Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors
Abstract
1. Introduction
2. Invasive Methods
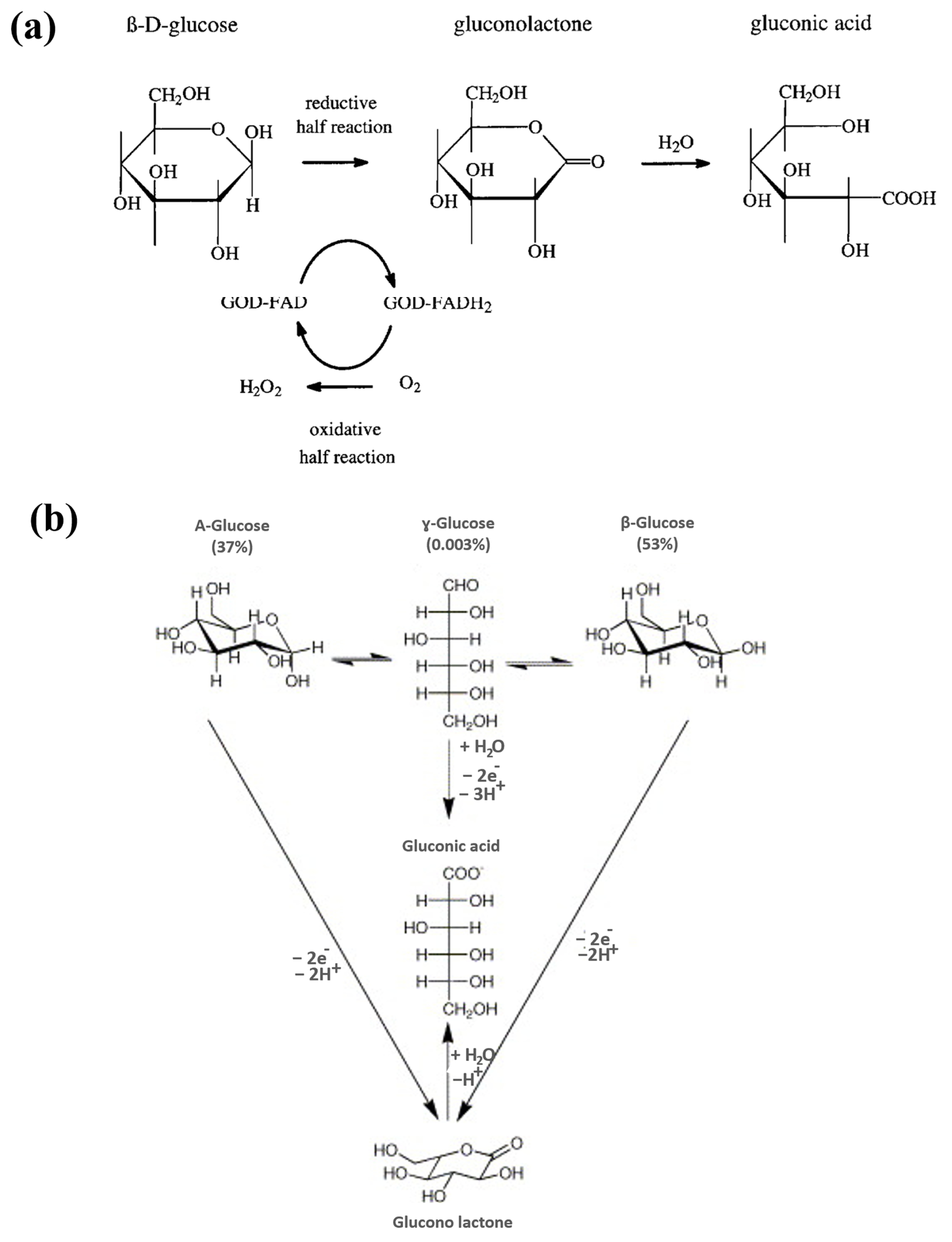
2.1. Electrochemical Glucose Sensors

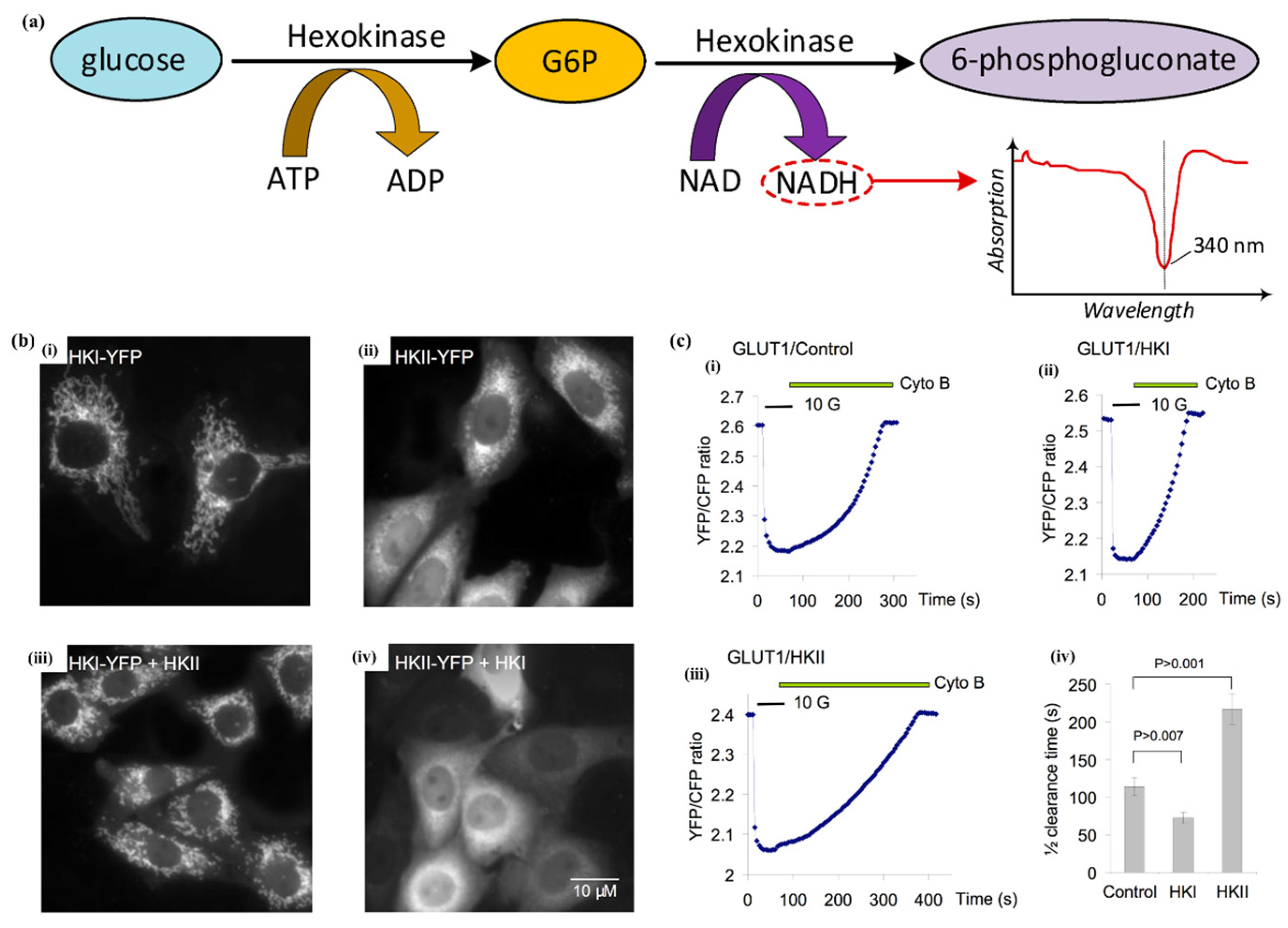
2.2. Hexokinase Method
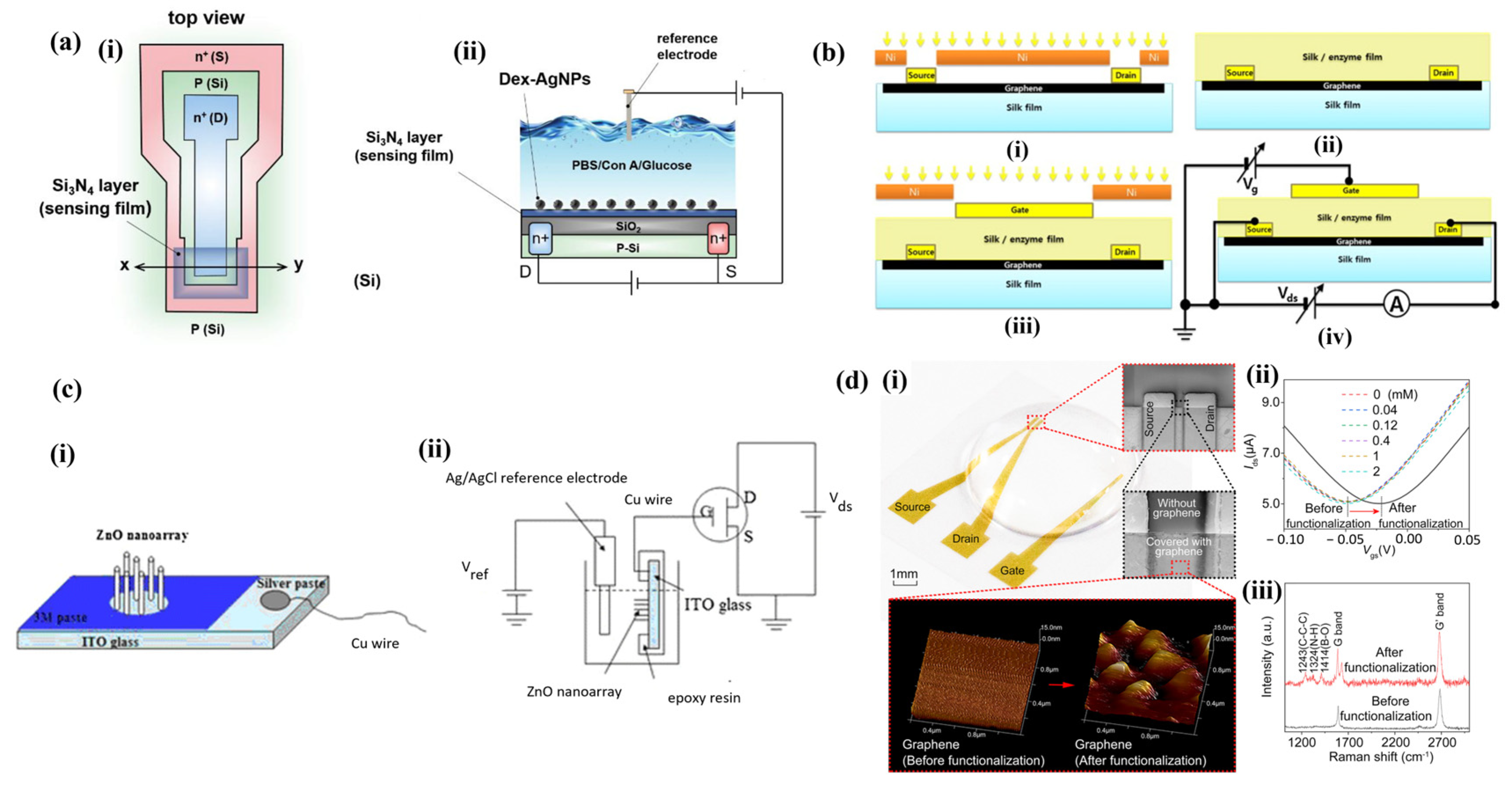
2.3. Ion-Selective Field-Effect Transistor (ISFET)
3. Minimally Invasive Methods
3.1. Fluorescence Methods
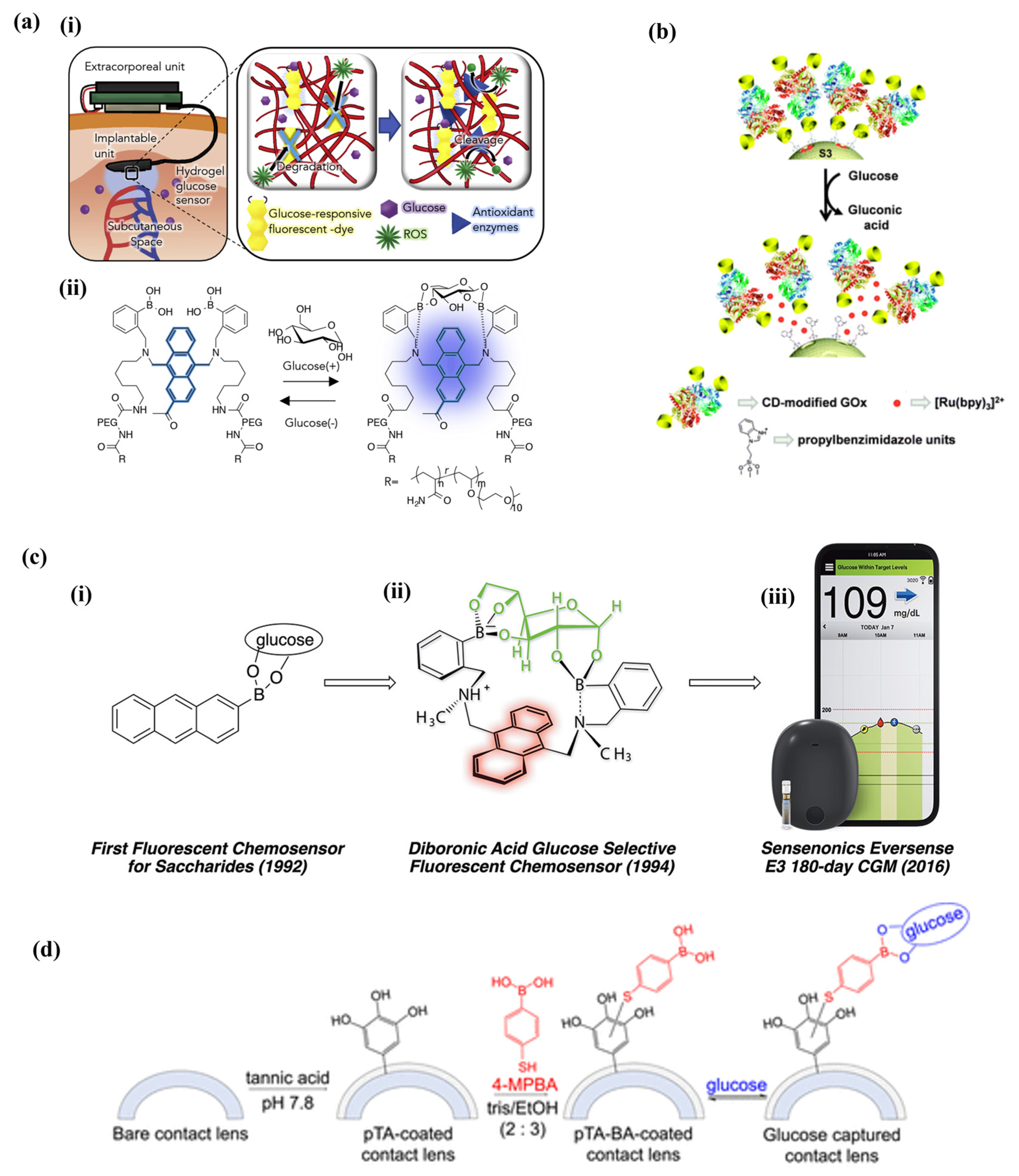
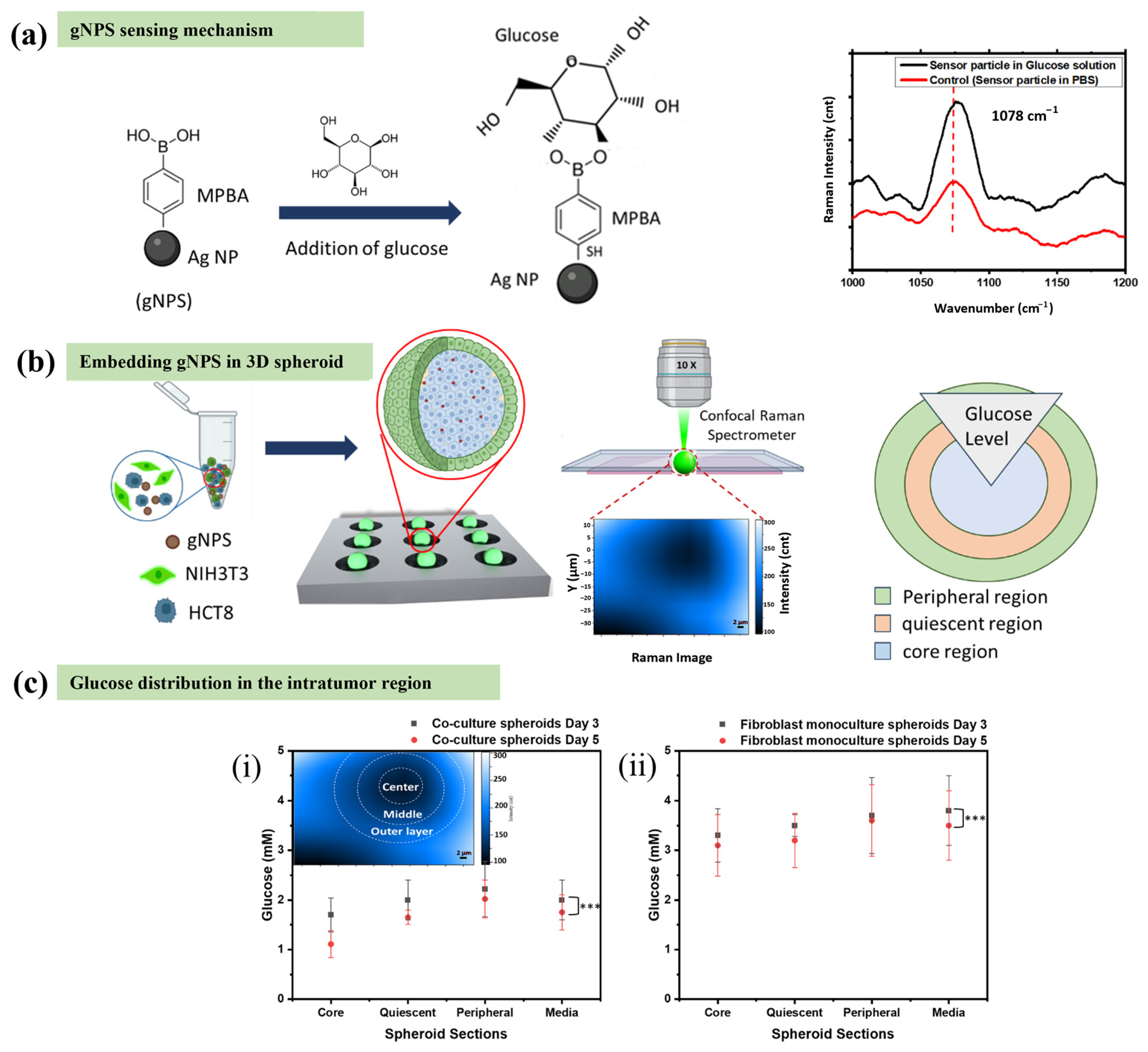
3.2. Raman Spectroscopy
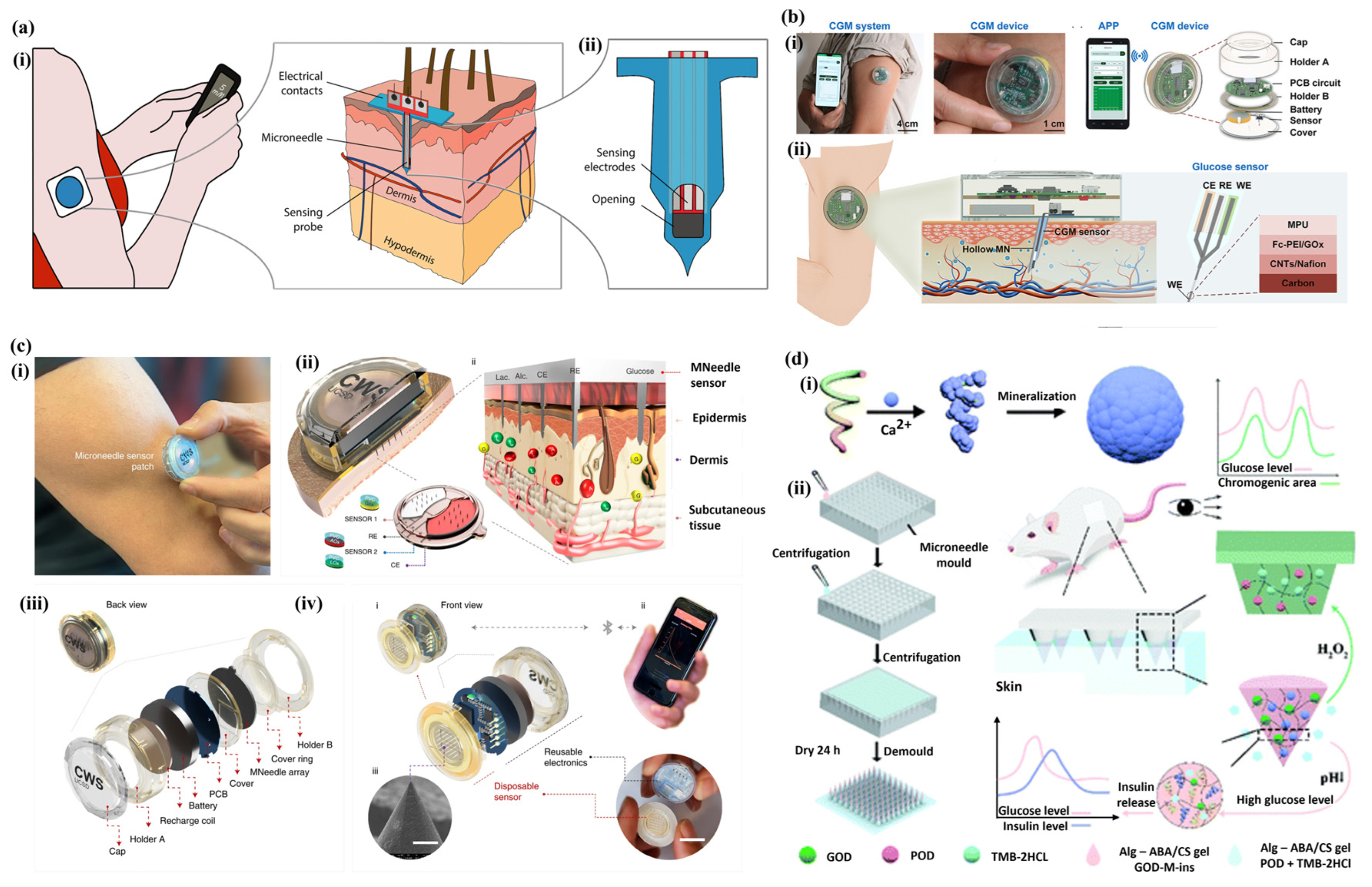
3.3. Microneedle Array
4. Non-Invasive Methods
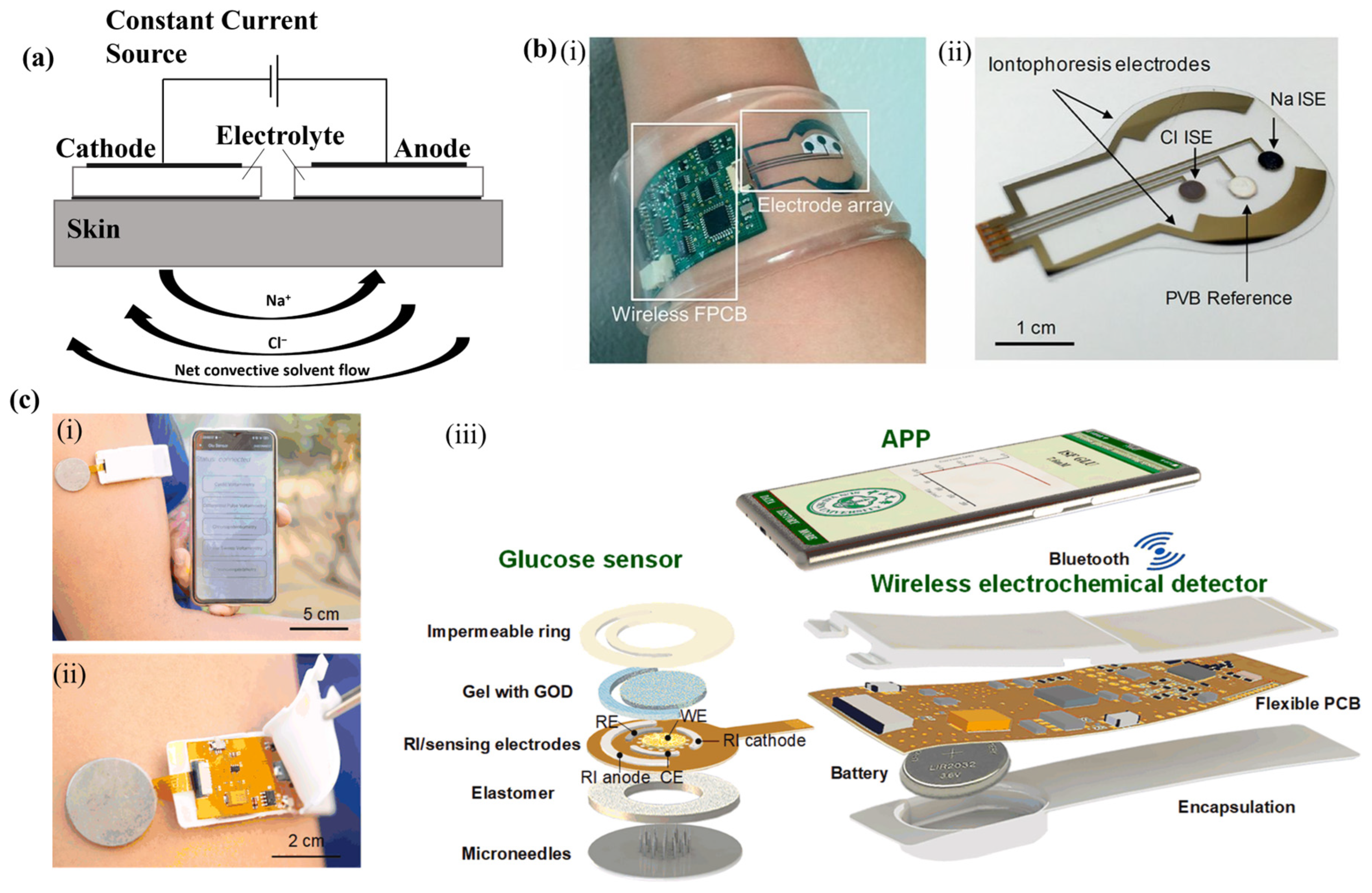
4.1. Reverse Iontophoresis
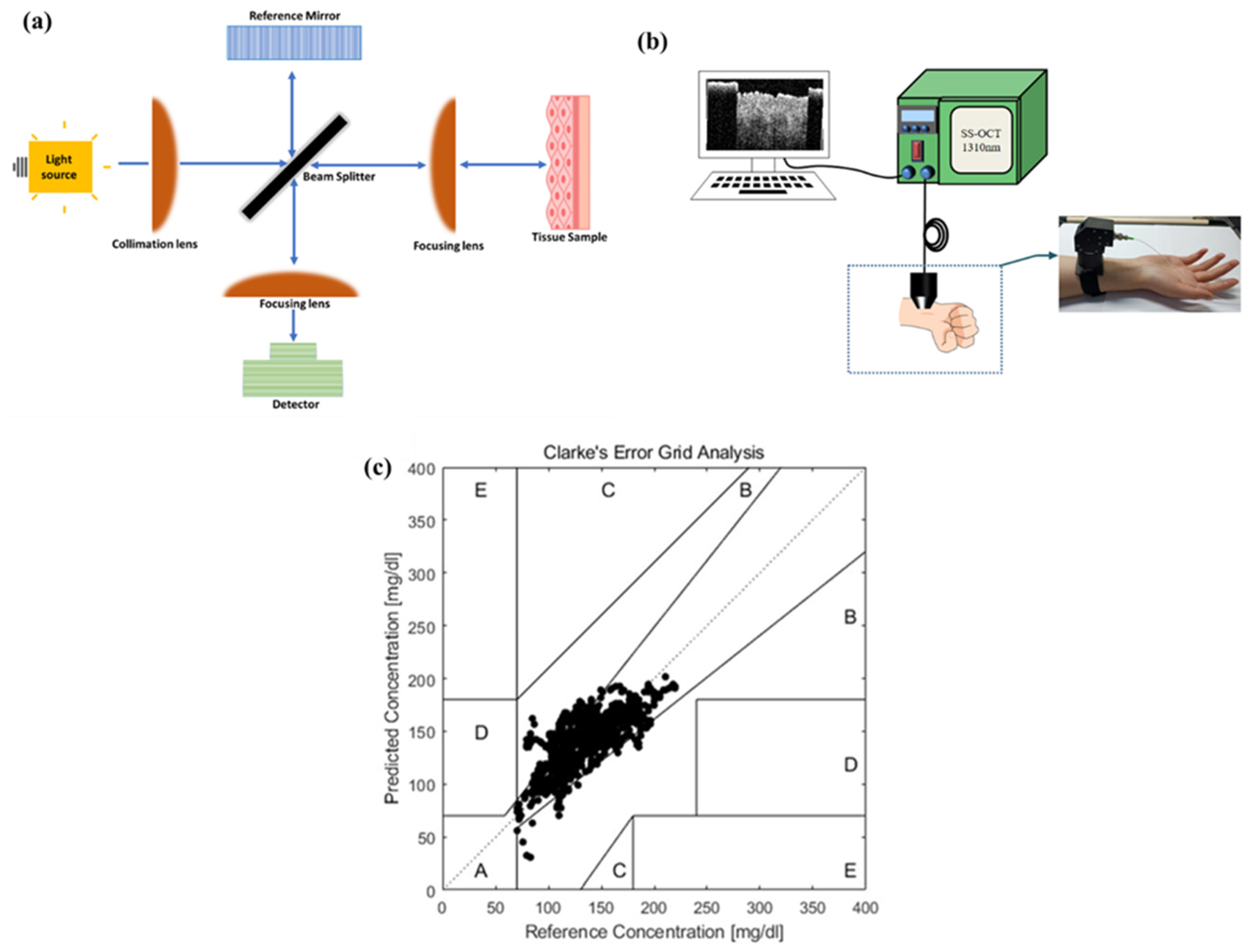
4.2. Optical Coherence Tomography (OCT)
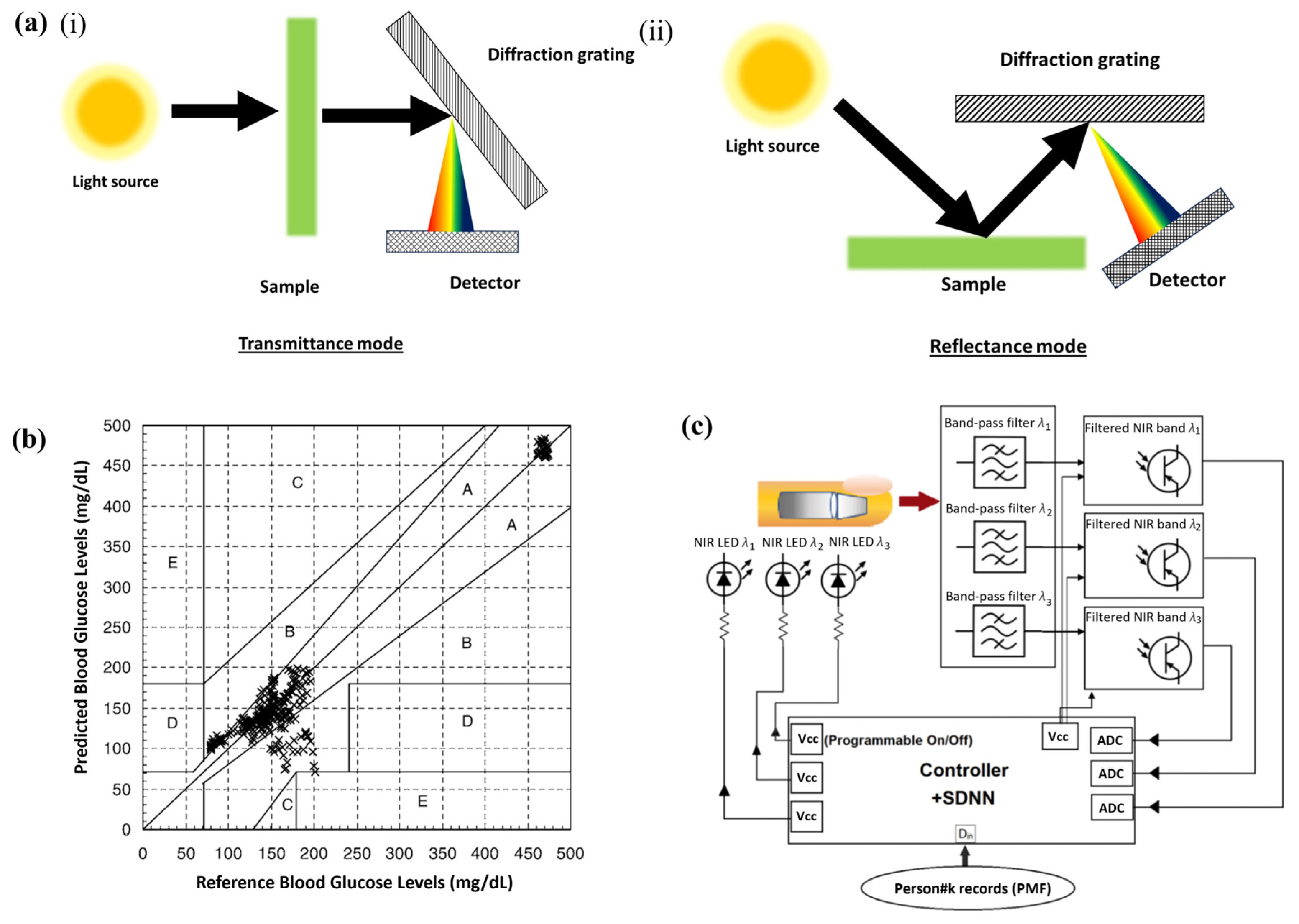
4.3. Infrared Absorption Spectroscopy
5. Limitation of Current Methodologies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SERS | surface-enhanced Raman spectroscopy |
| OCT | optical coherence tomography |
| MOFs | metal–organic frameworks |
| ATP | adenosine triphosphate |
| G6P | glucose-6-phosphate |
| ADP | adenosine diphosphate |
| NAD | nicotinamide adenine dinucleotide |
| HK | hexokinase |
| ConA | concanavalin A |
| SOD | superoxide dismutase |
| 4-MPBA | 4-Mercaptophenylboronic acid |
| MH | mercaptohexanol |
| RSD | relative standard deviation |
| CGM | continuous glucose monitoring |
| RSD | relative standard deviation |
| Gox | glucose oxidase |
| RI | reverse iontophoresis |
| ISF | interstitial fluid |
| FPCB | flexible printed circuit board |
| GOD | glucose oxidase |
| GEDP | Glucose Electrochemical Detection Platform |
| MA | microneedle array |
| ARD | absolute relative difference |
| ANN | Artificial Neural Networks |
| mbNIR | Multiple photonic band near-infrared |
| NN | Neural networks |
| GTT | glucose tolerance tests |
| SEP | standard error of prediction |
| CEG | Clarke error grid |
| NIR | near-infrared |
| ARD | absolute relative difference |
| T2D | Type 2 Diabetes |
| T1D | Type 1 Diabetes |
References
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as Regulators of Energy Balance and Glucose Homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef]
- L’Heveder, R.; Nolan, T. International Diabetes Federation. Diabetes Res. Clin. Pract. 2013, 101, 349–351. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A Review of Recent Advances in Nonenzymatic Glucose Sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF Nanosheet Array: A High-Performance Electrochemical Sensor for Non-Enzymatic Glucose Detection. Sens. Actuators B Chem. 2019, 278, 126–132. [Google Scholar] [CrossRef]
- D’Auria, S.; DiCesare, N.; Staiano, M.; Gryczynski, Z.; Rossi, M.; Lakowicz, J.R. A Novel Fluorescence Competitive Assay for Glucose Determinations by Using a Thermostable Glucokinase from the Thermophilic Microorganism Bacillus Stearothermophilus. Anal. Biochem. 2002, 303, 138–144. [Google Scholar] [CrossRef]
- Barone, P.W.; Parker, R.S.; Strano, M.S. In Vivo Fluorescence Detection of Glucose Using a Single-Walled Carbon Nanotube Optical Sensor: Design, Fluorophore Properties, Advantages, and Disadvantages. Anal. Chem. 2005, 77, 7556–7562. [Google Scholar] [CrossRef]
- Dey, K.; Goudar, V.S.; Kaladharan, K.; Santra, T.S.; Tseng, F.-G. Continuous In-Vitro Physiological Glucose Sensing for Co-Cultured 3D Tumour Spheroids Using SERS Based Nano Particle Sensors (SERS-gNPS). Sens. Actuators B Chem. 2024, 404, 135243. [Google Scholar] [CrossRef]
- Yonzon, C.R.; Lyandres, O.; Shah, N.C.; Dieringer, J.A.; Duyne, R.P. Glucose Sensing with Surface-Enhanced Raman Spectroscopy. In Surface-Enhanced Raman Scattering; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2006; Volume 103, pp. 367–379. ISBN 978-3-540-33566-5. [Google Scholar]
- Bode, K.J.; Mueller, S.; Schweinlin, M.; Metzger, M.; Brunner, T. A Fast and Simple Fluorometric Method to Detect Cell Death in 3D Intestinal Organoids. BioTechniques 2019, 67, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, L.E.; Harrison, D.J.; Campbell, C.J. Raman Spectroscopy Investigation of Biochemical Changes in Tumor Spheroids with Aging and after Treatment with Staurosporine. J. Biophotonics 2019, 12, e201800201. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, L.E.; Camus, V.L.; Bagnaninchi, P.O.; Fisher, K.M.; Stewart, G.D.; Nailon, W.H.; McLaren, D.B.; Harrison, D.J.; Campbell, C.J. Targeted SERS Nanosensors Measure Physicochemical Gradients and Free Energy Changes in Live 3D Tumor Spheroids. Nanoscale 2016, 8, 16710–16718. [Google Scholar] [CrossRef] [PubMed]
