Lactic Acid Bacteria from Kiwi: Antifungal and Biofilm-Inhibitory Activities Against Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacteria
2.2. Bacterial Cultivation Conditions and Preparation of Metabolites
2.3. Broth Microdilution Method
2.4. Scanning Electron Microscope (SEM)
2.5. Biofilm Formation Crystal Violet Assay
2.6. Antioxidant Activity
2.7. Cell Culture and Viability
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Adhesion Assay
2.10. Acid Tolerance
2.11. Statistical Analysis
3. Results
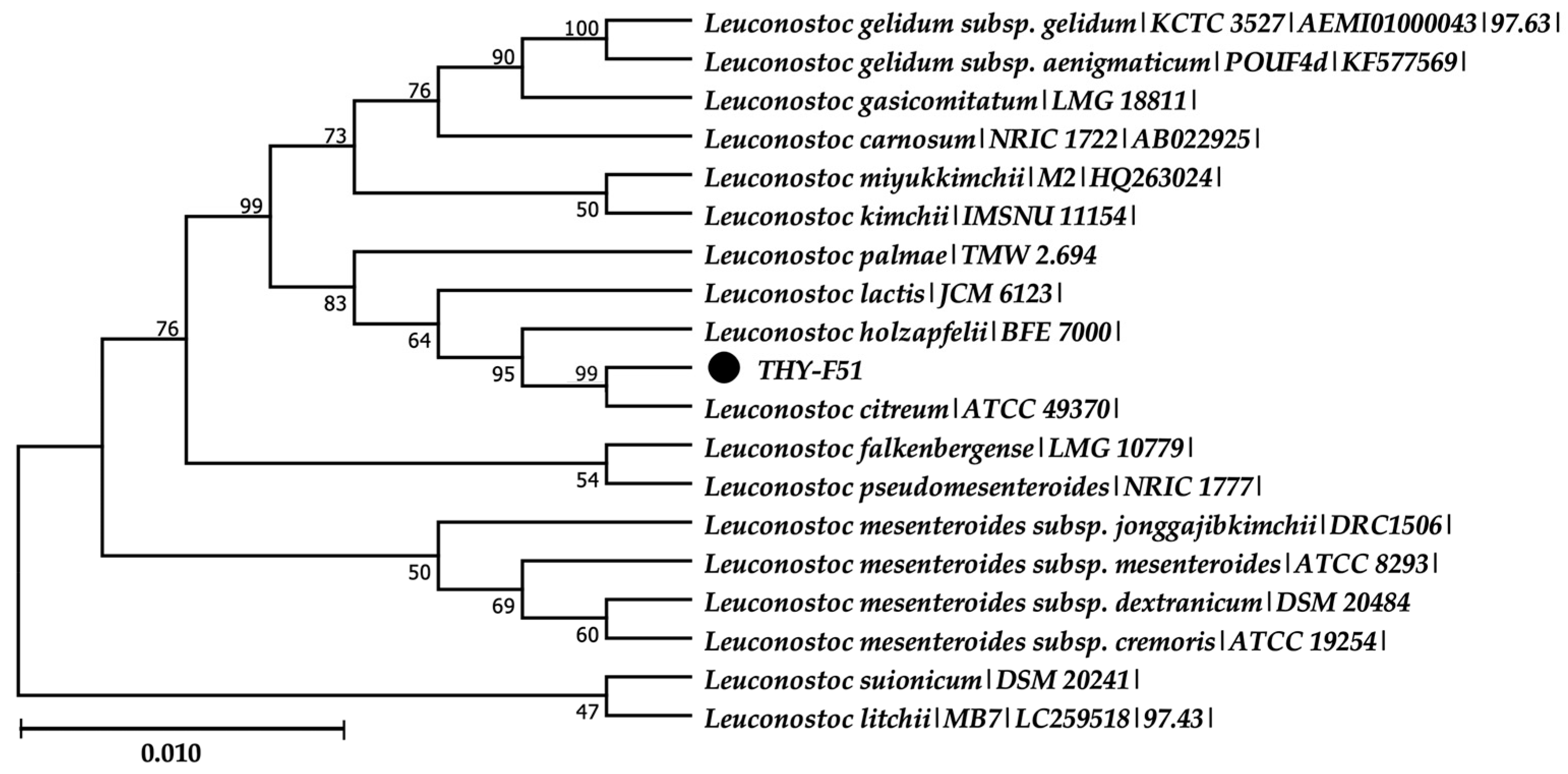
3.1. Isolation and Identification of Leuconostoc citreum THY-F51
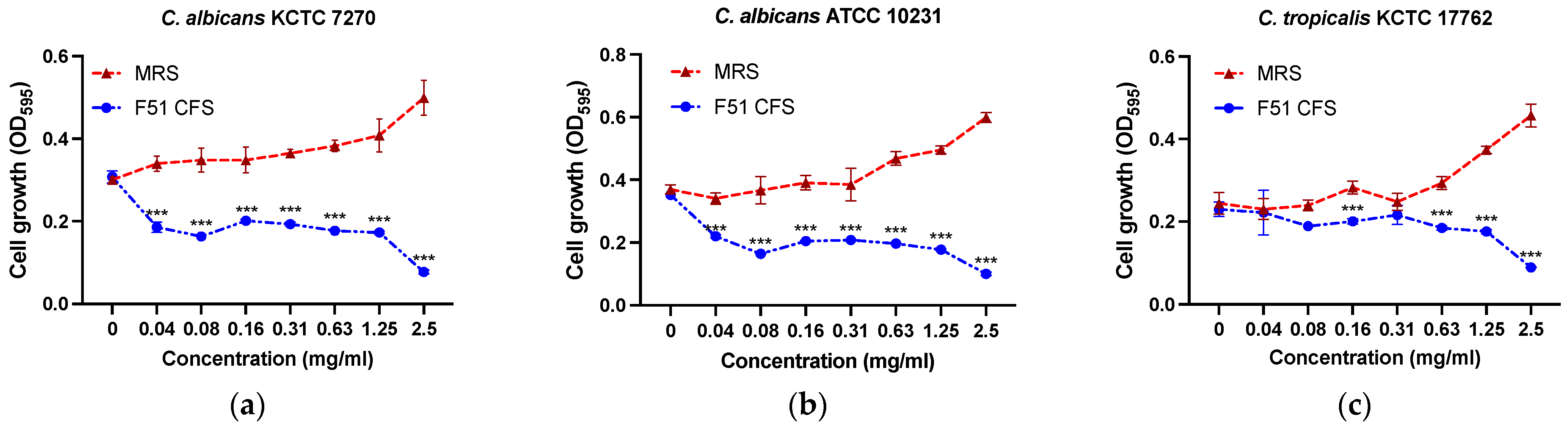
