Exploring the Clinical Utility of Cardiorespiratory Optimal Point in Heart Failure Patients: Creating a New Research Gap
Abstract
:1. Introduction
| Prognostic Variable | Number of Patients | Cut-Point Values for Poor Prognostic | |
|---|---|---|---|
| Peak V′O2 | Mancini et al. [3] | 114 | <14 mL/kg.min−1 |
| Myers et al. [4] | 644 | <10 mL/kg.min−1 | |
| Stelken et al. [2] | 181 | <50% of predicted | |
| V′E-V′CO2 slope | Chua et al. [5] | 173 | >34 |
| Corra et al. [7] | 600 | >34 | |
| Periodic breathing | Corra et al. [6] | 323 | PB > 60% of time, amplitude > 14% |
| Leite et al. [8] | 84 | More than 2 regular oscillations, amplitude > 5 L | |
| ΔV′O2/ΔWR | Koike et al. [9] | 385 | <7 |
| V′O2 kinetics in the recovery | Groote et al. [10] | 153 | Peak V′O2 > 39% of predicted and R V′O2 < 2.5 |
| Scrutinio et al. [11] | 196 | T1/2 V′O2 peak > 200 s | |
| Circulatory power | Cohen-Solal et al. [12] | 175 | <3.047 mL/(kg.min−1) mm Hg |
| Scharf et al. [13] | 154 | <5.000% mm Hg | |
| COP | Silva et al. [20] | 2205 | >23.3 |
| Laukkanen et al. [21] | 3160 | >24.3 | |
| Laukkanen et al. [23] | 2190 | >29 | |
| Peterman et al. [24] | 3160 | >24 | |
| Ferreira et al. [25] | 2190 | >23.3 | |
| Wernhart et al. [26] | 2205 | >26 | |
| Ramos et al. [17] | 277 | Low: <26.0; Moderate: 26.0–30.7; high: >30.7 | |
| Charitonidis et al. [27] | 30 | ≥36 |
2. Methods
3. Is the COP a New and Reliable Marker?
4. Does COP Have Independent Prognostic Value?
5. Is the COP Concept Comparable to That of the Oxygen-Uptake-Efficiency Slope?
6. Clinical and Prognostic Applications
7. Future Studies
8. Perspectives and Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrovszki, Z.; Czimbalmos, O.; Gal, V.; Korosi, G.; Nagy, E.; Mikulan, R.; Horvath, G. Age- and Post-Based Complex Analyses of Heart Rate Variability in Young Male Handball Players for Potential Prevention of Overload-induced Injuries. Phys. Act. Health 2025, 9, 67–82. [Google Scholar] [CrossRef]
- Stelken, A.M.; Younis, L.T.; Jennison, S.H.; Miller, D.D.; Miller, L.W.; Shaw, L.J.; Kargl, D.; Chaitman, B.R. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J. Am. Coll. Cardiol. 1996, 27, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Gullestad, L.; Vagelos, R.; Do, D.; Bellin, D.; Ross, H.; Fowler, M.B. Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am. Heart J. 2000, 139, 78–84. [Google Scholar] [CrossRef]
- Chua, T.P.; Ponikowski, P.; Harrington, D.; Anker, S.D.; Webb-Peploe, K.; Clark, A.L.; Poole-Wilson, P.A.; Coats, A.J. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J. Am. Coll. Cardiol. 1997, 29, 1585–1590. [Google Scholar] [CrossRef]
- Corra, U.; Giordano, A.; Bosimini, E.; Mezzani, A.; Piepoli, M.; Coats, A.J.; Giannuzzi, P. Oscillatory ventilation during exercise in patients with chronic heart failure: Clinical correlates and prognostic implications. Chest 2002, 121, 1572–1580. [Google Scholar] [CrossRef]
