An HPLC and LC-MS Method for Analyzing 2,2′,4,4′,6,6′-Hexanitrostilbene
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Methods
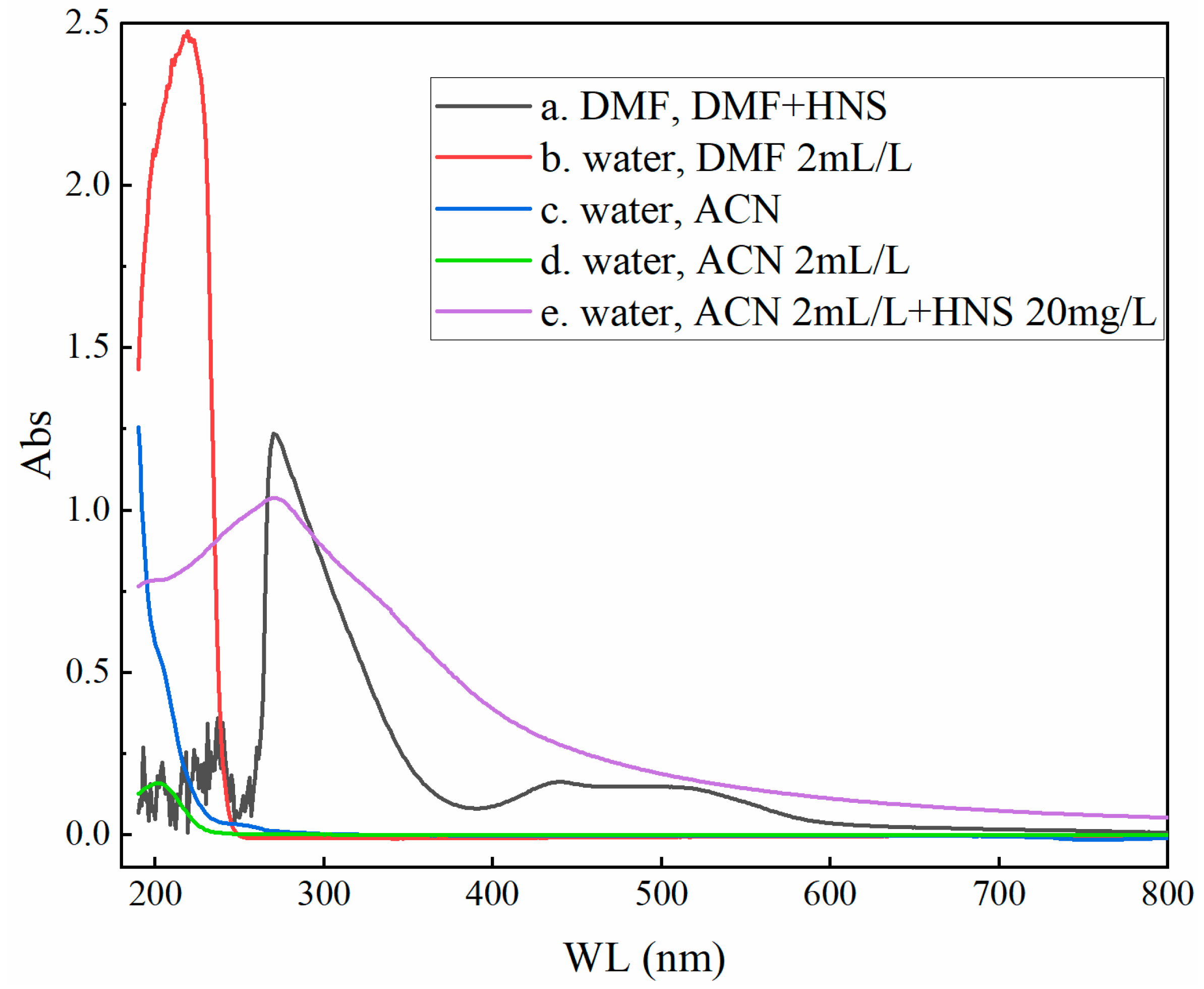
2.2. UV Full-Wavelength Analysis
3. Results and Discussion
3.1. HPLC Detection of HNS
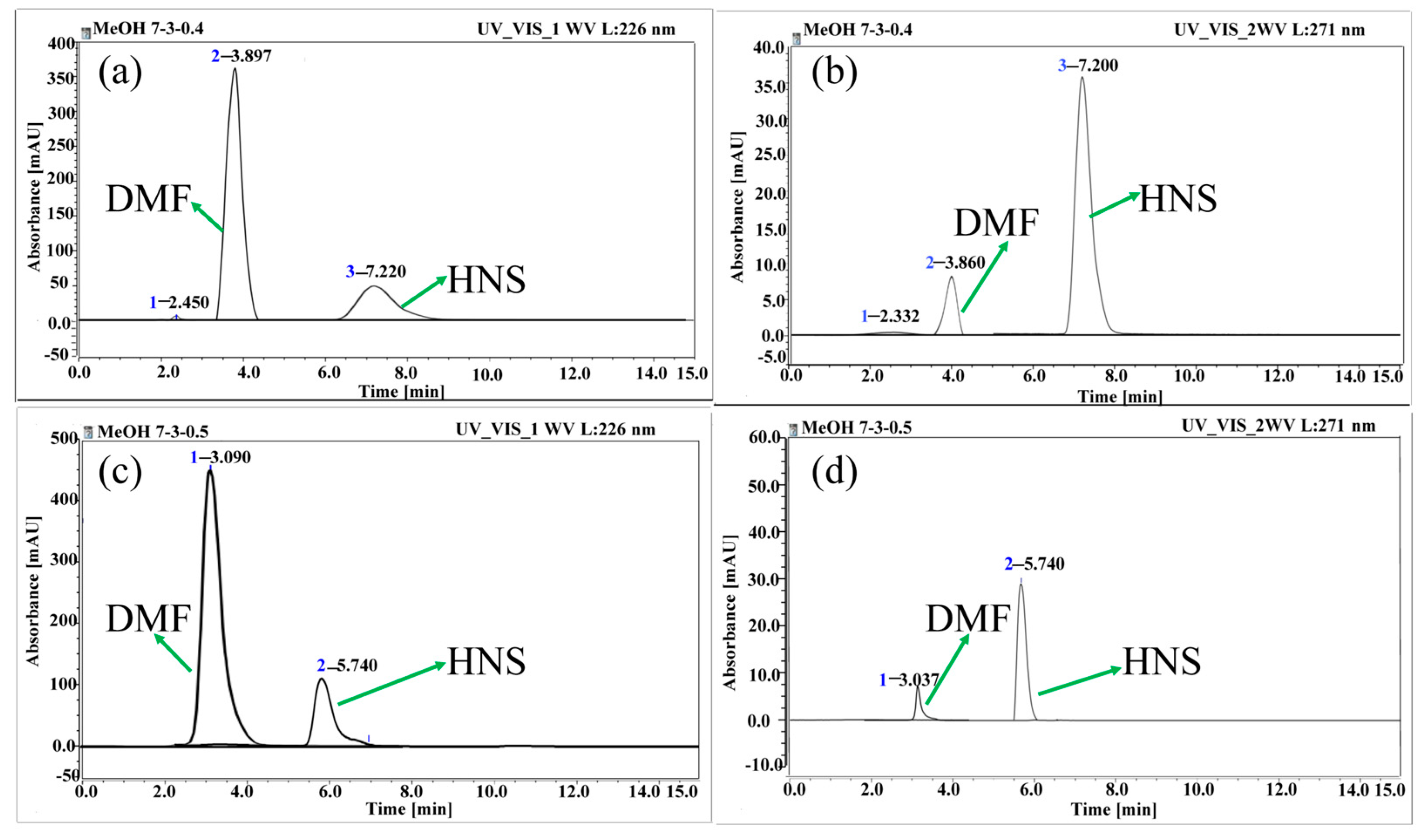
3.1.1. Determination of the Mobile Phase
3.1.2. Determination of Flow Velocity
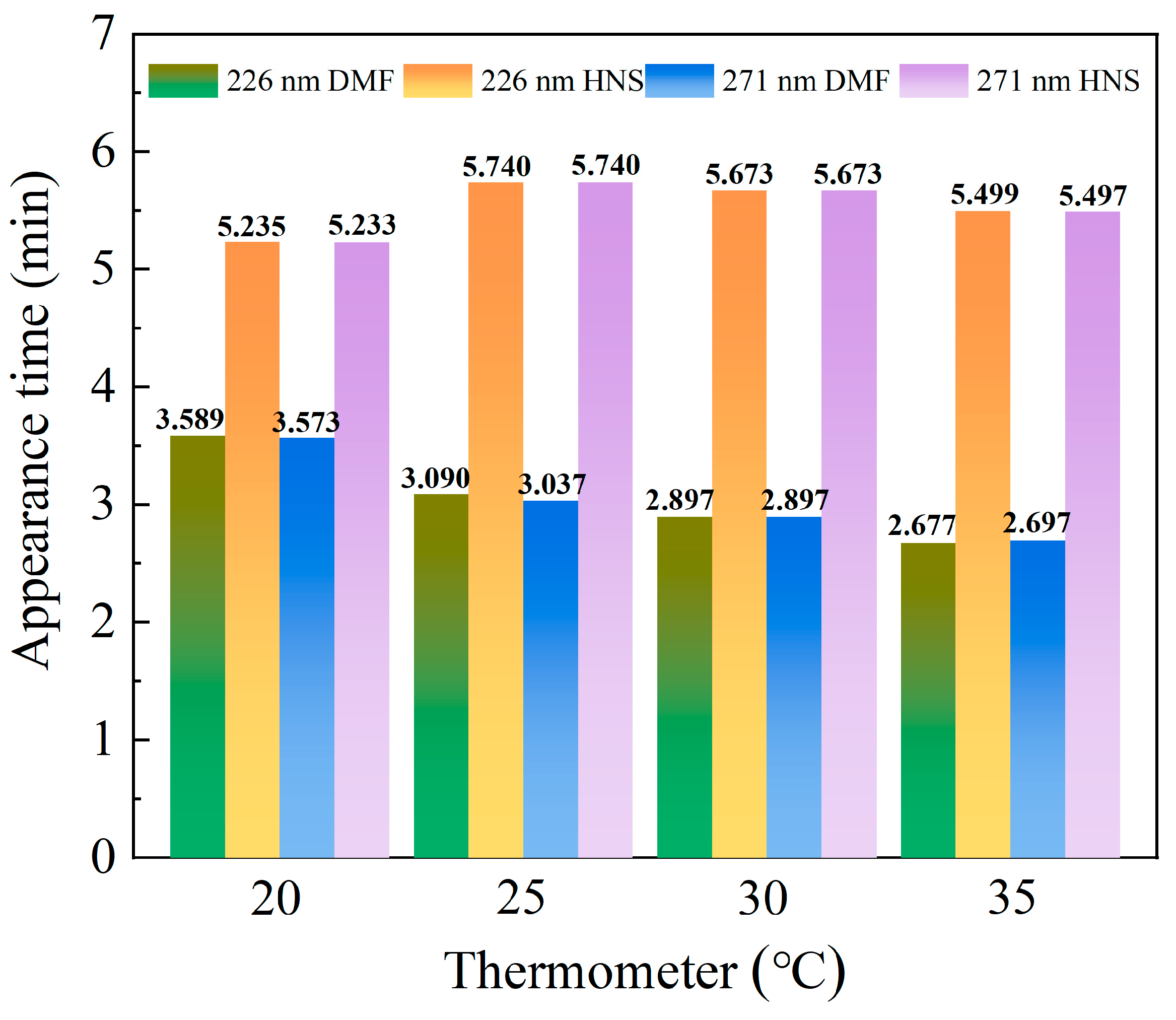
3.1.3. Determination of Column Temperature
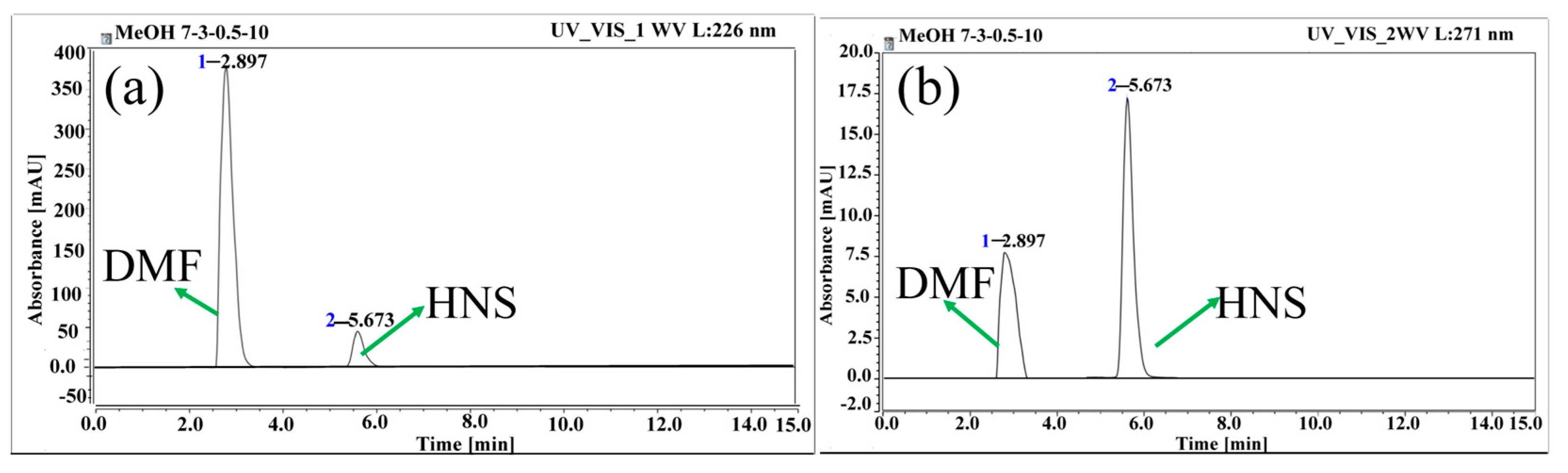
3.1.4. Determination of Sample Volume
3.2. Validation of the HPLC Analytical Method Performance
3.2.1. Sensitivity and Linearity of the Test Method
3.2.2. Accuracy of the Test Method
3.2.3. Precision of the Test Method
3.2.4. Repeatability and Stability of the Test Method
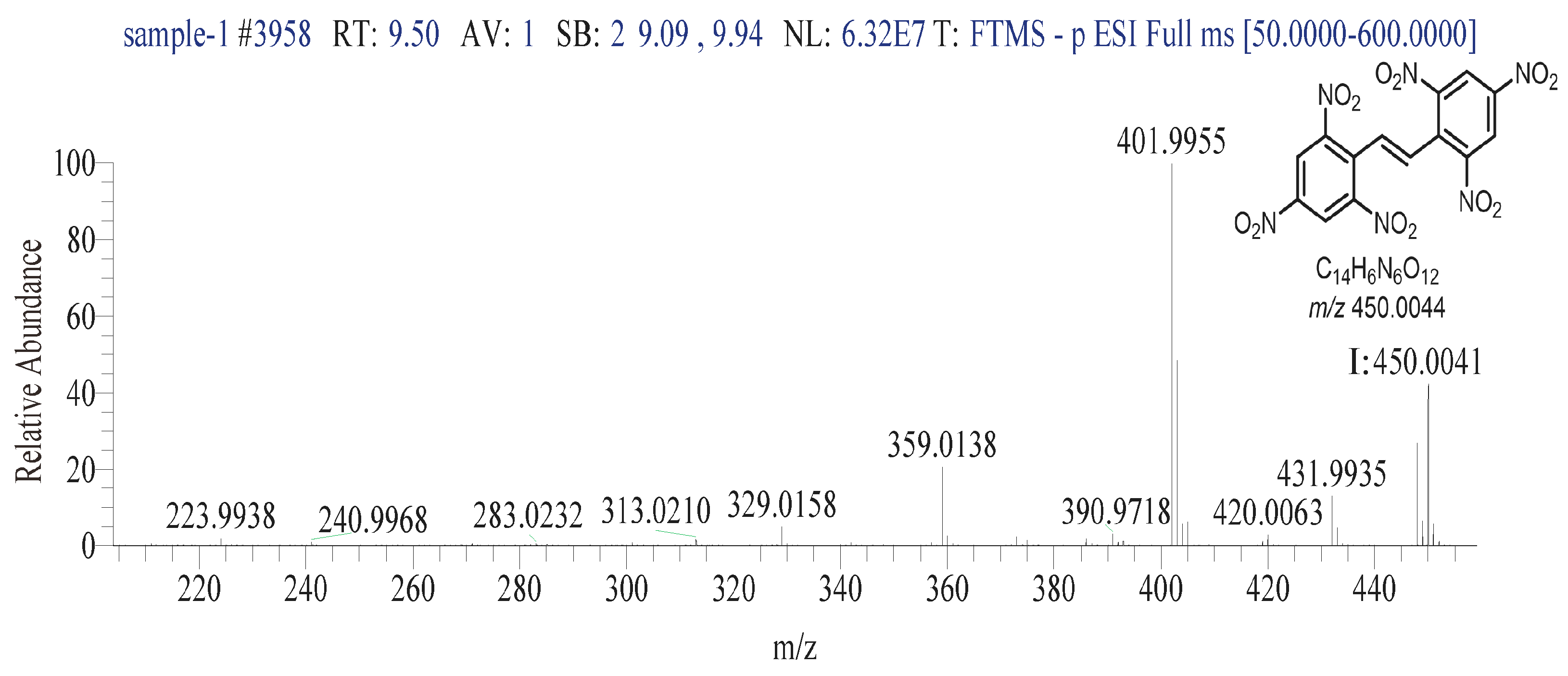
3.2.5. Liquid Chromatography–Mass Spectrometry Analysis Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, B.; Wang, Q.; Wang, H.; Zong, Q.; Gao, P.-J. Research on the mechanism and application of wedge cutting blasting with hole-inner delay. Sci. Rep. 2024, 14, 11383. [Google Scholar] [CrossRef]
- Oliveira, T.A.B.D.; Silva, G.D.; Mattos, E.D.C. Trends and prospects in the use of energetic materials: A comprehensive analysis of cyclotrimethylenetrinitramine, cyclotetramethylene-tetranitramine, and hexanitrohexaazaisowurtzitane. J. Aerosp. Technol. Manag. 2024, 16, 1326. [Google Scholar] [CrossRef]
- Sueska, M. Application of Explosive Equivalency Approach in Blast-Induced Seismic Effect Prediction Using EXPLO5 Thermochemical Code. Appl. Sci. 2024, 14, 7408. [Google Scholar] [CrossRef]