3.2. Antibacterial Activity
3.3. SEM Result of Pathogens with THY-F51 Treatment
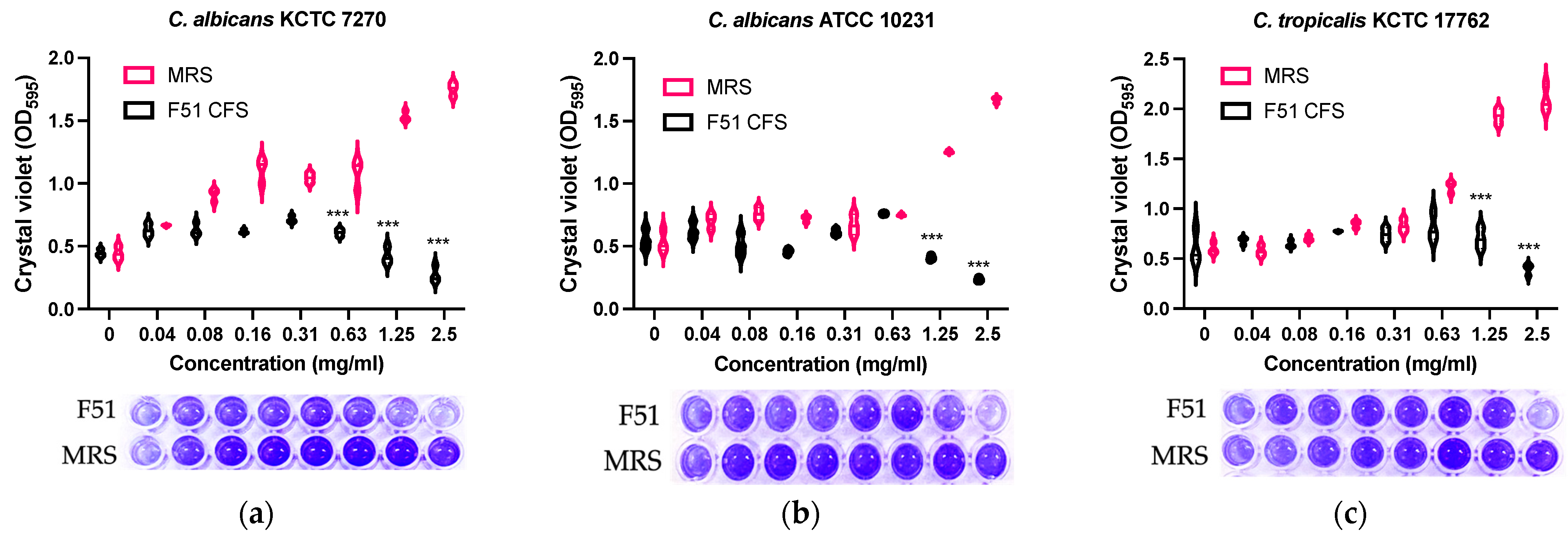
3.4. Inhibition of Biofilm Formation by E. faecalis HM20
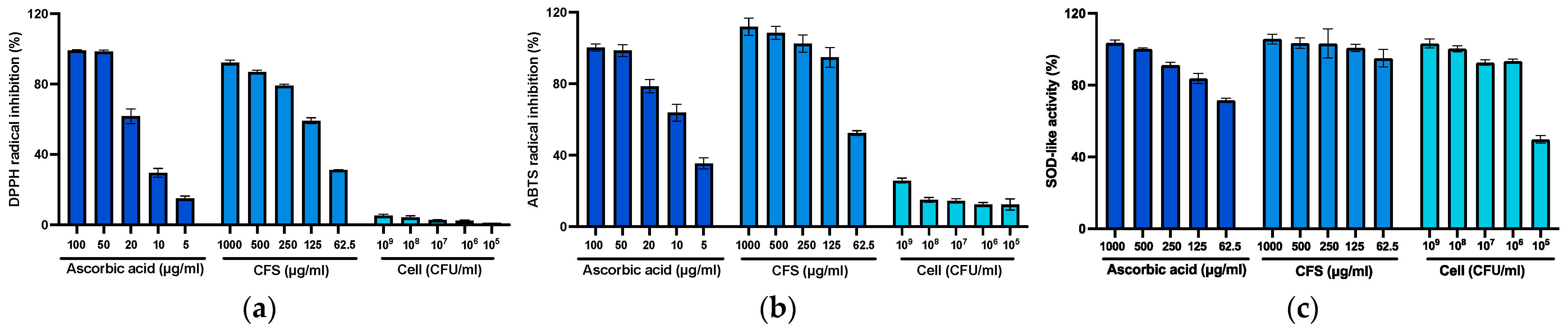
3.5. Effect of Radical Scavenging Activity and Superoxide Dismutase-Like Assay
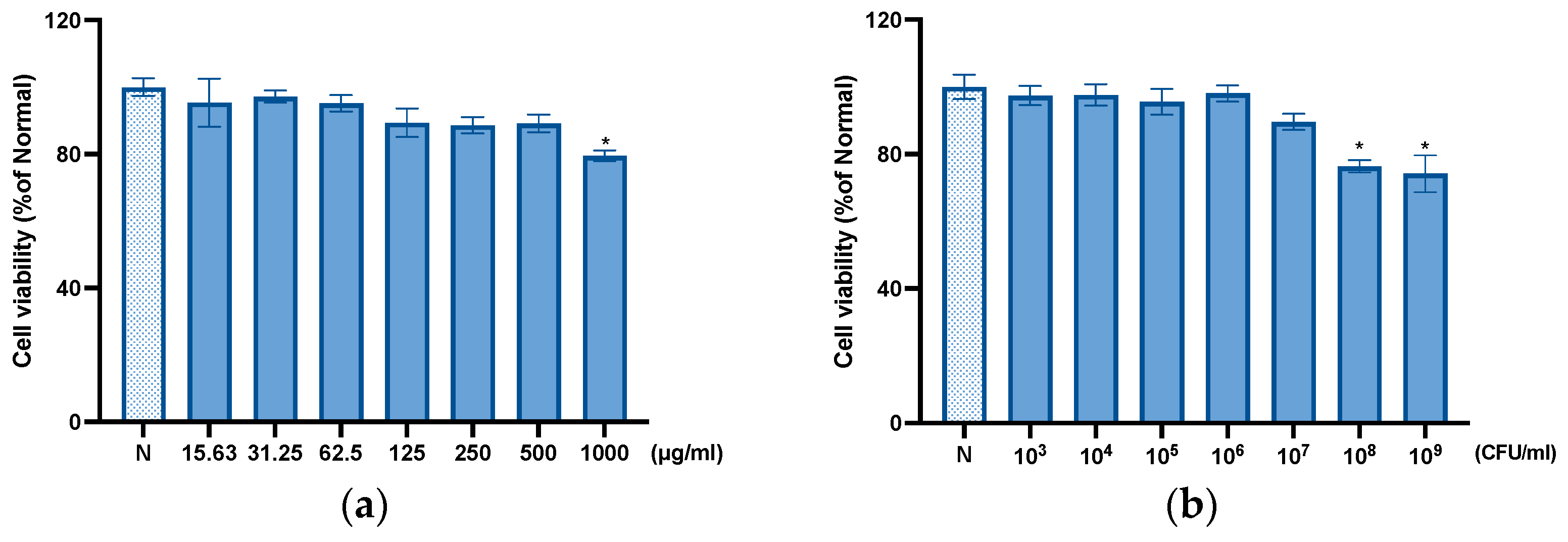
