Drug Delivery Systems Utilizing Essential Oils and Their Compounds—A Promising Approach to Fight Pathogens
Abstract
:1. Introduction
| Compound Name | Density [g/cm3] | Vapor Pressure [mm/Hg] | Solubility in Water | Boiling Point | Selected Biological Properties | Plant Sources | References |
|---|---|---|---|---|---|---|---|
| Geraniol | 0.870–0.885 | 0.03 | 100 mg/L at 25 °C | 230 °C | AM, AI | Cymbopogon spp. Rosa spp. | [18,19] |
| Farnesol | 0.884–0.889 | 0.0000394 | Insoluble in water | 110–113 °C | AM, AI | Cymbopogon spp. | [20,21,22] |
| Linalyl acetate | 0.895 | 0.11 | Insoluble in water | 221 °C | AM, AI, AO | Lavandula spp. Citrus bergamia | [23,24] |
| Carvacrol | 0.974–0.979 | - | Insoluble in water | 237–238 °C | AM, AO | Origanum spp. Thymus spp. | [25,26] |
| α-Pinene | 0.8592 at 20 °C/4 °C | 4.75 at 25 °C | 2.49 mg/L at 25 °C | 156 °C | AM | Pinus spp. Picea spp. | [27,28] |
| α-Terpineol | 0.930–0.936 | 0.0423 at 24 °C | 7100 mg/L at 25 °C | 218–221 °C | AO, AC, AH | Origanum vulgare Ocimum canum | [29,30] |
| 1,8-Cineole | 0.921–0.924 | 1.90 at 25 °C | Insoluble in water | 176–177 °C | AM, AI, AO | Eucalyptus spp. | [31,32] |
| Eugenol | 1.064–1.070 | 0.0221 at 25 °C | Insoluble in water | 252–253 °C | AM, AI, AO | Syzygium spp. | [33,34,35] |
| Cinnamaldehyde | 1.048–1.052 | 0.0289 at 25 °C | Insoluble in water | 248.0 °C | AM | Cinnamomum spp. | [36,37,38] |
| p-Cymene | 0.853–0.855 | 1.50 at 25 °C | Insoluble in water | 176.0–178.0 °C | AM, AI | Cuminum cyminum Thymus spp. | [39,40] |
| Vanillin | 1.06 | 0.000118 at 25 °C | Insoluble in water | 285 °C | AM, AI, AO | Vanilla spp. | [41,42] |
| Menthol | 0.9131 | 0.06 | 420 mg/L at 25 °C | 214.6 °C | AM | Mentha spp. | [43,44] |
| trans-Anethole | 0.983–0.988 | 0.05 | Insoluble in water | 234.0 °C | AM, AI, AO | Pimpinella spp. Foeniculum spp. | [45,46] |
2. Problems Arising from the Chemical Properties of EOCs
2.1. Volatility
2.2. Water Insolubility
2.3. Thermal Instability
2.4. Light Susceptibility
2.5. Susceptibility to Oxidation
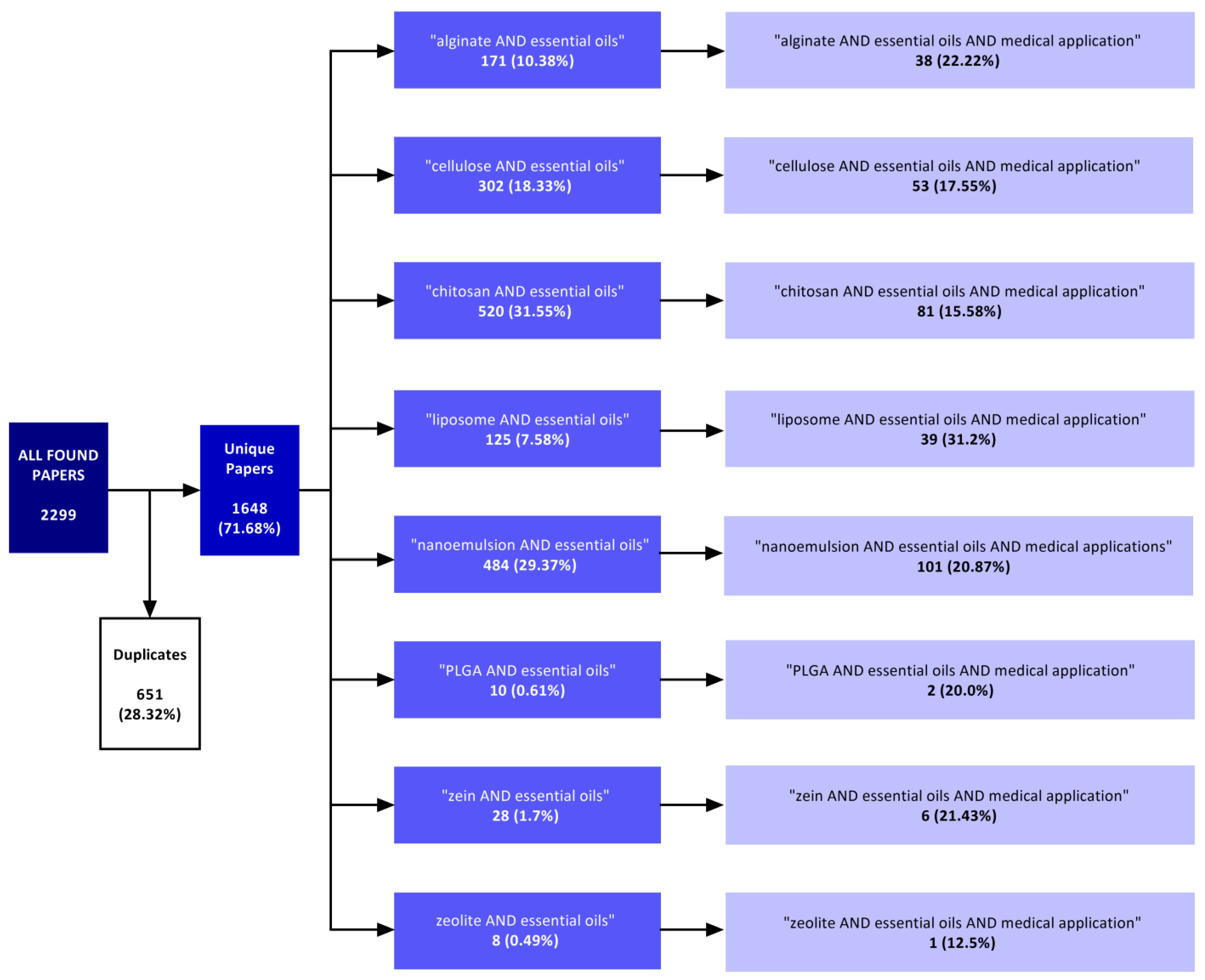
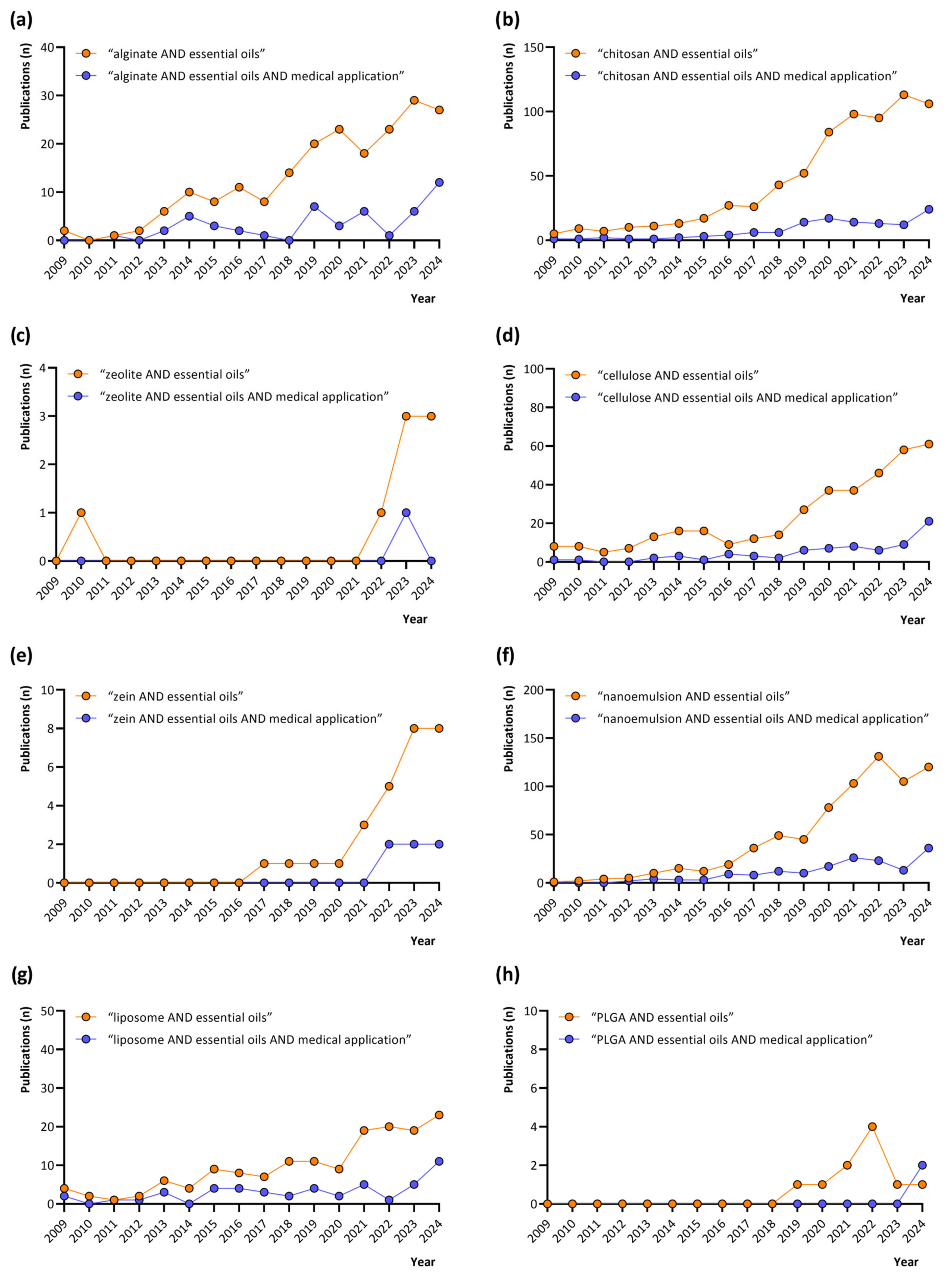
3. Search Strategy
4. Search Results
5. Drug Delivery Systems
5.1. Zeolites
5.2. Liposomes
5.3. Nanoemulsions
