Research on the Microscopic Adsorption Characteristics of Methane by Coals with Different Pore Sizes Based on Monte Carlo Simulation
Abstract
1. Introduction
2. Low-Temperature Liquid Nitrogen Adsorption Experiment
2.1. Preparation of Coal Samples and Experiment
2.2. Experimental Results
3. Construction and Simulation of Adsorption Models
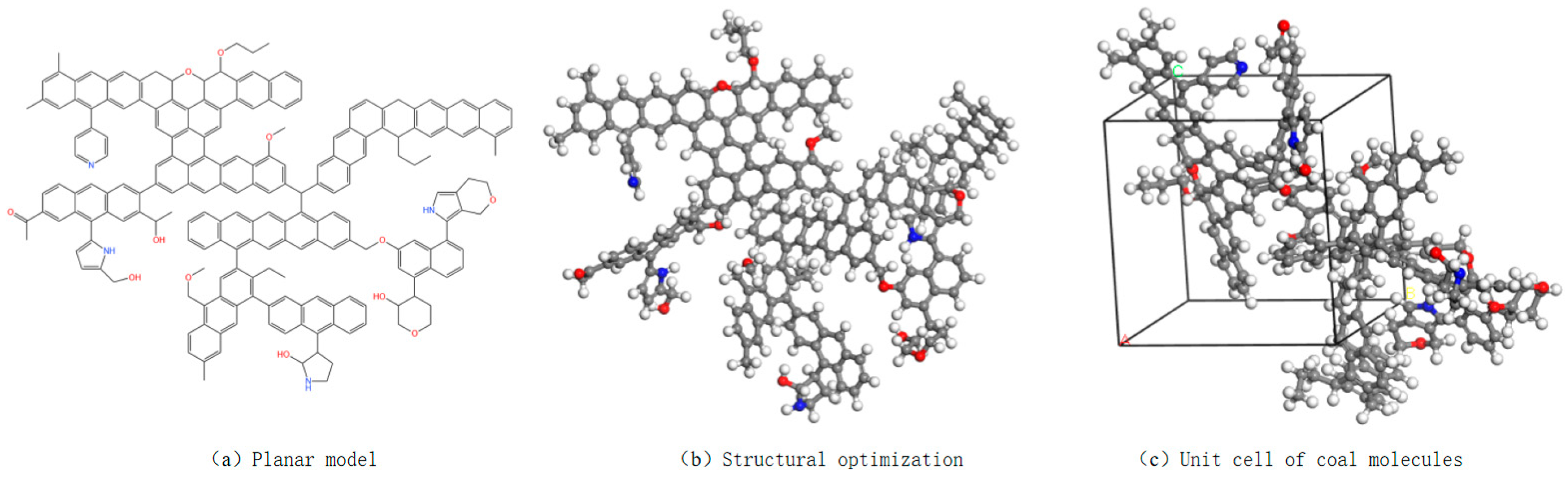
3.1. Elemental Analysis and Construction of Coal Samples
3.2. Simulation Parameter Settings and Model Configurations
- (1)
- In the Sorption module, the task type was set as “Fixed pressure”, the method was “Metropolis”, the simulation accuracy was “Customized”, and the forcefield (Forcefield) was selected as the COMPASS II forcefield. For charges, the “Forcefield assigned” method was chosen. For the electrostatic interaction between atoms, the “Atom Based” method was selected, and for both van der Waals interaction and hydrogen bonding, the “Atom Based” method was also adopted. The number of equilibration steps was set to 1 × 10⁶, the number of production steps was set to 1 × 10⁶, and the number of escape steps was set to 20.
- (2)
- In the Sorption module, when the task type was selected as the “Locate” task, its corresponding parameter settings were the same as those of the “Fixed pressure” module.
- (3)
- In the Forcite module, the task type was set as “Dynamics”, the temperature was chosen as 298 K, the NVT ensemble was selected, the simulation time was set to 500 ps, the time step length was set to 1.0 fs, and the temperature control method was selected as “Nose”. The “Analysis” function was used to choose the diffusion coefficient (MSD).