- Camus, V.L.; Stewart, G.; Nailon, W.H.; McLaren, D.B.; Campbell, C.J. Measuring the Effects of Fractionated Radiation Therapy in a 3D Prostate Cancer Model System Using SERS Nanosensors. Analyst 2016, 141, 5056–5061. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campion, A.; Kambhampati, P. Surface-Enhanced Raman Scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, W.; Best, M.D.; Camden, J.P.; Crozier, K.B. Directional Raman Scattering from Single Molecules in the Feed Gaps of Optical Antennas. Nano Lett. 2013, 13, 2194–2198. [Google Scholar] [CrossRef] [PubMed]
- Weinzimer, S.A. Analysis: PENDRA: The Once and Future Noninvasive Continuous Glucose Monitoring Device? Diabetes Technol. Ther. 2004, 6, 442–444. [Google Scholar] [CrossRef]
- Davison, N.B.; Gaffney, C.J.; Kerns, J.G.; Zhuang, Q.D. Recent Progress and Perspectives on Non-Invasive Glucose Sensors. Diabetology 2022, 3, 56–71. [Google Scholar] [CrossRef]
- Villena Gonzales, W.; Mobashsher, A.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Ridhuan, N.S.; Abdul Razak, K. Progress of Enzymatic and Non-Enzymatic Electrochemical Glucose Biosensor Based on Nanomaterial-Modified Electrode. Biosensors 2022, 12, 1136. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose Oxidase—An Overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Witt, S.; Wohlfahrt, G.; Schomburg, D.; Hecht, H.-J.; Kalisz, H.M. Conserved Arginine-516 of Penicillium Amagasakiense Glucose Oxidase Is Essential for the Efficient Binding of β-d-Glucose. Biochem. J. 2000, 347, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, C.F.B.; Veenhuis, M.; Visser, J. Localization of Glucose Oxidase and Catalase Activities in Aspergillus Niger. Appl. Environ. Microbiol. 1992, 58, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Heitbaum, J.; Hamann, C.H. The Electrooxidation of Glucose in Phosphate Buffer Solutions: Kinetics and Reaction Mechanism. Ber. Bunsenges. Phys. Chem. 1980, 84, 50–55. [Google Scholar] [CrossRef]
- Wang, C.-C.; Hennek, J.W.; Ainla, A.; Kumar, A.A.; Lan, W.-J.; Im, J.; Smith, B.S.; Zhao, M.; Whitesides, G.M. A Paper-Based “Pop-up” Electrochemical Device for Analysis of Beta-Hydroxybutyrate. Anal. Chem. 2016, 88, 6326–6333. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Lubrano, G.J. An Enzyme Electrode for the Amperometric Determination of Glucose. Anal. Chim. Acta 1973, 64, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Chung, C.-H. Gold Nanowire Array Electrode for Non-Enzymatic Voltammetric and Amperometric Glucose Detection. Sens. Actuators B Chem. 2009, 142, 216–223. [Google Scholar] [CrossRef]
- Koike, K.; Sasaki, T.; Hiraki, K.; Ike, K.; Hirofuji, Y.; Yano, M. Characteristics of an Extended Gate Field-Effect Transistor for Glucose Sensing Using an Enzyme-Containing Silk Fibroin Membrane as the Bio-Chemical Component. Biosensors 2020, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; AlGhamdi, W.S.; Faber, H.; Lin, Y.-H.; Liu, C.-H.; Hsu, E.-K.; Lin, W.-Z.; Naphade, D.; Mandal, S.; Heeney, M.; et al. Non-Invasive, Ultrasensitive Detection of Glucose in Saliva Using Metal Oxide Transistors. Biosens. Bioelectron. 2023, 237, 115448. [Google Scholar] [CrossRef]
- You, X.; Pak, J.J. Graphene-Based Field Effect Transistor Enzymatic Glucose Biosensor Using Silk Protein for Enzyme Immobilization and Device Substrate. Sens. Actuators B Chem. 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Shehab, M.; Ebrahim, S.; Soliman, M. Graphene Quantum Dots Prepared from Glucose as Optical Sensor for Glucose. J. Lumin. 2017, 184, 110–116. [Google Scholar] [CrossRef]
- Zhai, Q.; Gong, S.; Wang, Y.; Lyu, Q.; Liu, Y.; Ling, Y.; Wang, J.; Simon, G.P.; Cheng, W. Enokitake Mushroom-like Standing Gold Nanowires toward Wearable Noninvasive Bimodal Glucose and Strain Sensing. ACS Appl. Mater. Interfaces 2019, 11, 9724–9729. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical Non-Enzymatic Glucose Sensors: Recent Progress and Perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef] [PubMed]
