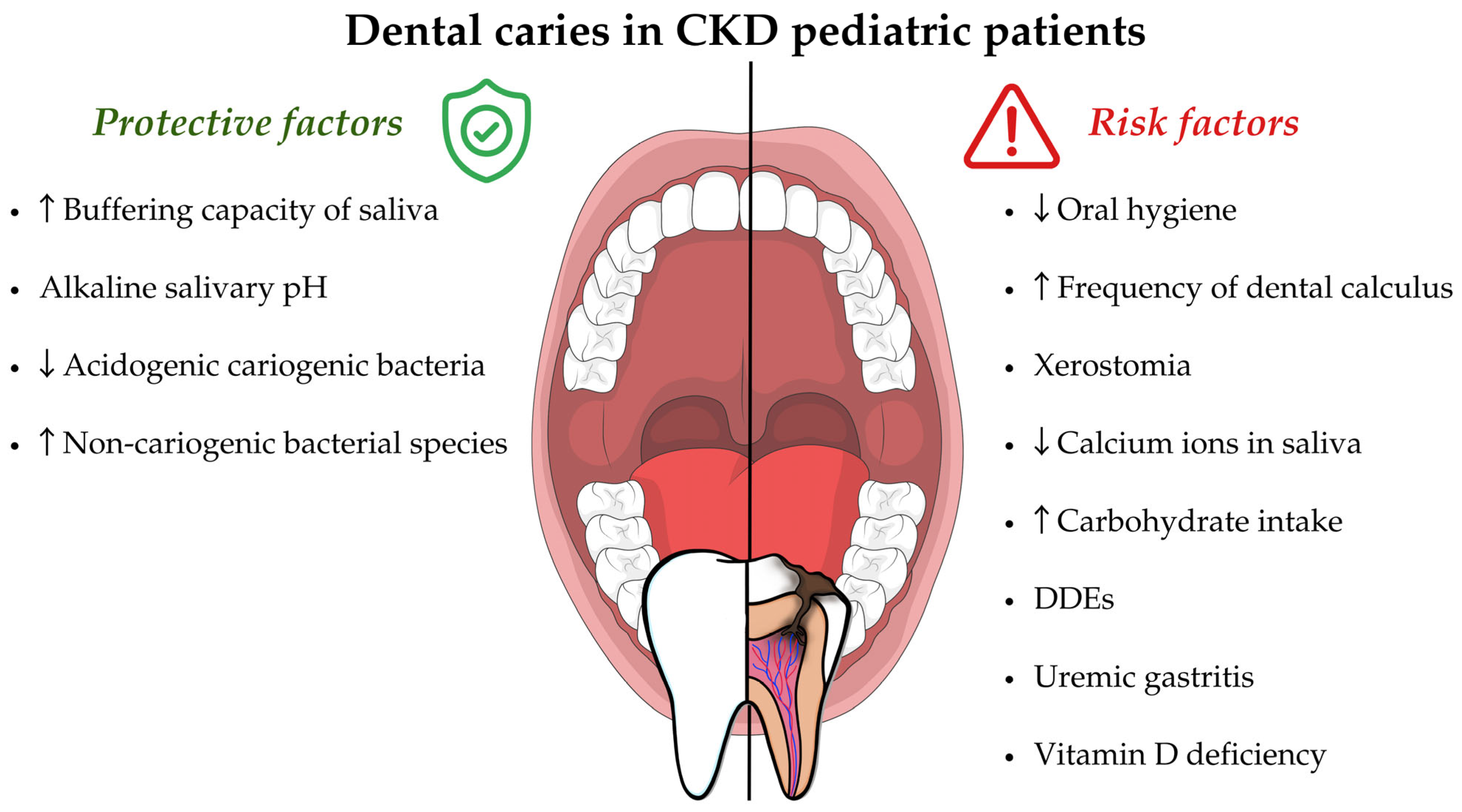
- Oral hygiene. Oral hygiene in CKD pediatric patients is poor compared to the control group of healthy subjects [61,66,84]. Some authors underline how there are differences in oral hygiene between CKD patients in the different stages of the disease. Indeed, the highest mean of the simplified oral hygiene index (OHI-S) score was observed in stage 4–5 children (2.10 ± 1.08), followed by kidney-transplanted children (1.46 ± 1.19), by stage 1–3 (1.27 ± 0.61) and by healthy children (0.45 ± 0.44), respectively [62]. Poor oral hygiene is directly related to the increased risk of dental caries formation.
- Dental calculus. Dental calculus in CKD patients is more frequent than in healthy subjects [62] and can also occur in 57.5% of CKD pediatric patients [67], especially in dialysis patients [105]. Dental calculus accumulation can cause difficulties in home oral hygiene, represent a retentive factor for dental plaque, and thus, can increase the risk of dental caries and periodontal disease. Dental calculus formation is determined by the precipitation of salivary calcium–phosphorus and calcium oxalate due to the high salivary pH and the changes in mineral balance with high levels of salivary urea and phosphate [105].It is crucial to emphasize that CKD patients should be included and monitored in a preventive oral hygiene program. Frequent dental check-ups and non-surgical periodontal therapy sessions are important not only for adult CKD patients but also for pediatric ones. Indeed, the pediatric CKD patients may also exhibit alterations related to the periodontal attachment apparatus, such as gingival inflammation, gingival overgrowth, gingival recessions and, rarely, periodontal attachment loss [106]. Although these diseases are beyond the aim of this review, they must be diagnosed and treated early in order to ensure oral health. The oral prophylaxis intervention is related to the baseline degree of inflammation, assessed by periodontal indices (like gingival index, papilla bleeding index and periodontal screening index) and includes professional mechanical plaque removal, the local application of chlorhexidine gel, mouth rinses, oral hygiene instructions/motivation and periodic follow-ups [107].Moreover, the oral prophylaxis intervention for CKD children/adolescents with an accelerated rate of dental calculus formation should include the professional mechanical calculus removal (scaling). The scaling must be performed at least every three months [108].
- Xerostomia. In CKD patients, xerostomia (decreased salivary flow) can be caused by a restricted fluid intake and by a side effect of drug therapy [109]. Several studies also state that salivary flow tends to decrease in CKD and in hemodialysis patients [49,110]. The decreased salivary flow could cause important alterations of its function [111]. In fact, saliva exerts various protective functions against caries development through the dilution and the clearing of the oral cavity, the buffering capacity, the antimicrobial and immune activity and the ion exchange capacity on tooth surfaces (re-mineralization) [112].
