Investigating the Effects of Fish Effluents as Organic Fertilisers on Basil (Ocimum basilicum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outline
2.2. Crop Choice
2.3. Treatments
2.4. Substrate Mixes
2.5. Experimental Setup and Growth Conditions
2.6. Measurements and Analyses
2.7. Statistical Analyses
3. Results
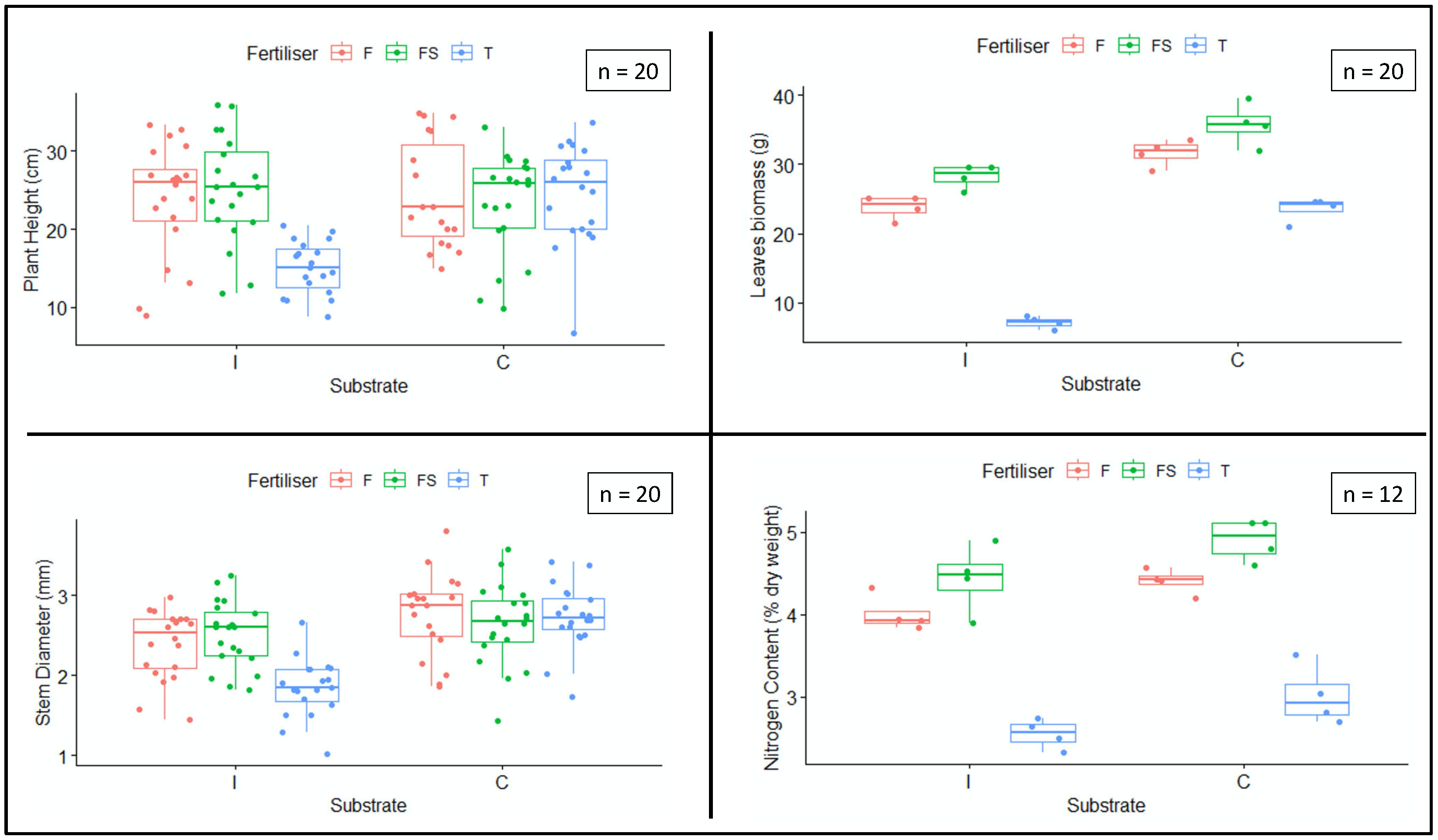
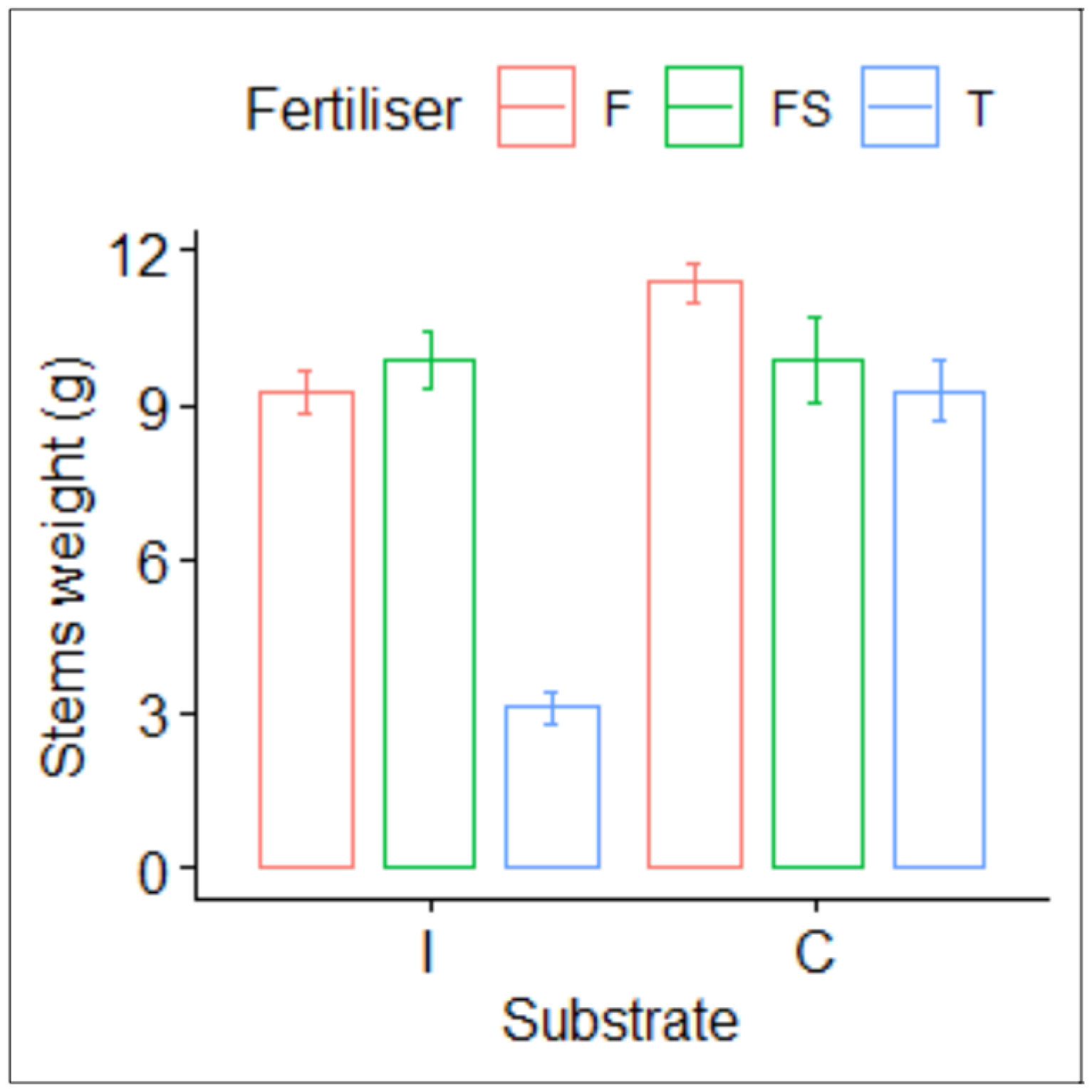
3.1. Plant Growth and Yield