- Imran, H.; Manikandan, P.N.; Dharuman, V. Highly Selective and Rapid Non-Enzymatic Glucose Sensing at Ultrathin Layered Nb Doped C3N4 for Extended Linearity Range. Microchem. J. 2021, 160, 105774. [Google Scholar] [CrossRef]
- Imran, H.; Alam, A.; Dharuman, V.; Lim, S. Fabrication of Enzyme-Free and Rapid Electrochemical Detection of Glucose Sensor Based on ZnO Rod and Ru Doped Carbon Nitride Modified Gold Transducer. Nanomaterials 2022, 12, 1778. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Guo, X.; Li, Y.; Li, L.; Pan, L. Recent Advances in Conductive Polymers-Based Electrochemical Sensors for Biomedical and Environmental Applications. Polymers 2024, 16, 1597. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Zhang, Z.; Miao, Z.; Zhang, X.; Su, Z. Fabrication of Hollow CuO/PANI Hybrid Nanofibers for Non-Enzymatic Electrochemical Detection of H2O2 and Glucose. Sens. Actuators B Chem. 2019, 286, 370–376. [Google Scholar] [CrossRef]
- Najmi, A.; Saidi, M.S.; Shahrokhian, S.; Hosseini, H.; Kazemzadeh Hannani, S. Fabrication of a Microdialysis-Based Nonenzymatic Microfluidic Sensor for Regular Glucose Measurement. Sens. Actuators B Chem. 2021, 333, 129569. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S.; Shi, Q.; Cheng, W. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Burrin, J.M.; Price, C.P. Measurement of Blood Glucose. Ann. Clin. Biochem. 1985, 22, 327–342. [Google Scholar] [CrossRef]
- John, S.; Weiss, J.N.; Ribalet, B. Subcellular Localization of Hexokinases I and II Directs the Metabolic Fate of Glucose. PLoS ONE 2011, 6, e17674. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, C.; Hu, H.; Li, Z.; Xiao, G.; Yang, Q.; Sun, P.; Cheng, L.; Niu, W.; Bi, J.; et al. ISFET and Dex-AgNPs Based Portable Sensor for Reusable and Real-Time Determinations of Concanavalin A and Glucose on Smartphone. Biosens. Bioelectron. 2020, 151, 111962. [Google Scholar] [CrossRef] [PubMed]
- Volotovsky, V.; Soldatkin, A.P.; Shul’ga, A.A.; Rossokhaty, V.K.; Strikha, V.I.; El’skaya, A.V. Glucose-Sensitive Ion-Sensitive Field-Effect Transistor-Based Biosensor with Additional Positively Charged Membrane. Dynamic Range Extension and Reduction of Buffer Concentration Influence on the Sensor Response. Anal. Chim. Acta 1996, 322, 77–81. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, H.; Ji, Z.; Xu, M.; Zhang, Y. ZnO Nano-Array-Based EGFET Biosensor for Glucose Detection. Appl. Phys. A 2015, 119, 807–811. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Wang, Z.; Zhao, X.; Wang, H.; Li, F.; Liu, S.; Pan, Y. A Fully Integrated Graphene-Polymer Field-Effect Transistor Biosensing Device for on-Site Detection of Glucose in Human Urine. Mater. Today Chem. 2022, 23, 100635. [Google Scholar] [CrossRef]
- Klonoff, D.C. Overview of Fluorescence Glucose Sensing: A Technology with a Bright Future. J. Diabetes Sci. Technol. 2012, 6, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Lyandres, O.; Shah, N.C.; Yonzon, C.R.; Walsh, J.T.; Glucksberg, M.R.; Van Duyne, R.P. Real-Time Glucose Sensing by Surface-Enhanced Raman Spectroscopy in Bovine Plasma Facilitated by a Mixed Decanethiol/Mercaptohexanol Partition Layer. Anal. Chem. 2005, 77, 6134–6139. [Google Scholar] [CrossRef] [PubMed]
- Ribet, F.; Stemme, G.; Roxhed, N. Real-Time Intradermal Continuous Glucose Monitoring Using a Minimally Invasive Microneedle-Based System. Biomed. Microdevices 2018, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- DiCesare, N.; Lakowicz, J.R. Evaluation of Two Synthetic Glucose Probes for Fluorescence-Lifetime-Based Sensing. Anal. Biochem. 2001, 294, 154–160. [Google Scholar] [CrossRef]
- Chen, L.; Hwang, E.; Zhang, J. Fluorescent Nanobiosensors for Sensing Glucose. Sensors 2018, 18, 1440. [Google Scholar] [CrossRef]
- Zhai, H.; Bai, Y.; Qin, J.; Feng, F. Colorimetric and Ratiometric Fluorescence Dual-Mode Sensing of Glucose Based on Carbon Quantum Dots and Potential UV/Fluorescence of o-Diaminobenzene. Sensors 2019, 19, 674. [Google Scholar] [CrossRef]
- Fang, H.; Kaur, G.; Wang, B. Progress in Boronic Acid-Based Fluorescent Glucose Sensors. J. Fluoresc. 2004, 14, 481–489. [Google Scholar] [CrossRef]