3.6. Cytotoxic Effect of THY-F51
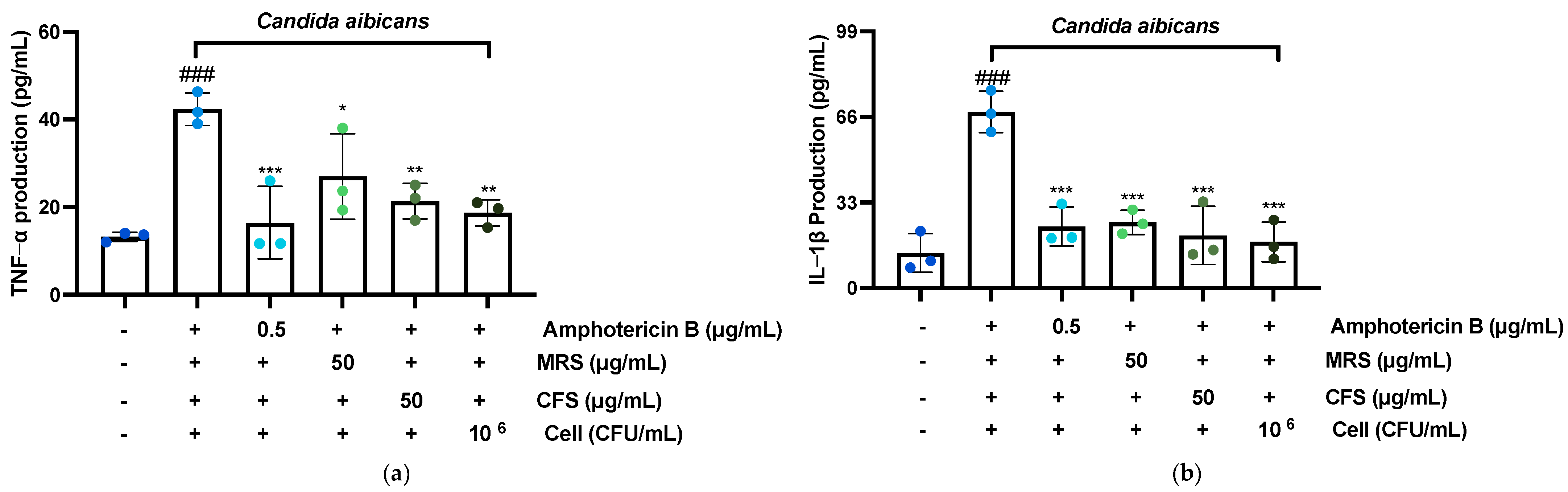
3.7. Effect of F51 on TNF-α, IL-1β, IL-6, and IL-8 Production
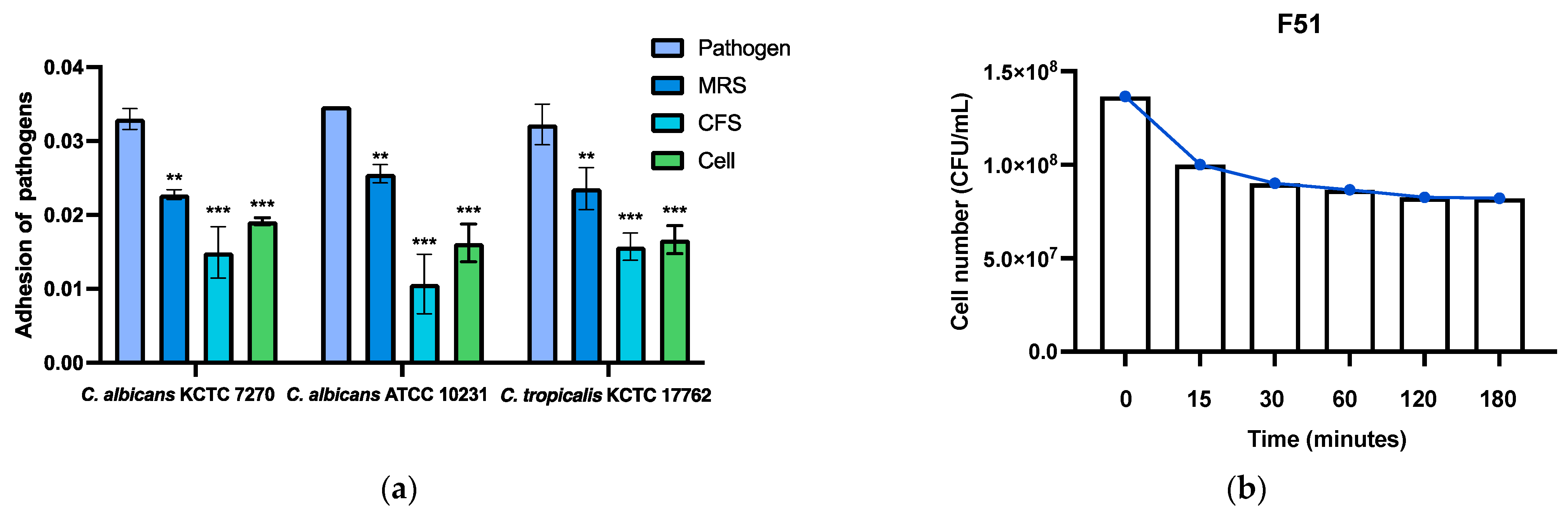
3.8. Anti-Adhesion Ability and Acid Tolerance Assays of THY-F51
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neal, C.M.; Martens, M.G. Clinical Challenges in Diagnosis and Treatment of Recurrent Vulvovaginal Candidiasis. SAGE Open Med. 2022, 10, 20503121221115201. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Vulvovaginal Candidiasis. Available online: https://www.cdc.gov/std/treatment-guidelines/candidiasis.htm (accessed on 7 January 2025).