- Jiang, N.; Zhu, B.; Yao, Y.; Luo, X.-D. SC-CO2 and emulsion explosives equivalence of rock-breaking effect in granite and mudstone sites. J. CO2 Util. 2024, 83, 102806. [Google Scholar] [CrossRef]
- Ferr, O.G.E.B.; Da, C.D.M.B.; Naked, H.A.; Dos, S.I.J.A.L. Risk Assessment for Hexamine Nitration into RDX. J. Aerosp. Technol. Manag. 2014, 6, 373–388. [Google Scholar] [CrossRef]
- Shree, M.D.; Sangeetha, A.; Krishnan, P. Design and Analysis of FBG Sensor for Explosive Detection Applications. Plasmonics 2020, 15, 813–819. [Google Scholar] [CrossRef]
- Kucewicz, M.; Ukasz, M.; Baranowski, P.; Maachowski, J.; Fuawka, K.; Mertuszka, P.; Szumny, M. Numerical modeling of blast-induced rock fragmentation in deep mining with 3D and 2D FEM method approaches. Journal of Rock Mechanics and Geotechnical Engineering. J. Rock Mech. Geotech. Eng. 2024, 16, 4532–4553. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Yang, R.; Xiao, L.; Zhang, G.; Feng, X.; Zhang, K.; Jiang, W.; Hao, G. An Overview on Synthesis, Explosion, Catalysis, Modification, and Application of Dihydroxylammonium 5,5′-Bistetrazole-1,1′-diolate (TKX-50). Chem. Mat. 2024, 36, 3496–3535. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Trache, D.; Tarchoun, A.F.; Boukeciat, H.; Kadri, D.E.; Hassam, H.; Ouahioune, S.; Sahnoun, N.; Thakur, S.; Klapoetke, T.M. Application of co-crystallization method for the production of ammonium perchlorate/ammonium nitrate oxidizer for solid rocket propellants. Chem. Eng. J. 2024, 487, 150654. [Google Scholar] [CrossRef]
- Sikde, A.K.; Sikder, N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard. Mater. 2004, 112, 1–15. [Google Scholar] [CrossRef]