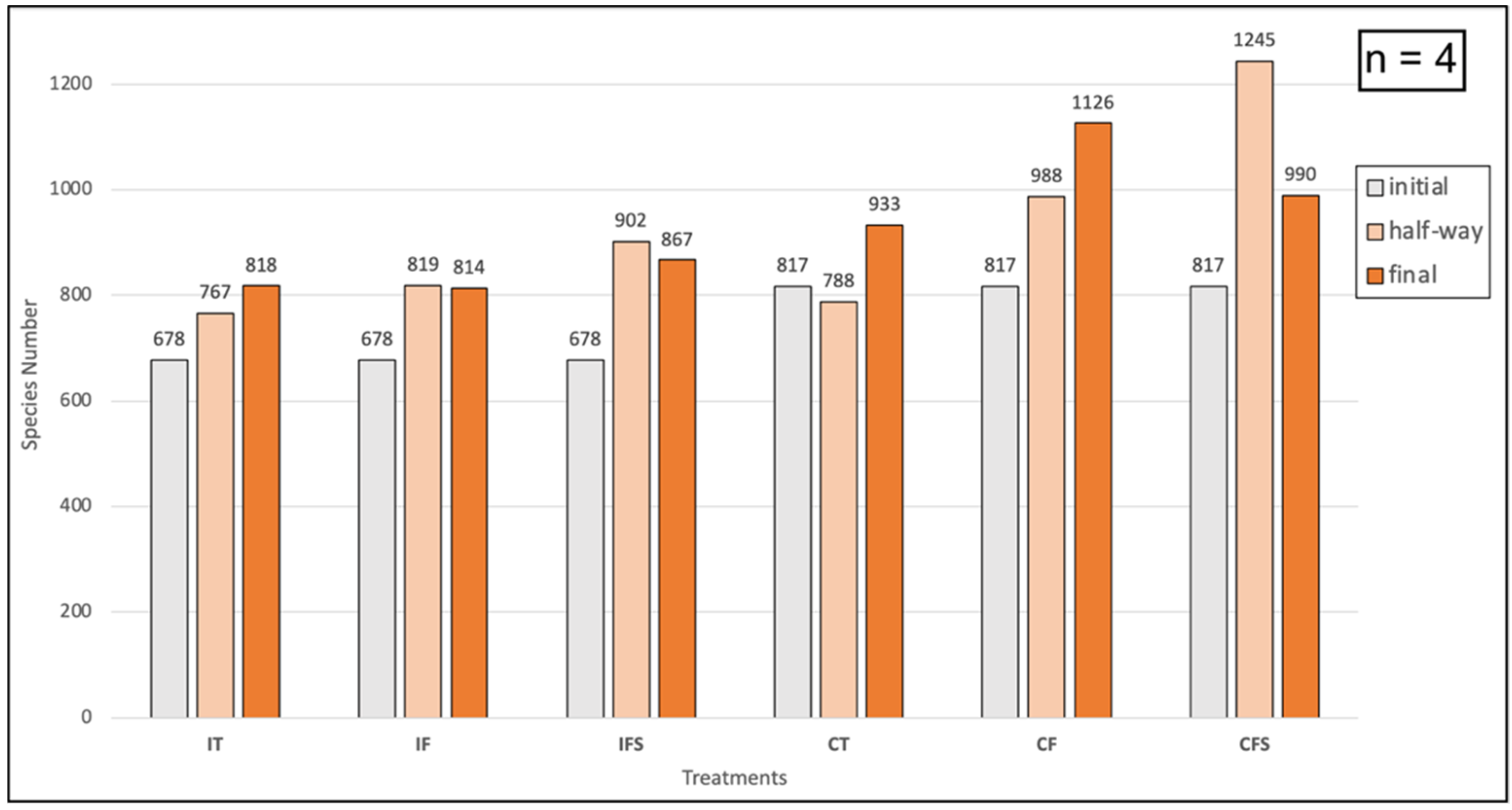
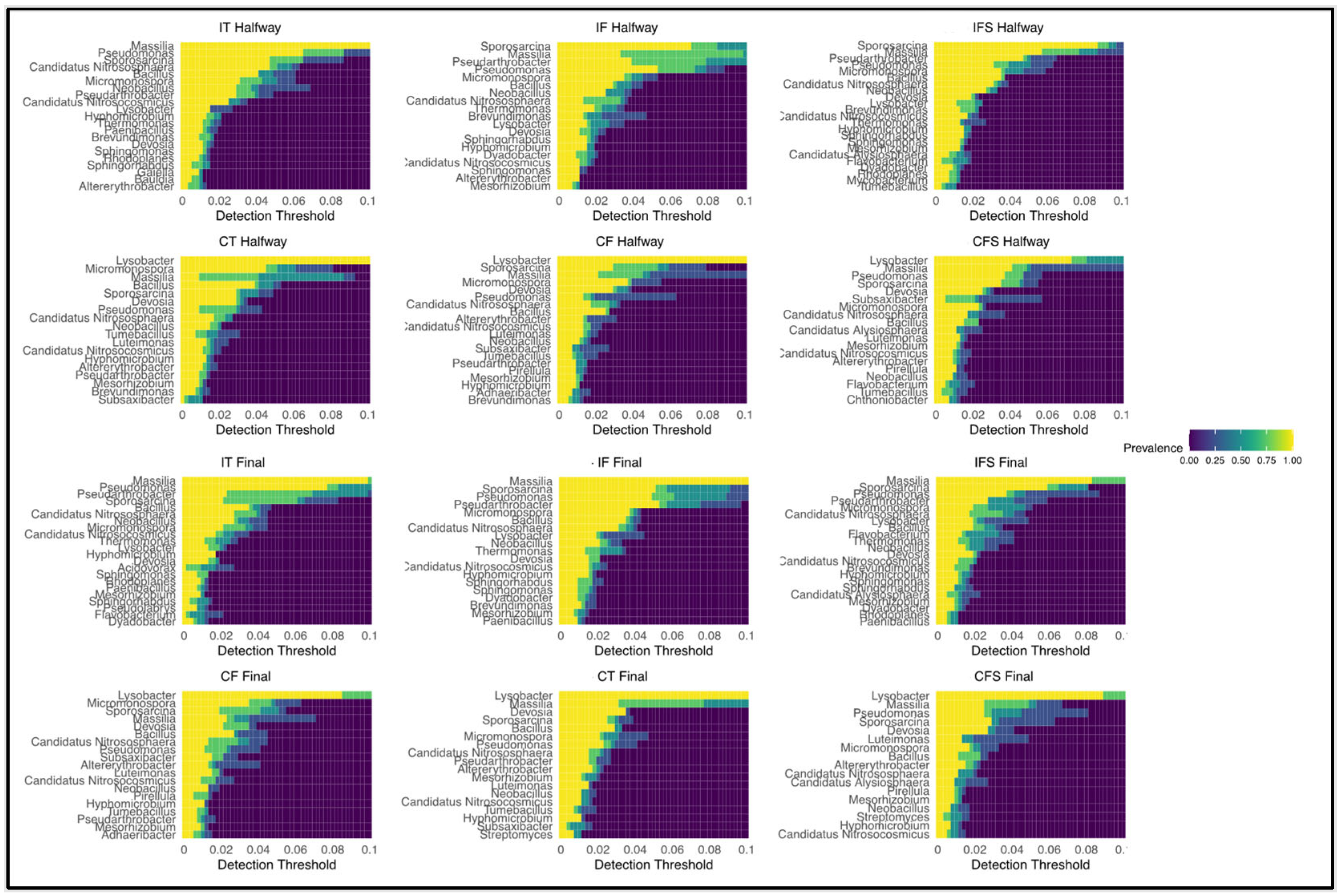
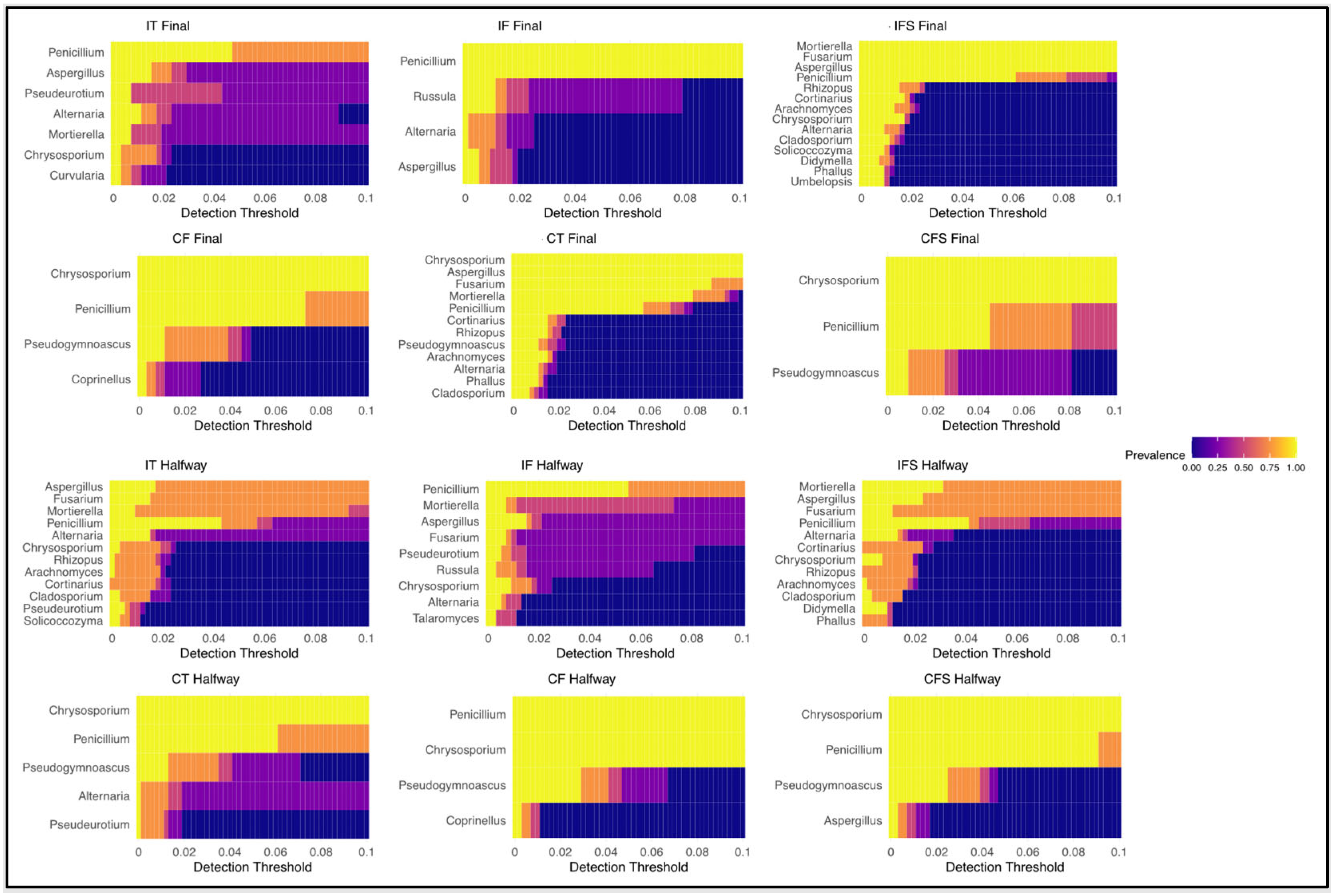
3.2. Soil Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Substrate—ammonium nitrate extractable calcium and sodium
- Substrate—available sulphate
- Substrate—DTPA-extractable manganese, iron, copper and zinc
- Substrate—hot water-soluble boron
- Substrate—organic matter content by loss on ignition at 430 °C
- Substrate—mineral nitrogen (available N)
- Substrate—pH and lime requirement
- Substrate—Olsen’s extractable phosphorus
- Substrate—ammonium nitrate-extractable potassium and magnesium
- Fish effluent—nitrate nitrogen
- Fish effluent—dissolved elements
- Fish effluent—electrical conductivity
- Fish effluent—pH
- Fish effluent—total dissolved solids
References
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Available online: https://eur-lex.europa.eu/eli/reg/2018/848/oj/eng (accessed on 31 March 2024).
- Commission Implementing Regulation (EU) 2023/121 of 17 January 2023 amending and correcting Implementing Regulation (EU) 2021/1165 authorising certain products and substances for use in organic production and establishing their lists. Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/121/oj/eng (accessed on 31 March 2024).