- Diet. A diet characterized by a controlled protein intake and a high caloric intake derived from carbohydrate, that is recommended for CKD conservative patients, could be another risk factor for caries disease [50,114], whereas in hemodialysis patients the nutritional-dietetic treatment is characterized by a high protein intake (1.1–1.2 g/kg of ideal body weight/day) and a normal-high caloric intake (30–35 kcal/kg of ideal body weight/day) [115,116].In this regard, a study conducted on CKD patients undergoing hemodialysis highlighted how a higher sugar intake, more frequent in CKD patients than healthy controls, was associated with an altered oral microbiota (characterized by an increase in the supragingival plaque samples of Streptococcus mutans, Lactobacillus salivarius, Lactobacillus fermentum, Lactobacillus vaginalis, Scardovia wiggsiae F0424 and Actinomyces naeslundii) and a higher DMFT [101].
- DDEs. The DDEs are risk factors for the development of carious lesions [91]. Enamel defects with their rough and irregular surfaces can promote the accumulation of plaque and the determination of a cariogenic bacterial ecological niche [117]. Furthermore, teeth with DDEs have a lower enamel hardness and are subject to wear, especially when the thin enamel layer is subjected to masticatory loads [48]. Moreover, they are shown to have a lower resistance to acid insult when compared to healthy enamel [81].On the contrary, CKD patients show a lower percentage of dental caries despite having a high prevalence of DDEs compared to healthy subjects [62]. This observation could be explained by analyzing the protective factors of dental caries in CKD subjects.
- Uremic gastritis. CKD patients, especially in more advanced stages, are often characterized by a condition known as uremic gastritis, that among the possible consequences, may induce gastroesophageal reflux and vomiting. These manifestations can lead to enamel erosion, with a consequent increased risk of dental caries [118].
- Vitamin D Deficiency. Several cross-sectional studies state that vitamin D deficiency is associated with an increased incidence of dental caries in the primary dentition [117,119] and in permanent dentition [120,121]. Moreover, the recent systematic reviews point out a relationship between low serum vitamin D levels and dental caries in children and adolescents in primary dentition [122] and in permanent dentition [123]. In a study by Seminario and Velan [113], several mechanisms are proposed to explain this relationship: (1) the alteration of enamel mineralization with consequent presence of DDEs, which constitute a risk factor for dental caries (as described above); (2) the reduced activation of antimicrobial (AMPs) peptides, such as cathelicidins and defensins, which have antimicrobial properties against several bacteria and participate in the innate immune response [74,124,125,126]; (3) decreased saliva flow and the alteration of its composition by reducing the amount of calcium ions.
- Buffer system and salivary pH. In CKD patients, an increased buffering capacity of saliva and an alkaline salivary pH are observed, probably due to the higher concentration of ammonia obtained from the urea hydrolyzation in the mouth through bacterial ureases [84,127]. In fact, the qualitative composition of saliva in CKD patients tends to change compared to healthy subjects; namely, it is characterized by a higher concentration of urea, creatinine, sodium, potassium, chloride and phosphorus [128].Menezes et al. also report that the salivary levels of urea and the IgA anti-Streptococcus mutans are higher in CKD patients (especially in chronic maintained hemodialysis patients) than in the healthy group and it may be a protective factor against dental caries [83]. According to the study by Al-Nowaiser et al., conducted on CKD children (aged 4–13.6), the mean salivary urea level was significantly higher (11.6 mmol/L) compared to the healthy controls (3.6 mmol/L); a significant difference was also highlighted between the buffering capacity (pH 6.4) compared to the healthy controls (pH 5.6) [66]. Moreover, in a case–control study conducted on hemodialysis pediatric patients (aged 4–17), it was observed that 89.5% of them showed a high salivary buffer capacity [129].The alkaline salivary pH determines the acids neutralization and protects the tooth from demineralization [130]. In fact, the tooth surfaces are continuously subjected to acid attack from organic acids formed by the bacterial fermentation of refined carbohydrates [112] or by an excessive consumption of acidic beverages and foods [131].
- Oral microbiota. The alkaline salivary pH creates an unfavorable environment for acidogenic cariogenic bacteria and ecological shift, modifying the salivary microbiota to non-cariogenic bacterial species [48]. In a study conducted on hemodialysis patients (aged 4–17), the salivary levels of Streptococcus mutans and Lactobacilli were significantly lower than in the control group of healthy subjects [129]. The same evidence was highlighted by a further study conducted on hemodialyzed children [84]. On the contrary, in kidney-transplanted patients, a decrease in oral pH is observed, which could modify the oral microbiota and promote the onset of caries pathology, especially in those subjects who have poor eating habits (rich in free sugars) [48]. Furthermore, kidney-transplanted patients may take immunosuppressive drugs (such as cyclosporine A) and antihypertensive calcium-blocking agents, which can determine gingival overgrowth (Drug-Induced Gingival Overgrowth—DIGO) and complicate daily tooth brushing maneuvers for the removal of dental plaque [132]. In a recent study, it was found that 16.7% of pediatric subjects undergoing kidney transplant had DIGO, especially grade 1 [67].
5. Natural Bioactive Compounds as Innovative Tools in the Prevention and Adjuvant Treatment of Remineralization and Dental Caries in CKD Pediatric Patients
6. Conclusions
- -
- Developmental defects of enamel related to an altered amelogenesis, both primary and permanent teeth.
- -
- Rachitic teeth with anomalies of enamel mineralization, dentin hypomineralization and various degrees of interglobular dentine.