5.4. Chitosan
5.5. Cellulose Nanomaterials
5.6. Zein
5.7. Alginate
5.8. Poly(D,L-lactic-co-glycolic) Acid (PLGA)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, M.K.; Cha, J.Y.; Kang, M.; Jang, H.W.; Choi, Y. The Effects of Different Extraction Methods on Essential Oils from Orange and Tangor: From the Peel to the Essential Oil. Food Sci. Nutr. 2024, 12, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.C.; Franciscato, L.M.S.S.; Mendes, S.S.; Barizon, F.M.A.; Gonçalves, D.D.; Barbosa, L.N.; Faria, M.G.I.; Valle, J.S.; Casalvara, R.F.A.; Gonçalves, J.E.; et al. Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential. Molecules 2024, 29, 469. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.M.; Naser, A.; Ghorbanpour, M. Chemical Composition and Antimicrobial Activity of the Essential Oils in Different Populations of Coriandrum sativum L. (Coriander) from Iran and Iraq. Food Sci. Nutr. 2024, 12, 3872–3882. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Tanveer, M.; Wagner, C.; ul Haq, M.I.; Ribeiro, N.C.; Rathinasabapathy, T.; Butt, M.S.; Shehzad, A.; Komarnytsky, S. Spicing up Gastrointestinal Health with Dietary Essential Oils. Phytochem. Rev. 2020, 19, 243–263. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; De Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential Oils as a Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef]
- Neagu, R.; Popovici, V.; Ionescu, L.-E.; Ordeanu, V.; Biță, A.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Phytochemical Screening and Antibacterial Activity of Commercially Available Essential Oils Combinations with Conventional Antibiotics against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2024, 13, 478. [Google Scholar] [CrossRef]
- Karlberg, A.; Magnusson, K.; Nilsson, U. Air Oxidation of d-limonene (the Citrus Solvent) Creates Potent Allergens. Contact Dermat. 1992, 26, 332–340. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Saleh, M.A. Effect of Exposure to Light Emitted Diode (LED) Lights on Essential Oil Composition of Sweet Mint Plants. J. Environ. Sci. Health Part A 2019, 54, 435–440. [Google Scholar] [CrossRef]