- Lan, G.; Zhang, G.; Shen, J.; Jin, G.; Wang, J.; Li, J. Ameliorating the sensitivities, thermal and combustion properties of RDX byin situ self-assembly TA-Pb/Cu shells to RDX surface. Arab. J. Chem. 2023, 16, 104497. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, W.; Du, P.; Liu, J.; Hao, G.; Gao, H.; Xiao, L.; Ke, X. Facile preparation of 1,3,5,7-tetranitro-1,3,5,7-tetrazocane/glycidylazide polymer energetic nanocomposites with enhanced thermolysis activity and low impact sensitivity. RSC Adv. 2017, 7, 5957–5965. [Google Scholar] [CrossRef]
- Duan, B.; Shu, Y.; Liu, N.; Wang, B.; Lu, X.; Lu, Y. Direct insight into the formation driving force, sensitivity and detonation performance of the observed CL-20-based energetic cocrystals. CrystEngComm 2018, 20, 5790–5800. [Google Scholar] [CrossRef]
- Cao, X.; Guan, Y.; Yang, L.; Yan, M.; Ma, Q.; Fan, G.; Liu, Y.; Huang, S. An energetic derivative of 2,2′,4,4′,6,6′-hexanitrostilbene (HNS) and its DMF solvate crystallized from HNS solution with tertiary amine additives. J. Energ. Mater. 2019, 37, 90–97. [Google Scholar] [CrossRef]
- Wang, J.; Huang, H.; Xu, W.-Z.; Zhang, Y.-R.; Lu, B.; Xie, R.-Z.; Wang, P.; Yun, N. Prefilming twin-fluid nozzle assisted precipitation method for preparing nanocrystalline HNS and its characterization. J. Hazard. Mater. 2009, 162, 842–847. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.-S.; Zhao, F.-Q.; Xu, S.-Y.; Ju, X.-H. Revealing the decomposition behavior of hexanitrostilbene and aluminum nanoparticles composites: A reactive molecular dynamics simulation. Acta Astronaut. 2020, 177, 320–331. [Google Scholar] [CrossRef]