- -
- Dental caries related to poor oral hygiene, dental calculus accumulation, xerostomia, the alteration of the remineralization mechanism, vitamin D deficiency and DDEs; on the other hand, the CKD could determine the presence of protective factors for dental caries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Lamb, E.J.; Levey, A.S.; Stevens, P.E. The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: Evolution not revolution. Clin. Chem. 2013, 59, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Warady, B.A.; Chadha, V. Chronic kidney disease in children: The global perspective. Pediatr. Nephrol. 2007, 22, 1999–2009. [Google Scholar] [CrossRef]
- Harambat, J.; van Stralen, K.J.; Kim, J.J.; Tizard, E.J. Epidemiology of chronic kidney disease in children. Pediatr. Nephrol. 2012, 27, 363–373. [Google Scholar] [CrossRef]
- Furth, S.L.; Abraham, A.G.; Jerry-Fluker, J.; Schwartz, G.J.; Benfield, M.; Kaskel, F.; Wong, C.; Mak, R.H.; Moxey-Mims, M.; Warady, B.A. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2132–2140. [Google Scholar] [CrossRef]
- Haffner, D. Strategies for Optimizing Growth in Children With Chronic Kidney Disease. Front. Pediatr. 2020, 8, 399. [Google Scholar] [CrossRef]
- Santos, F.; Carbajo-Perez, E.; Rodriguez, J.; Fernandez-Fuente, M.; Molinos, I.; Amil, B.; Garcia, E. Alterations of the growth plate in chronic renal failure. Pediatr. Nephrol. 2005, 20, 330–334. [Google Scholar] [CrossRef]
- Silverstein, D.M. Growth and Nutrition in Pediatric Chronic Kidney Disease. Front. Pediatr. 2018, 6, 205. [Google Scholar] [CrossRef]
- Rees, L.; Mak, R.H. Nutrition and growth in children with chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Norman, L.J.; Coleman, J.E.; Macdonald, I.A.; Tomsett, A.M.; Watson, A.R. Nutrition and growth in relation to severity of renal disease in children. Pediatr. Nephrol. 2000, 15, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Waziri, B.; Duarte, R.; Naicker, S. Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Current Perspectives. Int. J. Nephrol. Renovasc. Dis. 2019, 12, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, D.; Rusu, C.; Kacso, I.M.; Potra, A.; Patiu, I.M.; Gherman-Caprioara, M. Mineral and bone disorders, morbidity and mortality in end-stage renal failure patients on chronic dialysis. Clujul Med. 2016, 89, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.; Drueke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G.; et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef]
- Moe, S.M.; Drueke, T.; Lameire, N.; Eknoyan, G. Chronic kidney disease-mineral-bone disorder: A new paradigm. Adv. Chronic Kidney Dis. 2007, 14, 3–12. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Bricker, N.S. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N. Engl. J. Med. 1972, 286, 1093–1099. [Google Scholar] [CrossRef]
- Jowsey, J.; Reiss, E.; Canterbury, J.M. Long-term effects of high phosphate intake on parathyroid hormone levels and bone metabolism. Acta Orthop. Scand. 1974, 45, 801–808. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Pastor, J.; Nakatani, T.; Lanske, B.; Razzaque, M.S.; Rosenblatt, K.P.; Baum, M.G.; Kuro-o, M.; et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010, 24, 3438–3450. [Google Scholar] [CrossRef]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Klotho and kidney disease. J. Nephrol. 2010, 23 (Suppl. 16), S136–S144. [Google Scholar] [PubMed]
- Hsu, A.C.; Kooh, S.W.; Fraser, D.; Cumming, W.A.; Fornasier, V.L. Renal osteodystrophy in children with chronic renal failure: An unexpectedly common and incapactating complication. Pediatrics 1982, 70, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, K.; Bakkaloglu, S.; Salusky, I. Chronic kidney disease mineral and bone disorder in children. Pediatr. Nephrol. 2008, 23, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Denburg, M.R.; Kumar, J.; Jemielita, T.; Brooks, E.R.; Skversky, A.; Portale, A.A.; Salusky, I.B.; Warady, B.A.; Furth, S.L.; Leonard, M.B. Fracture Burden and Risk Factors in Childhood CKD: Results from the CKiD Cohort Study. J. Am. Soc. Nephrol. 2016, 27, 543–550. [Google Scholar] [CrossRef]
- Groothoff, J.W.; Offringa, M.; Van Eck-Smit, B.L.; Gruppen, M.P.; Van De Kar, N.J.; Wolff, E.D.; Lilien, M.R.; Davin, J.C.; Heymans, H.S.; Dekker, F.W. Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int. 2003, 63, 266–275. [Google Scholar] [CrossRef]
- Denburg, M.R.; Tsampalieros, A.K.; de Boer, I.H.; Shults, J.; Kalkwarf, H.J.; Zemel, B.S.; Foerster, D.; Stokes, D.; Leonard, M.B. Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J. Clin. Endocrinol. Metab. 2013, 98, 1930–1938. [Google Scholar] [CrossRef]
- Sanchez, C.P. Secondary hyperparathyroidism in children with chronic renal failure: Pathogenesis and treatment. Paediatr. Drugs 2003, 5, 763–776. [Google Scholar] [CrossRef]
- Waller, S. Parathyroid hormone and growth in chronic kidney disease. Pediatr. Nephrol. 2011, 26, 195–204. [Google Scholar] [CrossRef]
- Waller, S.C.; Ridout, D.; Cantor, T.; Rees, L. Parathyroid hormone and growth in children with chronic renal failure. Kidney Int. 2005, 67, 2338–2345. [Google Scholar] [CrossRef]
- Klaus, G.; Watson, A.; Edefonti, A.; Fischbach, M.; Ronnholm, K.; Schaefer, F.; Simkova, E.; Stefanidis, C.J.; Strazdins, V.; Vande Walle, J.; et al. Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr. Nephrol. 2006, 21, 151–159. [Google Scholar] [CrossRef]
- Kemper, M.J.; van Husen, M. Renal osteodystrophy in children: Pathogenesis, diagnosis and treatment. Curr. Opin. Pediatr. 2014, 26, 180–186. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Salusky, I.B.; Malluche, H.; Nickolas, T.L. Renal osteodystrophy: Something old, something new, something needed. Curr. Opin. Nephrol. Hypertens. 2023, 32, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kari, J.A.; El Desoky, S.M.; El-Morshedy, S.M.; Habib, H.S. Vitamin D insufficiency and deficiency in children with chronic kidney disease. Ann. Saudi Med. 2012, 32, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Solarin, A.U.; Nourse, P.; Gajjar, P. Vitamin D status of children with moderate to severe chronic Kidney Disease at a Tertiary Pediatric Center in Cape Town. Saudi J. Kidney Dis. Transpl. 2019, 30, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Franca Gois, P.H.; Wolley, M.; Ranganathan, D.; Seguro, A.C. Vitamin D Deficiency in Chronic Kidney Disease: Recent Evidence and Controversies. Int. J. Environ. Res. Public Health 2018, 15, 1773. [Google Scholar] [CrossRef]