4. Simulation and Analysis of Coal’s Adsorption of Gas
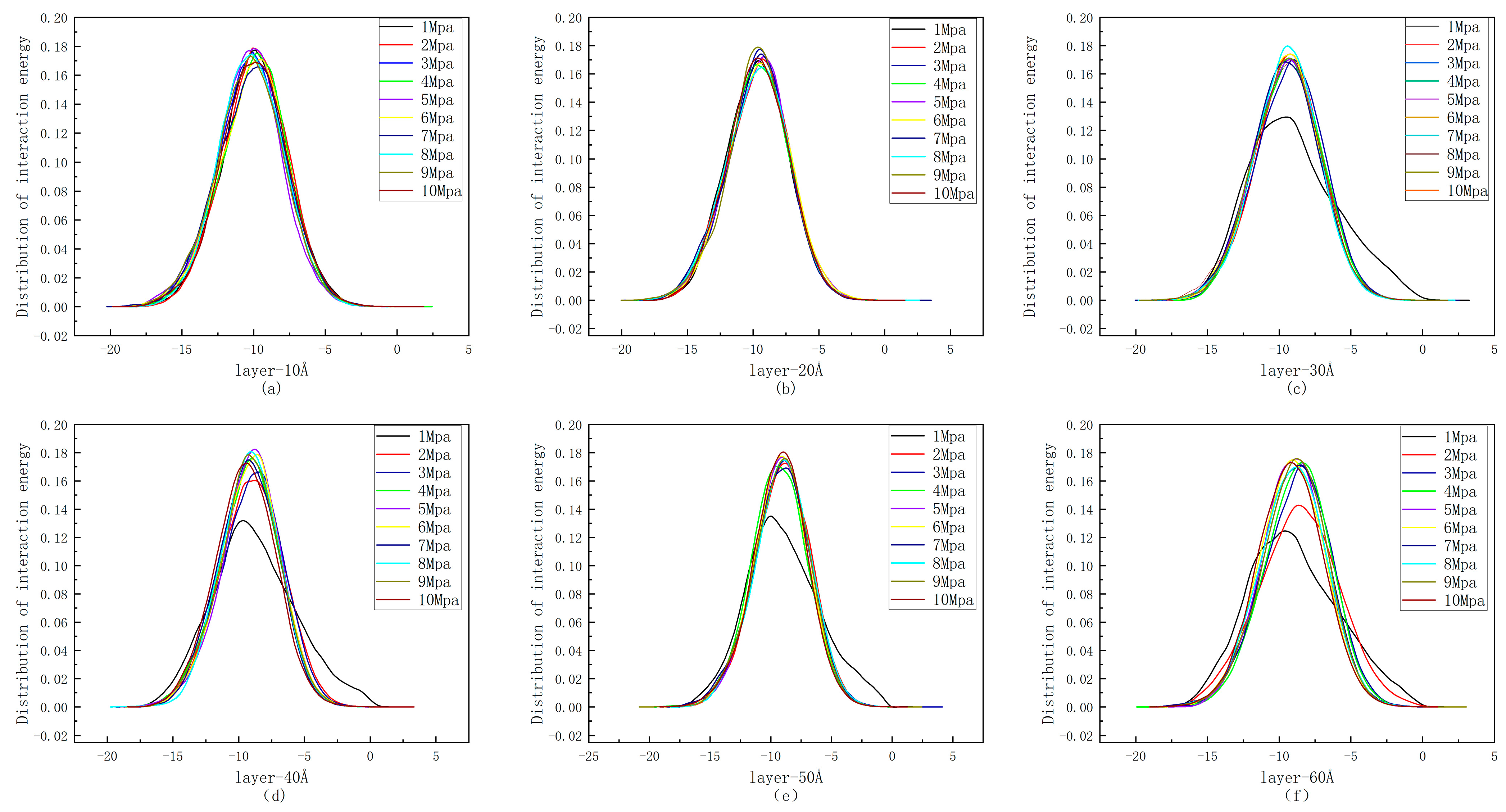
4.1. Distribution of Interaction Energy
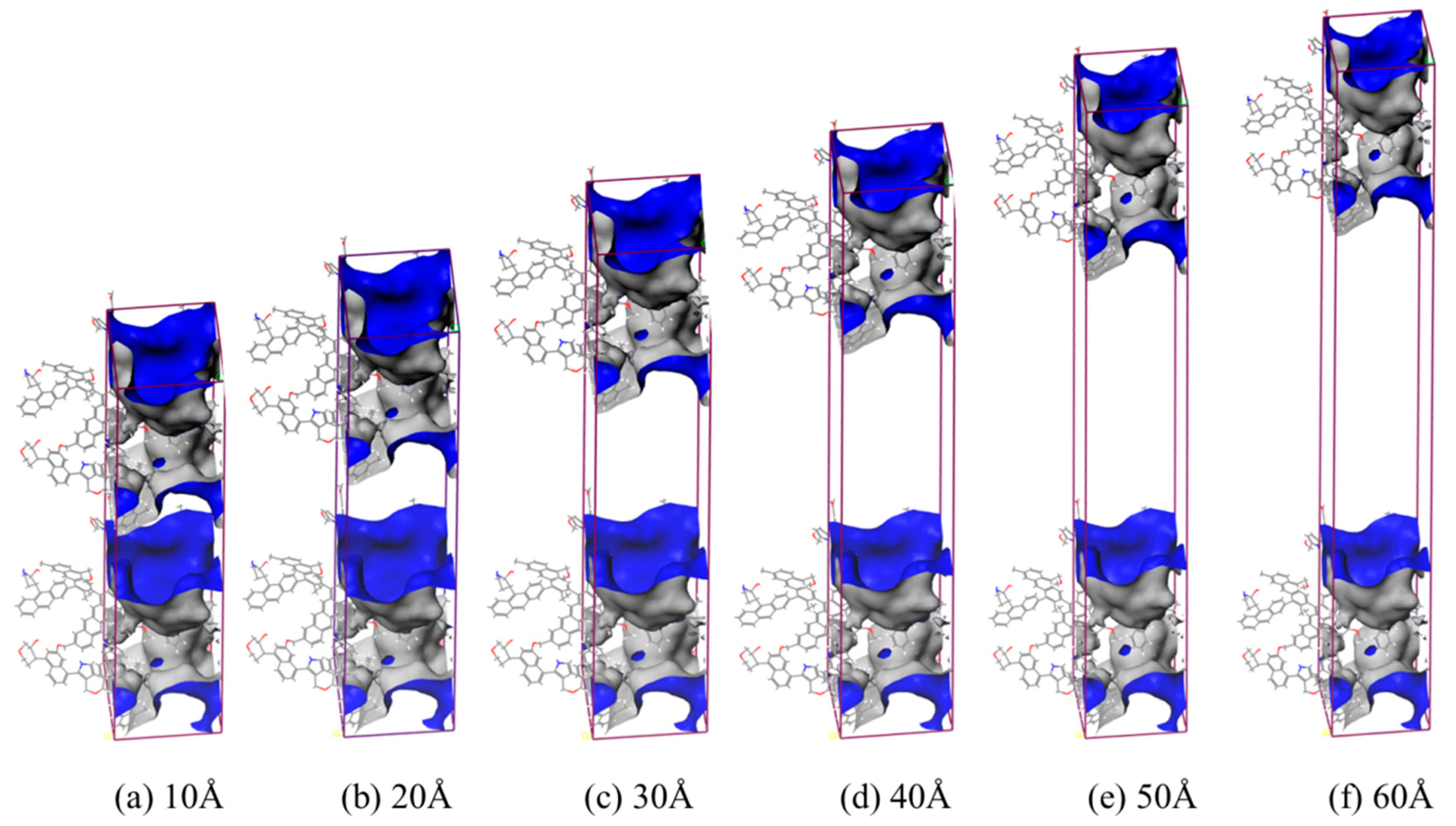
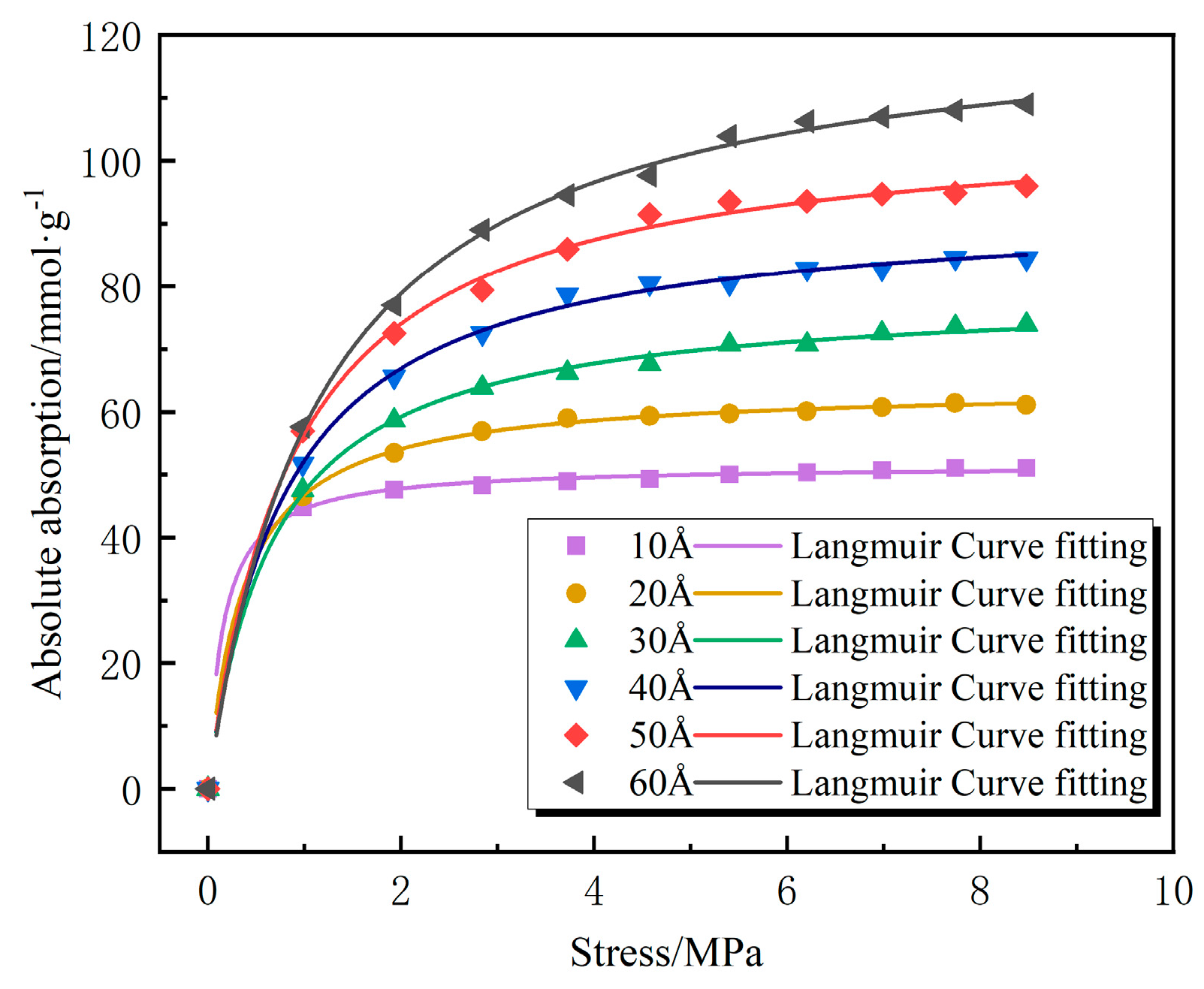
4.2. Influence of Coal with Different Pore Diameters on the Isothermal Adsorption of CH4
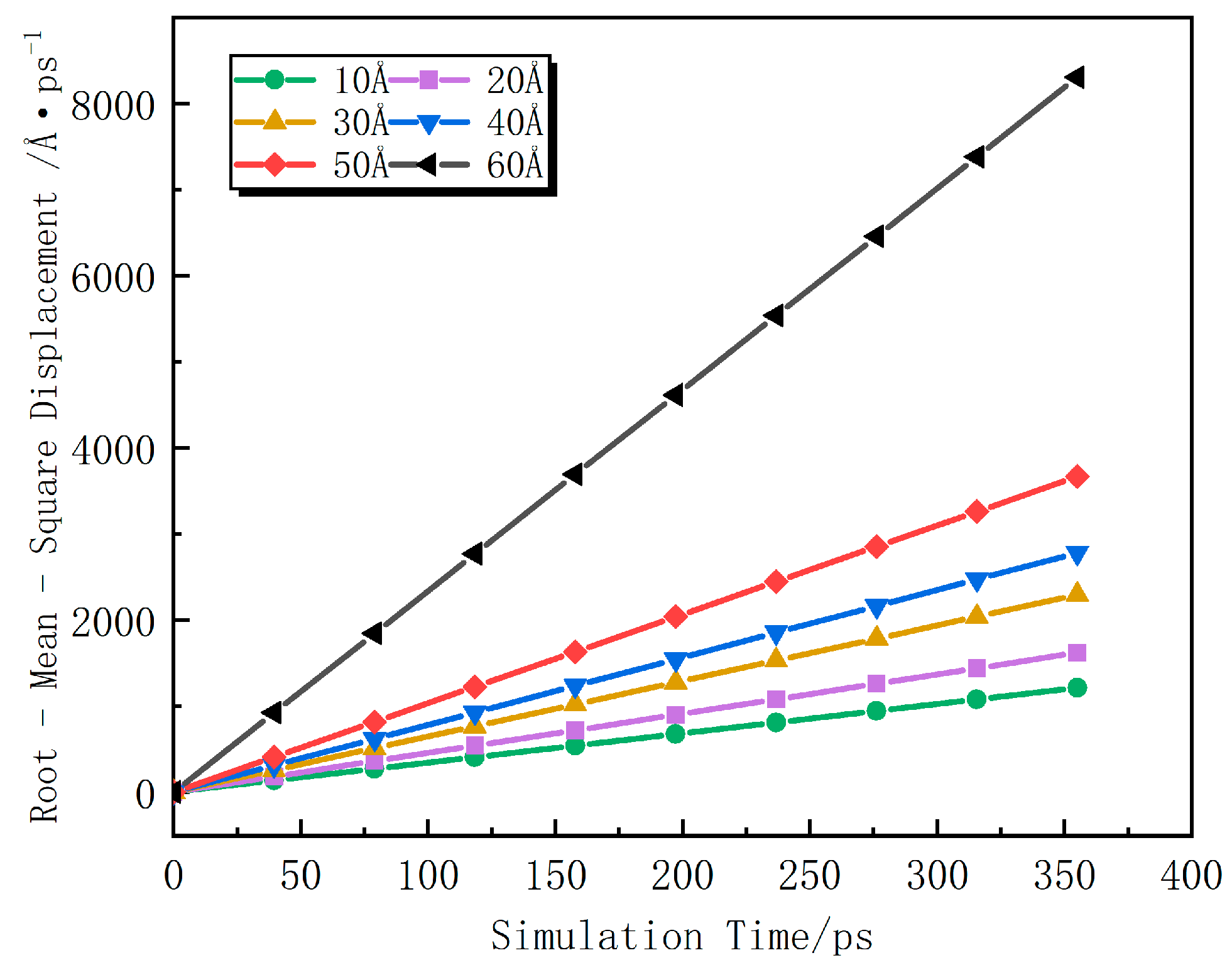
4.3. Influence of Coal with Different Pore Diameters on the Diffusion of CH4
5. Conclusions
- (1)
- The size of the pore diameter in coal directly affects the interaction energy during the adsorption of CH4. Due to the relatively stronger van der Waals force generated between the upper and lower layers of smaller pores, the force exerted on CH4 is more significant than that in other larger pores. As the pore diameter increases, the van der Waals forces between the inner walls of the pores decrease, and the interaction energy between CH4 and coal molecules gradually weakens.
- (2)
- The adsorption processes of anthracite with different pore diameters all conform to the Langmuir model. There is a positive correlation between the pore diameter size and the adsorption amount. Under the same temperature and pressure conditions, with every 10 Å increase in pore diameter, the absolute adsorption amount of CH4 increases by an average of 16.43%. After reaching the critical pressure of CH4, the absolute adsorption amount increases slowly and tends to reach a saturated state.
- (3)
- The diffusion coefficient of CH4 in the pores of anthracite increases linearly with the change in diffusion time. When the pore diameter is less than 50 Å, the diffusion coefficient of CH4 in the pores increases at a uniform rate of 32.25%. When the pore diameter reaches 50 Å, the diffusion of CH4 changes from being mainly in a free state to being mainly in an adsorbed state, and the diffusion coefficient increases rapidly at a rate of 126% at 50 Å.
