Validating Accelerated Shelf Life Testing Methodology for Predicting Shelf Life in High-Pressure-Processed Meat Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples and HPP Application
2.2. Experimental Design
2.3. Physicochemical Analysis
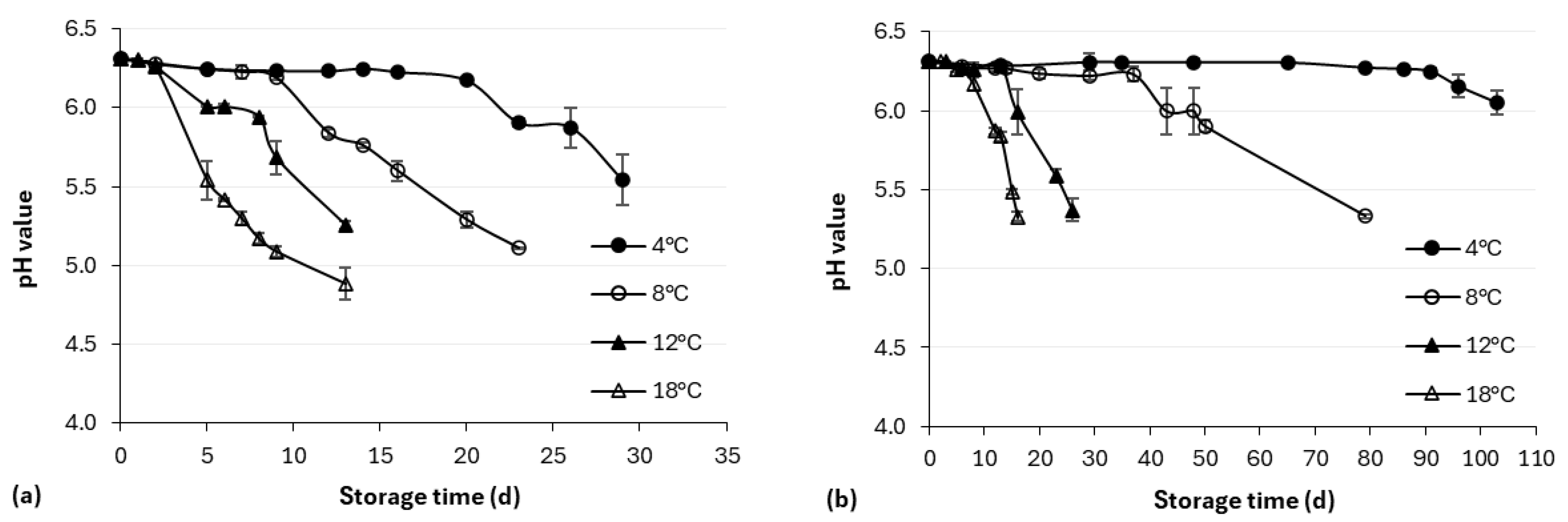
2.3.1. pH Measurement
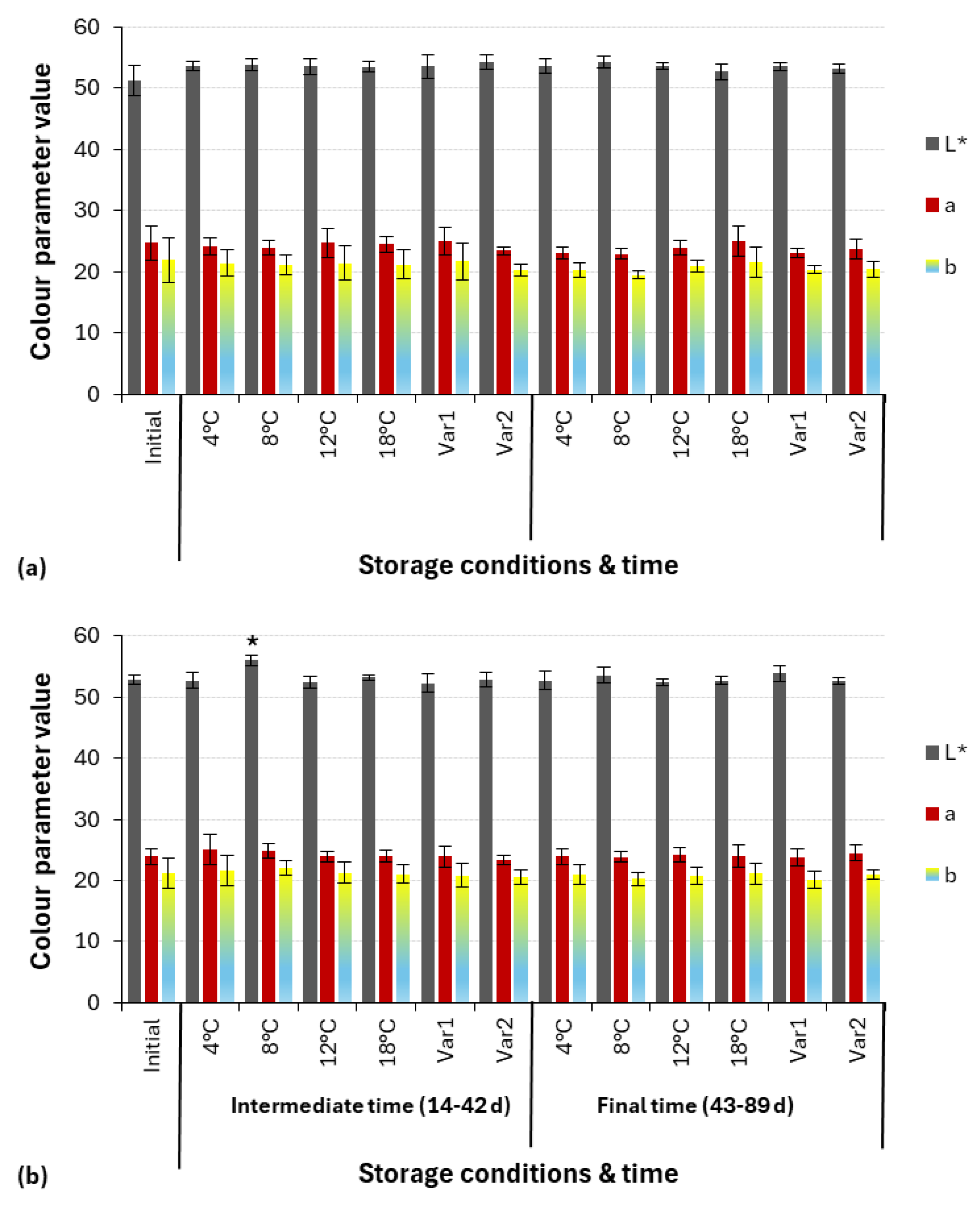
2.3.2. Color Measurement
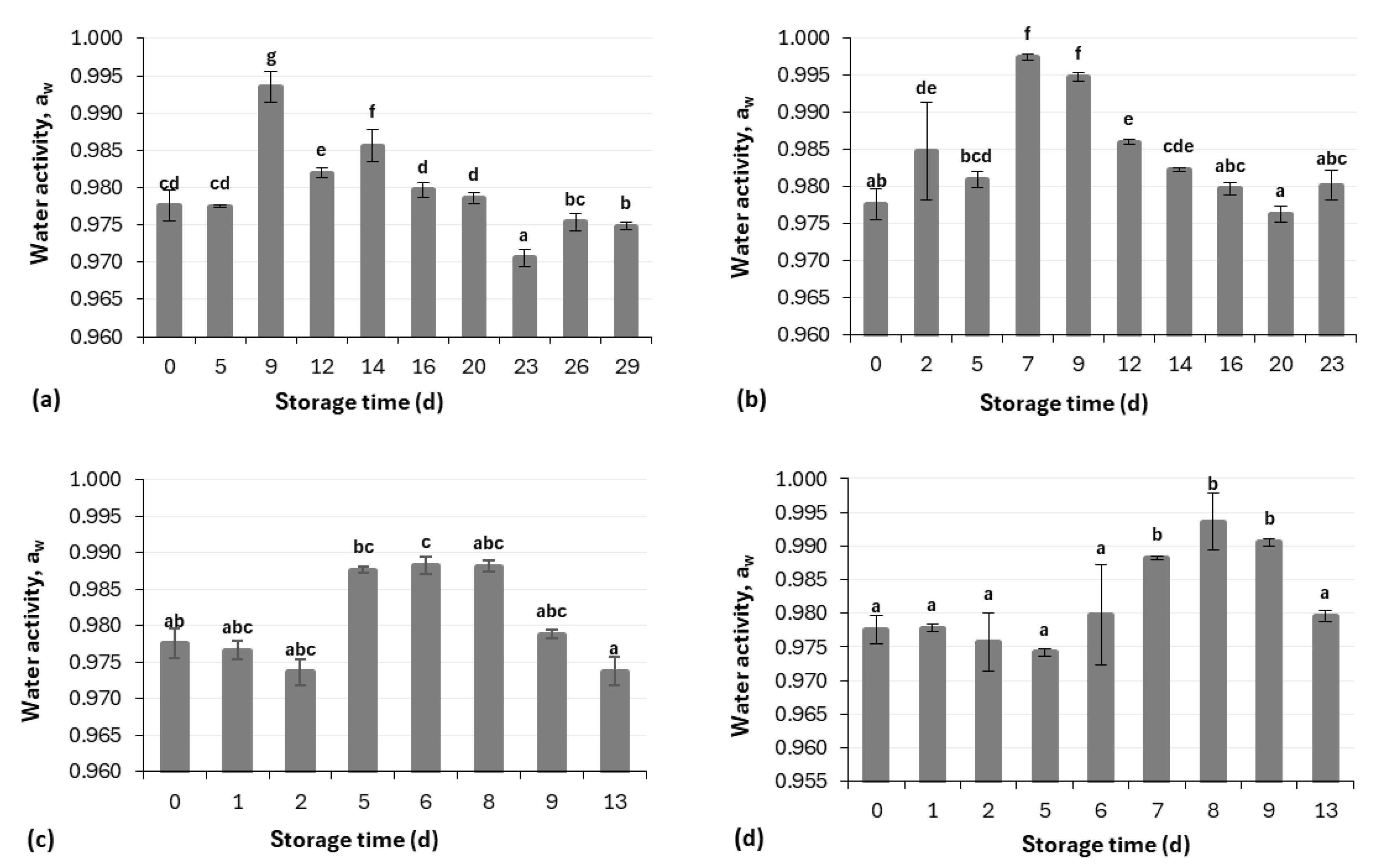
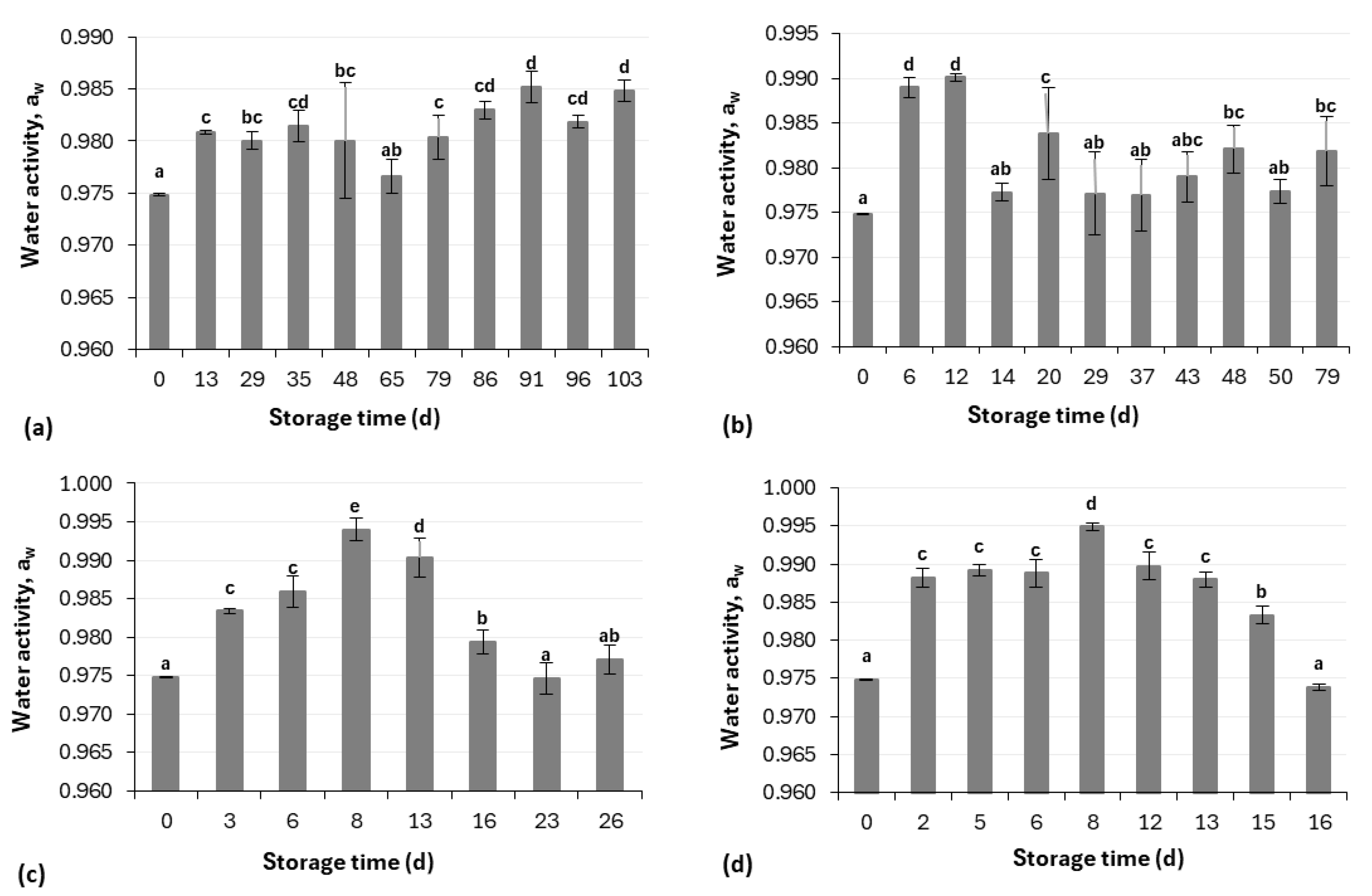
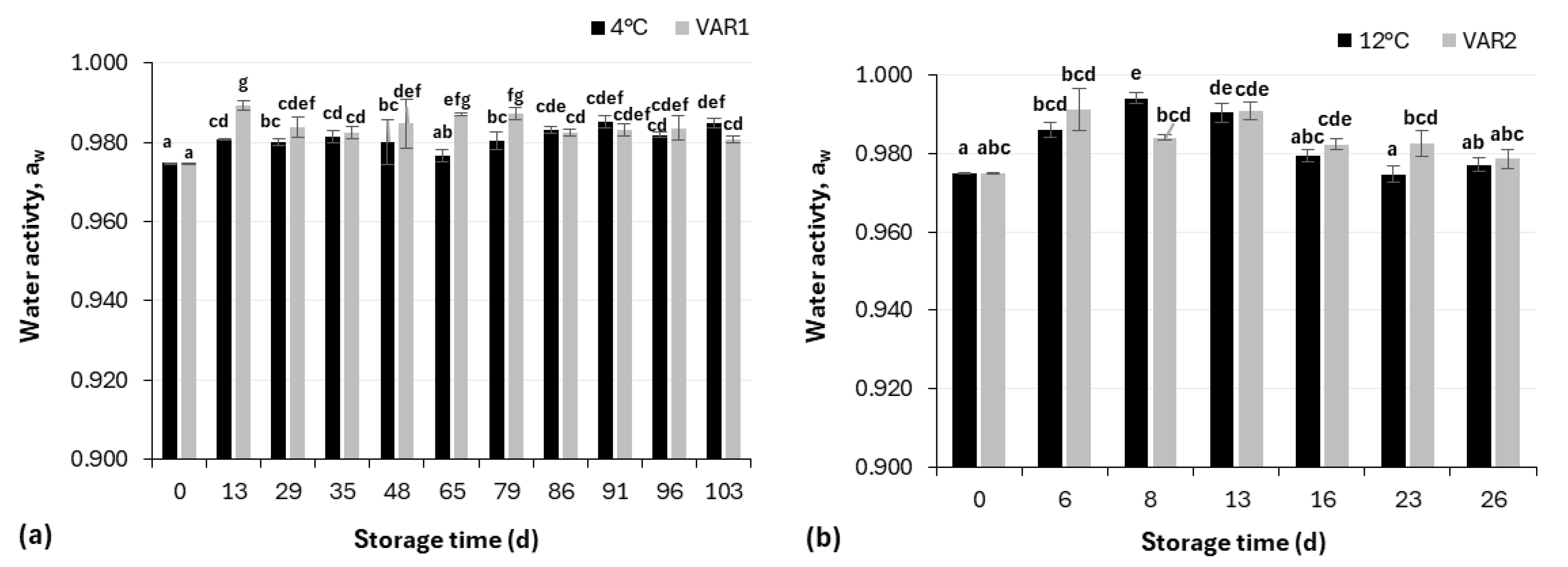
2.3.3. Water Activity (aw) Measurement
2.3.4. Texture Analysis
2.3.5. Lipid Oxidation
2.4. Microbiological Analysis
2.5. Data Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis
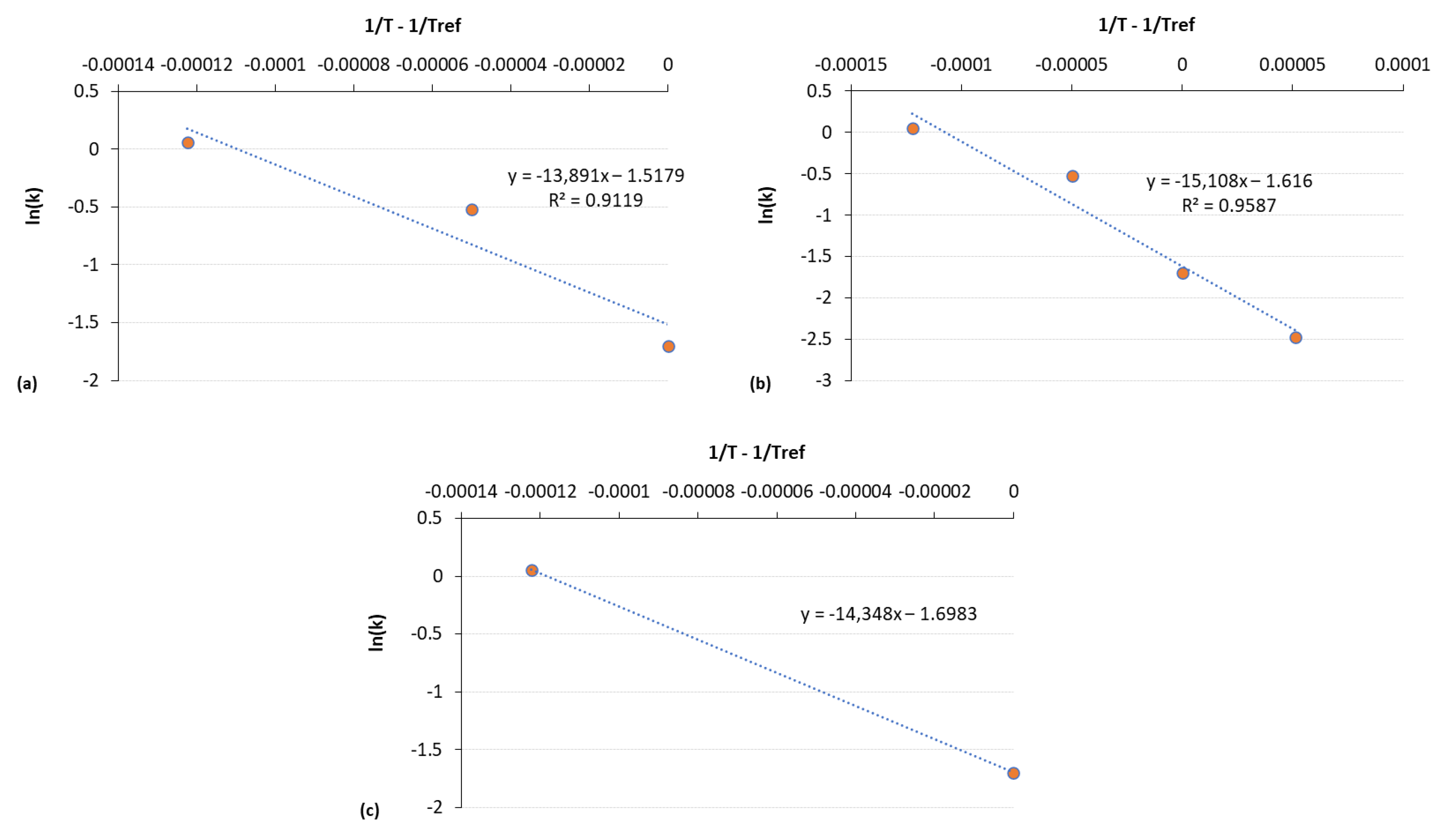
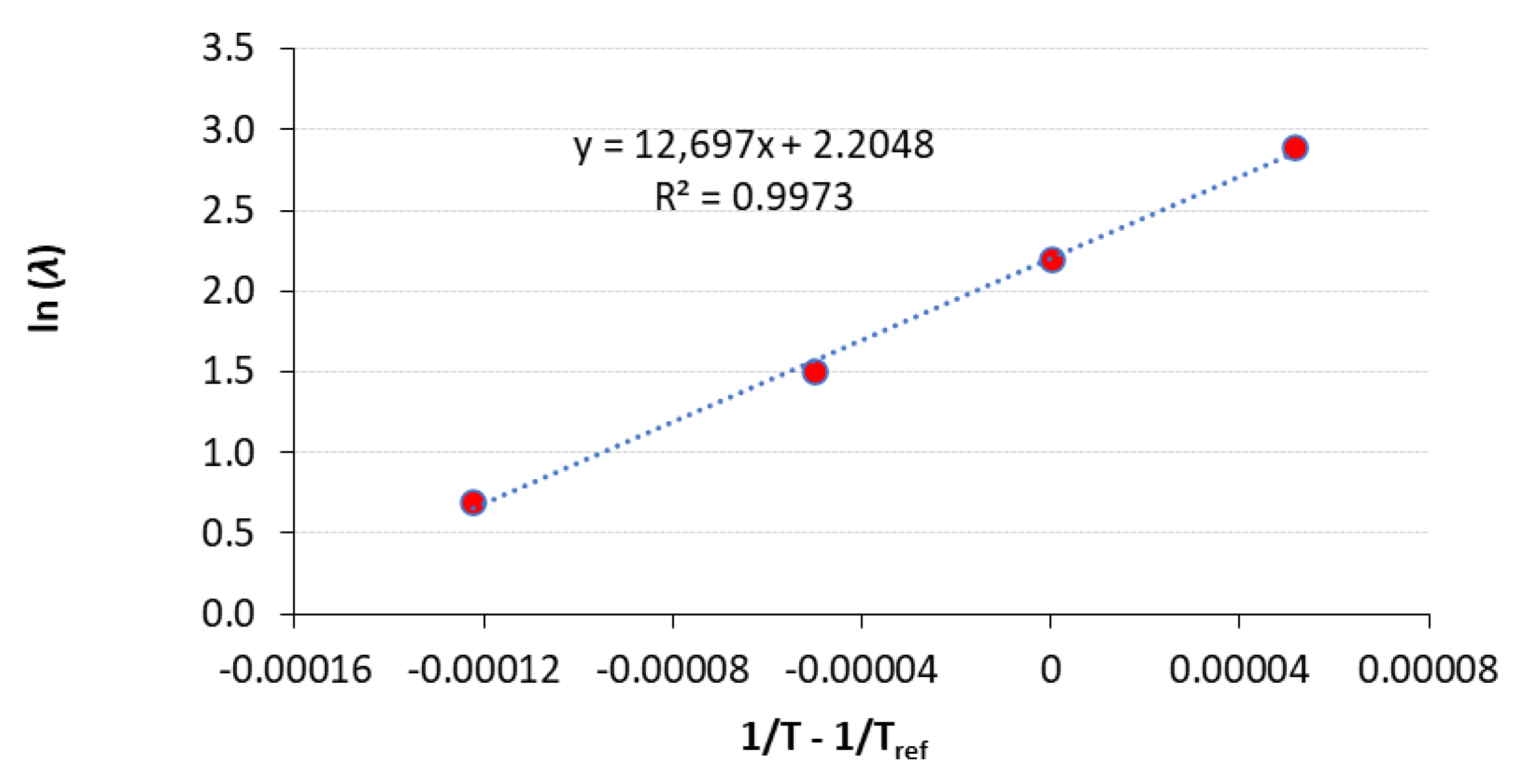
3.2. Estimation of Microbial Growth Parameters and Shelf Life
3.2.1. Microbial Growth Curves of Reference (CNT) Samples
3.2.2. Microbial Growth Curves of HPP Samples
3.2.3. Shelf Life Estimation
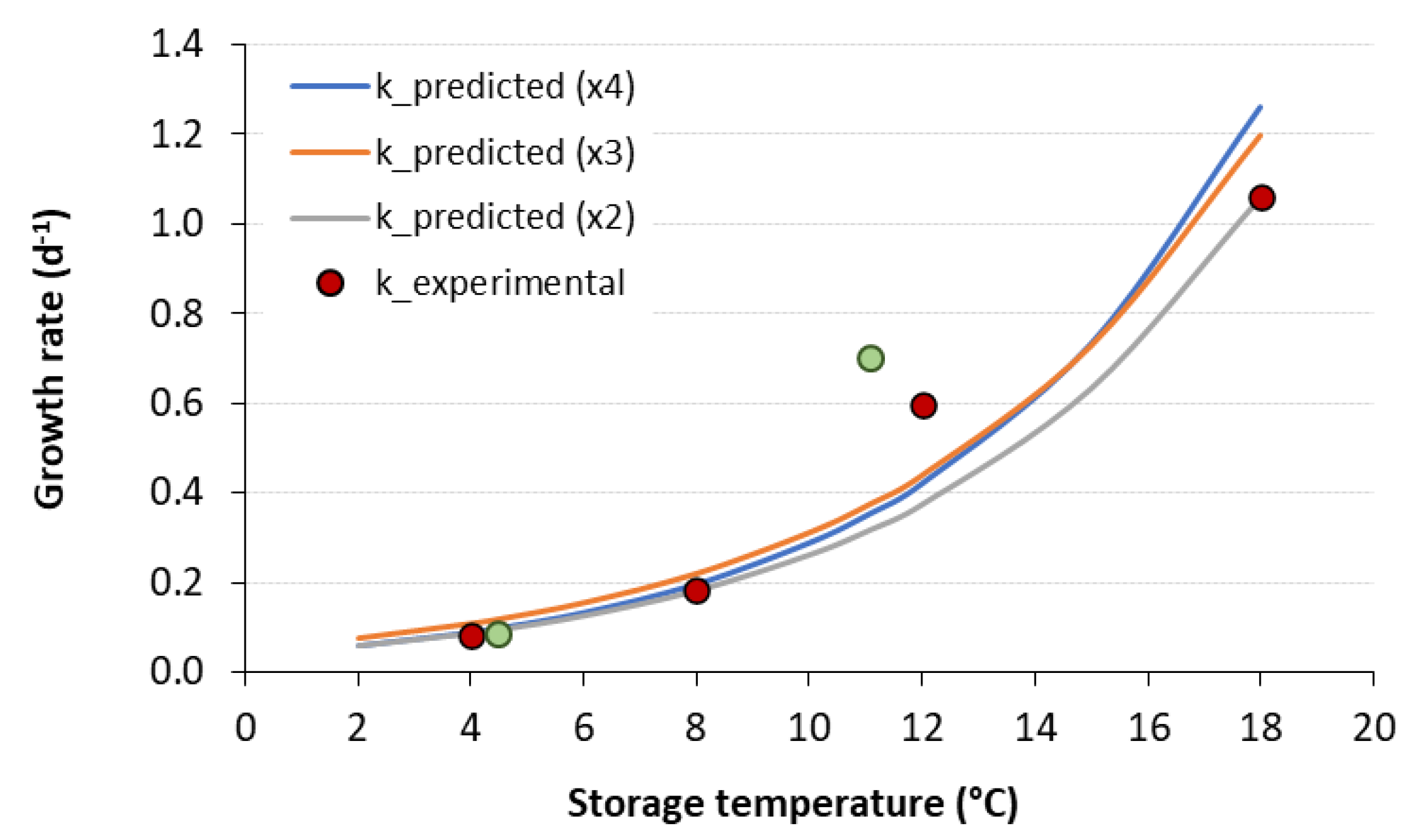
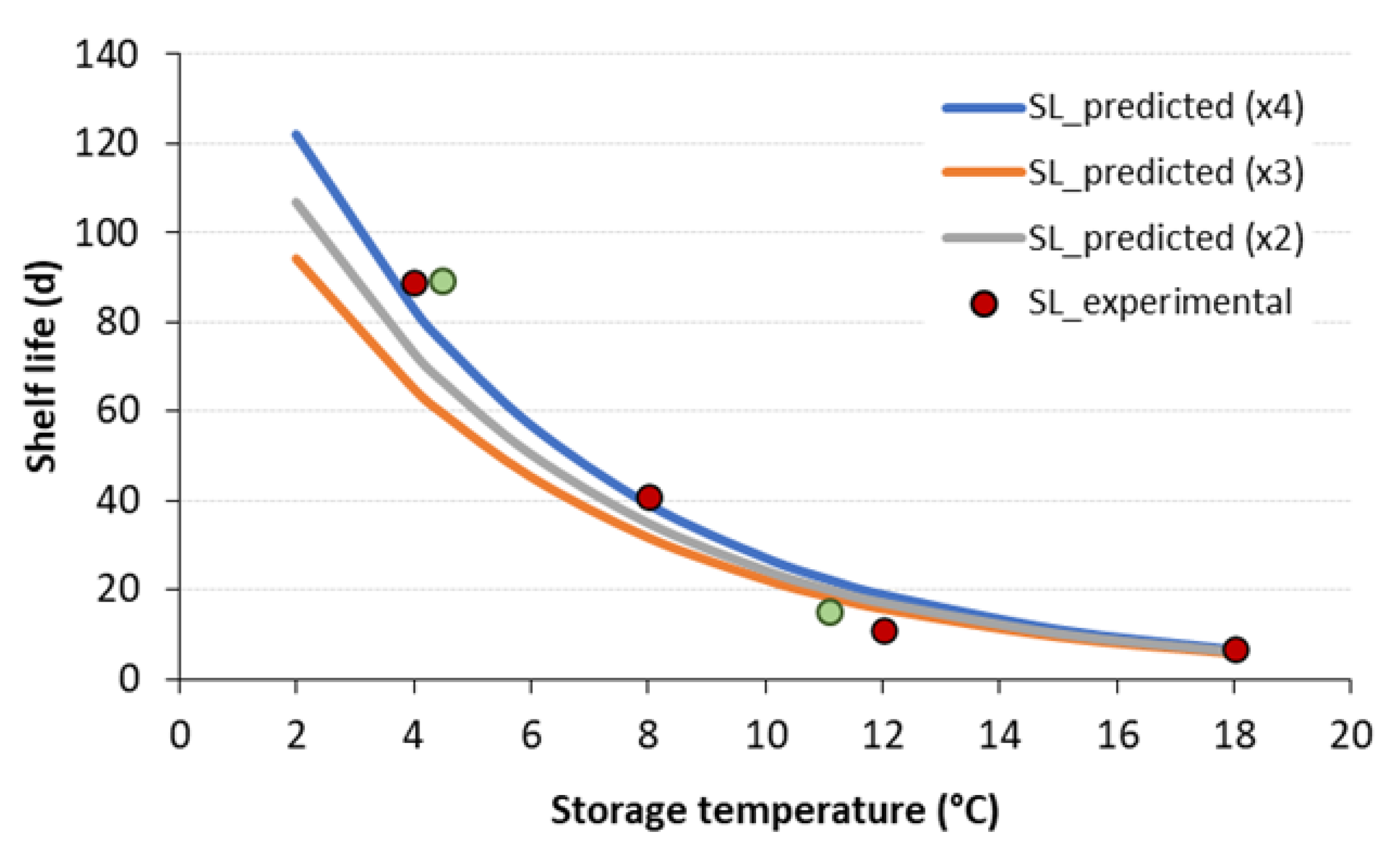
3.3. Validation of the ASLT Methodology in Predicting the Shelf Life of Frankfurter-Type Sausages
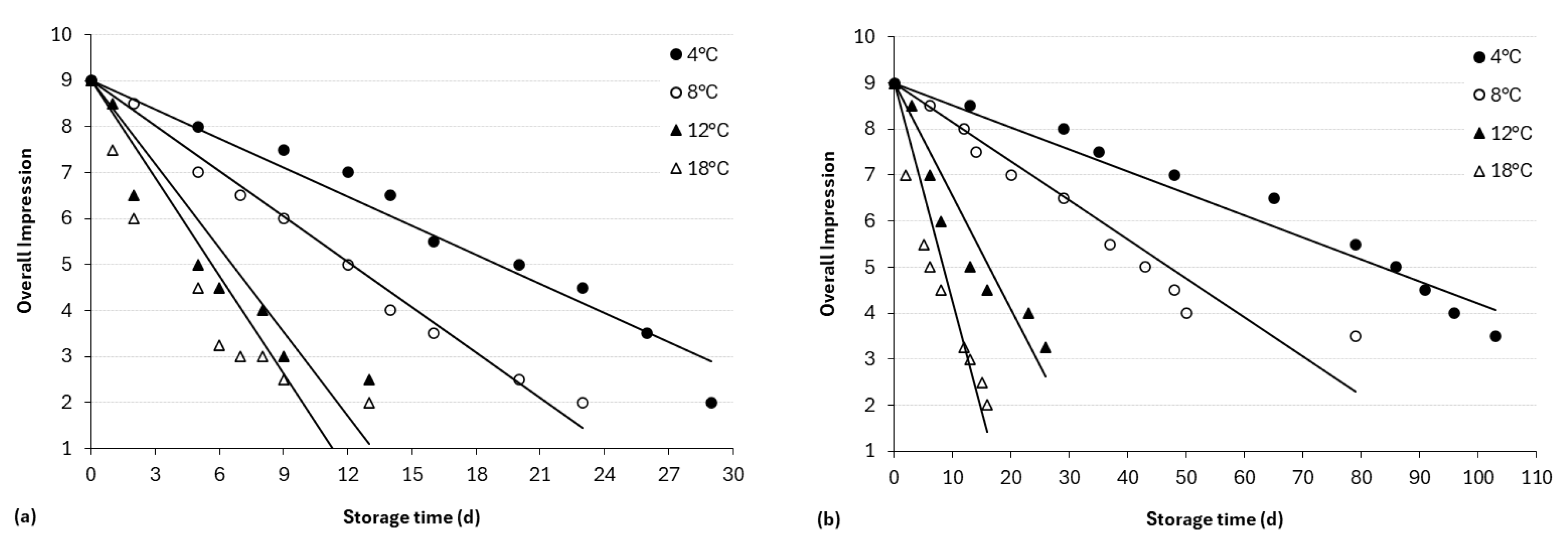
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different preservative strategies on the shelf-life of fresh meat products. Appl. Sci. 2020, 10, 8340. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; Carneiro de Almeida, P.L. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kelemen, L.; Ikonić, P.; Džinić, N.; Jokanović, M. Antioxidant effects of Juniperus communis L. essential oil in cooked pork sausages. Food Control 2019, 98, 68–74. [Google Scholar]
- Katiyo, W.; Coorey, R.; Buys, E.M. Consumers’ perceptions of intrinsic and extrinsic attributes as indicators of safety and quality of chicken meat: Actionable information for public health authorities and the chicken industry. J. Food Sci. 2020, 85, 1845–1855. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Kerry, J.P.; Tiwari, B.K.; McDonnell, C.K. Novel processing technologies and ingredient strategies for the reduction of phosphate additives in processed meat. Trends Food Sci. 2019, 94, 43–53. [Google Scholar] [CrossRef]
- Kapetanakou, A.; Karyotis, D.; Skandamis, P.N. Control of Listeria monocytogenes by applying ethanol-based antimicrobial edible films on ham slices and microwave-reheated frankfurters. Food Microbiol. 2016, 54, 80–90. [Google Scholar] [CrossRef]
- Fuentes, V.; Ventanas, J.; Morcuende, D.; Estévez, M.; Ventanas, S. Lipid and protein oxidation and sensory properties of vacuum-packaged dry-cured ham subjected to high hydrostatic pressure. Meat Sci. 2010, 85, 506–514. [Google Scholar] [CrossRef]