- Corra, U.; Mezzani, A.; Bosimini, E.; Scapellato, F.; Imparato, A.; Giannuzzi, P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am. Heart J. 2002, 143, 418–426. [Google Scholar] [CrossRef]
- Leite, J.J.; Mansur, A.J.; de Freitas, H.F.; Chizola, P.R.; Bocchi, E.A.; Terra-Filho, M.; Neder, J.A.; Lorenzi-Filho, G. Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. J. Am. Coll. Cardiol. 2003, 41, 2175–2181. [Google Scholar] [CrossRef]
- Koike, A.; Itoh, H.; Kato, M.; Sawada, H.; Aizawa, T.; Fu, L.T.; Watanabe, H. Prognostic power of ventilatory responses during submaximal exercise in patients with chronic heart disease. Chest 2002, 121, 1581–1588. [Google Scholar] [CrossRef]
- de Groote, P.; Millaire, A.; Decoulx, E.; Nugue, O.; Guimier, P.; Ducloux, G. Kinetics of oxygen consumption during and after exercise in patients with dilated cardiomyopathy. New markers of exercise intolerance with clinical implications. J. Am. Coll. Cardiol. 1996, 28, 168–175. [Google Scholar] [CrossRef]
- Scrutinio, D.; Passantino, A.; Lagioia, R.; Napoli, F.; Ricci, A.; Rizzon, P. Percent achieved of predicted peak exercise oxygen uptake and kinetics of recovery of oxygen uptake after exercise for risk stratification in chronic heart failure. Int. J. Cardiol. 1998, 64, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, A.; Tabet, J.Y.; Logeart, D.; Bourgoin, P.; Tokmakova, M.; Dahan, M. A non-invasively determined surrogate of cardiac power (’circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur. Heart J. 2002, 23, 806–814. [Google Scholar] [CrossRef]
- Scharf, C.; Merz, T.; Kiowski, W.; Oechslin, E.; Schalcher, C.; Brunner-La Rocca, H.P. Noninvasive assessment of cardiac pumping capacity during exercise predicts prognosis in patients with congestive heart failure. Chest 2002, 122, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Abella, J.; Pinkstaff, S.; Brubaker, P.; Kitzman, D.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Prognostic significance of the oxygen uptake efficiency slope: Percent-predicted versus actual value. Am. J. Cardiol. 2010, 105, 757–758. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Stein, R.; Chiappa, G.R. Beyond peak oxygen uptake: New prognostic markers from gas exchange exercise tests in chronic heart failure. J. Cardiopulm. Rehabil. 2006, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, W. Historical remarks on the development of the aerobic-anaerobic threshold up to 1966. Int. J. Sports Med. 1985, 6, 109–116. [Google Scholar] [CrossRef]
- Ramos, P.S.; Ricardo, D.R.; Araujo, C.G. Cardiorespiratory optimal point: A submaximal variable of the cardiopulmonary exercise testing. Arq. Bras. Cardiol. 2012, 99, 988–996. [Google Scholar] [CrossRef]