- Padda, R.S.; Wang, C.-Y.; Hughes, J.B.; Razia, K.; Bennett, G.N. Mutagenicity of nitroaromatic degradation compounds. Environ. Toxicol. Chem. 2003, 22, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-H.; Ye, Z.; Li, H.; Che, R.; Cui, L. Biological treatment of hexanitrostilbene (HNS) produced wastewater using an anaerobic-aerobic immobilized microbial system. Chem. Eng. J. 2012, 213, 118–124. [Google Scholar] [CrossRef]
- Lima, D.R.S.; Bezerra, M.L.S.; Neves, E.B.; Moreira, F.R. Impact of ammunition and military explosives on human health and the environment. Rev. Environ. Health 2011, 26, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Maser, E.; Buenning, T.H.; Strehse, J.S. Environmental and human toxicology studies on explosive chemicals leaking from submerged munitions. Propellants Explos. Pyrotech. 2024, 49, e202300181. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol. 2014, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Darrach, M.R.; Chutjian, A.; Plett, G.A. Trace Explosives Signatures from World War II Unexploded Undersea Ordnance. Environ. Sci. Technol. 1998, 32, 1354–1358. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Nicolai, B.M.; Hertog, M. Optimization of HS SPME Fast GC-MS for High-Throughput Analysis of Strawberry Aroma. Food Anal. Meth. 2013, 6, 512–520. [Google Scholar] [CrossRef]
- Nikalje, A. Liquid Chromatography-Mass Spectrometry and Its Applications: A Brief Review. Arch. Org. Inorg. Chem. Sci. 2018, 1, 001–009. [Google Scholar] [CrossRef]
- Can, Z.; Uzer, A.; Tekdemir, Y.; Ercag, E.; Turker, L.; Apak, R. Spectrophotometric and chromatographic determination of insensitive energetic materials: HNS and NTO, in the presence of sensitive nitro-explosives. Talanta 2012, 90, 69–76. [Google Scholar] [CrossRef]