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Thoman, E.S. Update on tilapia and vegetable production in the UVI aquaponic system. In New Dimensions on Farmed Tilapia, Proceedings of the Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; Bolivar, R., Mair, G., Fitzsimmons, K., Eds.; Creative Unlimited: Clovelly, NSW, Australia, 2004; pp. 298–312. [Google Scholar]
- Graber, A.; Junge, R. Aquaponic systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Tyson, R.V.; Treadwell, D.D.; Simonne, E.H. Opportunities and challenges to sustainability in aquaponic systems. HortTechnology 2011, 21, 6–13. [Google Scholar] [CrossRef]
- Naylor, S.J.; Moccia, R.D.; Durant, G.M. The chemical composition of settleable solid fish waste (manure) from commercial rainbow trout farms in Ontario, Canada. N. Am. J. Aquac. 1999, 61, 21–26. [Google Scholar] [CrossRef]
- Castro, R.S.; Borges Azevedo, C.M.S.; Bezerra-Neto, F. Increasing cherry tomato yield using fish effluent as irrigation water in Northeast Brazil. Sci. Hortic. 2006, 110, 44–50. [Google Scholar] [CrossRef]
- Gravel, V.; Dorais, M.; Dey, D.; Vandenberg, G. Fish effluents promote root growth and suppress fungal diseases in tomato transplants. Can. J. Plant Sci. 2015, 95, 427–436. [Google Scholar] [CrossRef]
- Mangmang, J.S.; Deaker, R.; Rogers, G. Azospirillum brasilense enhances recycling of fish effluent to support growth of tomato seedlings. Horticulturae 2015, 1, 14–26. [Google Scholar] [CrossRef]
- Delaide, B.; Teerlinck, S.; Decombel, A.; Bleyaert, P. Effect of wastewater from a pikeperch (Sander lucioperca L.) recirculated aquaculture system on hydroponic tomato production and quality. Agric. Water Manag. 2019, 226, 105814. [Google Scholar] [CrossRef]
- Pattillo, A.D.; Foshee, W.G.; Blythe, E.K.; Pickens, J.; Wells, D.; Monday, T.A.; Hanson, T.R. Performance of aquaculture effluent for tomato production in outdoor raised beds. HortTechnology 2020, 30, 624–631. [Google Scholar] [CrossRef]
- Pickens, J.M.; Danaher, J.J.; Sibley, J.L.; Chappell, J.A.; Hanson, T.R. Integrating greenhouse cherry tomato production with biofloc tilapia production. Horticulturae 2020, 6, 44. [Google Scholar] [CrossRef]
- Diatta, A.A.; Manga, A.G.B.; Bassène, C.; Mbow, C.; Battaglia, M.; Sambou, M.; Babur, E.; Uslu, Ö.S. Sustainable production of tomato using fish effluents improved plant growth, yield components, and yield in northern Senegal. Agronomy 2023, 13, 2696. [Google Scholar] [CrossRef]
- Mechouma, A.; Mezerdi, F. Impact of the use of water from fish farming in the irrigation of pepper crops (Capsicum annuum L.) in the greenhouse in the Biskra region. Int. J. Environ. Stud. 2024, 81, 734–750. [Google Scholar] [CrossRef]
- Lenz, G.L.; Loss, A.; Lourenzi, C.R.; Lopes, D.L.A.; Siebeneichler, L.M.; Brunetto, G. Common chicory production in aquaponics and in soil fertilized with aquaponics sludge. Sci. Hortic. 2021, 281, 109946. [Google Scholar] [CrossRef]
- Mangmang, J.S.; Deaker, R.S.; Rogers, G. Response of lettuce seedlings fertilized with fish effluent to Azospirillum brasilense inoculation. Biol. Agric. Hortic. 2015, 31, 61–71. [Google Scholar] [CrossRef]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The effect of anaerobic and aerobic fish sludge supernatant on hydroponic lettuce. Agronomy 2016, 6, 37. [Google Scholar] [CrossRef]
- Delaide, B.; Panana, E.; Teerlinck, S.; Bleyaert, P. Suitability of supernatant of aerobic and anaerobic pikeperch (Sander lucioperca L.) sludge treatments as a water source for hydroponic production of lettuce (Lactuca sativa L. var. capitata). Aquac. Int. 2021, 29, 1721–1735. [Google Scholar] [CrossRef]
- Lenz, G.L.; Loss, A.; Lourenzi, C.R.; Lopes, D.L.A.; Siebeneichler, L.M.; Brunetto, G. Lettuce growth in aquaponic system and in soil fertilized with fish sludge. Aquac. Res. 2021, 52, 5008–5021. [Google Scholar] [CrossRef]
- Mangmang, J.S.; Deaker, R.S.; Rogers, G. Response of cucumber seedlings fertilized with fish effluent to Azospirillum brasilense. Int. J. Veg. Sci. 2016, 22, 129–140. [Google Scholar] [CrossRef]
- Abdelraouf, R.E. Reuse of fish farm drainage water in irrigation. In Unconventional Water Resources and Agriculture in Egypt; Negm, A., Ed.; Springer: Cham, Switzerland, 2017; pp. 393–410. [Google Scholar]
- Chen, S.; Coffin, D.E.; Malone, R.F. Sludge production and management for recirculating aquacultural systems. J. World Aquac. Soc. 1997, 28, 303–315. [Google Scholar] [CrossRef]
- Montanhini Neto, R.; Ostrensky, A. Nutrient load estimation in the waste of Nile tilapia Oreochromis niloticus (L.) reared in cages in tropical climate conditions. Aquac. Res. 2013, 46, 1309–1322. [Google Scholar] [CrossRef]
- Brod, E.; Oppen, J.; Kristoffersen, A.Ø.; Haraldsen, T.K.; Krogstad, T. Drying or anaerobic digestion of fish sludge: Nitrogen fertilisation effects and logistics. Ambio 2017, 46, 852–864. [Google Scholar] [CrossRef]
- Rafiee, G.; Saad, C.R. Nutrient cycle and sludge production during different stages of red tilapia (Oreochromis sp.) growth in a recirculating aquaculture system. Aquaculture 2005, 244, 109–118. [Google Scholar] [CrossRef]
- Cripps, S.J.; Bergheim, A. Solids management and removal for intensive land-based aquaculture production systems. Aquac. Eng. 2000, 22, 33–56. [Google Scholar] [CrossRef]