- Ramos, P.S.; Araujo, C.G. Cardiorespiratory optimal point during exercise testing as a predictor of all-cause mortality. Rev. Port. Cardiol. 2017, 36, 261–269. [Google Scholar] [CrossRef]
- Oyarzo-Aravena, A.; Arce-Alvarez, A.; Salazar-Ardiles, C.; Ramirez-Campillo, R.; Alvarez, C.; Toledo, C.; Izquierdo, M.; Andrade, D.C. Cardiorespiratory optimal point as a submaximal evaluation tool in endurance athletes: An exploratory study. Front. Physiol. 2023, 14, 1087829. [Google Scholar] [CrossRef]
- Silva, C.G.D.S.E.; Castro, C.L.B.D.; Franca, J.F.; Bottino, A.; Myers, J.; Araújo, C.G.S.D. Cardiorespiratory Optimal Point in Professional Soccer Players: A Novel Submaximal Variable During Exercise. Int. J. Cardiovasc. Sci. 2018, 31, 323–332. [Google Scholar]
- Laukkanen, J.A. Is Cardiorespiratory Optimal Point Measured During the Maximal Cardiopulmonary Exercise Test a Relevant Indicator of Sports Performance? Int. J. Cardiovasc. Sci. 2018, 31, 320–322. [Google Scholar]
- Kroesen, S.H.; Snoek, J.A.; RRJ, V.A.N.K.; Molinger, J.; Araujo, C.G.; Hopman, M.T.E.; Eijsvogels, T.M.H.; Bakker, E.A. Comparison of Cardiopulmonary Exercise Test Variables to Predict Adverse Events in Patients with Heart Failure. Med. Sci. Sports Exerc. 2024, 56, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Savonen, K.; Hupin, D.; Araujo, C.G.S.; Kunutsor, S.K. Cardiorespiratory optimal point during exercise testing and sudden cardiac death: A prospective cohort study. Prog. Cardiovasc. Dis. 2021, 68, 12–18. [Google Scholar] [CrossRef]
- Peterman, J.E.; Harber, M.P.; Fleenor, B.S.; Whaley, M.H.; Araujo, C.G.; Kaminsky, L.A. Cardiorespiratory Optimal Point Is a Submaximal Exercise Test Variable and a Predictor of Mortality Risk: THE BALL STATE ADULT FITNESS LONGITUDINAL LIFESTYLE STUDY (BALL ST). J. Cardiopulm. Rehabil. Prev. 2022, 42, E90–E96. [Google Scholar] [CrossRef]
- Ferreira Reis, J.; Goncalves, A.; Bras, P.; Moreira, R.; Pereira-da-Silva, T.; Timoteo, A.T.; Soares, R.; Cruz Ferreira, R. The prognostic value of the cardiorespiratory optimal point during submaximal exercise testing in heart failure. Rev. Port. Cardiol. 2022, 41, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Wernhart, S.; Mincu, R.; Balcer, B.; Rammos, C.; Muentjes, C.; Rassaf, T. The cardiorespiratory optimal point as a discriminator of lesion severity in adults with congenital heart disease. J. Sports Med. Phys. Fitness 2023, 63, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Charitonidis, K.; Koutlianos, N.; Anagnostaras, K.; Anifanti, M.; Kouidi, E.; Deligiannis, A. Combination of novel and traditional cardiorespiratory indices for the evaluation of adolescent volleyball players. Hippokratia 2019, 23, 70–74. [Google Scholar]