- Garofolo, F.; Longo, A.; Migliozzi, V.; Tallarico, C. Quantitative Analysis of Thermostable Explosive Compounds by Combined Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 960819. [Google Scholar] [CrossRef]
- Sandoval, J. Improvement of Water Soluble Test for HNS. Period Covered: January–March 1977; Mason and Hanger-Silas Mason Co., Inc.: Amarillo, TX, USA, 1977. [Google Scholar] [CrossRef]
- Chen, L.; Song, L.; Gao, Y.; Zhu, A.; Cao, D. Experimental determination of solubilities and supersolubilities of 2,2′, 4,4′, 6,6′-hexanitrostilbene in different organic solvents. Chin. J. Chem. Eng. 2017, 25, 809–814. [Google Scholar] [CrossRef]
- Ochsenbein, U.; Zeh, M.; Berset, J.-D. Comparing solid phase extraction and direct injection for the analysis of ultra-trace levels of relevant explosives in lake water and tributaries using liquid chromatography-electrospray tandem mass spectrometry. Chemosphere 2008, 72, 974–980. [Google Scholar] [CrossRef]
- DeTata, D.; Collins, P.; McKinley, A. A fast liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QToF-MS) method for the identification of organic explosives and propellants. Forensic Sci. Int. 2013, 233, 63–74. [Google Scholar] [CrossRef]
- Irlam, R.C.; Parkin, M.C.; Brabazon, D.P.; Beardah, M.S.; O’Donnell, M.; Barron, L.P. Improved determination of femtogram-level organic explosives in multiple matrices using dual-sorbent solid phase extraction and liquid chromatography-high resolution accurate mass spectrometry. Talanta 2019, 203, 65–76. [Google Scholar] [CrossRef] [PubMed]



| Reagent Name | Specification | Manufacturer |
|---|---|---|