- Cummins, B.M.; Garza, J.T.; Coté, G.L. Optimization of a Concanavalin A-Based Glucose Sensor Using Fluorescence Anisotropy. Anal. Chem. 2013, 85, 5397–5404. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.J.; Pickup, J.C.; Rolinski, O.J.; Birch, D.J.S. Near-Infrared Fluorescence Lifetime Assay for Serum Glucose Based on Allophycocyanin-Labeled Concanavalin A. Anal. Biochem. 2001, 292, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.Q.; Srivastava, R.; Zhu, H.; McShane, M.J. Enzymatic Fluorescent Microsphere Glucose Sensors:Evaluation of Response Under Dynamic Conditions. Diabetes Technol. Ther. 2006, 8, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, N.; Li, D.; Zhang, M.; Lin, Y.; Liu, Z.; Lin, X.; Shui, L. Cesium-Doped Graphene Quantum Dots as Ratiometric Fluorescence Sensors for Blood Glucose Detection. ACS Appl. Nano Mater. 2021, 4, 8437–8446. [Google Scholar] [CrossRef]
- Karachevtsev, V.A.; Glamazda, A.Y.; Leontiev, V.S.; Lytvyn, O.S.; Dettlaff-Weglikowska, U. Glucose Sensing Based on NIR Fluorescence of DNA-Wrapped Single-Walled Carbon Nanotubes. Chem. Phys. Lett. 2007, 435, 104–108. [Google Scholar] [CrossRef]
- Tang, B.; Cao, L.; Xu, K.; Zhuo, L.; Ge, J.; Li, Q.; Yu, L. A New Nanobiosensor for Glucose with High Sensitivity and Selectivity in Serum Based on Fluorescence Resonance Energy Transfer (FRET) between CdTe Quantum Dots and Au Nanoparticles. Chem. Eur. J. 2008, 14, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Sawayama, J.; Okitsu, T.; Nakamata, A.; Kawahara, Y.; Takeuchi, S. Hydrogel Glucose Sensor with In Vivo Stable Fluorescence Intensity Relying on Antioxidant Enzymes for Continuous Glucose Monitoring. iScience 2020, 23, 101243. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Villalonga, R.; Giménez, C.; Sancenón, F.; Marcos, M.D.; Martínez-Máñez, R.; Díez, P.; Pingarrón, J.M.; Amorós, P. Glucose-Triggered Release Using Enzyme-Gated Mesoporous Silica Nanoparticles. Chem. Commun. 2013, 49, 6391–6393. [Google Scholar] [CrossRef]
- Chen, L.; Tse, W.H.; Chen, Y.; McDonald, M.W.; Melling, J.; Zhang, J. Nanostructured Biosensor for Detecting Glucose in Tear by Applying Fluorescence Resonance Energy Transfer Quenching Mechanism. Biosens. Bioelectron. 2017, 91, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dotzert, M.; Melling, C.W.J.; Zhang, J. Tunable Photoluminescence of Carbon Dots Used for Homogeneous Glucose Sensing Assay. Biochem. Eng. J. 2020, 159, 107580. [Google Scholar] [CrossRef]
- Ge, M.; Bai, P.; Chen, M.; Tian, J.; Hu, J.; Zhi, X.; Yin, H.; Yin, J. Utilizing Hyaluronic Acid as a Versatile Platform for Fluorescence Resonance Energy Transfer-Based Glucose Sensing. Anal. Bioanal. Chem. 2018, 410, 2413–2421. [Google Scholar] [CrossRef]
- Czarnik, A.W.; James, T.D. Fluorescent Chemosensors in the Creation of a Commercially Available Continuous Glucose Monitor. ACS Sens. 2024, 9, 6320–6326. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Song, G.; Zhong, K.; Wang, Z.; Xia, X.; Tian, Y. Wearable Fluorescent Contact Lenses for Monitoring Glucose via a Smartphone. Sens. Actuators B Chem. 2022, 352, 131067. [Google Scholar] [CrossRef]
- Seo, J.; Kang, J.; Kim, J.; Han, H.; Park, M.; Shin, M.; Lee, K. Smart Contact Lens for Colorimetric Visualization of Glucose Levels in the Body Fluid. ACS Biomater. Sci. Eng. 2024, 10, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Mal, D.K.; Pal, H.; Chakraborty, G. A Comprehensive Review on Recent Advances in Fluorescence-Based Bio-Analytes Sensing. TrAC Trends Anal. Chem. 2024, 171, 117493. [Google Scholar] [CrossRef]
- Setchfield, K.; Gorman, A.; Simpson, A.H.R.W.; Somekh, M.G.; Wright, A.J. Effect of Skin Color on Optical Properties and the Implications for Medical Optical Technologies: A Review. J. Biomed. Opt. 2024, 29, 010901. [Google Scholar] [CrossRef]
- Stuart, D.A.; Yuen, J.M.; Shah, N.; Lyandres, O.; Yonzon, C.R.; Glucksberg, M.R.; Walsh, J.T.; Van Duyne, R.P. In Vivo Glucose Measurement by Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2006, 78, 7211–7215. [Google Scholar] [CrossRef]