- Ramos, P.S.; Sardinha, A.; Nardi, A.E.; de Araujo, C.G. Cardiorespiratory optimal point: A submaximal exercise variable to assess panic disorder patients. PLoS ONE 2014, 9, e104932. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Kunutsor, S.K.; Araujo, C.G.; Savonen, K. Cardiorespiratory optimal point during exercise testing is related to cardiovascular and all-cause mortality. Scand. J. Med. Sci. Sports 2021, 31, 1949–1961. [Google Scholar] [CrossRef]
- Agostoni, P.; Corra, U.; Emdin, M. Periodic Breathing during Incremental Exercise. Ann. Am. Thorac. Soc. 2017, 14, S116–S122. [Google Scholar] [CrossRef]
- Karsten, M.; Salvioni, E.; Palermo, P.; Mattavelli, I.; Scatigna, M.; Mapelli, M.; Grilli, G.; Pezzuto, B.; Apostolo, A.; Ribeiro, G.D.S.; et al. Periodic breathing during exercise in heart failure: Beyond the classic risk factors. Eur. Heart J. Suppl. 2025, 27, i103–i108. [Google Scholar] [CrossRef]
- Chiappa, G.R.; Borghi-Silva, A.; Ferreira, L.F.; Carrascosa, C.; Oliveira, C.C.; Maia, J.; Gimenes, A.C.; Queiroga, F., Jr.; Berton, D.; Ferreira, E.M.; et al. Kinetics of muscle deoxygenation are accelerated at the onset of heavy-intensity exercise in patients with COPD: Relationship to central cardiovascular dynamics. J. Appl. Physiol. 2008, 104, 1341–1350. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Alencar, M.C.; Arbex, F.; Souza, A.; Sperandio, P.; Medina, L.; Medeiros, W.M.; Hirai, D.M.; O’Donnell, D.E.; Neder, J.A. Effects of heart failure on cerebral blood flow in COPD: Rest and exercise. Respir. Physiol. Neurobiol. 2016, 221, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, P.A.; Borghi-Silva, A.; Barroco, A.; Nery, L.E.; Almeida, D.R.; Neder, J.A. Microvascular oxygen delivery-to-utilization mismatch at the onset of heavy-intensity exercise in optimally treated patients with CHF. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1720–H1728. [Google Scholar] [CrossRef]
- Polkey, M.I. Respiratory Muscle Assessment in Clinical Practice. Clin. Chest Med. 2019, 40, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, S.H.R.; Cipriano Junior, G.; Vieira, P.J.C.; Nakano, E.Y.; Winkelmann, E.R.; Callegaro, C.C.; Chiappa, G.R. Inspiratory muscle strength and six-minute walking distance in heart failure: Prognostic utility in a 10 years follow up cohort study. PLoS ONE 2019, 14, e0220638. [Google Scholar] [CrossRef]
- Milani, J.; Milani, M.; Cipriano, G.F.B.; de Castro, I.; Hansen, D.; Cipriano Junior, G. Oxygen Uptake Efficiency Slope in South American Healthy Adults: COMPREHENSIVE REFERENCE VALUES AND INTERNATIONAL COMPARISONS. J. Cardiopulm. Rehabil. Prev. 2023, 43, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, M.; Tager, I.B. Oxygen uptake efficiency slope: An index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J. Am. Coll. Cardiol. 2000, 36, 194–201. [Google Scholar] [CrossRef]