- Ghosh, I.N.; Patil, S.D.; Sharma, T.K.; Srivastava, S.K.; Pathania, R.; Navani, N.K. Synergistic Action of Cinnamaldehyde with Silver Nanoparticles against Spore-Forming Bacteria: A Case for Judicious Use of Silver Nanoparticles for Antibacterial Applications. Int. J. Nanomed. 2013, 8, 4721–4731. [Google Scholar] [CrossRef]
- Gagné, F.; André, C.; Skirrow, R.; Gélinas, M.; Auclair, J.; van Aggelen, G.; Turcotte, P.; Gagnon, C. Toxicity of Silver Nanoparticles to Rainbow Trout: A Toxicogenomic Approach. Chemosphere 2012, 89, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Mnichowska-Polanowska, M.; Pruss, A.; Masiuk, H.; Dzięcioł, M.; Giedrys-Kalemba, S.; Sienkiewicz, M. The Effect of Fennel Essential Oil in Combination with Antibiotics on Staphylococcus aureus Strains Isolated from Carriers. Burns 2017, 43, 1544–1551. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial Activity of Selected Essential Oil Compounds Alone and in Combination with β-Lactam Antibiotics Against MRSA Strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
- Simbu, S.; Orchard, A.; van Vuuren, S. Essential Oil Compounds in Combination with Conventional Antibiotics for Dermatology. Molecules 2024, 29, 1225. [Google Scholar] [CrossRef] [PubMed]
- Ermenlieva, N.; Tsankova, G.; Nedelcheva, G.; Stamova, S.; Laleva, K.; Georgieva, E. Antimicrobial Effects of Antibiotics in Combination with Oregano Essential Oil against Staphylococcus aureus. Preprints 2024. [Google Scholar] [CrossRef]
- Zych, S.; Adaszyńska-Skwirzyńska, M.; Szewczuk, M.A.; Szczerbińska, D. Interaction between Enrofloxacin and Three Essential Oils (Cinnamon Bark, Clove Bud and Lavender Flower)—A Study on Multidrug-Resistant Escherichia coli Strains Isolated from 1-Day-Old Broiler Chickens. Int. J. Mol. Sci. 2024, 25, 5220. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 637566, Geraniol” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/geraniol (accessed on 30 November 2024).
- Su, Y.-W.; Chao, S.-H.; Lee, M.-H.; Ou, T.-Y.; Tsai, Y.-C. Inhibitory Effects of Citronellol and Geraniol on Nitric Oxide and Prostaglandin E2 Production in Macrophages. Planta Medica 2010, 76, 1666–1671. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3327, Farnesol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/farnesol (accessed on 30 November 2024).