- Argyri, A.A.; Tassou, C.C.; Samaras, F.; Mallidis, C.; Chorianopoulos, N. Effect of high hydrostatic pressure processing on microbiological shelf-life and quality of fruits pretreated with ascorbic acid or SnCl2. BioMed Res. Int. 2014, 2014, 819209. [Google Scholar] [CrossRef]
- Katsaros, G.; Taoukis, P. Microbial control by high pressure processing for shelf-life extension of packed meat products in the cold chain: Modeling and case studies. Appl. Sci. 2021, 11, 1317. [Google Scholar] [CrossRef]
- Andres, A.I.; Adamsen, C.E.; Møller, J.K.S.; Ruiz, J.; Skibsted, L.H. High-pressure treatment of dry-cured Iberian ham. Effect on colour and oxidative stability during chill storage packed in modified atmosphere. Eur. Food Res. Technol. 2006, 222, 486–491. [Google Scholar] [CrossRef]
- Rivas-Cañedo, A.; Fernández-García, E.; Nuñez, M. Volatile compounds in fresh meats subjected to high pressure processing: Effect of the packaging material. Meat Sci. 2009, 81, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Simonin, H.; Duranton, F.; de Lamballerie, M. New insights into the high-pressure processing of meat and meat products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- Taoukis, P.; Labuza, T.P. Summary: Integrative Concepts (Shelf-life testing and modelling). In Food Chemistry, 3rd ed.; Fennema, O., Ed.; Marcel Dekker: New York, NY, USA, 1996; Chapter 17; pp. 1013–1042. [Google Scholar]