- Ju, J.; Hsieh, C.-M.; Tian, Y.; Kang, J.; Chia, R.; Chang, H.; Bai, Y.; Xu, C.; Wang, X.; Liu, Q. Surface Enhanced Raman Spectroscopy Based Biosensor with a Microneedle Array for Minimally Invasive In Vivo Glucose Measurements. ACS Sens. 2020, 5, 1777–1785. [Google Scholar] [CrossRef]
- Yang, D.; Afroosheh, S.; Lee, J.O.; Cho, H.; Kumar, S.; Siddique, R.H.; Narasimhan, V.; Yoon, Y.-Z.; Zayak, A.T.; Choo, H. Glucose Sensing Using Surface-Enhanced Raman-Mode Constraining. Anal. Chem. 2018, 90, 14269–14278. [Google Scholar] [CrossRef] [PubMed]
- Borah, R.; Ninakanti, R.; Bals, S.; Verbruggen, S.W. Plasmon Resonance of Gold and Silver Nanoparticle Arrays in the Kretschmann (Attenuated Total Reflectance) vs. Direct Incidence Configuration. Sci. Rep. 2022, 12, 15738. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of Silver Nanoparticles on Human Cells: Effect of Particle Size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver Nanoparticles Outperform Gold Nanoparticles in Radiosensitizing U251 Cells in Vitro and in an Intracranial Mouse Model of Glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef]
- Tong, L.; Xu, H.; Käll, M. Nanogaps for SERS Applications. MRS Bull. 2014, 39, 163–168. [Google Scholar] [CrossRef]
- Huang, X.; Yao, C.; Huang, S.; Zheng, S.; Liu, Z.; Liu, J.; Wang, J.; Chen, H.; Xie, X. Technological Advances of Wearable Device for Continuous Monitoring of In Vivo Glucose. ACS Sens. 2024, 9, 1065–1088. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Chen, S.; Zheng, Y.; Peng, L.; Liu, B.; Chen, Z.; Xie, X.; Yi, C.; Jiang, L. Development of Smartphone-Controlled and Microneedle-Based Wearable Continuous Glucose Monitoring System for Home-Care Diabetes Management. ACS Sens. 2023, 8, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Aghavali, R.; Hosseini-Toudeshki, H.; Brown, C.; Zhang, F.; et al. An Integrated Wearable Microneedle Array for the Continuous Monitoring of Multiple Biomarkers in Interstitial Fluid. Nat. Biomed. Eng. 2022, 6, 1214–1224. [Google Scholar] [CrossRef]
- Sun, X.; Ji, W.; Zhang, B.; Ma, L.; Fu, W.; Qian, W.; Zhang, X.; Li, J.; Sheng, E.; Tao, Y.; et al. A Theranostic Microneedle Array Patch for Integrated Glycemia Sensing and Self-Regulated Release of Insulin. Biomater. Sci. 2022, 10, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.; Liu, Q. Hollow Agarose Microneedle with Silver Coating for Intradermal Surface-Enhanced Raman Measurements: A Skin-Mimicking Phantom Study. J. Biomed. Opt. 2015, 20, 061102. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable Non-Invasive Epidermal Glucose Sensors: A Review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D. Skinside out. Drug Delivery in Reverse Could Be a Diabetic’s Boon. Sci. Am. 1991, 265, 128–130. [Google Scholar] [CrossRef]
- Giri, T.K.; Chakrabarty, S.; Ghosh, B. Transdermal Reverse Iontophoresis: A Novel Technique for Therapeutic Drug Monitoring. J. Control. Release 2017, 246, 30–38. [Google Scholar] [CrossRef]
- Potts, R.O.; Tamada, J.A.; Tierney, M.J. Glucose Monitoring by Reverse Iontophoresis. Diabetes Metab. Res. Rev. 2002, 18, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Jovanovic, L.; Garg, S. Clinical Evaluation of the GlucoWatch® Biographer: A Continual, Non-Invasive Glucose Monitor for Patients with Diabetes. Biosens. Bioelectron. 2001, 16, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Charro, M.B. Recent Advances on Transdermal Iontophoretic Drug Delivery and Non-Invasive Sampling. J. Drug Deliv. Sci. Technol. 2009, 19, 75–88. [Google Scholar] [CrossRef]
- The Diabetes Research in Children Network (DirecNet) Study Group. Accuracy of the GlucoWatch G2 Biographer and the Continuous Glucose Monitoring System During Hypoglycemia. Diabetes Care 2004, 27, 722–726. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous Sweat Extraction and Analysis Applied to Cystic Fibrosis and Glucose Monitoring Using a Fully Integrated Wearable Platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A Touch-Actuated Glucose Sensor Fully Integrated with Microneedle Array and Reverse Iontophoresis for Diabetes Monitoring. Biosens. Bioelectron. 