- Lopes, A.P.; Branco, R.R.d.O.C.; Oliveira, F.A.d.A.; Campos, M.A.S.; Sousa, B.d.C.; Agostinho, Í.R.C.; Gonzalez, A.G.M.; Rocha, J.A.; Pinheiro, R.E.E.; Araújo, A.R.; et al. Antimicrobial, Modulatory, and Antibiofilm Activity of Tt-Farnesol on Bacterial and Fungal Strains of Importance to Human Health. Bioorg. Med. Chem. Lett. 2021, 47, 128192. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8294, Linalyl Acetate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/linalyl-acetate (accessed on 30 November 2024).
- Oliveira, M.S.; Paula, M.S.A.; Cardoso, M.M.; Silva, N.P.; Tavares, L.C.D.; Gomes, T.V.; Porto, D.L.; Aragão, C.F.S.; Fabri, R.L.; Tavares, G.D.; et al. Exploring the Antimicrobial Efficacy of Tea Tree Essential Oil and Chitosan against Oral Pathogens to Overcome Antimicrobial Resistance. Microb. Pathog. 2024, 196, 107006. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10364, Carvacrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/carvacrol (accessed on 30 November 2024).
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6654, Alpha-PINENE. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-pinene (accessed on 30 November 2024).
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and Antimicrobial Activity of Essential Oils of Industrial Hemp Varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 17100, Alpha-Terpineol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-terpineol (accessed on 30 November 2024).
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2758, Eucalyptol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/eucalyptol (accessed on 30 November 2024).
- Juergens, U.R. Anti-Inflammatory Properties of the Monoterpene 1.8-Cineole: Current Evidence for Co-Medication in Inflammatory Airway Diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3314, Eugenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/eugenol (accessed on 30 November 2024).
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 637511, Cinnamaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/cinnamaldehyde (accessed on 30 November 2024).
- Wang, B.; Zhao, M.-Z.; Huang, L.-Y.; Zhang, L.-J.; Yu, X.-J.; Liu, Y.; Li, J. Exploring Cinnamaldehyde: Preparation Methods, Biological Functions, Efficient Applications, and Safety. Food Rev. Int. 2024, 1–28. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7463, p-CYMENE. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/p-cymene (accessed on 30 November 2024).
- Balahbib, A.; Omari, N.E.; Hachlafi, N.E.; Lakhdar, F.; Menyiy, N.E.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of P-Cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1183, Vanillin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/vanillin (accessed on 30 November 2024).
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A Food Additive with Multiple Biological Activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1254, Menthol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/menthol (accessed on 30 November 2024).
- Freires, I.A.; Denny, C.; Benso, B.; De Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 637563, Anethole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/anethole (accessed on 30 November 2024).
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Qiu, X.-Y.; Yan, L.-S.; Kang, J.-Y.; Gu, C.Y.; Cheng, B.C.-Y.; Wang, Y.-W.; Luo, G.; Zhang, Y. Eucalyptol, Limonene and Pinene Enteric Capsules Attenuate Airway Inflammation and Obstruction in Lipopolysaccharide-Induced Chronic Bronchitis Rat Model via TLR4 Signaling Inhibition. Int. Immunopharmacol. 2024, 129, 111571. [Google Scholar] [CrossRef]
- Galan, D.M.; Ezeudu, N.E.; Garcia, J.; Geronimo, C.A.; Berry, N.M.; Malcolm, B.J. Eucalyptol (1,8-Cineole): An Underutilized Ally in Respiratory Disorders? J. Essent. Oil Res. 2020, 32, 103–110. [Google Scholar] [CrossRef]
- Unusan, N. Essential Oils and Microbiota: Implications for Diet and Weight Control. Trends Food Sci. Technol. 2020, 104, 60–71. [Google Scholar] [CrossRef]