- Taoukis, P.; Labuza, T.P.; Saguy, I. Kinetics of food deterioration and shelf-life prediction. In The Handbook of Food Engineering Practice; Valentas, K.J., Rotstein, E., Singh, R.P., Eds.; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 1997; Chapter 10; pp. 361–403. [Google Scholar]
- Mizrahi, S. Accelerated shelf life testing of foods. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing: Oxford, UK, 2011; Chapter 15; pp. 482–506. [Google Scholar]
- Taoukis, P.; Giannakourou, M.C. Temperature and food stability: Analysis and control. In Understanding and Measuring the Shelf-Life of Food; Steele, R., Ed.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Boca Raton, FL, USA, 2004; Chapter 3; pp. 42–68. [Google Scholar]
- López, Y.G.; Vega, J.T.V.; Rosillo, F.F.; Alayo, E.M.C.; Ríos, M.A.C.; Huatangari, L.Q.; Mendoza, M.M. Predicting the shelf life of cup chocolate using the Arrhenius model based on peroxide value. Math. Model. Eng. Probl. 2024, 11, 517–522. [Google Scholar] [CrossRef]
- Puangwerakul, Y.; Chaisakdanukul, C.; Soithongsuk, S.; Sajjaut, S.; Pewlong, W. Comparative effects of HPP and irradiation on plant-based whole hard-boiled eggs. J. Curr. Sci. Technol. 2024, 14, 66. [Google Scholar] [CrossRef]
- Baka, A.M.; Papavergou, E.J.; Pragalaki, T.; Bloukas, J.G.; Kotzekidou, P. Effect of selected starter cultures on the microbiological, physicochemical, and sensory attributes of Greek dry-fermented sausages. Food Cont 2014, 37, 5–14. [Google Scholar]