| 2,2′,4,4′,6,6′-hexanitrostilbene (HNS) | AR, ≥99.5 | North University of China (Taiyuan, China) |
| N,N-Dimethylformamide (DMF) | AR, ≥99.9 | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Methanol (MeOH) | AR, ≥99.9 | Shanghai Aladdin Biochemical Technology Co., Ltd. |
| Acetonitrile (ACN) | AR, ≥99.9 | Shanghai Aladdin Biochemical Technology Co., Ltd. |
| Ultrapure water | AR, ≥99.9 | self-preparation |
| Instrument Name | Model | Manufacturer |
|---|---|---|
| Electronic balance | AL204 | Mettler Toledo Instruments (Shanghai, China) Co., Ltd. |
| UV spectrophotometer | UV-8000S | Shanghai Yuan analysis Instrument Co., Ltd. (Shanghai, China) |
| Ultrasonic cleaner | SB-5200DT | Ningbo Xinzhi Biotechnology Co., Ltd. (Ningbo, China) |
| Vacuum drying oven | DZG-6050SA | Shanghai Senxin Experimental Instrument Co., Ltd. (Shanghai, China) |
| Long axis rotary mixer | ROM-80pro | Jiangyin elite experimental instrument Co., Ltd. (Jiangyin, China) |
| Liquid chromatography (HPLC) | Ultimate3000 UHPLC | Shanghai Si instrument Biochemical Technology Co., Ltd. (Shanghai, China) |
| Chromatographic Parameter | Chromatographic Condition |
|---|---|
| Chromatographic instrument | Ultimate 3000 UHPLC |
| chromatographic column | ACCUCORE C30,2.6UM 150 × 4.6 MM |
| UV detection channel wavelength | 226 nm and 271 nm |
| Liquid phase mobile phase ratio | MeOH:water = 70:30 |
| Liquid flow rate | 0.5 mL min−1 |
| Column temperature | 30 °C |
| Liquid phase injection quantity | 10 μL |
| Sample test time | 6.5 min |
| Sample Preparation | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ |
|---|---|---|---|---|---|---|
| Sampling ① (mL) | 0.000 | 0.000 | 0.000 | 0.000 | 0.600 | 1.500 |