- Cuppari, L.; Garcia-Lopes, M.G. Hypovitaminosis D in chronic kidney disease patients: Prevalence and treatment. J. Ren. Nutr. 2009, 19, 38–43. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Jo, C.H.; Cockrell, G.E.; Moreau, C.S.; Fowlkes, J.L. Enhanced excretion of vitamin D binding protein in type 1 diabetes: A role in vitamin D deficiency? J. Clin. Endocrinol. Metab. 2011, 96, 142–149. [Google Scholar] [CrossRef]
- Andress, D.L. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006, 69, 33–43. [Google Scholar] [CrossRef]
- Jacob, A.I.; Sallman, A.; Santiz, Z.; Hollis, B.W. Defective photoproduction of cholecalciferol in normal and uremic humans. J. Nutr. 1984, 114, 1313–1319. [Google Scholar] [CrossRef]
- DeLuca, H.F. The metabolism and functions of vitamin D. Adv. Exp. Med. Biol. 1986, 196, 361–375. [Google Scholar] [CrossRef]
- Dahash, B.A.; Sankararaman, S. Rickets; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wharton, B.; Bishop, N. Rickets. Lancet 2003, 362, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Makitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. Horm. Res. Paediatr. 2016, 85, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.Y.; Gordon, C.M. Vitamin D deficiency in children and adolescents: Epidemiology, impact and treatment. Rev. Endocr. Metab. Disord. 2008, 9, 161–170. [Google Scholar] [CrossRef]
- Kanjevac, T.; Bijelic, B.; Brajkovic, D.; Vasovic, M.; Stolic, R. Impact of Chronic Kidney Disease Mineral and Bone Disorder on Jaw and Alveolar Bone Metabolism: A Narrative Review. Oral Health Prev. Dent. 2018, 16, 79–85. [Google Scholar] [CrossRef]
- Yamashita, S.; Kondo, Y.; Watanabe, C.; Nodai, T.; Munemasa, T.; Mukaibo, T.; Masaki, C.; Shibata, Y.; Hosokawa, R. Chronic kidney disease compromises structural and mechanical properties of maxillary cortical bone in a rat model. J. Prosthodont. Res. 2024, 68, 264–272. [Google Scholar] [CrossRef]
- Velan, E.; Sheller, B. Oral health in children with chronic kidney disease. Pediatr. Nephrol. 2021, 36, 3067–3075. [Google Scholar] [CrossRef]
- Albagieh, H.; Alosimi, A.; Aldhuhayan, A.; AlAbdulkarim, A.; Fatani, B.; Alabood, A. Dental management of patients with renal diseases or undergoing renal transplant. Saudi Dent. J. 2024, 36, 270–276. [Google Scholar] [CrossRef]
- Elhusseiny, G.A.; Saleh, W. Oral Health in Children with Chronic Kidney Disease, Hemodialysis, and Renal Transplantation: A Comprehensive Narrative Review of the Oral Manifestations and Dental Implications. Clin. Med. Insights Pediatr. 2024, 18, 11795565241271689. [Google Scholar] [CrossRef]
- Yepes, J.F. Dental Manifestations of Pediatric Bone Disorders. Curr. Osteoporos. Rep. 2017, 15, 588–592. [Google Scholar] [CrossRef]
- Yuan, Q.; Xiong, Q.C.; Gupta, M.; Lopez-Pintor, R.M.; Chen, X.L.; Seriwatanachai, D.; Densmore, M.; Man, Y.; Gong, P. Dental implant treatment for renal failure patients on dialysis: A clinical guideline. Int. J. Oral Sci. 2017, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shavlokhova, V.; Goeppert, B.; Gaida, M.M.; Saravi, B.; Weichel, F.; Vollmer, A.; Vollmer, M.; Freudlsperger, C.; Mertens, C.; Hoffmann, J. Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism: A Case Report with 5 Years Follow-Up and Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 7370. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chang, Z.F.; Chau, Y.P.; Chen, A.; Lee, O.K.; Yang, A.H. Uremia Induces Dental Pulp Ossification but Reciprocally Inhibits Adjacent Alveolar Bone Osteogenesis. Calcif. Tissue Int. 2015, 97, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Costacurta, M.; Basilicata, M.; Marrone, G.; Di Lauro, M.; Campolattano, V.; Bollero, P.; Docimo, R.; Di Daniele, N.; Noce, A. The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients 2022, 14, 2002. [Google Scholar] [CrossRef]
- Basilicata, M.; Di Lauro, M.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Di Daniele, N.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, M.; Abhishek. Oral conditions in renal disorders and treatment considerations—A review for pediatric dentist. Saudi Dent. J. 2015, 27, 113–119. [Google Scholar] [CrossRef]
- Basilicata, M.; Pieri, M.; Marrone, G.; Nicolai, E.; Di Lauro, M.; Paolino, V.; Tomassetti, F.; Vivarini, I.; Bollero, P.; Bernardini, S.; et al. Saliva as Biomarker for Oral and Chronic Degenerative Non-Communicable Diseases. Metabolites 2023, 13, 889. [Google Scholar] [CrossRef]
- Lucas, V.S.; Roberts, G.J. Oro-dental health in children with chronic renal failure and after renal transplantation: A clinical review. Pediatr. Nephrol. 2005, 20, 1388–1394. [Google Scholar] [CrossRef]
- Silva, T.M.C.; Alves, L.A.C.; Garrido, D.; Watanabe, A.; Mendes, F.M.; Ciamponi, A.L. Health and oral health-related quality of life of children and adolescents with chronic kidney disease: A cross-sectional study. Qual. Life Res. 2019, 28, 2481–2489. [Google Scholar] [CrossRef]
- Sezer, B.; Kaya, R.; Kodaman Dokumacigil, N.; Siddikoglu, D.; Guven, S.; Yildiz, N.; Alpay, H.; Kargul, B. Assessment of the oral health status of children with chronic kidney disease. Pediatr. Nephrol. 2023, 38, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Andaloro, C.; Sessa, C.; Bua, N.; Mantia, I. Chronic kidney disease in children: Assessment of oral health status. Dent. Med. Probl. 2018, 55, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Limeira, F.I.R.; Yamauti, M.; Moreira, A.N.; Galdino, T.M.; de Magalhaes, C.S.; Abreu, L.G. Dental caries and developmental defects of enamel in individuals with chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019, 25, 1446–1464. [Google Scholar] [CrossRef] [PubMed]