- Monsees, H.; Keitel, J.; Paul, M.; Kloas, W.; Wuertz, S. Potential of aquacultural sludge treatment for aquaponics: Evaluation of nutrient mobilization under aerobic and anaerobic conditions. Aquac. Environ. Interact. 2017, 9, 9–18. [Google Scholar] [CrossRef]
- Wotton, R.; Malmqvist, B. Feces in aquatic ecosystems. BioScience 2001, 51, 537–544. [Google Scholar] [CrossRef]
- Kledal, P.R.; König, B.; Matulić, D. Aquaponics: The ugly duckling in organic regulation. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G., Eds.; SpringerOpen: Cham, Switzerland, 2019; pp. 487–500. [Google Scholar] [CrossRef]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Vaccari, D.A. Phosphorus: A looming crisis. Sci. Am. 2009, 300, 54–59. [Google Scholar] [CrossRef]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable use of phosphorus: A finite resource. Sci. Total Environ. 2013, 461–462, 799–803. [Google Scholar] [CrossRef]
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. Acta Hortic. 2004, 648, 63–69. [Google Scholar] [CrossRef]
- Xie, K.; Rosentrater, K. Life cycle assessment (LCA) and techno-economic analysis (TEA) of tilapia-basil aquaponics. In Proceedings of the ASABE Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015; Available online: https://dr.lib.iastate.edu/server/api/core/bitstreams/566003c3-9fe9-4735-a8c8-b4dfa8a4c32c/content (accessed on 31 March 2024).
- Ferrarezi, R.S.; Bailey, D.S. Basil performance evaluation in aquaponics. HortTechnology 2019, 29, 85–93. [Google Scholar] [CrossRef]
- Knaus, U.; Pribbernow, M.; Xu, L.; Appelbaum, S.; Palm, H.W. Basil (Ocimum basilicum) cultivation in decoupled aquaponics with three hydro-components (grow pipes, raft, gravel) and African catfish (Clarias gariepinus) production in Northern Germany. Sustainability 2020, 12, 8745. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing nutrient composition and concentration in tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Pasch, J.; Ratajczak, B.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of basil (Ocimum basilicum) in DRF, raft, and grow pipes with effluents of African catfish (Clarias gariepinus) in decoupled aquaponics. Agric. Eng. 2021, 3, 92–109. [Google Scholar] [CrossRef]
- JohnnySeeds. Hydroponic & Container Basil Variety Comparison Charts. Available online: https://www.johnnyseeds.com/growers-library/herbs/basil/hydroponic-container-basil-varieties-comparison-chart.html (accessed on 31 March 2024).
- Royal Horticultural Society. Onions. Available online: https://www.rhs.org.uk/advice/grow-your-own/vegetables/onions (accessed on 31 March 2024).
- RStudio Team. RStudio: Integrated Development for R. Available online: https://posit.co/download/rstudio-desktop/ (accessed on 31 March 2024).
- Fruscella, L.; Kotzen, B.; Paradelo, M.; Milliken, S. Investigating the effects of fish effluents as organic fertilisers on onion (Allium cepa) yield, soil nutrients, and soil microbiome. Sci. Hortic. 2023, 321, 112297. [Google Scholar] [CrossRef]
- Omeir, M.K.; Jafari, A.; Shirmardi, M.; Roosta, H. Effects of irrigation with fish farm effluent on nutrient content of basil and purslane. Proc. Natl. Acad. Sci. India B 2020, 90, 825–831. [Google Scholar] [CrossRef]
- Valkovszki, N.J.; Jancsó, M.; Székely, Á.; Szalóki, T.; Kolozsvári, I.; Kun, Á. Influence of agricultural effluent irrigation on common purslane (Portulaca oleracea l.) and garden basil (Ocimum basilicum L.): Preliminary results. J. Agric. Environ. Sci. 2022, 9, 71–81. [Google Scholar] [CrossRef]
- Kimera, F.; Sewilam, H.; Fouad, W.M.; Suloma, A. Efficient utilization of aquaculture effluents to maximize plant growth, yield, and essential oils composition of Origanum majorana cultivation. Ann. Agric. Sci. 2021, 66, 1–7. [Google Scholar] [CrossRef]
- Aboagye, D.A.; Adjadeh, W.T.; Nartey, E.K.; Asuming-Brempong, S. Co-application of biochar compost and inorganic nitrogen fertilizer affects the growth and nitrogen uptake by lowland rice in northern Ghana. Nitrogen 2022, 3, 414–425. [Google Scholar] [CrossRef]
- Bi, G.; Li, T.; Gu, M.; Evans, W.B.; Williams, M. Effects of fertilizer source and rate on zinnia cut flower production in a high tunnel. Horticulturae 2021, 7, 333. [Google Scholar] [CrossRef]
- Al-Dulaimi, O.I.M.; Al-Rawi, A.R.M.; Al-Qaisi, E.K.K.; El-Moursy, R.S.A. Response of some wheat cultivars to organic, mineral and foliar fertilization. J. Plant Prod. 2015, 6, 1755–1770. [Google Scholar] [CrossRef]
- Antoun, L.W.; Zakaria, S.M.; Rafla, H.H. Influence of compost, N-mineral and humic acid on yield and chemical composition of wheat plants. J. Soil Sci. Agric. Eng. 2010, 1, 1131–1143. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Rastogi, M.; Nandal, M.; Khosla, B. Microbes as vital additives for solid waste composting. Heliyon 2020, 6, e03343. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Inbar, E.; Michel, F.C.; Hadar, Y.; Minz, D. Succession of bacterial communities during early plant development: Transition from seed to root and effect of compost amendment. Appl. Environ. Microbiol. 2006, 72, 3975–3983. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.C.; Hoitink, H.A.J.; Hadar, Y.; Minz, D. Microbial Communities Active in Soil-Induced Systemic Plant Disease Resistance; United States Department of Agriculture: Washington, DC, USA, 2005.