- Sun, Z.; Ge, X.; Qiu, B.; Xiang, Z.; Jiang, C.; Wu, J.; Li, Y. Vulvovaginal Candidiasis and Vaginal Microflora Interaction: Microflora Changes and Probiotic Therapy. Front. Cell. Infect. Microbiol. 2023, 13, 1123026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adolfsson, A.; Hagander, A.; Mahjoubipour, F.; Larsson, P.G. How Vaginal Infections Impact Women’s Everyday Life: Women’s Lived Experiences of Bacterial Vaginosis and Recurrent Vulvovaginal Candidiasis. Adv. Sex. Med. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Gigi, R.M.S.; Buitrago-Garcia, D.; Taghavi, K.; Dunaiski, C.M.; van de Wijgert, J.H.H.M.; Peters, R.P.H.; Low, N. Vulvovaginal Yeast Infections during Pregnancy and Perinatal Outcomes: Systematic Review and Meta-Analysis. BMC Womens Health 2023, 23, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, W.; Filler, S.G. Interactions of Candida Albicans with Epithelial Cells. Cell Microbiol. 2010, 12, 273–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida Albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, F.; Liu, Y.; Liu, Y.; Lv, R.; Sun, W.; Ding, W.; Cai, Y.; Li, W.; Liu, X.; Qu, W. Candida Albicans Biofilms: Antifungal Resistance, Immune Evasion, and Emerging Therapeutic Strategies. Int. J. Antimicrob. Agents 2022, 60, 106673. [Google Scholar] [CrossRef] [PubMed]
- Kumwenda, P.; Cottier, F.; Hendry, A.C.; Kneafsey, D.; Keevan, B.; Gallagher, H.; Tsai, H.J.; Hall, R.A. Estrogen Promotes Innate Immune Evasion of Candida Albicans through Inactivation of the Alternative Complement System. Cell Rep. 2022, 38, 110183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrick, E.J.; Patel, P.; Hashmi, M.F. Antifungal Ergosterol Synthesis Inhibitors. 2024 Mar 1. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Te Welscher, Y.M.; van Leeuwen, M.R.; de Kruijff, B.; Dijksterhuis, J.; Breukink, E. Polyene Antibiotic That Inhibits Membrane Transport Proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 11156–11159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, E.H.; Qi, L.W.; Li, B.; Peng, Y.B.; Li, P.; Li, C.Y.; Cao, J. High-Speed Separation and Characterization of Major Constituents in Radix Paeoniae Rubra by Fast High-Performance Liquid Chromatography Coupled with Diode-Array Detection and Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Lavallée, P.; Deschênes, M. Dendroarchitecture and Lateral Inhibition in Thalamic Barreloids. J. Neurosci. 2004, 24, 6098–6105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hudson, B.S.; Kohler, B.E.; Schulten, K. Linear Polyene Electronic Structure and Potential Surfaces. In Excited States; Elsevier: Amsterdam, The Netherlands, 1982; Volume 6, pp. 1–95. [Google Scholar] [CrossRef]
- Gupta, A.K.; Tomas, E. New Antifungal Agents. Dermatol. Clin. 2003, 21, 565–576. [Google Scholar] [CrossRef]
- Oerlemans, E.F.; Bellen, G.; Claes, I.; Henkens, T.; Allonsius, C.N.; Wittouck, S.; van den Broek, M.F.L.; Wuyts, S.; Kiekens, F.; Donders, G.G.G.; et al. Impact of a Lactobacilli-Containing Gel on Vulvovaginal Candidosis and the Vaginal Microbiome. Sci. Rep. 2020, 10, 7976. [Google Scholar] [CrossRef]
- Mastromarino, P.; Cassetta, M.I.; Capobianco, D.; Trinchieri, V.; Ristori, M. Lactobacillus Rhamnosus GR1 and Lactobacillus Reuteri RC-14 in the Prevention and Treatment of Recurrent Vulvovaginal Candidiasis: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Microbiol. 2013, 51, 1245–1251. [Google Scholar]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In Vitro Model of the Genital Tract to Study the Interaction of Lactobacillus Strains with Candida Albicans in the Vaginal Environment. FEMS Immunol. Med. Microbiol. 2013, 39, 33–41. [Google Scholar] [CrossRef]