2022, 203, 114026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Su, H.; Sun, F.; Lu, Z.; Su, A. A Wearable Self-Powered Biosensor System Integrated with Diaper for Detecting the Urine Glucose of Diabetic Patients. Sens. Actuators B Chem. 2021, 341, 130046. [Google Scholar] [CrossRef]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and Challenges in the Diagnostic Utility of Dermal Interstitial Fluid. Nat. Biomed. Eng. 2023, 7, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Pu, Z.; Wu, H.; Li, C.; Zhang, X.; Li, D. Reverse Iontophoresis with the Development of Flexible Electronics: A Review. Biosens. Bioelectron. 2023, 223, 115036. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Quinones, S. Review of Non-Invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Eledrisi, M.S.; Motamedi, M.; Esenaliev, R.O. Noninvasive Blood Glucose Monitoring With Optical Coherence Tomography. Diabetes Care 2002, 25, 2263–2267. [Google Scholar] [CrossRef] [PubMed]
- Esenaliev, R.O.; Larin, K.V.; Larina, I.V.; Motamedi, M. Noninvasive Monitoring of Glucose Concentration with Optical Coherence Tomography. Opt. Lett. 2001, 26, 992. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Jiang, N.; Shao, X.; Elsherif, M.; Alam, F.; Salih, A.; Butt, H.; Yetisen, A.K. Recent Advances in Optical Sensors for Continuous Glucose Monitoring. Sens. Diagn. 2022, 1, 1098–1125. [Google Scholar] [CrossRef]
- Gabbay, R.A.; Sivarajah, S. Optical Coherence Tomography-Based Continuous Noninvasive Glucose Monitoring in Patients with Diabetes. Diabetes Technol. Ther. 2008, 10, 188–193. [Google Scholar] [CrossRef]
- Su, Y.; Cai, K.; Sun, J.; Hao, P.; Zhang, Y.; Dong, Y.; Xue, Y.; Guo, Z.; Yao, X.S. Three-Dimensional Correlation Method for Non-Invasive Blood Glucose Monitoring with Optical Coherence Tomography. Opt. Lasers Eng. 2025, 186, 108821. [Google Scholar] [CrossRef]
- Jernelv, I.L.; Milenko, K.; Fuglerud, S.S.; Hjelme, D.R.; Ellingsen, R.; Aksnes, A. A Review of Optical Methods for Continuous Glucose Monitoring. Appl. Spectrosc. Rev. 2019, 54, 543–572. [Google Scholar] [CrossRef]
- Olesberg, J.T.; Liu, L.; Zee, V.V.; Arnold, M.A. In Vivo Near-Infrared Spectroscopy of Rat Skin Tissue with Varying Blood Glucose Levels. Anal. Chem. 2006, 78, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Liu, J.; Liu, R.; Chen, W.; Yao, M.; Liu, X.; Ge, Q.; Zhang, Z.; Li, C.; Wang, Y.; et al. In Vivo Near-Infrared Noninvasive Glucose Measurement and Detection in Humans. Appl. Spectrosc. 2022, 76, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Maruo, K.; Oota, T.; Tsurugi, M.; Nakagawa, T.; Arimoto, H.; Hayakawa, M.; Tamura, M.; Ozaki, Y.; Yamada, Y. Noninvasive Near-Infrared Blood Glucose Monitoring Using a Calibration Model Built by a Numerical Simulation Method: Trial Application to Patients in an Intensive Care Unit. Appl. Spectrosc. 2006, 60, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Cano-Garcia, H.; Kshirsagar, R.; Pricci, R.; Teyeb, A.; O’Brien, F.; Saha, S.; Kosmas, P.; Kallos, E. Enhancing the Accuracy of Non-Invasive Glucose Sensing in Aqueous Solutions Using Combined Millimeter Wave and Near Infrared Transmission. Sensors 2021, 21, 3275. [Google Scholar] [CrossRef]
- Srichan, C.; Srichan, W.; Danvirutai, P.; Ritsongmuang, C.; Sharma, A.; Anutrakulchai, S. Non-Invasively Accuracy Enhanced Blood Glucose Sensor Using Shallow Dense Neural Networks with NIR Monitoring and Medical Features. Sci. Rep. 2022, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Shokrekhodaei, M.; Cistola, D.P.; Roberts, R.C.; Quinones, S. Non-Invasive Glucose Monitoring Using Optical Sensor and Machine Learning Techniques for Diabetes Applications. IEEE Access 2021, 9, 73029–73045. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tajima, T.; Seyama, M.; Waki, K. Differential Continuous Wave Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring. IEEE Sens. J. 2020, 20, 4453–4458. [Google Scholar] [CrossRef]
- Baghelani, M.; Abbasi, Z.; Daneshmand, M.; Light, P.E. Non-Invasive Continuous-Time Glucose Monitoring System Using a Chipless Printable Sensor Based on Split Ring Microwave Resonators. Sci. Rep. 2020, 10, 12980. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, R.; Kim, E.S.; Jo, Y.-H.; Kim, S.-S.; Kim, N.-Y. Characterization of Micro-Resonator Based on Enhanced Metal Insulator Semiconductor Capacitor for Glucose Recognition. Med. Eng. Phys. 2017, 41, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Wang, C.; Meng, F.-Y.; Zhou, Z.-L.; Zhao, M.; Yan, G.-F.; Kim, E.-S.; Kim, N.-Y. High-Sensitivity, Quantified, Linear and Mediator-Free Resonator-Based Microwave Biosensor for Glucose Detection. Sensors 2020, 20, 4024. [Google Scholar] [CrossRef]