- (4)
- In this paper, the microscopic adsorption law of methane by coal with pore diameters ranging from 10 Å to 60 Å has been studied. However, there are still many factors that affect the adsorption of methane by coal, such as the influence of different specific surface areas of pores and changes in air pressure. For such issues, further in-depth research should be continued.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.P.; Zhang, Q.Y.; Zhao, C.H. Characteristics of natural gas, coalbed methane and shale gas formation and explorationand development. China Min. Ind. 2020, 29, 398–401. [Google Scholar]
- Qin, Y.J.; Chen, Y.P.; Jiang, W.Z. Current status and prospect of research on the mechanism of gas storage in deep coal seams. Coal Mine Saf. 2020, 51, 10–15. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 2013, 54, 2201–2218. [Google Scholar] [CrossRef]
- Cheng, Y.P.; Hu, B. A new pore classification method based on methane accumulation and transportation characteristics in coal. J. Coal Sci. 2023, 48, 212–225. [Google Scholar]
- Wang, B.; Liu, X.; Tu, Q. Pore structure characteristics of tectonic coal and its influence on gas extraction. Min. Technol. 2023, 23, 163–170. [Google Scholar]
- Li, J.; Liu, D.; Yao, Y. Control of CO2 permeabilitychange in different rank coals during pressure depletion: An experimentalstudy. Energy Fuels 2014, 28, 987–996. [Google Scholar] [CrossRef]
- Li, Z.B.; Ren, T.; Li, X.; Qiao, M.; Yang, X.; Tan, L.; Nie, B. Multi-scale pore fractal characteristics of differently ranked coal and its impact on gas adsorption. Int. J. Min. Sci. Technol. 2023, 33, 389–401. [Google Scholar] [CrossRef]
- Han, Q.; Deng, C.; Jin, Z.; Gao, T. Molecular Simulation of the Adsorption Characteristics of Methane in Pores of Coal with Different Metamorphic Degrees. Molecules 2021, 26, 7217. [Google Scholar] [CrossRef]
- Hong, L.; Gao, D.M.; Zheng, D. Characterization of methane adsorption by carbon materials with different pore apertures. J. Saf. Environ. 2018, 18, 146–150. [Google Scholar]
- Han, Q.; Deng, C.; Gao, T. Molecular Simulation on Competitive Adsorption Differences of Gas with Different Pore Sizes in Coal. Molecules 2022, 27, 1594. [Google Scholar] [CrossRef]
- Hao, M.; Wei, C.; Zhang, H. Adsorption and diffusion of methane in coal slit pores: Insights into the molecular level. Energy Fuels 2022, 36, 880–886. [Google Scholar] [CrossRef]
- Jia, J.Z.; Xing, Y.H.; Li, B. Structural modeling and molecular simulation of anthracite macromolecules from a mine of Yang Coal. Chem. Eng. 2023, 51, 52–57. [Google Scholar]
- Fan, Z.H. Modeling and Adsorption Mechanism of Coal Macromolecules from Differentcoal Rank. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2020. [Google Scholar]
- Wang, Y.; Cheng, H.F.; Hu, Q.H. Adsorption of methane onto mudstones under supercritical conditions: Mechanisms, physical properties and thermodynamic parameters. Pet. Sci. 2023, 20, 34–47. [Google Scholar] [CrossRef]
- Qu, L.N.; Wang, I.U. ecular Simulation Study Based on Adsorption of Gas (CO2, O2, CH4) on Coal. Fire 2023, 6, 355. [Google Scholar] [CrossRef]
- Xu, T. Study on the effect of temperature on the methane adsorption characteristics of soft and hard anthracite based on molecular simulation method. Coal Mine Saf. 2022, 53, 158–165. [Google Scholar]
- Guang, H.; Dongfang, L.; Xihua, Z.; Gang, B. Mechanism of the effect of water on the adsorption of CH4 and CO2 on anthracite. J. Liaoning Univ. Eng. Technol. (Nat. Sci. Ed.) 2021, 40, 420–424. [Google Scholar]