- Dingstad, G.I.; Kubberød, E.; Næs, T.; Egelandsdal, B. Critical quality constraints of sensory attributes in frankfurter-type sausages, to be applied in optimization models. LWT-Food Sci Technol. 2005, 38, 665–676. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Tsironi, T.; Dermesonlouoglou, E.; Giannoglou, M.; Katsaros, G.; Taoukis, P. Shelf-life prediction models for ready-to-eat fresh cut salads: Testing in real cold chain. Int. J. Food Microbiol. 2017, 240, 131–140. [Google Scholar] [CrossRef]
- Lovaas, E.A. Sensitive spectrophotometric method for lipid hydroperoxide determination. J. Am. Oil Chem. Soc. 1992, 69, 777–783. [Google Scholar] [CrossRef]
- Speck, M.L. APHA Technical Committee on Microbiological Methods for Foods; American Public Health Association: Washington, DC, USA, 1984. [Google Scholar]
- ISO 6888-2:2021/Amd 1:2023; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive staphylococci (Staphylococcus aureus and Other Species)—Part 2: Method Using Rabbit Plasma Fibrinogen Agar Medium. Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Part 2: Colony-Count Technique. Published (Edition 2, 2017). International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 16140:2003; Microbiology of Food and Animal Feeding Stuffs—Protocol for the Validation of Alternative Methods. International Organization for Standardization: Geneva, Switzerland, 2003.