- Su, X.; Li, B.; Chen, S.; Wang, X.; Song, H.; Shen, B.; Zheng, Q.; Yang, M.; Yue, P. Pore Engineering of Micro/Mesoporous Nanomaterials for Encapsulation, Controlled Release and Variegated Applications of Essential Oils. J. Control. Release 2024, 367, 107–134. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Alzahrani, E.; Shaltout, A.A.; Emam, H.E. Temperature-Controlled-Release of Essential Oil via Reusable Mesoporous Composite of Microcrystalline Cellulose and Zeolitic Imidazole Frameworks. J. Ind. Eng. Chem. 2021, 94, 134–144. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.-J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in Drug Delivery: Progress, Challenges and Opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Almeida-Aguiar, C.; Costa, S.P.G.; Neves, I.C. Essential Oils Encapsulated in Zeolite Structures as Delivery Systems (EODS): An Overview. Molecules 2022, 27, 8525. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Almeida-Aguiar, C.; Parpot, P.; Fonseca, A.M.; Neves, I.C. Preparation and Assessment of Antimicrobial Properties of Bimetallic Materials Based on NaY Zeolite. RSC Adv. 2015, 5, 37188–37195. [Google Scholar] [CrossRef]
- Costa, S.P.G.; Soares, O.S.G.P.; Aguiar, C.A.; Neves, I.C. Fragrance Carriers Obtained by Encapsulation of Volatile Aromas into Zeolite Structures. Ind. Crops Prod. 2022, 187, 115397. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J.; Setzer, W.N.; Changbunjong, T. Encapsulation of Eucalyptus Largiflorens Essential Oil by Mesoporous Silicates for Effective Control of the Cowpea Weevil, Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae). Molecules 2022, 27, 3531. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Ye, L.; Lv, Y.; Zhou, Z.; Shen, Y.; He, Y.; Jiang, L. Encapsulation of Highly Volatile Fragrances in Y Zeolites for Sustained Release: Experimental and Theoretical Studies. ACS Omega 2020, 5, 31925–31935. [Google Scholar] [CrossRef]
- Mallard, I.; Bourgeois, D.; Fourmentin, S. A Friendly Environmental Approach for the Controlled Release of Eucalyptus Essential Oil. Colloids Surf. Physicochem. Eng. Asp. 2018, 549, 130–137. [Google Scholar] [CrossRef]
- Iin, H.; Sugiarto; Fahma, F. Production of Zeolite-Cellulose Nanocomposites with Garlic Essential Oil for Antimicrobial Tablets. IOP Conf. Ser. Earth Environ. Sci. 2022, 1034, 012016. [Google Scholar] [CrossRef]
- Mojarab-Mahboubkar, M.; Afrazeh, Z.; Azizi, R.; Sendi, J.J. Efficiency of Artemisia Annua L. Essential Oil and Its Chitosan/Tripolyphosphate or Zeolite Encapsulated Form in Controlling Sitophilus oryzae L. Pestic. Biochem. Physiol. 2023, 195, 105544. [Google Scholar] [CrossRef]
- Milićević, Z.; Krnjajić, S.; Stević, M.; Ćirković, J.; Jelušić, A.; Pucarević, M.; Popović, T. Encapsulated Clove Bud Essential Oil: A New Perspective as an Eco-Friendly Biopesticide. Agriculture 2022, 12, 338. [Google Scholar] [CrossRef]
- Barbieri, N.; Sanchez-Contreras, A.; Canto, A.; Cauich-Rodriguez, J.V.; Vargas-Coronado, R.; Calvo-Irabien, L.M. Effect of Cyclodextrins and Mexican Oregano (Lippia graveolens Kunth) Chemotypes on the Microencapsulation of Essential Oil. Ind. Crops Prod. 2018, 121, 114–123. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins Enhance the Antioxidant Activity of Essential Oils from Three Lamiaceae Species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Kasperkowiak, M.; Strzemiecka, B.; Voelkel, A. Characteristics of Natural and Synthetic Molecular Sieves and Study of Their Interactions with Fragrance Compounds. Physicochem Probl Min. Process 2016, 52, 789–802. [Google Scholar] [CrossRef]
- Hao, J. Effects of Zeolite as a Drug Delivery System on Cancer Therapy: A Systematic Review. Molecules 2021, 26, 6196. [Google Scholar] [CrossRef]
- Kihara, T.; Zhang, Y.; Hu, Y.; Mao, Q.; Tang, Y.; Miyake, J. Effect of Composition, Morphology and Size of Nanozeolite on Its in Vitro Cytotoxicity. J. Biosci. Bioeng. 2011, 111, 725–730. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; De Vietro, N.; Iannaccone, G.; Baruzzi, F.; De Giglio, E. A Green Approach to Develop Zeolite-Thymol Antimicrobial Composites: Analytical Characterization and Antimicrobial Activity Evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef]
- Binay, M.I.; Kart, D.; Akata, B. Investigating Antimicrobial Behavior of Thymol/Zn Encapsulated Hierarchically Structured Zeolite and Thymol Release Kinetics. Microporous Mesoporous Mater. 2024, 376, 113188. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Shelf Life of Minced Pork in Vacuum-Adsorbed Carvacrol@Natural Zeolite Nanohybrids and Poly-Lactic Acid/Triethyl Citrate/Carvacrol@Natural Zeolite Self-Healable Active Packaging Films. Antioxidants 2024, 13, 776. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in Essential Oils Encapsulation: Development, Characterization and Release Mechanisms. Polym. Bull. 2024, 81, 3837–3882. [Google Scholar] [CrossRef]
- Bedoya-Agudelo, J.P.; López-Carvajal, J.E.; Quiguanás-Guarín, E.S.; Cardona, N.; Padilla-Sanabria, L.; Castaño-Osorio, J.C. Assessment of Antimicrobial and Cytotoxic Activities of Liposomes Loaded with Curcumin and Lippia Origanoides Essential Oil. Biomolecules 2024, 14, 851. [Google Scholar] [CrossRef]