- Sun, Z.X.; Min, C.; Zhang, W.L. Molecular simulation study on the effect of CO2/N2 binary gas on methane adsorption in coal. Coalf. Geol. Explor. 2022, 50, 127–136. [Google Scholar]
- Jing, T.L.; Tao, C.Q.; Wang, Y.B. Energy Variation Features during the Isothermal Adsorption of Coal under High-Temperature and High-Pressure Conditions. Processes 2023, 11, 2524. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.Y.; Li, B. Molecular simulation of the effect of pore structure on the wettability of low-rank coal. J. China Univ. Min. Technol. 2022, 51, 1117–1127+1192. [Google Scholar]
- Russian, A.; Riva, M.; Russo, E.R. Stochastic inverse modeling and global sensitivity analysis to assist interpretation of drilling mud losses in fractured formations. Stoch Environ. Res Risk Assess 2019, 33, 1681–1697. [Google Scholar] [CrossRef]
- Schiavo, M. Entropy, fractality, and thermodynamics of groundwater pathways. J. Hydrol. 2023, 623, 129824. [Google Scholar] [CrossRef]
- Wen, H.; Liu, M.Y.; Tang, R. Molecular simulation study of C2H4 adsorption in bituminous coal. Coal Mine Saf. 2023, 54, 77–82. [Google Scholar]
- Hu, B. Characterization of Methane Adsorption Behavior of Multi-Scale Pore Structure in Coal and Its Microscopic Influence Mechanism. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2022. [Google Scholar]
| Pore Size/nm | 0~0.2 | 0.2~1 | 1~2 | 2~3 | 3~4 | 4~5 | 5~6 | 6~10 |
|---|---|---|---|---|---|---|---|---|
| Specific surface area (m2·g−1) | 1.126 | 3.402 | 0.558 | 0.491 | 0.324 | 0.468 | 0.265 | 0.096 |
| Pore volume/(cm3·g−1) | 0.00158 | 0.00247 | 0.00156 | 0.00096 | 0.00077 | 0.00082 | 0.00069 | 0.00349 |
| Sample Elements | C | H | O | N |
|---|---|---|---|---|
| Mass fraction/% | 85.81 | 5.64 | 6.63 | 1.93 |
| Pore Diameter/Å | Surface Area/Å2 | Free Volume/Å3 | Occupied Volume/Å3 | Porosity% |
|---|---|---|---|---|
| 10 | 2468.89 | 8355.84 | 8116.71 | 50.72 |
| 20 | 2460.12 | 10,631.16 | 8118.79 | 56.69 |
| 30 | 2470.55 | 12,920.03 | 8107.32 | 61.44 |
| 40 | 2463.97 | 15,189.34 | 8115.41 | 65.17 |
| 50 | 2459.37 | 17,461.22 | 8120.93 | 68.25 |
| 60 | 2468.89 | 19,744.05 | 8115.49 | 70.86 |
| Adsorbate | Pore Diameter/Å | a/(mmol·g−1) | b/(MPa−1) | R2 |
|---|---|---|---|---|
| CH4 | 10 | 51.55 | 6.40 | 0.9542 |
| 20 | 64.00 | 2.73 | 0.9943 | |
| 30 | 78.97 | 1.50 | 0.9928 | |
| 40 | 92.87 | 1.29 | 0.9933 | |
| 50 | 106.80 | 1.13 | 0.9906 | |
| 60 | 124.60 | 0.86 | 0.9961 |
| Pore Spacing/Å | Curve Slope | Diffusion Coefficient/Å·ps−1 | R2 |
|---|---|---|---|
| 10 | 3.40 | 0.567 | 0.9996 |
| 20 | 4.56 | 0.760 | 0.9906 |
| 30 | 6.46 | 1.077 | 0.9976 |
| 40 | 7.83 | 1.305 | 0.9980 |
| 50 | 10.33 | 1.722 | 0.9951 |
| 60 | 23.35 | 3.892 | 0.9977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhai, Y. Research on the Microscopic Adsorption Characteristics of Methane by Coals with Different Pore Sizes Based on Monte Carlo Simulation. Appl. Sci. 2025, 15, 2349. https://doi.org/10.3390/app15052349
Zhang C, Zhai Y. Research on the Microscopic Adsorption Characteristics of Methane by Coals with Different Pore Sizes Based on Monte Carlo Simulation. Applied Sciences. 2025; 15(5):2349. https://doi.org/10.3390/app15052349
Chicago/Turabian StyleZhang, Chunhua, and Yuqi Zhai. 2025. "Research on the Microscopic Adsorption Characteristics of Methane by Coals with Different Pore Sizes Based on Monte Carlo Simulation" Applied Sciences 15, no. 5: 2349. https://doi.org/10.3390/app15052349
APA StyleZhang, C., & Zhai, Y. (2025). Research on the Microscopic Adsorption Characteristics of Methane by Coals with Different Pore Sizes Based on Monte Carlo Simulation. Applied Sciences, 15(5), 2349. https://doi.org/10.3390/app15052349