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. The efficacy and safety of high-pressure processing of food. EFSA J. 2022, 20, 7128–7323. [Google Scholar]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.-S.; de Lamballerie, M.; Hertel, C.; Brügge-mann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef] [PubMed]
- ISO 13300-1; Sensory Analysis—General Guidance for the Staff of a Sensory Evaluation Laboratory—Part 1: Staff Responsibilities. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 13300-2; Sensory Analysis—General Guidance for the Staff of a Sensory Evaluation Laboratory—Part 2: Recruitment and Training of Panel Leaders. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 6658; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 20613; Sensory Analysis—General Guidance for the Application of Sensory Analysis in Quality Control. International Organization for Standardization: Geneva, Switzerland, 2019.
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Effect of orange dietary fibre, oregano essential oil and packaging conditions on shelf-life of bologna sausages. Food Control 2010, 21, 436–443. [Google Scholar] [CrossRef]
- Shao, X.; Xu, B.; Chen, C.; Li, P.; Luo, H. The function and mechanism of lactic acid bacteria in the reduction of toxic substances in food: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 5950–5963. [Google Scholar] [CrossRef]
- Borch, E.; Berg, H.; Holst, O. Heterolactic fermentation by a homofermentative Lactobacillus sp. during glucose limitation in anaerobic continuous culture with complete cell recycle. J. Appl. Bacteriol. 1991, 71, 265–269. [Google Scholar] [CrossRef]
- Pexara, E.S.; Metaxopoulos, J.; Drosinos, E.H. Evaluation of shelf life of cured, cooked, sliced turkey fillets and cooked pork sausages-‘piroski’- Stored under vacuum and modified atmospheres at+ 4 and+ 10° C. Meat Sci. 2002, 62, 33–43. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Xu, X.; Sun, X.; Xu, B.; Zhou, G. Effect of high pressure treatment on microbial populations of sliced vacuum-packed cooked ham. Meat Sci. 2011, 88, 682–688. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, M.; Xu, B.; Chen, C.; Deng, J.; Li, P. Combined effect of high-pressure processing with spice extracts on quality of low-salt sausage during refrigerated storage. Foods 2021, 10, 2610. [Google Scholar] [CrossRef]
- Mor-Mur, M.; Yuste, J. High pressure processing applied to cooked sausage manufacture: Physical properties and sensory analysis. Meat Sci. 2003, 65, 1187–1191. [Google Scholar] [CrossRef]
- Jeremiah, L.E. Packaging alternatives to deliver fresh meat using short- or long-term distribution. Food Res. Int. 2001, 34, 749–772. [Google Scholar] [CrossRef]
- Souza, M.A.; Visentainer, J.V.; Carvalho, R.H.; Garcia, F.; Ida, E.I.; Shimokomaki, M. Lipid and protein oxidation in charqui meat and jerked beef. Braz. Arch. Biol. Technol. 2013, 56, 107–112. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Cava, R. Protein oxidation in frankfurters with increasing levels of added rosemary essential oil: Effect on colour and texture deterioration. J. Food Sci. 2005, 70, 427–432. [Google Scholar] [CrossRef]
- O’ Neill, C.M.; Cruz-Romero, M.C.; Duffy, G.; Kerry, J.P. Shelf-life extension of vacuum-packed salt reduced frankfurters and cooked ham through the combined application of high pressure processing and organic acids. Food Packag. Shelf Life. 2018, 17, 120–128. [Google Scholar] [CrossRef]
- Jensen, C.; Skibsted, L.H.; Bertelsen, G. Oxidative stability of chilled meats. In Antioxidants in Muscle Foods; Springer: Boston, MA, USA, 1998; pp. 57–73. [Google Scholar]