- Rizzo, J.; Caggiano, G. Role of Probiotics in the Prevention and Treatment of Recurrent Vulvovaginal Candidiasis. J. Appl. Microbiol. 2018, 125, 940–951. [Google Scholar]
- Kovachev, S.; Todorova, I.; Yordanov, D. Effectiveness of Lactobacillus Supplementation in Reducing the Recurrence of Vulvovaginal Candidiasis by Restoring the Vaginal Microbiome and Inhibiting Candida Overgrowth. J. Clin. Microbiol. 2020, 58, e01720-19. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M07-A10: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2018. [Google Scholar] [CrossRef]
- Stewart, P.S.; William, M.R. The Use of Crystal Violet to Assess Biofilm Formation in the Laboratory. J. Microbiol. Methods 2001, 44, 369–373. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Li, Y. Evaluation of the Effect of Lactobacillus Strains on the Adhesion of Candida Albicans to Vaginal Epithelial Cells. J. Appl. Microbiol. 2017, 123, 1041–1050. [Google Scholar] [CrossRef]
- Bizzini, A. Acid Tolerance of Lactic Acid Bacteria: Methods and Applications in the Food Industry. Int. J. Food Microbiol. 2010, 137, 145–152. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In Vitro Antioxidant and Free Radical Scavenging Activity of Different Parts of Tabebuia Pallida Growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Asma, U.; Bertotti, M.L.; Zamai, S.; Arnold, M.; Amorati, R.; Scampicchio, M. A Kinetic Approach to Oxygen Radical Absorbance Capacity (ORAC): Restoring Order to the Antioxidant Activity of Hydroxycinnamic Acids and Fruit Juices. Antioxidants 2024, 13, 222. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. In Vivo Total Antioxidant Capacity: Comparison of Different Analytical Methods. Free Radic. Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G. Role of Cytokines in the Host Defense against Candida Species. Clin. Microbiol. Rev. 2006, 19, 27–54. [Google Scholar] [CrossRef]
- Salari, S.; Ghasemi Nejad Almani, P. Antifungal Effects of Lactobacillus Acidophilus and Lactobacillus Plantarum against Different Oral Candida Species Isolated from HIV/AIDS Patients: An in Vitro Study. J. Oral Microbiol. 2020, 12, 1769386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Thienpont, B.; Sapuru, V.; Hite, R.K.; Dittman, J.S.; Sturgis, J.N.; Scheuring, S. Membrane-Mediated Protein Interactions Drive Membrane Protein Organization. Nat. Commun. 2022, 13, 7373. [Google Scholar] [CrossRef]
- Morici, P.; Fais, R.; Rizzato, C.; Tavanti, A.; Lupetti, A. Inhibition of Candida albicans biofilm formation by the synthetic lactoferricin derived peptide hLF1-11. PLoS ONE 2016, 11, e0167470. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal Candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Noverr, M.C.; Huffnagle, G.B. Pathogenesis of Candida Albicans Biofilms. Curr. Opin. Microbiol. 2004, 7, 309–314. [Google Scholar] [CrossRef]
- Netea, M.G.; Kullberg, B.J.; Van Der Meer, J.W. Cytokine Response to Candida Albicans in Health and Disease. FEMS Immunol. Med. Microbiol. 2006, 47, 1–12. [Google Scholar] [CrossRef]
- Gaffen, S.L. T-Helper Cell Differentiation in the Presence of Candida Albicans. Curr. Opin. Microbiol. 2005, 8, 334–338. [Google Scholar]
- Koh, A.Y.; Pier, G.B. The Impact of Candida Albicans on Host Immune Responses. Microbes Infect. 2009, 11, 1371–1378. [Google Scholar]
- Jiang, Y.; Zhao, Y. Candidalysin Triggers a Cascade of Immune Responses That Promote Inflammation in Candida Albicans Infection. Front. Immunol. 2014, 5, 537. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H. Bacterial Modulation of Host Immune Responses through Pattern Recognition Receptors. Front. Immunol. 2017, 8, 117. [Google Scholar] [CrossRef]