- Zhao, J.; Wang, X.W.; Fan, H.; Fan, C.P.; Shao, S.G.; Xie, J.B. Screening and application of microbial inoculants for sludge composting in expressway service area of northwest China. E3S Web Conf. 2021, 248, 01037. [Google Scholar] [CrossRef]
- Partanen, P.; Hultman, J.; Paulín, L.; Auvinen, P.; Romantschuk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010, 10, 94. [Google Scholar] [CrossRef]
- Neher, D.A.; Weicht, T.R.; Bates, S.T.; Leff, J.W.; Fierer, N. Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 2013, 8, e79512. [Google Scholar] [CrossRef]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Maul, J.E.; Smith, J.L.; Collins, H.P.; Halvorson, J.J.; et al. Understanding and enhancing soil biological health: The solution for reversing soil degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Ofek, M.; Hadar, Y.; Minz, D. Ecology of root colonizing Massilia (Oxalobacteraceae). PLoS ONE 2012, 7, e40117. [Google Scholar] [CrossRef]
- Yan, S.; Zeng, M.; Wang, H.; Zhang, H. Micromonospora: A prolific source of bioactive secondary metabolites with therapeutic potential. J. Med. Chem. 2022, 65, 8735–8771. [Google Scholar] [CrossRef]
- Schmautz, Z.; Walser, J.-C.; Espinal, C.A.; Gartmann, F.; Scott, B.; Pothier, J.F.; Frossard, E.; Junge, R.; Smits, T.H.M. Microbial diversity across compartments in an aquaponic system and its connection to the nitrogen cycle. Sci. Total Environ. 2022, 852, 158426. [Google Scholar] [CrossRef]
- Eck, M.; Sare, A.R.; Massart, S.; Schmautz, Z.; Junge, R.; Smits, T.H.M.; Jijakli, M.H. Exploring bacterial communities in aquaponic systems. Water 2019, 11, 260. [Google Scholar] [CrossRef]
- Reichenbach, H. The Lysobacter genus. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 939–957. [Google Scholar]
- Chen, L.; Feng, Q.; Li, C.; Wei, Y.; Zhao, Y.; Feng, Y.; Zheng, H.; Li, F.; Li, H. Impacts of aquaculture wastewater irrigation on soil microbial functional diversity and community structure in arid regions. Sci. Rep. 2017, 7, 11193. [Google Scholar] [CrossRef]
- Guan, W.; Li, K.; Li, K. Bacterial communities in co-cultured fish intestines and rice field soil irrigated with aquaculture wastewater. AMB Express 2022, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Marcos, M.S.; González, M.C.; Vallejos, M.B.; Barrionuevo, C.G.; Olivera, N.L. Impact of irrigation with fish-processing effluents on nitrification and ammonia-oxidizer abundances in Patagonian arid soils. Arch. Microbiol. 2021, 203, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, M.B.; Marcos, M.S.; Barrionuevo, C.; Olivera, N.L. Fish-processing effluent discharges influenced physicochemical properties and prokaryotic community structure in arid soils from Patagonia. Sci. Total Environ. 2020, 714, 136882. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Mi, W.; Qu, Z.; Mu, W.; Wang, J.; Zhang, J.; Wang, Q. Effects of irrigation using activated brackish water on the bacterial community structure of rhizosphere soil. J. Soil Sci. Plant Nutr. 2022, 22, 4008–4023. [Google Scholar] [CrossRef]



| Parameter | Tap Water 1 | Fish Water 2 | Fish Sludge 2 |
|---|---|---|---|
| pH | 7.60 3 | 6.7 | 5.8 |
| Calcium carbonate concentration | 323 ppm | - | - |
| Conductivity at 20 °C | 594 μS/cm | - | - |
| Conductivity at 25 °C | - | 791 μS/cm | 1020 μS/cm |
| Turbidity | <0.09 FTU | - | - |
| Ammonium as NH4 | 0.12 mg/L | - | - |
| Nitrate as NO3 | 27.2 mg/L | 44.3 mg/L | 69.6 mg/L |
| Nitrate/nitrite calculation | 0.55 mg/L | - | - |