- Ahn, D.U.; Nam, K.C. Effects of ascorbic acid and antioxidants on color, lipid oxidation, and volatiles of irradiated ground beef. J. Food Sci. 2004, 69, C499–C505. [Google Scholar]
- Parra, V.; Viguera, J.; Sánchez, J.; Peinado, J.; Espárrago, F.; Gutierrez, J.I.; Andrés, A.I. Modified atmosphere packaging and vacuum packaging for long period chilled storage of dry-cured Iberian ham. Meat Sci. 2010, 84, 760–768. [Google Scholar] [CrossRef]
- Ospina-E, J.C.; Rojano, B.; Ochoa, O.; Pérez-Álvarez, J.A.; Fernández-López, J. Development of frankfurter-type sausages with healthy lipid formulation and their nutritional, sensory and stability properties. Eur. J. Lipid Sci. Technol. 2015, 117, 122–131. [Google Scholar] [CrossRef]
- Andres, A.I.; Moller, J.K.S.; Adamsen, C.E.; Skibsted, L.H. High pressure treatment of dry-cured Iberian ham. Effect on radical formation, lipid oxidation and colour. Eur. Food Res. Technol. 2004, 219, 205–210. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barnaba, C.; Barbosa-Cánovas, G.V. Effects of high pressure processing on lipid oxidation: A review. Innov. Food Sci. Emerg. Technol. 2014, 22, 1–10. [Google Scholar] [CrossRef]
- Rohaya, S.; Rahmat, F.; Sulaiman, I. Shelf-life prediction of chicken nuggets based on the thickness level of composite plastic packaging using accelerated shelf-life testing (ASLT) method by the Arrhenius equation approach. IOP Conf. Ser. Earth Environ. Sci. 2024, 1297, 012078. [Google Scholar] [CrossRef]
- McArdle, R.A.; Marcos, B.; Kerry, J.P.; Mullen, A.M. Influence of HPP conditions on selected beef quality attributes and their stability during chilled storage. Meat Sci. 2011, 87, 274–281. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Kagkli, D.M.; Nychas, G.J.E. Spoilage microbiota associated with the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef]
- Remenant, B.; Jaffrès, E.; Dousset, X.; Pilet, M.F.; Zagorec, M. Bacterial spoilers of food: Behavior, fitness, and functional properties. Food Microbiol. 2015, 45, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Grau, R.; Sarraga, C.; Blesa, E. The effect of modified atmosphere and vacuum packaging on the spoilage microorganisms of beef meat. Meat Sci. 2019, 153, 43–48. [Google Scholar]
- Campus, M. High-pressure processing of meat, meat products and seafood. Food Eng. Rev. 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Da, C.; Liang, P.; Jin, S.; Tang, C. Effects of Cyclocarya paliurus (Batal.) extracts on oxidative stability and sensory quality in meat products (Frankfurters). Foods 2022, 11, 3721. [Google Scholar] [CrossRef]
- Vercammen, A.; Vanoirbeek, K.G.A.; Lurquin, I.; Steen, L.; Goemaere, O.; Szczepaniak, S.; Paelinck, H.; Hendrickx, M.E.G.; Michiels, C.W. Shelf-life extension of cooked ham model product by high hydrostatic pressure and natural preservatives. Innov. Food Sci. Emerg. Technol. 2011, 12, 407–415. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Grèbol, N.; Garriga, M. Efficiency of high hydrostatic pressure at 600 MPa against food-borne microorganisms by challenge tests on convenience meat products. LWT-Food Sci. Technol. 2009, 42, 924–928. [Google Scholar] [CrossRef]
- Myers, K.; Cannon, J.; Mantoya, D.; Dickson, J.; Lonergan, S.; Sebranek, J. Effects of high hydrostatic pressure and varying concentrations of sodium nitrite from traditional vegetable-based sources on the growth of Listeria monocytogenes on ready-to-eat (RTE) sliced ham. Meat Sci. 2013, 86, 95–102. [Google Scholar] [CrossRef]
- Pietrzak, D.; Fonberg-Broczek, M.; Mucka, A.; Windyga, B. Effects of high pressure treatment on the quality of cooked pork ham prepared with different levels of curing ingredients. High Press. Res. 2007, 27, 27–31. [Google Scholar] [CrossRef]