- Li, Q.; Ran, C.; Chen, J.; Jin, J.; He, J.; Li, Y.; Wang, Q. Chitosan-Coated Double-Loaded Liposomes as a Promising Delivery System for Clove Essential Oil. J. Food Eng. 2024, 376, 112084. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, S.A.; Misaghi, A.; Moosavy, M.H.; Akhondzadeh Basti, A.; Mohamadian, S.; Khanjari, A. Effect of Nanoliposomes Containing Zataria Multiflora Boiss. Essential Oil on Gene Expression of Shiga Toxin 2 in Escherichia coli O157:H7. J. Appl. Microbiol. 2018, 124, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ellboudy, N.M.; Elwakil, B.H.; Shaaban, M.M.; Olama, Z.A. Cinnamon Oil-Loaded Nanoliposomes with Potent Antibacterial and Antibiofilm Activities. Molecules 2023, 28, 4492. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, F.; Swing, C.J.; Xia, S.; Zhang, X. Modulation Effect of Core-Wall Ratio on the Stability and Antibacterial Activity of Cinnamaldehyde Liposomes. Chem. Phys. Lipids 2019, 223, 104790. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome Containing Cinnamon Oil with Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus Biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.-V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of Essential Oils as Efficient Tools against Antimicrobial Resistance: A Review of the Type and Physical-Chemical Properties of the Delivery Systems and Applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Martínez-Velázquez, M.; Castillo-Herrera, G.A.; Espinosa-Andrews, H. Clove Essential Oil Nanoemulsions: Development, Physical Characterization, and Anticancer Activity Evaluation. J. Dispers. Sci. Technol. 2024, 1–9. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive Properties of Clove (Syzygium aromaticum) Essential Oil Nanoemulsion: A Comprehensive Review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef]
- Özakar, E.; Alparslan, L.; Adıgüzel, M.C.; Torkay, G.; Baran, A.; Bal-Öztürk, A.; Sevinç-Özakar, R. A Comprehensive Study on Peppermint Oil and Cinnamon Oil as Nanoemulsion: Preparation, Stability, Cytotoxicity, Antimicrobial, Antifungal, and Antioxidant Activity. Curr. Drug Deliv. 2024, 21, 603–622. [Google Scholar] [CrossRef]
- Yingngam, B.; Kacha, W.; Rungseevijitprapa, W.; Sudta, P.; Prasitpuriprecha, C.; Brantner, A. Response Surface Optimization of Spray-Dried Citronella Oil Microcapsules with Reduced Volatility and Irritation for Cosmetic Textile Uses. Powder Technol. 2019, 355, 372–385. [Google Scholar] [CrossRef]
- El-Sherbiny, G.M.; Kalaba, M.H.; Foda, A.M.; Shehata, M.E.; Youssef, A.S.E.-D.; Elsehemy, I.A.; Farghal, E.E.; El-Fakharany, E.M. Nanoemulsion of Cinnamon Oil to Combat Colistin-Resistant Klebsiella pneumoniae and Cancer Cells. Microb. Pathog. 2024, 192, 106705. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Su, W.; Zhang, X.; Yang, S.; Zhu, Y.; Liu, X. Self-Assembly of Sophorolipid and Eugenol into Stable Nanoemulsions for Synergetic Antibacterial Properties through Alerting Membrane Integrity. Colloids Surf. B Biointerfaces 2024, 234, 113749. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Tsai, W.-C.; Lu, H.-Y.; Fang, S.-Y.; Chan, H.-W.; Huang, C.-H. Enhancing Therapeutic Efficacy of Cinnamon Essential Oil by Nanoemulsification for Intravaginal Treatment of Candida Vaginitis. Int. J. Nanomed. 2024, 19, 4941–4956. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Doghish, A.S.; El-Dakroury, W.A.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Hashem, A.H. Antimicrobial, Antibiofilm, and Anticancer Activities of Syzygium Aromaticum Essential Oil Nanoemulsion. Molecules 2023, 28, 5812. [Google Scholar] [CrossRef]
- Miastkowska, M.; Sikora, E.; Kulawik-Pióro, A.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Szulc, J.; Piasecka-Zelga, J.; Zelga, P.; et al. Bioactive Lavandula Angustifolia Essential Oil-Loaded Nanoemulsion Dressing for Burn Wound Healing. In Vitro and in Vivo Studies. Biomater. Adv. 2023, 148, 213362. [Google Scholar] [CrossRef]
- Kakadia, P.G.; Conway, B.R. Design and Development of Essential Oil Based Nanoemulsion for Topical Application of Triclosan for Effective Skin Antisepsis. Pharm. Dev. Technol. 2022, 27, 554–564. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Nur Fatin Nazurah, R.; Noranizan, M.A.; Nor-Khaizura, M.A.R.; Nur Hanani, Z.A. Chitosan Nanoparticles Incorporate with Curry Leaf Essential Oil: Physicochemical Characterization and in vitro Release Properties. Int. J. Biol. Macromol. 2024, 273, 132972. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, Y.; Ma, S.; Li, L.; Yu, M.; Han, M.; Yuan, Z.; Zhang, J. Chitosan Nanoparticles Loaded with Eucommia Ulmoides Seed Essential Oil: Preparation, Characterization, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2024, 257, 128820. [Google Scholar] [CrossRef]