- Nunn, J.H.; Sharp, J.; Lambert, H.J.; Plant, N.D.; Coulthard, M.G. Oral health in children with renal disease. Pediatr. Nephrol. 2000, 14, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Al-Nowaiser, A.; Roberts, G.J.; Trompeter, R.S.; Wilson, M.; Lucas, V.S. Oral health in children with chronic renal failure. Pediatr. Nephrol. 2003, 18, 39–45. [Google Scholar] [CrossRef]
- Tuma, M.; Silva Andrade, N.; Correia Aires, R.; Cristelli, M.P.; Medina Pestana, J.O.; Gallottini, M. Oral findings in kidney transplant children and adolescents. Int. J. Paediatr. Dent. 2022, 32, 894–902. [Google Scholar] [CrossRef]
- Foster, B.L.; Chu, E.Y.; Hujoel, P.P. Vitamin D in dentoalveolar and oral health. In Feldman and Pike’ s Vitamin D, 5th ed.; Academic Press: London, UK, 2024; Volume 1, pp. 453–484. [Google Scholar]
- Collignon, A.M.; Vergnes, J.N.; Germa, A.; Azogui, S.; Breinig, S.; Hollande, C.; Bonnet, A.L.; Nabet, C. Factors and Mechanisms Involved in Acquired Developmental Defects of Enamel: A Scoping Review. Front. Pediatr. 2022, 10, 836708. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- De Menezes Oliveira, M.A.; Torres, C.P.; Gomes-Silva, J.M.; Chinelatti, M.A.; De Menezes, F.C.; Palma-Dibb, R.G.; Borsatto, M.C. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc. Res. Tech. 2010, 73, 572–577. [Google Scholar] [CrossRef]
- Warady, B.A.; Koch, M.; O’Neal, D.W.; Higginbotham, M.; Harris, D.J.; Hellerstein, S. Plasma fluoride concentration in infants receiving long-term peritoneal dialysis. J. Pediatr. 1989, 115, 436–439. [Google Scholar] [CrossRef]
- Mungai, L.N.W.; Mohammed, Z.; Maina, M.; Anjumanara, O. Vitamin D Review: The Low Hanging Fruit for Human Health. J. Nutr. Metab. 2021, 2021, 6335681. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Proenca, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Dura-Trave, T.; Gallinas-Victoriano, F. Dental caries in children and vitamin D deficiency: A narrative review. Eur. J. Pediatr. 2024, 183, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.L.; Nociti, F.H., Jr.; Somerman, M.J. The rachitic tooth. Endocr. Rev. 2014, 35, 1–34. [Google Scholar] [CrossRef] [PubMed]
- D’Ortenzio, L.; Kahlon, B.; Peacock, T.; Salahuddin, H.; Brickley, M. The rachitic tooth: Refining the use of interglobular dentine in diagnosing vitamin D deficiency. Int. J. Paleopathol. 2018, 22, 101–108. [Google Scholar] [CrossRef]
- D’Ortenzio, L.; Ribot, I.; Kahlon, B.; Bertrand, B.; Bocaege, E.; Raguin, E.; Schattmann, A.; Brickley, M. The rachitic tooth: The use of radiographs as a screening technique. Int. J. Paleopathol. 2018, 23, 32–42. [Google Scholar] [CrossRef]
- Tapalaga, G.; Bumbu, B.A.; Reddy, S.R.; Vutukuru, S.D.; Nalla, A.; Bratosin, F.; Fericean, R.M.; Dumitru, C.; Crisan, D.C.; Nicolae, N.; et al. The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review. Nutrients 2023, 15, 3863. [Google Scholar] [CrossRef]
- Bensi, C.; Costacurta, M.; Belli, S.; Paradiso, D.; Docimo, R. Relationship between preterm birth and developmental defects of enamel: A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2020, 30, 676–686. [Google Scholar] [CrossRef]
- Salanitri, S.; Seow, W.K. Developmental enamel defects in the primary dentition: Aetiology and clinical management. Aust. Dent. J. 2013, 58, 133–140. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Amadeu de Oliveira, F.; Kinoshita, Y.; Yalamanchili, R.R.; Eltilib, L.A.; Andras, N.L.; Narisawa, S.; Tani, T.; Chu, E.Y.; Millan, J.L.; et al. Dentoalveolar Alterations in an Adenine-Induced Chronic Kidney Disease Mouse Model. J. Bone Miner. Res. 2023, 38, 1192–1207. [Google Scholar] [CrossRef]
- Menezes, C.R.; Pereira, A.L.; Ribeiro, C.C.; Chaves, C.O.; Guerra, R.N.; Thomaz, E.B.; Monteiro-Neto, V.; Alves, C.M. Is there association between chronic kidney disease and dental caries? A case-controlled study. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e211–e216. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Moussa, S.; Mostafa, N. The Oral and Dental Health Status in Children Under Haemodialysis. Pediatr. Dent. 2019, 2, 145–156. [Google Scholar] [CrossRef]
- Khalifa, R.; Kammoun, R.; Mansour, L.; Ben Alaya, T.; Ghoul, S. Enamel renal syndrome: A case report with calcifications in pulp, gingivae, dental follicle and kidneys. Spec. Care Dent. 2024, 44, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Misgar, R.A.; Hassan, Z.; Wani, A.I.; Bashir, M.I. Amelogenesis Imperfecta with Distal Renal Tubular Acidosis: A Novel Syndrome? Indian J. Nephrol. 2017, 27, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Therssen, L.; Lambrecht, L.; Vansteenkiste, G.; Knops, N. Abnormal teeth and renal calcifications: Answers. Pediatr. Nephrol. 2023, 38, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Zyla, T.; Kawala, B.; Antoszewska-Smith, J.; Kawala, M. Black stain and dental caries: A review of the literature. Biomed. Res. Int. 2015, 2015, 469392. [Google Scholar] [CrossRef]
- Ribeiro, M.; Fonseca, L.; Anjos, J.S.; Capo-Chichi, J.C.C.; Borges, N.A.; Burrowes, J.; Mafra, D. Oral iron supplementation in patients with chronic kidney disease: Can it be harmful to the gut microbiota? Nutr. Clin. Pract. 2022, 37, 81–93. [Google Scholar] [CrossRef]
- Seow, W.K. Developmental defects of enamel and dentine: Challenges for basic science research and clinical management. Aust. Dent. J. 2014, 59 (Suppl. 1), 143–154. [Google Scholar] [CrossRef]
- Vargas-Ferreira, F.; Salas, M.M.; Nascimento, G.G.; Tarquinio, S.B.; Faggion, C.M., Jr.; Peres, M.A.; Thomson, W.M.; Demarco, F.F. Association between developmental defects of enamel and dental caries: A systematic review and meta-analysis. J. Dent. 2015, 43, 619–628. [Google Scholar] [CrossRef]
- Guerra, F.; Mazur, M.; Corridore, D.; Pasqualotto, D.; Nardi, G.M.; Ottolenghi, L. Evaluation of the esthetic properties of developmental defects of enamel: A spectrophotometric clinical study. Sci. World J. 2015, 2015, 878235. [Google Scholar] [CrossRef]