- Yeung, C.K.; Huang, S.C. Effects of high-pressure processing technique on the quality and shelf life of Chinese style sausages. J. Food Nutr. Res. 2016, 4, 442–447. [Google Scholar]
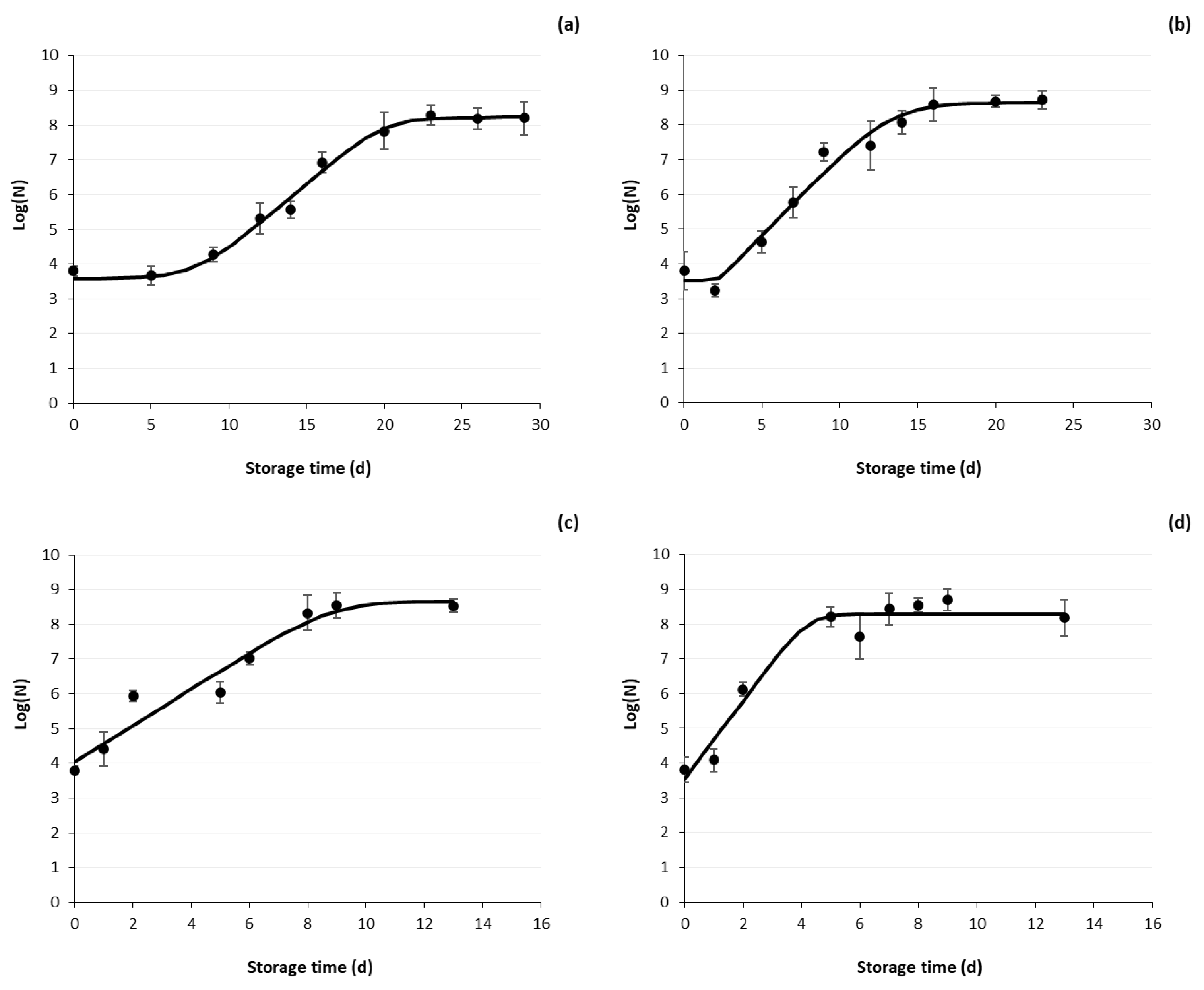

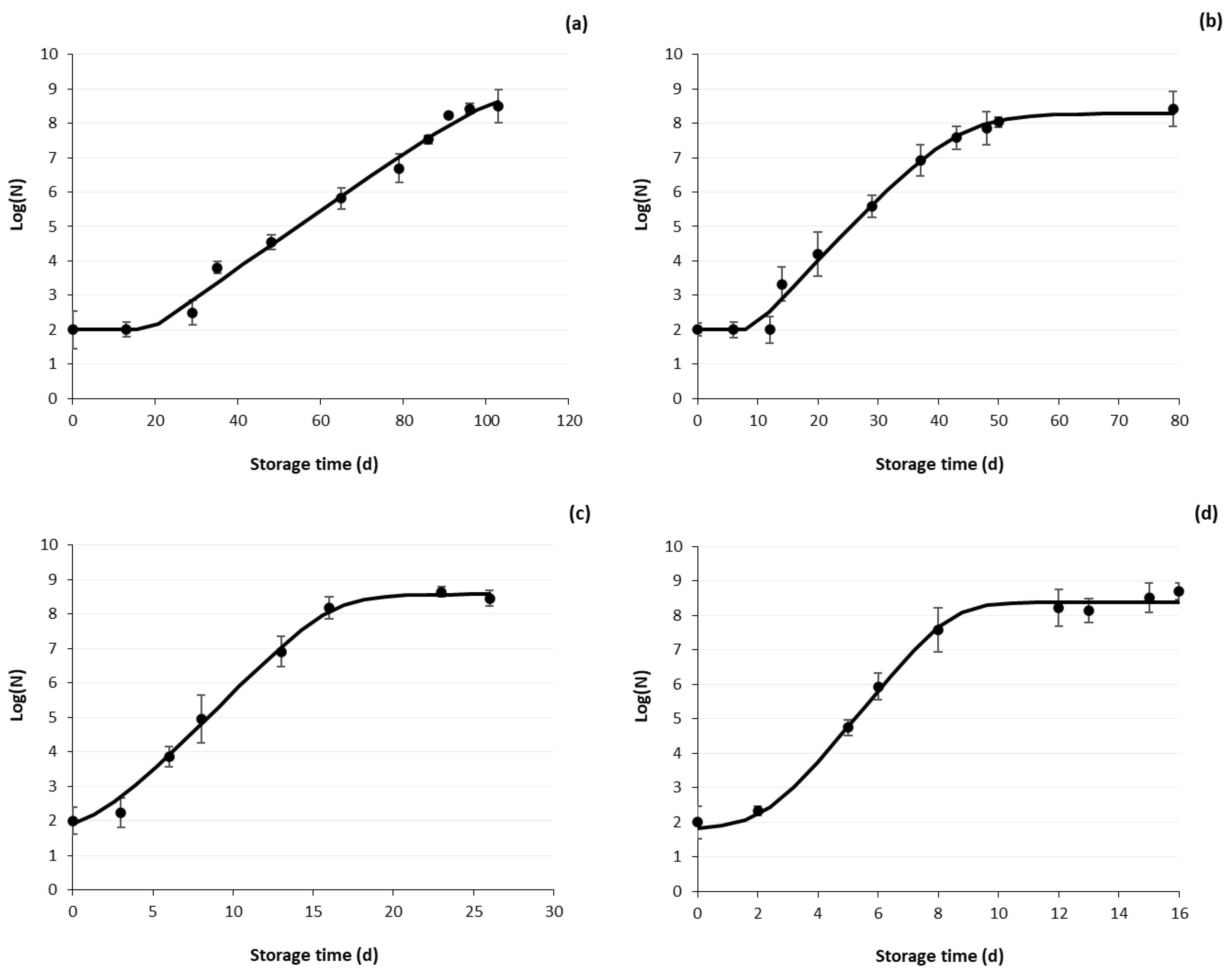
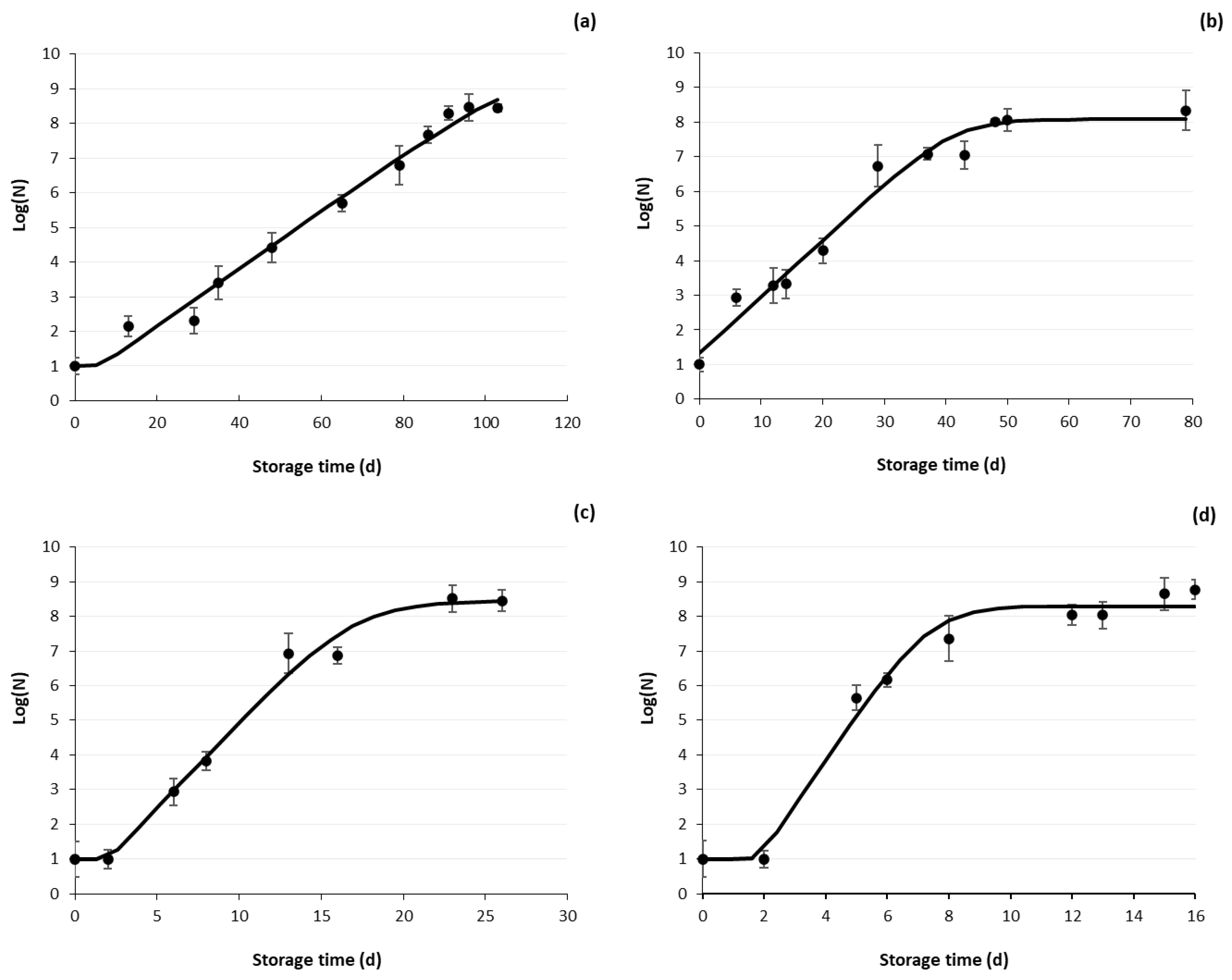
| Storage Temperature (°C) | Growth Rate k (d−1) | Lag Phase (d) | R2 | Doubling Time Td (d) |
|---|---|---|---|---|
| 4 | 0.384 ± 0.041 b | 8.27 ± 0.87 a | 0.984 | 1.8 |
| 8 | 0.465 ± 0.063 c | 2.15 ± 0.42 c | 0.971 | 1.4 |
| 12 | 0.521 ± 0.081 d | N.D. | 0.924 | 1.3 |
| 18 | 1.132 ± 0.301 e | N.D. | 0.947 | 0.6 |
| VAR1 (Teff = 3.8 °C) | 0.267 ± 0.032 a | 5.34 ± 1.57 b | 0.979 | 2.6 |
| VAR2 (Teff = 9.7 °C) | 0.853 ± 0.147 e | 2.05 ± 0.84 c | 0.981 | 0.8 |
| Storage Temperature (°C) | Growth Rate k (d−1) | Lag Phase (d) | R2 | Doubling Time Td (d) |
|---|---|---|---|---|
| 4 | 0.084 ± 0.007 a | 18.9 ± 5.43 a | 0.988 | 8.2 |
| 8 | 0.183 ± 0.018 b | 8.51 ± 0.89 b | 0.990 | 3.7 |
| 12 | 0.595 ± 0.037 c | 4.27 ± 0.70 c | 0.994 | 1.1 |
| 18 | 1.058 ± 0.102 d | 2.28 ± 0.48 d | 0.992 | 0.6 |
| VAR1 (Teff = 4.5 °C) | 0.084 ± 0.005 a | 18.1 ± 4.11 a | 0.994 | 8.3 |
| VAR2 (Teff = 11.1 °C) | 0.701 ± 0.110 c | 7.11 ± 1.21 b | 0.974 | 1.0 |
| Sample | Storage Temperature (°C) | Shelf Life (d) |
|---|---|---|
| CNT | 4 | 18 |
| 8 | 11 | |
| 12 | 8 | |
| 18 | 3 | |
| VAR1 | 20 | |
| VAR2 | 6 | |
| HPP | 4 | 89 |
| 8 | 41 | |
| 12 | 11 | |
| 18 | 7 | |
| VAR1 | 89 | |
| VAR2 | 15 |
| Estimated Parameters | Kinetic Data Whole Data Set | Kinetic Data 3 Temperatures | Kinetic Data 2 Temperatures |
|---|---|---|---|
| Ea (kJ /mol) | 125.6 ± 18.4 a | 116.2 ± 35.9 a | 119.3 a |
| kref (d−1) | 0.199 ± 0.031 a | 0.219 ± 0.074 ab | 0.183 a |
| R2 | 0.959 | 0.912 | - |
|
Estimated Parameters |
Kinetic Data Whole Data Set |
Kinetic Data 3 Temperatures |
Kinetic Data 2 Temperatures |
|---|---|---|---|
| Ea (kJ/mol) | 105.6 ± 3.9 a | 101.6 ± 6.1 a | 102.3 a |
| λref (d) | 9.07 ± 0.30 a | 8.73 ± 0.49 a | 9.00 a |
| R2 | 0.997 | 0.996 | - |
| Sample | Storage Temperature (°C) | Sensorial Deterioration Rate (d−1) | R2 |
Shelf Life
(d) |
|---|---|---|---|---|
| CNT | 4 | 0.211 ± 0.016 c | 0.970 | 21 |
| 8 | 0.328 ± 0.013 d | 0.988 | 13 | |
| 12 | 0.608 ± 0.082 f | 0.906 | 8 | |
| 18 | 0.709 ± 0.112 f | 0.861 | 6 | |
| HPP | 4 | 0.048 ± 0.003 a | 0.974 | 93 |
| 8 | 0.085 ± 0.007 b | 0.937 | 52 | |
| 12 | 0.245 ± 0.030 c | 0.922 | 18 | |
| 18 | 0.475 ± 0.052 e | 0.949 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntzimani, A.; Tsevdou, M.; Andrianos, E.; Gounaris, D.; Spiliotopoulos, T.; Taoukis, P.; Giannakourou, M.C. Validating Accelerated Shelf Life Testing Methodology for Predicting Shelf Life in High-Pressure-Processed Meat Products. Appl. Sci. 2025, 15, 1264. https://doi.org/10.3390/app15031264
Ntzimani A, Tsevdou M, Andrianos E, Gounaris D, Spiliotopoulos T, Taoukis P, Giannakourou MC. Validating Accelerated Shelf Life Testing Methodology for Predicting Shelf Life in High-Pressure-Processed Meat Products. Applied Sciences. 2025; 15(3):1264. https://doi.org/10.3390/app15031264
Chicago/Turabian StyleNtzimani, Athina, Maria Tsevdou, Evangelos Andrianos, Dimitrios Gounaris, Theodosios Spiliotopoulos, Petros Taoukis, and Maria C. Giannakourou. 2025. "Validating Accelerated Shelf Life Testing Methodology for Predicting Shelf Life in High-Pressure-Processed Meat Products" Applied Sciences 15, no. 3: 1264. https://doi.org/10.3390/app15031264
APA StyleNtzimani, A., Tsevdou, M., Andrianos, E., Gounaris, D., Spiliotopoulos, T., Taoukis, P., & Giannakourou, M. C. (2025). Validating Accelerated Shelf Life Testing Methodology for Predicting Shelf Life in High-Pressure-Processed Meat Products. Applied Sciences, 15(3), 1264. https://doi.org/10.3390/app15031264