| Sampling ② (mL) | 0.125 | 0.250 | 5.000 | 7.500 | 0.000 | 0.000 |
| Sampling ③ (mL) | 7.375 | 7.250 | 2.500 | 0.000 | 0.000 | 0.000 |
| Corresponding HNS concentration (mg/L) | 0.50 | 1.00 | 20.00 | 30.00 | 60.00 | 150.00 |
| Name | Concentration/(mg/L) | Spike Recovery/% | Average/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Basic | Added | 1 | 2 | 3 | 1 | 2 | 3 | ||
| HNS | 60.00 | 48.00 | 107.93 | 108.15 | 107.88 | 99.88 | 100.25 | 99.80 | 99.98 |
| 60.00 | 120.21 | 120.17 | 119.93 | 100.35 | 100.28 | 99.88 | 100.17 | ||
| 72.00 | 131.89 | 132.11 | 131.87 | 99.82 | 100.18 | 99.78 | 99.93 | ||
| Concentration/(mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 1 | 2 | 3 | 4 | 5 | 6 | SD | Intra-Day RSD/% | Inter-Day RSD/% | |
| Day 1 | 10.00 | 9.87 | 10.03 | 9.96 | 9.87 | 10.07 | 9.85 | 0.09 | 0.92 | 1.16 |
| Day 2 | 9.77 | 9.85 | 10.01 | 9.82 | 10.08 | 10.01 | 0.09 | 0.87 | ||
| Day 3 | 10.29 | 9.97 | 10.23 | 10.28 | 10.25 | 10.33 | 0.11 | 1.03 | ||
| Day 1 | 20.00 | 19.72 | 19.79 | 20.01 | 20.02 | 20.03 | 19.72 | 0.16 | 0.81 | 0.98 |
| Day 2 | 19.78 | 20.03 | 19.79 | 20.07 | 19.97 | 19.71 | 0.18 | 0.91 | ||
| Day 3 | 20.37 | 20.39 | 19.96 | 19.97 | 20.27 | 20.39 | 0.17 | 0.82 | ||
| Day 1 | 30.00 | 30.02 | 29.71 | 30.02 | 29.92 | 29.71 | 30.23 | 0.30 | 0.99 | 0.96 |
| Day 2 | 29.71 | 30.02 | 30.03 | 30.01 | 29.72 | 30.19 | 0.24 | 0.81 | ||
| Day 3 | 30.23 | 30.35 | 29.97 | 30.37 | 30.39 | 29.88 | 0.32 | 1.05 | ||
| Time (h) | 0 | 2 | 4 | 6 | 8 | 24 |
|---|---|---|---|---|---|---|
| Concentration (mg/L) | 19.98 | 20.05 | 19.87 | 20.19 | 19.93 | 20.21 |
| RDS % | 0.86 | |||||
| LC Conditions | MS Conditions |