- Silva, F.; Magno, M.B.; Neves, A.B.; Coqueiro, R.D.S.; Costa, M.C.; Maia, L.C.; Pithon, M.M. Aesthetic perceptions and social judgments about different enamel opacities. Braz. Oral Res. 2020, 34, e049. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, L.; Murri Dello Diago, A.; Silingardi, G.; Spinas, E. “Superficial infiltration to treat white hypomineralized defects of enamel: Clinical trial with 12-month follow-up. J. Biol. Regul. Homeost. Agents 2018, 32, 1335–1338. [Google Scholar] [PubMed]
- Luppieri, V.; Porrelli, D.; Ronfani, L.; Turco, G.; Cadenaro, M. A Resin Infiltration Technique for Molar Hypomineralization Treatment: A Preliminary Study in a Pediatric Population. Pediatr. Dent. 2022, 44, 322–325. [Google Scholar] [PubMed]
- Altan, H.; Yilmaz, R.E. Clinical evaluation of resin infiltration treatment masking effect on hypomineralised enamel surfaces. BMC Oral Health 2023, 23, 444. [Google Scholar] [CrossRef]
- Shah, Y.; Deshpande, A.; Jain, A.; Jaiswal, V.; Andharia, M. Effectiveness of resin infiltration (ICON) and microabrasion-remineralization technique with two remineralizing agents (Tooth Mousse and Toothmin) on permanent incisor hypoplasia—A randomized clinical trial. J. Indian Soc. Pedod. Prev. Dent. 2023, 41, 204–215. [Google Scholar] [CrossRef]
- Perdigao, J. Resin infiltration of enamel white spot lesions: An ultramorphological analysis. J. Esthet. Restor. Dent. 2020, 32, 317–324. [Google Scholar] [CrossRef]
- Brescia, A.V.; Montesani, L.; Fusaroli, D.; Docimo, R.; Di Gennaro, G. Management of Enamel Defects with Resin Infiltration Techniques: Two Years Follow Up Retrospective Study. Children 2022, 9, 1365. [Google Scholar] [CrossRef]
- Pakpour, A.H.; Kumar, S.; Fridlund, B.; Zimmer, S. A case-control study on oral health-related quality of life in kidney disease patients undergoing haemodialysis. Clin. Oral Investig. 2015, 19, 1235–1243. [Google Scholar] [CrossRef]
- Yue, Q.; Yin, F.T.; Zhang, Q.; Yuan, C.; Ye, M.Y.; Wang, X.L.; Li, J.J.; Gan, Y.H. Carious status and supragingival plaque microbiota in hemodialysis patients. PLoS ONE 2018, 13, e0204674. [Google Scholar] [CrossRef]
- Weraarchakul, W.; Weraarchakul, W. Oral Health Status in Pediatric Patients with Renal Disease at Srinagarind Hospital, Khon Kaen University, Thailand. J. Med. Assoc. Thai 2015, 98 (Suppl. 7), S185–S191. [Google Scholar]
- Andrade, M.R.; Antunes, L.A.; Soares, R.M.; Leao, A.T.; Maia, L.C.; Primo, L.G. Lower dental caries prevalence associated to chronic kidney disease: A systematic review. Pediatr. Nephrol. 2014, 29, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Tadakamadla, J.; Kumar, S.; Mamatha, G.P. Comparative evaluation of oral health status of chronic kidney disease (CKD) patients in various stages and healthy controls. Spec. Care Dent. 2014, 34, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, E.; Davidovits, M.; Peretz, B.; Shapira, J.; Aframian, D.J. The correlation between dental calculus and disturbed mineral metabolism in paediatric patients with chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, E.; Schwarz, Z.; Davidovitch, M.; Eidelman, E.; Bimstein, E. Oral findings and periodontal status in children, adolescents and young adults suffering from renal failure. J. Clin. Periodontol. 2005, 32, 1076–1082. [Google Scholar] [CrossRef]
- Hoefer, K.C.; Weber, L.T.; Barbe, A.G.; Graf, I.; Thom, S.; Ehren, R.; Nowag, A.; Wisplinghoff, H.; Noack, M.J.; Scholz, C.J.; et al. Intensive oral prophylaxis does not alter the tongue microbiome in young patients with chronic kidney disease: Longitudinal, randomized, controlled study. Front. Immunol. 2024, 15, 1430655. [Google Scholar] [CrossRef]
- Martins, C.; Siqueira, W.L.; Oliveira, E.; Nicolau, J.; Primo, L.G. Dental calculus formation in children and adolescents undergoing hemodialysis. Pediatr. Nephrol. 2012, 27, 1961–1966. [Google Scholar] [CrossRef]
- Proctor, R.; Kumar, N.; Stein, A.; Moles, D.; Porter, S. Oral and dental aspects of chronic renal failure. J. Dent. Res. 2005, 84, 199–208. [Google Scholar] [CrossRef]
- Lopez-Pintor, R.M.; Lopez-Pintor, L.; Casanas, E.; de Arriba, L.; Hernandez, G. Risk factors associated with xerostomia in haemodialysis patients. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e185–e192. [Google Scholar] [CrossRef]
- Su, N.; Marek, C.L.; Ching, V.; Grushka, M. Caries prevention for patients with dry mouth. J. Can. Dent. Assoc. 2011, 77, b85. [Google Scholar]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological factors in dental caries: Role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J. Clin. Pediatr. Dent. 2003, 28, 47–52. [Google Scholar] [CrossRef]
- Seminario, A.L.; Velan, E. Vitamin D and Dental Caries in Primary Dentition. J. Dent. Child. 2016, 83, 114–119. [Google Scholar]
- Noce, A.; Romani, A.; Bernini, R. Dietary Intake and Chronic Disease Prevention. Nutrients 2021, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Dessi, M.; Noce, A.; Bertucci, P.; Noce, G.; Rizza, S.; De Stefano, A.; di Villahermosa, S.M.; Bernardini, S.; De Lorenzo, A.; Di Daniele, N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef]
- Chhonkar, A.; Gupta, A.; Arya, V. Comparison of Vitamin D Level of Children with Severe Early Childhood Caries and Children with No Caries. Int. J. Clin. Pediatr. Dent. 2018, 11, 199–204. [Google Scholar] [CrossRef]
- Álamo, S.M.; Esteve, C.G.; Pérez, G.S. Dental considerations for the patient with renal disease. J. Clin. Exp. Dent. 2011, 3, 112–119. [Google Scholar] [CrossRef]
- Williams, T.L.; Boyle, J.; Mittermuller, B.A.; Carrico, C.; Schroth, R.J. Association between Vitamin D and Dental Caries in a Sample of Canadian and American Preschool-Aged Children. Nutrients 2021, 13, 4465. [Google Scholar] [CrossRef]
- Carvalho Silva, C.; Gavinha, S.; Manso, M.C.; Rodrigues, R.; Martins, S.; Guimaraes, J.T.; Santos, A.C.; Melo, P. Serum Levels of Vitamin D and Dental Caries in 7-Year-Old Children in Porto Metropolitan Area. Nutrients 2021, 13, 166. [Google Scholar] [CrossRef]