- Baba, R.; Nagashima, M.; Goto, M.; Nagano, Y.; Yokota, M.; Tauchi, N.; Nishibata, K. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996, 28, 1567–1572. [Google Scholar] [CrossRef]
- Baba, R.; Kubo, N.; Morotome, Y.; Iwagaki, S. Reproducibility of the oxygen uptake efficiency slope in normal healthy subjects. J. Sports Med. Phys. Fitness 1999, 39, 202–206. [Google Scholar]
- Pogliaghi, S.; Dussin, E.; Tarperi, C.; Cevese, A.; Schena, F. Calculation of oxygen uptake efficiency slope based on heart rate reserve end-points in healthy elderly subjects. Eur. J. Appl. Physiol. 2007, 101, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L.; Perrey, S.; Tordi, N.; Rouillon, J.D. Evaluation of fitness level by the oxygen uptake efficiency slope after a short-term intermittent endurance training. Int. J. Sports Med. 2004, 25, 85–91. [Google Scholar] [CrossRef]
- Baba, R.; Tsuyuki, K.; Kimura, Y.; Ninomiya, K.; Aihara, M.; Ebine, K.; Tauchi, N.; Nishibata, K.; Nagashima, M. Oxygen uptake efficiency slope as a useful measure of cardiorespiratory functional reserve in adult cardiac patients. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Chiappa, G.R.; Guths, H.; Dall’Ago, P.; Ribeiro, J.P. Inspiratory muscle training improves oxygen uptake efficiency slope in patients with chronic heart failure. J. Cardiopulm. Rehabil. Prev. 2009, 29, 392–395. [Google Scholar] [CrossRef]
- Defoor, J.; Schepers, D.; Reybrouck, T.; Fagard, R.; Vanhees, L. Oxygen uptake efficiency slope in coronary artery disease: Clinical use and response to training. Int. J. Sports Med. 2006, 27, 730–737. [Google Scholar] [CrossRef]
- Van de Veire, N.R.; Van Laethem, C.; Philippe, J.; De Winter, O.; De Backer, G.; Vanderheyden, M.; De Sutter, J. VE/VCO2 slope and oxygen uptake efficiency slope in patients with coronary artery disease and intermediate peakVO2. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Michelis, K.C.; Pandey, A.; Ayers, C.; Thibodeau, J.T.; Grodin, J.L.; Drazner, M.H. Oxygen Uptake Efficiency Slope and Prognosis in Heart Failure With Reduced Ejection Fraction. Am. J. Cardiol. 2023, 201, 273–280. [Google Scholar] [CrossRef]
- Peterman, J.E.; Novelli, D.S.; Fleenor, B.S.; Whaley, M.H.; Kaminsky, L.A.; Harber, M.P. Oxygen Uptake Efficiency Slope as a Predictor of Mortality Risk: The ball state adult fitness longitudinal lifestyle study (ball ST). J. Cardiopulm. Rehabil. Prev. 2023, 43, 282–289. [Google Scholar] [CrossRef]
- Cofre-Bolados, C.; Ferrari, G.; Valdivia-Moral, P.; Vidal-Diaz, F.; Ramirez-Velez, R.; Izquierdo-Redin, M. Sub Maximal Ergospirometry Parameters in Untrained Non-Frail Octogenarian Subjects. Medicina 2022, 58, 378. [Google Scholar] [CrossRef]