|---|---|
| Chromatograph: Dinonex Ultimate 3000 UHPLC | Mass spectrometer: Thermo Scientific Q Exactive |
| Column: Eclipse Plus C18 100 mm × 4.6 mm, 3.5 μm | Ion source: HESI |
| Column temperature: 30 °C | Sheath gas rate: 30 arb; Auxiliary gas rate: 5 arb |
| Injection volume: 10.0 μL | Spray voltage: negative ion 2.8 kV |
| Mobile phase: A: 5 mM ammonium acetate solution; B: acetonitrile | Capillary temperature: 320 °C; Auxiliary temperature: 300 °C |
| Gradient elution conditions: 45%A + 55%B | S-lens: 50% |
| Flow rate: 0.5 mL/min | Scan mode: Fullms/dd-ms2 top10 |
| Scanning range: First level scanning: resolution 70,000, range 50~600 m/z; | |
| Collision voltage: NCE15, 30, 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Jin, R.; Qiao, Y.; Jiang, Y.; Liu, J.; He, Z.; Liu, Z.; Gao, J. An HPLC and LC-MS Method for Analyzing 2,2′,4,4′,6,6′-Hexanitrostilbene. Appl. Sci. 2025, 15, 1486. https://doi.org/10.3390/app15031486
Jia M, Jin R, Qiao Y, Jiang Y, Liu J, He Z, Liu Z, Gao J. An HPLC and LC-MS Method for Analyzing 2,2′,4,4′,6,6′-Hexanitrostilbene. Applied Sciences. 2025; 15(3):1486. https://doi.org/10.3390/app15031486
Chicago/Turabian StyleJia, Mengye, Riya Jin, Yina Qiao, Yu Jiang, Jiaoqin Liu, Zengdi He, Zhaoguo Liu, and Jingshuai Gao. 2025. "An HPLC and LC-MS Method for Analyzing 2,2′,4,4′,6,6′-Hexanitrostilbene" Applied Sciences 15, no. 3: 1486. https://doi.org/10.3390/app15031486
APA StyleJia, M., Jin, R., Qiao, Y., Jiang, Y., Liu, J., He, Z., Liu, Z., & Gao, J. (2025). An HPLC and LC-MS Method for Analyzing 2,2′,4,4′,6,6′-Hexanitrostilbene. Applied Sciences, 15(3), 1486. https://doi.org/10.3390/app15031486