- Jang, C.; Lee, H.-J.; Yook, J.-G. Radio-Frequency Biosensors for Real-Time and Continuous Glucose Detection. Sensors 2021, 21, 1843. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.-Y.; Cheong, W.H.; Jang, J.; Park, Y.-G.; Na, K.; Kim, Y.-T.; Heo, J.H.; Lee, C.Y.; et al. Soft, Smart Contact Lenses with Integrations of Wireless Circuits, Glucose Sensors, and Displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.R.A.; Bain, S.C.; Luzio, S.; Handy, C.; Fowles, D.J.; Love, B.; Wareham, K.; Barlow, L.; Dunseath, G.J.; Crane, J.; et al. Using Artificial Intelligence to Improve the Accuracy of a Wrist-Worn, Noninvasive Glucose Monitor: A Pilot Study. J. Diabetes Sci. Technol. 2024, 19322968241252819. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Yang, Y.; Huang, Y.; Ma, G.; Luo, Y.; Huang, P.; Lin, J. Glucose Oxidase-Instructed Fluorescence Amplification Strategy for Intracellular Glucose Detection. ACS Appl. Mater. Interfaces 2019, 11, 10554–10558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jing, Y.; Zhai, Q.; Yu, Y.; Xing, H.; Li, J.; Wang, E. Point-of-Care Diagnoses: Flexible Patterning Technique for Self-Powered Wearable Sensors. Anal. Chem. 2018, 90, 11780–11784. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, J.; Wang, C.; Feng, L.; Song, Z.; Zhao, W.; Wang, Q.; Liu, C. The Application of Wearable Glucose Sensors in Point-of-Care Testing. Front. Bioeng. Biotechnol. 2021, 9, 774210. [Google Scholar] [CrossRef] [PubMed]
- Oyaert, M.; Delanghe, J.R. Semiquantitative, Fully Automated Urine Test Strip Analysis. Clin. Lab. Anal. 2019, 33, e22870. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cai, A.; Xu, T.; Zhang, X. Artificial Intelligence Biosensors for Continuous Glucose Monitoring. Interdiscip. Mater. 2023, 2, 290–307. [Google Scholar] [CrossRef]
- Wickramasinghe, Y.; Yang, Y.; Spencer, S.A. Current Problems and Potential Techniques in In Vivo Glucose Monitoring. J. Fluoresc. 2004, 14, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Sun, P.; Xiao, G.; Tang, Q.; Sun, X.; Zhao, H.; Zhao, S.; Lu, H.; Yue, Z. ISFET-based Sensors for (Bio)Chemical Applications: A Review. Electrochem. Sci. Adv. 2023, 3, e2100207. [Google Scholar] [CrossRef]
- Seo, H.-I.; Kim, C.-S.; Sohn, B.-K.; Yeow, T.; Son, M.-T.; Haskard, M. ISFET Glucose Sensor Based on a New Principle Using the Electrolysis of Hydrogen Peroxide. Sens. Actuators B Chem. 1997, 40, 1–5. [Google Scholar] [CrossRef]
- Park, K.-Y.; Choi, S.-B.; Lee, M.; Sohn, B.-K.; Choi, S.-Y. ISFET Glucose Sensor System with Fast Recovery Characteristics by Employing Electrolysis. Sens. Actuators B Chem. 2002, 83, 90–97. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose Biosensors in Clinical Practice: Principles, Limits and Perspectives of Currently Used Devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Wang, J.; Yang, X.; Fan, G.; Yu, X.; Wang, Y.; Wang, T.; Wang, Q.; Zhao, H. Improved Glucose Detection Limit Based on Phosphorescence from Protected Metalloporphyrin Triplet State. Anal. Chim. Acta 2024, 1315, 342825. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Ren, H.; Qiu, H.; Zhang, S.; Lu, Q.; Hu, Y. Highly Sensitive SERS Sensors for Glucose Detection Based on enzyme@MOFs and Ratiometric Raman. Talanta 2024, 271, 125647. [Google Scholar] [CrossRef] [PubMed]
- Kuranov, R.V.; Sapozhnikova, V.V.; Prough, D.S.; Cicenaite, I.; Esenaliev, R.O. Prediction Capability of Optical Coherence Tomography for Blood Glucose Concentration Monitoring. J. Diabetes Sci. Technol. 2007, 1, 470–477. [Google Scholar] [CrossRef]
- Kuranov, R.; Prough, D.; Sapozhnikova, V.; Cicenaite, I.; Esenaliev, R. In Vivo Application of 2-D Lateral Scanning Mode Optical Coherence Tomography for Glucose Sensing. In Smart Medical and Biomedical Sensor Technology III; Cullum, B.M., Carter, J.C., Eds.; SPIE Press: Bellingham, WA, USA, 2005; p. 60070K. [Google Scholar]