- Turovskiy, Y.; Talarico, S. The Role of Live Bacteria in Host Immune Modulation. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Sobel, J.D.; Faro, S. Vaginitis: Candida, Bacterial Vaginosis, and Other Pathogens. Clin. Obstet. Gynecol. 2019, 62, 91–98. [Google Scholar] [CrossRef]
- Muthusamy, V.P.; Sivakumar, V. Role of Nrf2-Keap1 Pathway in Inflammation and Tissue Repair. Biochem. Pharmacol. 2018, 154, 9–24. [Google Scholar] [CrossRef]
- Rabelo, A.P.; Silva, J.P.; Nunes, J.R. Antioxidant Properties of Microbial Metabolites and Their Role in Probiotic Therapy. Antioxidants 2020, 9, 574. [Google Scholar] [CrossRef]
- Persichetti, M.; Coccetti, P.; Cotugno, G. Antioxidant Properties of Lactobacillus Brevis 603 and Its Potential Therapeutic Applications. Microorganisms 2018, 6, 112. [Google Scholar] [CrossRef]
- Vinderola, G.; Ouwehand, A.; von Wright, M. Lactobacillus Plantarum: A Review of Its Antioxidant Activity. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef]
- Basso, M. Inhibition of Candida Albicans Adhesion and Biofilm Formation by Curcumin: Potential for the Treatment of Candida Infections. Mycoses 2017, 60, 97–106. [Google Scholar] [CrossRef]
- Hsieh, C. Differences in Immune Responses and Microbial Profiles in Individuals with Recurrent Vulvovaginal Candidiasis. Infect. Dis. Obstet. Gynecol. 2014, 2014, 395458. [Google Scholar]
- Cummings, J.H. The Role of the Gut Microbiota in Human Health and Disease: An Overview. Clin. Microbiol. Rev. 2019, 32, e00025-19. [Google Scholar]
- Roberfroid, M.B. Prebiotics and Health: An Overview. In Prebiotics and Probiotics Science and Technology; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Sobel, J.D. Fungal Infections in Women: Clinical Considerations and the Role of Probiotics. Infect. Dis. Clin. N. Am. 2007, 21, 563–577. [Google Scholar]
- Hemalatha, S. Efficacy of Probiotics in the Management of Vaginal Candidiasis: A Systematic Review. J. Clin. Diagn. Res. 2014, 8, 157–161. [Google Scholar]


| THY-F51 | Minimum Inhibitory Concentration (mg/mL) | ||
| C. albicans KCTC 7270 | C. albicans ATCC 10231 | C. tropicalis KCTC 17762 | |
| 1.25 | 1.25 | 1.25 | |
| Minimal Fungicidal Concentration (mg/mL) | |||
| C. albicans KCTC 7270 | C. albicans ATCC 10231 | C. tropicalis KCTC 17762 | |
| 2.5 | 2.5 | 2.5 | |
| Ascorbic Acid | THY-F51 CFS | THY-F51 Cell | |
|---|---|---|---|
| DPPH IC50 value (μg/mL) | 0.22 | 2.00 | 9.11 |
| ABTS IC50 value (μg/mL) | 9.12 | 61.24 | 297.01 |
| SOD-like IC50 value (μg/mL) | 31.55 | 55.51 | 62.60 |
| ORAC value (μmol TE/g DW) | 898.5 | 220.45 | 163.65 |
| T-AOC value (U/mL) | 223.2 | 5.27 | 2.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Zheng, Q.; Nguyen, T.T.M.; Yang, S.-J.; Park, S.-J.; Yi, G.-S.; Yi, T.-H. Lactic Acid Bacteria from Kiwi: Antifungal and Biofilm-Inhibitory Activities Against Candida albicans. Appl. Sci. 2025, 15, 1647. https://doi.org/10.3390/app15031647
Jin X, Zheng Q, Nguyen TTM, Yang S-J, Park S-J, Yi G-S, Yi T-H. Lactic Acid Bacteria from Kiwi: Antifungal and Biofilm-Inhibitory Activities Against Candida albicans. Applied Sciences. 2025; 15(3):1647. https://doi.org/10.3390/app15031647
Chicago/Turabian StyleJin, Xiangji, Qiwen Zheng, Trang Thi Minh Nguyen, Su-Jin Yang, Se-Jig Park, Gyeong-Seon Yi, and Tae-Hoo Yi. 2025. "Lactic Acid Bacteria from Kiwi: Antifungal and Biofilm-Inhibitory Activities Against Candida albicans" Applied Sciences 15, no. 3: 1647. https://doi.org/10.3390/app15031647
APA StyleJin, X., Zheng, Q., Nguyen, T. T. M., Yang, S.-J., Park, S.-J., Yi, G.-S., & Yi, T.-H. (2025). Lactic Acid Bacteria from Kiwi: Antifungal and Biofilm-Inhibitory Activities Against Candida albicans. Applied Sciences, 15(3), 1647. https://doi.org/10.3390/app15031647