- Stickland, M.K.; Butcher, S.J.; Marciniuk, D.D.; Bhutani, M. Assessing exercise limitation using cardiopulmonary exercise testing. Pulm. Med. 2012, 2012, 824091. [Google Scholar] [CrossRef]
- Song, J.; Chen, X.; Wang, B.; Cheng, Y.; Wang, Y. Effect of Exercise-Based Cardiac Rehabilitation on Patients With Chronic Heart Failure After Transcatheter Aortic Valve Replacement: A Randomized Controlled Trial. J. Cardiopulm. Rehabil. Prev. 2025, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Telford, R.D. Low physical activity and obesity: Causes of chronic disease or simply predictors? Med. Sci. Sports Exerc. 2007, 39, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Caldirola, D.; Namia, C.; Micieli, W.; Carminati, C.; Bellodi, L.; Perna, G. Cardiorespiratory response to physical exercise and psychological variables in panic disorder. Braz. J. Psychiatry 2011, 33, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, A.; Freire, R.C.; Zin, W.A.; Nardi, A.E. Respiratory manifestations of panic disorder: Causes, consequences and therapeutic implications. J. Bras. Pneumol. 2009, 35, 698–708. [Google Scholar] [CrossRef]



| Author, Year | Sample | Number Size | Age, Years (Mean ± SD) | VO2 | Results and Cut-Off Point Values |
|---|---|---|---|---|---|
| Ferreira Reis et al. [25] | Adult patients with HFrEF | 442 (80% male) | 56.2 ± 12.5 | 17.9 ± 6.1 mL/kg.min−1 | COP had the highest predictive power of all parameters analyzed in submaximal CPET, with a value ≥36 achieving a sensitivity of 100% and a specificity of 89% for the primary outcome (death and heart transplantation). Mean 29.6 ± 7.4—>36 (Worse prognostic) 20% reduction in survival at 30 months of follow-up |
| Ramos et al. [18] | Healthy (18%) or with chronic disease (81%) | 3331 | 58 ± 11.1 | 23.9 ± 10.1 mL/kg.min−1 | The all-cause mortality rate increased from 1.4% for COP < 22 to 17.1% in subjects with COP > 30. That is, COP is a good predictor of all-cause mortality, as it indicates that individuals with COP > 30 exhibited approximately six times greater risk of mortality than those with COP < 22. The combination of high COP (>30) + low VO2max showed high mortality (30.9%) and low COP (<22) + VO2max mortality was only 0.5%. COP: 22–30 HR 2.15 [95% CI 1.15–4.03] >30—HR 3.72 [1.98–6.98] |
| Peterman et al. [24] | Healthy adults (46% females) | 3160 | 44.0 ± 12.5 | 32.8 ± 10.5 mL/kg.min−1 | The cardiorespiratory optimum was significantly lower in participants identified as alive compared to those who died at follow-up (24.3 ± 4.7 and 26.0 ± 5.4, respectively). In conclusion, COP is predictive of all-cause mortality in men, regardless of traditional risk factors including VO2. For women, however, COP is unrelated to mortality after adjusting for possible confounders. Mean 24.6 ± 4.9; Survivor 24.3 ± 4.7; Deceased 26.0 ± 5.4 |