- Kuranov, R.V.; Sapozhnikova, V.V.; Prough, D.S.; Cicenaite, I.; Esenaliev, R.O. In Vivo Study of Glucose-Induced Changes in Skin Properties Assessed with Optical Coherence Tomography. Phys. Med. Biol. 2006, 51, 3885–3900. [Google Scholar] [CrossRef]
- Aloraynan, A.; Rassel, S.; Xu, C.; Ban, D. A Single Wavelength Mid-Infrared Photoacoustic Spectroscopy for Noninvasive Glucose Detection Using Machine Learning. Biosensors 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeon, H.-J.; Park, S.; Lee, D.Y.; Chung, E. Tear Glucose Measurement by Reflectance Spectrum of a Nanoparticle Embedded Contact Lens. Sci. Rep. 2020, 10, 8254. [Google Scholar] [CrossRef] [PubMed]


| Technologies | Advantages | Disadvantages | Measuring Site | Sensitivity | Cost | Ref |
|---|---|---|---|---|---|---|
| Electrochemical glucose sensors | Simpler concept, predictive accuracy, and stability upon changing conditions | Pain and invasiveness, risk of infections, and biocompatibility | Skin | 1.3 nM | Low | [6,34,118] |
| Microdialysis | Improved real-time monitoring | Pain and invasiveness | Skin | 21 μM | Moderate | [20,39] |
| Hexokinase method | High specificity | Enzyme instability | In vitro | 3.3 mM or 60 mg/dL | High | [42,105] |
| Ion-Selective Field-Effect Transistor (ISFET) | Fast reproducibility with repeat measurements | Low sensitivity and slow response, electrical noise and signal interference, and sensitivity to environmental factors | In vitro | 0.1 mM | High | [119,120,121,122] |
| Extended gate electrode field-effect transistor (EGFET) | Improved sensitivity as compared to ISFET | Electrical noise and signal interference and sensitivity to environmental factors | Potential application in saliva and sweat | 20 μM | High | [29,45] |
| Polymer functionalized graphene field-effect transistor (P-GFET) | Excellent reusability, maintaining its performance over 20 cycles | Invasiveness and biocompatibility | Ex vivo | 1.9 μM | [46] | |
| Fluorescence methods | Non-invasive measurement and real-time monitoring | Photobleaching, autofluorescence, and signal fluctuations due to various skin conditions such as pigmentation, redness, tattoo, and epidermal thickness. | In vivo | 800 μM | Moderate | [66,123,124] |
| Raman spectroscopy | Non-invasive measurements, sharp spectral features, and signal multiplexing options | Signal may decay with epidermal thickness and background noise | In vitro, aqueous humor, finger, and wrist | 0.03 μM | Moderate | [9,72,123,125] |
| Reverse iontophoresis | Real-time monitoring and low risk of infection | Low glucose concentration in ISF and risk of interference from sweat or other skin conditions | Skin | 25 μM | Moderate | [85,89,90,93] |
| Optical coherence tomography (OCT) | Real-time monitoring, high resolution, and high penetration depth | Sensitive to motion artifacts and environmental factors like varied skin conditions, temperature, and humidity | Skin | 1.1 mM or 20 mg/dL | Moderate | [123,126,127,128] |
| Infrared absorption spectroscopy | Skin penetration up to 100 mm | Sensitive to varied skin conditions | Skin | 1.6 mM or 30 mg/dL | Low | [101,123] |
| Photoacoustic spectroscopy | Faster response and contactless | Sensitive to varied skin conditions and physical interference from temperature and pressure changes | Skin and aqueous humor | 1.3 mM or 25 mg/dL | Moderate | [107,123,129] |
| Microwave | Non-invasive, real-time monitoring and flexible substrate | Lower precision | In vitro | 5 mM | High | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, K.; Santra, T.S.; Tseng, F.G. Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors. Appl. Sci. 2025, 15, 2523. https://doi.org/10.3390/app15052523
Dey K, Santra TS, Tseng FG. Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors. Applied Sciences. 2025; 15(5):2523. https://doi.org/10.3390/app15052523
Chicago/Turabian StyleDey, Koyel, Tuhin Subhra Santra, and Fan Gang Tseng. 2025. "Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors" Applied Sciences 15, no. 5: 2523. https://doi.org/10.3390/app15052523
APA StyleDey, K., Santra, T. S., & Tseng, F. G. (2025). Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors. Applied Sciences, 15(5), 2523. https://doi.org/10.3390/app15052523