- Soyeon, C.; Deog-Gyu, S.; Ji-Yun, H. Serum 25-hydroxyvitamin D levels are associated with dental caries experience in Korean adolescents: The 2010~2014 Korean National Health and Nutrition Examination Surveys. J. Nutr. Health 2018, 51, 287–294. [Google Scholar] [CrossRef]
- Bumbu, B.A.; Luca, M.M.; Buzatu, R. Examining the Role of Vitamin D in Caries Susceptibility in Children’s Deciduous Teeth: A Systematic Review. Nutrients 2023, 15, 4826. [Google Scholar] [CrossRef]
- Buzatu, R.; Luca, M.M.; Bumbu, B.A. A Systematic Review of the Relationship between Serum Vitamin D Levels and Caries in the Permanent Teeth of Children and Adolescents. Dent. J. 2024, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinol 2011, 3, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, S.; Diza, E.; Menexes, G.; Kalfas, S. Salivary concentration of the antimicrobial peptide LL-37 in children. Arch. Oral Biol. 2012, 57, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ye, X.J.; Ng, T.B. Cathelicidins: Peptides with antimicrobial, immunomodulatory, anti-inflammatory, angiogenic, anticancer and procancer activities. Curr. Protein Pept. Sci. 2013, 14, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.R.; Salazar, S.L.; de Sa, L.F.; Portela, M.; Ferreira-Pereira, A.; Soares, R.M.; Leao, A.T.; Primo, L.G. Role of saliva in the caries experience and calculus formation of young patients undergoing hemodialysis. Clin. Oral Investig. 2015, 19, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Tomas, I.; Marinho, J.S.; Limeres, J.; Santos, M.J.; Araujo, L.; Diz, P. Changes in salivary composition in patients with renal failure. Arch. Oral Biol. 2008, 53, 528–532. [Google Scholar] [CrossRef]
- Ertugrul, F.; Elbek-Cubukcu, C.; Sabah, E.; Mir, S. The oral health status of children undergoing hemodialysis treatment. Turk. J. Pediatr. 2003, 45, 108–113. [Google Scholar]
- Kamel, D.; Elghazawy, R.; Khattab, N.; Hassan, A. Oral Manifestations of Children with Chronic Kidney Disease: A Review Article. Ahram Can. Dent. J. 2024, 3, 42–54. [Google Scholar] [CrossRef]
- Saads Carvalho, T.; Lussi, A. Chapter 9: Acidic Beverages and Foods Associated with Dental Erosion and Erosive Tooth Wear. Monogr. Oral Sci. 2020, 28, 91–98. [Google Scholar] [CrossRef]
- Tungare, S.; Paranjpe, A.G. Drug-Induced Gingival Overgrowth; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Catalfamo, L.M.; Marrone, G.; Basilicata, M.; Vivarini, I.; Paolino, V.; Della-Morte, D.; De Ponte, F.S.; Di Daniele, F.; Quattrone, D.; De Rinaldis, D.; et al. The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review. Int. J. Environ. Res. Public Health 2022, 19, 11187. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78 (Suppl. 1), A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Budala, D.G.; Martu, M.A.; Maftei, G.A.; Diaconu-Popa, D.A.; Danila, V.; Luchian, I. The Role of Natural Compounds in Optimizing Contemporary Dental Treatment-Current Status and Future Trends. J. Funct. Biomater. 2023, 14, 273. [Google Scholar] [CrossRef]
- Yu, H.; Xu, L.X.; Oho, T.; Morioka, T. The effect of a tannin-fluoride mixture on human dental enamel. Caries Res. 1993, 27, 161–168. [Google Scholar] [CrossRef]
- Eymirli, P.S.; Gultekin, I.M.; Ozler, C.O.; Ozyurek, E.U. Evaluation of the efficacy of various remineralization agents and grape seed extract on microhardness and lesion depth of primary tooth enamel: An in vitro study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2024, 18, 182–188. [Google Scholar] [CrossRef]
- Golfeshan, F.; Mosaddad, S.A.; Ghaderi, F. The Effect of Toothpastes Containing Natural Ingredients Such As Theobromine and Caffeine on Enamel Microhardness: An In Vitro Study. Evid. Based Complement. Altern. Med. 2021, 2021, 3304543. [Google Scholar] [CrossRef]
- Cheng, L.; Li, J.; He, L.; Zhou, X. Natural products and caries prevention. Caries Res. 2015, 49 (Suppl. 1), 38–45. [Google Scholar] [CrossRef]
- Philip, N.; Leishman, S.; Walsh, L. Potential Role for Natural Products in Dental Caries Control. Oral Health Prev. Dent. 2019, 17, 479–485. [Google Scholar] [CrossRef]
- Alghutaimel, H.; Matoug-Elwerfelli, M.; Alhaji, M.; Albawardi, F.; Nagendrababu, V.; Dummer, P.M.H. Propolis Use in Dentistry: A Narrative Review of Its Preventive and Therapeutic Applications. Int. Dent. J. 2024, 74, 365–386. [Google Scholar] [CrossRef]
- Ellam, S.; Williamson, G. Cocoa and human health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef]
- Fideles, S.O.M.; Ortiz, A.C.; Reis, C.H.B.; Buchaim, D.V.; Buchaim, R.L. Biological Properties and Antimicrobial Potential of Cocoa and Its Effects on Systemic and Oral Health. Nutrients 2023, 15, 3927. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, K.; Antoniadou, M.; Varzakas, T.; Voidarou, C.C. Plant-Derived Compounds: A Promising Tool for Dental Caries Prevention. Curr. Issues Mol. Biol. 2024, 46, 5257–5290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Agnello, M.; Dinis, M.; Chien, K.C.; Wang, J.; Hu, W.; Shi, W.; He, X.; Zou, J. Lollipop containing Glycyrrhiza uralensis extract reduces Streptococcus mutans colonization and maintains oral microbial diversity in Chinese preschool children. PLoS ONE 2019, 14, e0221756. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Basilicata, M.; Di Lauro, M.; Vita, C.; Masci, C.; Klinger, F.G.; Cornali, K.; Maddaloni, G.; Bollero, P.; De Lorenzo, A.; et al. Healthy Effects of Pomegranate (Punica granatum L.) in Internal Medicine and Dentistry. Appl. Sci. 2024, 14, 1570. [Google Scholar] [CrossRef]
- Mahd, M.A.; Aref, P.; Emadi, F.; Javadi, F.; Kharazi Fard, M.J.; Tavassoli-Hojjati, S. Effect of hydroalcoholic extract of whole pomegranate fruit on cariogenic bacteria and its clinical effect on dental plaque formation in 8-10-year-old children. Dent. Res. J. 2023, 20, 114. [Google Scholar]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proenca, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An umbrella review of the evidence linking oral health and systemic noncommunicable diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef]
 , organ where the event occurs;
, organ where the event occurs;  , activation;
, activation;  , inhibition.