- Demirhan, I.; Korkmaz, A.; Oner, E.; Gumuscu, N.; Erbil, Y.; Babaarslan, O.; Kurutas, E.B. Synthesis, Characterization, and Antibacterial Effect of St. John’s Wort Oil Loaded Chitosan Hydrogel. Int. J. Biol. Macromol. 2024, 260, 129444. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.; da Silva, J.T.; Martins, J.A.; Rocha, V.L.; de Menezes, L.B.; Amaral, A.C. Chitosan Nanoparticles Encapsulating Farnesol Evaluated in vivo against Candida albicans. Braz. J. Microbiol. 2024, 55, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Jatomea, M.; Cortez-Rocha, M.O.; Rodríguez-Félix, F.; Mouriño-Pérez, R.R.; Lizardi-Mendoza, J.; Sánchez-Maríñez, R.I.; López-Meneses, A.K. Synthesis and Toxicological Study of Chitosan–Pirul (Schinus molle L.) Essential Oil Nanoparticles on Aspergillus flavus. Arch. Microbiol. 2024, 206, 133. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Afsarian, M.H.; Alipanah, H.; Yarian, F.; Moradi, H.; Khalaf, H.-E.; Osanloo, M. Development of Alginate and Chitosan Nanoparticles as Carriers of Zingiber officinale Essential Oil for Enhancement of Its Anticancer, Antibacterial, and Antifungal Activities. BioNanoScience 2024, 14, 3301–3312. [Google Scholar] [CrossRef]
- Hao, L.; Zheng, Q.; Zhuang, Q.; Guan, M.; Yin, Z.; Zeng, J.; Chen, H.; Wu, W.; Zhou, H.; Zhou, X. Antibacterial Microfibrillated Cellulose as Stimuli-Responsive Carriers with Enhanced UV Stability for Sustained Release of Essential Oils and Pesticides. ACS Sustain. Chem. Eng. 2024, 12, 6666–6681. [Google Scholar] [CrossRef]
- Simsek, M.; Eke, B.; Demir, H. Characterization of Carboxymethyl Cellulose-Based Antimicrobial Films Incorporated with Plant Essential Oils. Int. J. Biol. Macromol. 2020, 163, 2172–2179. [Google Scholar] [CrossRef]
- Darpentigny, C.; Marcoux, P.R.; Menneteau, M.; Michel, B.; Ricoul, F.; Jean, B.; Bras, J.; Nonglaton, G. Antimicrobial Cellulose Nanofibril Porous Materials Obtained by Supercritical Impregnation of Thymol. ACS Appl. Bio Mater. 2020, 3, 2965–2975. [Google Scholar] [CrossRef]
- Shin, J.; Na, K.; Shin, S.; Seo, S.-M.; Youn, H.J.; Park, I.-K.; Hyun, J. Biological Activity of Thyme White Essential Oil Stabilized by Cellulose Nanocrystals. Biomolecules 2019, 9, 799. [Google Scholar] [CrossRef]
- Kumineck, S.R., Jr.; Silveira, V.F.; Silva, D.A.K.; Garcia, M.C.F.; Apati, G.P.; Schneider, A.L.d.S.; Pezzin, A.P.T.; Baratto Filho, F. Development of Bacterial Cellulose Incorporated with Essential Oils for Wound Treatment. Polímeros 2023, 33, e20230026. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, J.; Zhu, Y.; Duan, C.; Sun, P.; Chen, Q.; Kong, B.; Wang, H. Oregano Essential Oil Encapsulated in Zein-Pectin-Chitosan Nanoparticles to Improve the Storage Quality of Harbin Red Sausage. Int. J. Biol. Macromol. 2024, 266, 131322. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Guarí-Borràs, G.; Ruiz-Rico, M.; Morellá-Aucejo, Á.; Aznar, E.; Barat, J.M.; Martínez-Máñez, R.; Marcos, M.D.; Bernardos, A. Towards the Enhancement of Essential Oil Components’ Antimicrobial Activity Using New Zein Protein-Gated Mesoporous Silica Microdevices. Int. J. Mol. Sci. 2021, 22, 3795. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lu, Y.; Sheng, J.; Song, Y. Zein-Functionalized MCM-41 Silica Nanoparticles with Enzyme-Responsive for Controlled Release in Antibacterial Activity. Coatings 2023, 13, 57. [Google Scholar] [CrossRef]
- Antunes, M.D.; Dannenberg, G.d.S.; Fiorentini, Â.M.; Pinto, V.Z.; Lim, L.-T.; Zavareze, E.d.R.; Dias, A.R.G. Antimicrobial Electrospun Ultrafine Fibers from Zein Containing Eucalyptus Essential Oil/Cyclodextrin Inclusion Complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef]
- Bilenler, T.; Gokbulut, I.; Sislioglu, K.; Karabulut, I. Antioxidant and Antimicrobial Properties of Thyme Essential Oil Encapsulated in Zein Particles. Flavour Fragr. J. 2015, 30, 392–398. [Google Scholar] [CrossRef]
- Alsakhawy, S.A.; Baghdadi, H.H.; El-Shenawy, M.A.; El-Hosseiny, L.S. Enhancement of Lemongrass Essential Oil Physicochemical Properties and Antibacterial Activity by Encapsulation in Zein-Caseinate Nanocomposite. Sci. Rep. 2024, 14, 17278. [Google Scholar] [CrossRef]