| Nitrite as NO2 | 0.013 mg/L | - | - |
| Hardness (total) as CaCO3 | 244 mg/L | - | - |
| Iron as Fe | 3.6 μg/l | - | - |
| Magnesium | 4.3 mg/L | 12.33 mg/L | 18.34 mg/L |
| Alkalinity (HCO3) | - | 36 mg/L | 13 mg/L |
| Sulphate (SO4) | - | 161.8 mg/L | 204.4 mg/L |
| Boron | - | 0.01 mg/L | 0.03 mg/L |
| Sodium | - | 44.0 mg/L | 50.5 mg/L |
| Chloride | - | 59.2 mg/L | 69.8 mg/L |
| Phosphorus as P | - | 4.4 mg/L | 12.8 mg/L |
| Potassium | - | 1.5 mg/L | 3.5 mg/L |
| Calcium | - | 112.6 mg/L | 136.4 mg/L |
| Carbonate | - | <10 mg/L | <10 mg/L |
| Total dissolved solids | - | 553.7 mg/L | 714 mg/L |
| Parameter | Compost-Free Substrate | Compost Substrate |
|---|---|---|
| pH | 8 | 7.81 |
| P | 18 mg/L | 36.80 mg/L |
| K | 218.35 mg/L | 538.20 mg/L |
| Mg | 55.90 mg/L | 111.65 mg/L |
| Mn | 7.88 mg/L | 7.32 mg/L |
| Cu | 1.56 mg/L | 1.97 mg/L |
| B | 0.97 mg/L | 1.35 mg/L |
| Na | 37.50 mg/L | 85 mg/L |
| Zn | 2.03 mg/L | 5.40 mg/L |
| Ca | 1835 mg/L | 1700 mg/L |
| Av Fe | 11.96 mg/L | 23.70 mg/L |
| Nitrate-N | 61.44 kg/ha | 361 kg/ha |
| Ammonium-N | 2.80 kg/ha | 7.96 kg/ha |
| Soil mineral nitrogen | 64 kg/ha | 369 kg/ha |
| Organic matter LOI 1 | 5.44% | 6.22% |
| Av SO4 | 47.02 mg/L | 56.86 mg/L |
| Cation exchange capacity | 13.60 meq/100 g | 14.7 meq/100 g |
| Treatment | Height (cm) | Stem Diameter (mm) | Leaf Biomass (g) | Nitrogen (%) |
|---|---|---|---|---|
| IT | 15.06 ± 1.75 b | 1.84 ± 0.13 b | 7.12 ± 0.85 d | 2.55 ± 0.18 c |
| IF | 23.85 ± 2.43 a | 2.39 ± 0.23 ab | 23.75 ± 1.66 c | 4.00 ± 0.21 b |
| IFS | 25.19 ± 2.85 a | 2.51 ± 0.35 a | 28.25 ± 1.66 bc | 4.44 ± 0.42 ab |
| CT | 24.58 ± 2.83 a | 2.73 ± 0.29 a | 23.50 ± 1.69 c | 3.01 ± 0.36 c |
| CF | 24.56 ± 1.21 a | 2.75 ± 0.36 a | 31.62 ± 1.93 ab | 4.40 ± 0.15 ab |
| CFS | 23.24 ± 2.42 a | 2.64 ± 0.28 a | 35.75 ± 3.07 a | 4.90 ± 0.26 a |
| Measurement | Substrate/Fertiliser | df | F-Value | p-Value |
|---|---|---|---|---|
| Substrate | 1 | 5.032 | 0.027 | |
| Plant height | Fertiliser | 2 | 5.973 | 0.003 |
| Substrate/Fertiliser | 2 | 8.820 | <0.001 | |
| Substrate | 1 | 181.56 | <0.001 | |
| Leaf biomass | Fertiliser | 2 | 162.18 | <0.001 |
| Substrate/Fertiliser | 2 | 13.61 | <0.001 | |
| Substrate | 1 | 31.870 | <0.001 | |
| Stem diameter | Fertiliser | 2 | 5.454 | 0.005 |
| Substrate/Fertiliser | 2 | 7.458 | 0.001 | |
| Substrate | 1 | 14.959 | 0.001 | |
| Nitrogen weight % | Fertiliser | 2 | 98.907 | <0.001 |
| Substrate/Fertiliser | 2 | 0.035 | 0.966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruscella, L.; Kotzen, B.; Paradelo Perez, M.; Milliken, S. Investigating the Effects of Fish Effluents as Organic Fertilisers on Basil (Ocimum basilicum). Appl. Sci. 2025, 15, 1563. https://doi.org/10.3390/app15031563
Fruscella L, Kotzen B, Paradelo Perez M, Milliken S. Investigating the Effects of Fish Effluents as Organic Fertilisers on Basil (Ocimum basilicum). Applied Sciences. 2025; 15(3):1563. https://doi.org/10.3390/app15031563
Chicago/Turabian StyleFruscella, Lorenzo, Benz Kotzen, Marcos Paradelo Perez, and Sarah Milliken. 2025. "Investigating the Effects of Fish Effluents as Organic Fertilisers on Basil (Ocimum basilicum)" Applied Sciences 15, no. 3: 1563. https://doi.org/10.3390/app15031563
APA StyleFruscella, L., Kotzen, B., Paradelo Perez, M., & Milliken, S. (2025). Investigating the Effects of Fish Effluents as Organic Fertilisers on Basil (Ocimum basilicum). Applied Sciences, 15(3), 1563. https://doi.org/10.3390/app15031563