, inhibition.
 , organ where the event occurs;
, organ where the event occurs;  , activation;
, activation;  , inhibition.
, inhibition.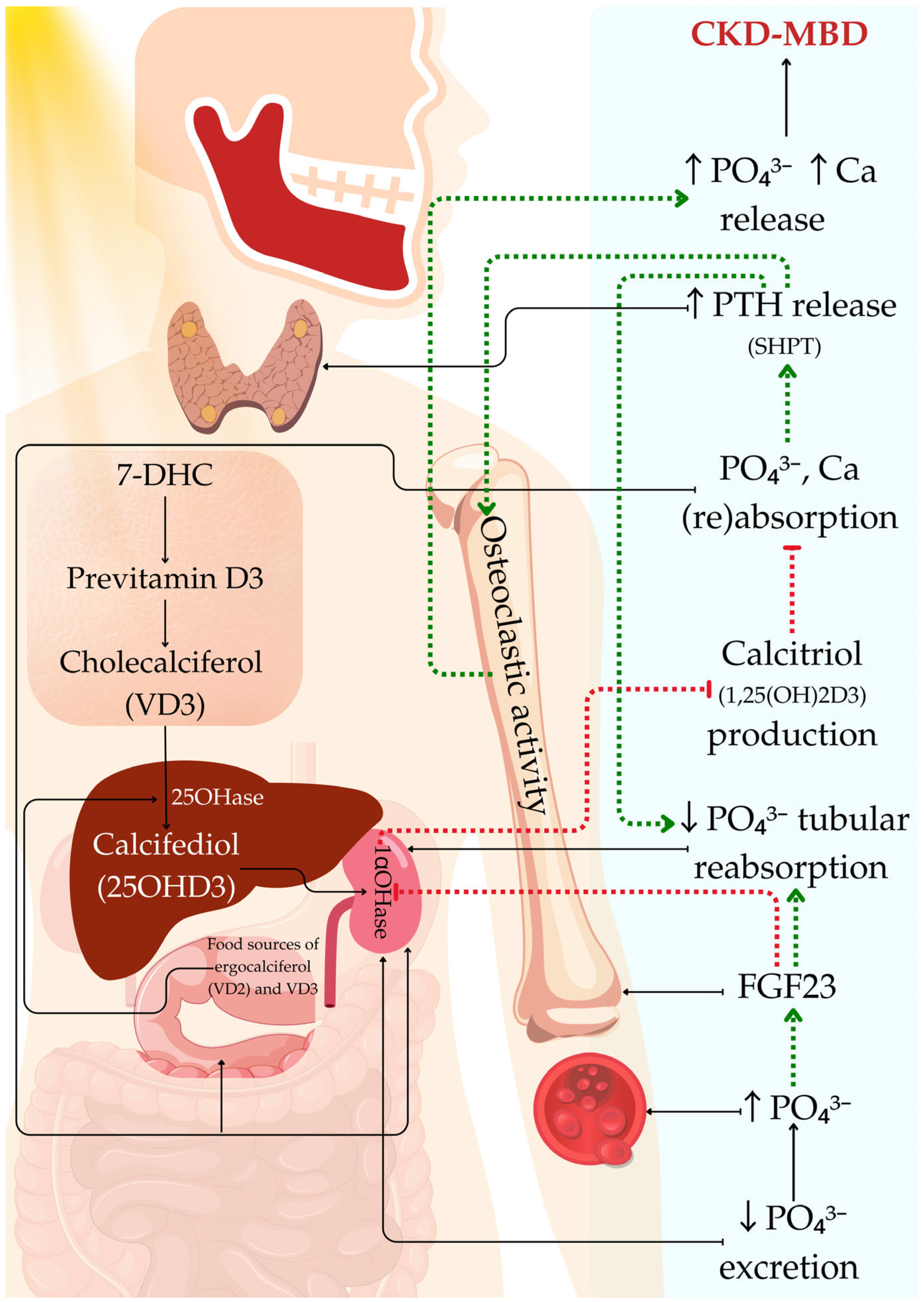
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costacurta, M.; Di Lauro, M.; Cornali, K.; Docimo, R.; Noce, A. Developmental Defects of Enamel and Dental Caries in Pediatric Patients with Chronic Kidney Disease–Mineral Bone Disorders. Appl. Sci. 2025, 15, 1164. https://doi.org/10.3390/app15031164
Costacurta M, Di Lauro M, Cornali K, Docimo R, Noce A. Developmental Defects of Enamel and Dental Caries in Pediatric Patients with Chronic Kidney Disease–Mineral Bone Disorders. Applied Sciences. 2025; 15(3):1164. https://doi.org/10.3390/app15031164
Chicago/Turabian StyleCostacurta, Micaela, Manuela Di Lauro, Kevin Cornali, Raffaella Docimo, and Annalisa Noce. 2025. "Developmental Defects of Enamel and Dental Caries in Pediatric Patients with Chronic Kidney Disease–Mineral Bone Disorders" Applied Sciences 15, no. 3: 1164. https://doi.org/10.3390/app15031164
APA StyleCostacurta, M., Di Lauro, M., Cornali, K., Docimo, R., & Noce, A. (2025). Developmental Defects of Enamel and Dental Caries in Pediatric Patients with Chronic Kidney Disease–Mineral Bone Disorders. Applied Sciences, 15(3), 1164. https://doi.org/10.3390/app15031164