- Yahya, E.B.; Jummaat, F.; Amirul, A.A.; Adnan, A.S.; Olaiya, N.G.; Abdullah, C.K.; Rizal, S.; Mohamad Haafiz, M.K.; Khalil, H.P.S.A. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- Nqoro, X.; Adeyemi, S.A.; Ubanako, P.; Ndinteh, D.T.; Kumar, P.; Choonara, Y.E.; Aderibigbe, B.A. Wound Healing Potential of Sodium Alginate-Based Topical Gels Loaded with a Combination of Essential Oils, Iron Oxide Nanoparticles and Tranexamic Acid. Polym. Bull. 2024, 81, 3459–3478. [Google Scholar] [CrossRef]
- Rosa, J.M.; Bonato, L.B.; Mancuso, C.B.; Martinelli, L.; Okura, M.H.; Malpass, G.R.P.; Granato, A.C. Antimicrobial Wound Dressing Films Containing Essential Oils and Oleoresins of Pepper Encapsulated in Sodium Alginate Films. Ciênc. Rural 2018, 48, e20170740. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and Characterization of Sodium Alginate Based Active Edible Films Incorporated with Essential Oils of Some Medicinal Plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and Antimicrobial Properties of Sodium Alginate/Carboxymethyl Cellulose Films Incorporated with Cinnamon Essential Oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Saffarian, H.; Rahimi, E.; Khamesipour, F.; Hashemi Dehkordi, S.M. Antioxidant and Antimicrobial Effect of Sodium Alginate Nanoemulsion Coating Enriched with Oregano Essential Oil (Origanum vulgare L.) and Trachyspermum ammi Oil (Carum cupticum) on Food Pathogenic Bacteria. Food Sci. Nutr. 2024, 12, 2985–2997. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.S.; Costa, A.M.M.; Cabral, L.M.C.; Freitas-Silva, O.; Tonon, R.V. Physical and Biological Properties of Alginate-Based Cinnamon Essential Oil Nanoemulsions: Study of Two Different Production Strategies. Int. J. Biol. Macromol. 2024, 275, 133627. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.S.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A.; Hussain, M.D.; Khan, M.A.; Habib, M.J. Formulation of Anastrozole Microparticles as Biodegradable Anticancer Drug Carriers. AAPS PharmSciTech 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, I.; Piotrowska, M. Anastrozole as Aromatase Inhibitor—New Approaches to Breast Cancer Treatment in Postmenopausal Women. Nowotw. J. Oncol. 2019, 69, 26–35. [Google Scholar] [CrossRef]
- Said, S.S.; Aloufy, A.K.; El-Halfawy, O.M.; Boraei, N.A.; El-Khordagui, L.K. Antimicrobial PLGA Ultrafine Fibers: Interaction with Wound Bacteria. Eur. J. Pharm. Biopharm. 2011, 79, 108–118. [Google Scholar] [CrossRef]
- Folle, C.; Marqués, A.M.; Mallandrich, M.; Suñer-Carbó, J.; Halbaut, L.; Sánchez-López, E.; López-Machado, A.L.; Díaz-Garrido, N.; Badia, J.; Baldoma, L.; et al. Colloidal Hydrogel Systems of Thymol-Loaded PLGA Nanoparticles Designed for Acne Treatment. Colloids Surf. B Biointerfaces 2024, 234, 113678. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Mallandrich, M.; Suñer-Carbó, J.; Sánchez-López, E.; Halbaut, L.; Marqués, A.M.; Espina, M.; Badia, J.; Baldoma, L.; et al. Hydrogel of Thyme-Oil-PLGA Nanoparticles Designed for Skin Inflammation Treatment. Gels 2024, 10, 149. [Google Scholar] [CrossRef]
- Costa-Fernandez, S.; Matos, J.K.R.; Scheunemann, G.S.; Salata, G.C.; Chorilli, M.; Watanabe, I.-S.; de Araujo, G.L.B.; Santos, M.F.; Ishida, K.; Lopes, L.B. Nanostructured Lipid Carriers Containing Chitosan or Sodium Alginate for Co-Encapsulation of Antioxidants and an Antimicrobial Agent for Potential Application in Wound Healing. Int. J. Biol. Macromol. 2021, 183, 668–680. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartman, K.; Świerczyńska, M.; Wieczorek, A.; Baszuk, P.; Wojciechowska-Koszko, I.; Sienkiewicz, M.; Kwiatkowski, P. Drug Delivery Systems Utilizing Essential Oils and Their Compounds—A Promising Approach to Fight Pathogens. Appl. Sci. 2025, 15, 1287. https://doi.org/10.3390/app15031287
Hartman K, Świerczyńska M, Wieczorek A, Baszuk P, Wojciechowska-Koszko I, Sienkiewicz M, Kwiatkowski P. Drug Delivery Systems Utilizing Essential Oils and Their Compounds—A Promising Approach to Fight Pathogens. Applied Sciences. 2025; 15(3):1287. https://doi.org/10.3390/app15031287
Chicago/Turabian StyleHartman, Kacper, Maja Świerczyńska, Amelia Wieczorek, Piotr Baszuk, Iwona Wojciechowska-Koszko, Monika Sienkiewicz, and Paweł Kwiatkowski. 2025. "Drug Delivery Systems Utilizing Essential Oils and Their Compounds—A Promising Approach to Fight Pathogens" Applied Sciences 15, no. 3: 1287. https://doi.org/10.3390/app15031287
APA StyleHartman, K., Świerczyńska, M., Wieczorek, A., Baszuk, P., Wojciechowska-Koszko, I., Sienkiewicz, M., & Kwiatkowski, P. (2025). Drug Delivery Systems Utilizing Essential Oils and Their Compounds—A Promising Approach to Fight Pathogens. Applied Sciences, 15(3), 1287. https://doi.org/10.3390/app15031287