| Laukkanen et al. [29] | Men Participants of the Kuopio Ischaemic Heart Disease risk factor study was used for the current analysis | 2.190 | 52.8 ± 5. 1 | 2427 ± 623 mL/min | In age-adjusted analysis, the HR (95% CI) per 1 SD increase in COP for SCD was 5.02 (2.85–8.85), which was attenuated to 2.51 (1.36–4.62) after further adjustment for the conventional risk factors (smoking status, history of type 2 diabetes, systolic blood pressure, total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein cholesterol, body mass index, fasting plasma glucose, alcohol consumption, prevalent coronary heart disease, family history of coronary heart disease, use of cholesterol medication, total physical activity and socioeconomic status. Mean: 23.3 ± 4.5; COP adjusted for age: <24.6 = HR 1.30 [0.92–1.84] (p = 0.14) >24.7 = HR 2.11 [1.52–2.94] (p < 0.001) COPD adjusted for other risk factors <24.6 = HR 1.23 [0.87–1.75] (p = 0.24) >24.7 = HR 1.55 [1.10–2.19] (p < 0.001) |
| Laukkanen et al. [23] | Participants of the Kuopio Ischaemic Heart Disease | 2.205 | 52.8 ± 5.2 | 30.5 ± 7.9 mL/kg.min−1 | During a median follow-up of 28.8 years, there were 402 CHD deaths, and risk of CHD mortality increased continuously with increasing COP from 26. During the follow-up period, there were 607 CVD deaths and the risk of CVD mortality increased continuously with increasing COP from 25. A total of 1348 all-cause deaths occurred during the follow-up period, and the risk of all-cause mortality increased continuously with increasing COP from 25 onwards. all causes in dose–response modes. COP during exercise may improve long-term risk prediction for CVD mortality. Mean 23.3; COP to all-cause mortality 1 SD increase HR 3.46 [2.66–4.51] Quintile (23.97–26.30): HR 1.35 [1.12–1.61]; Quintile (>26.30) HR 1.99 [1.67–2.37] |
| Kroesen et al. [22] | HF patients | 277 | 67 [58–74] Years | 13.9 [11.0–17.5] mL/kg.min−1 | Median COP was 28.2 [24.9–32.1] and was reached at 51 ± 15% of VO2peak. Lower age, female sex, higher body mass index, the absence of a pacemaker or the absence of chronic obstructive pulmonary disease and lower NT-pro-BNP concentrations were associated with a lower COP. Participation in cardiac rehabilitation (CR) reduced COP (−0.8, 95% confidence interval (CI): −1.3; −0.3). Low COP had a reduced risk (adjusted hazard ratio 0.53, 95%CI 0.33; 0.84) for adverse clinical outcomes as compared to high COP. The low COP group (18.0–26.0) had a 0.53 times risk (95% CI: 0.34; 0.85) of clinical outcomes compared to the high COP group (30.7–57.7). HRs of the moderate COP group (26.0–30.7) did not differ from the high COP group |
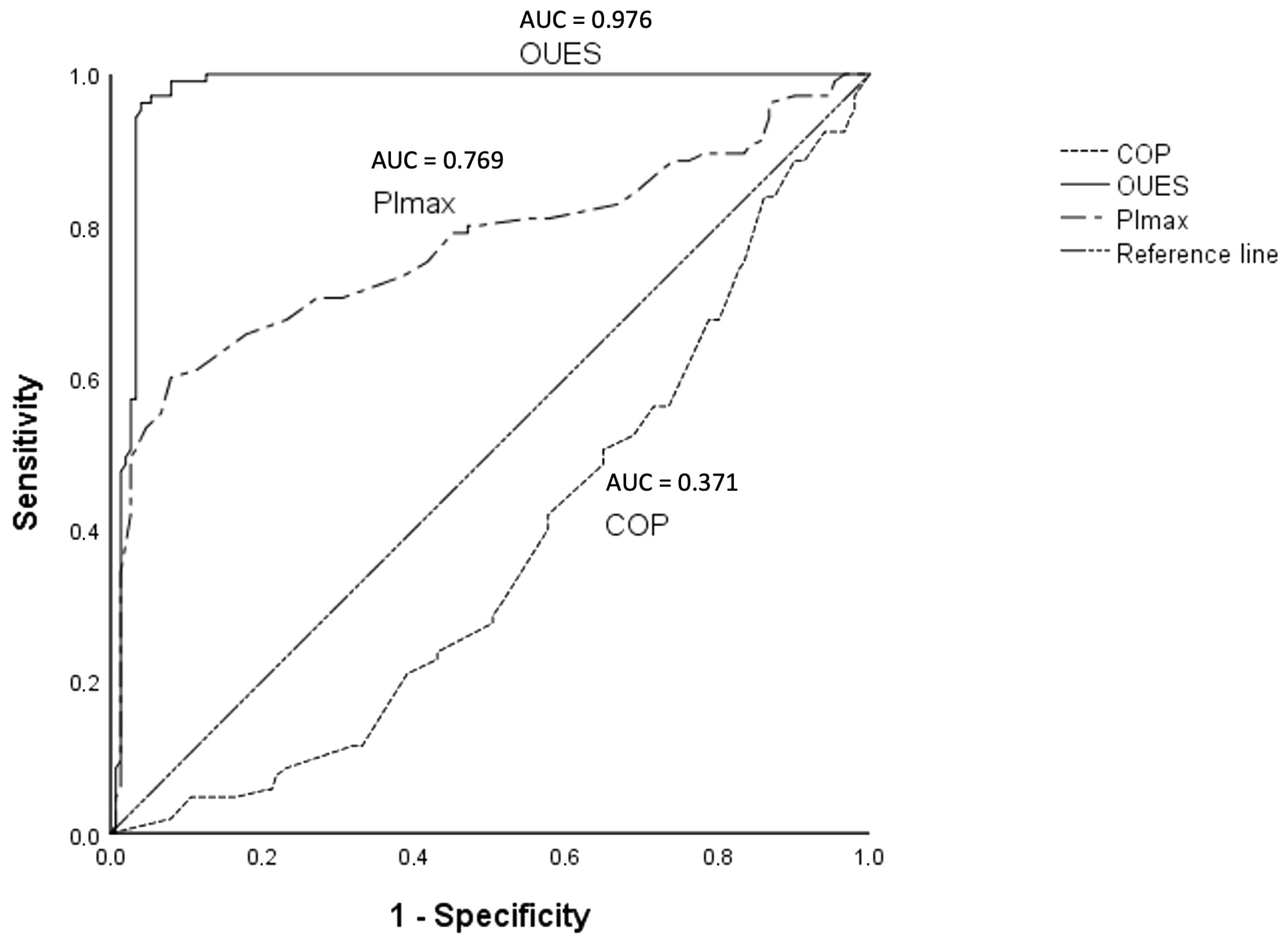
| Variables | AUC |
|---|---|
| COP | 0.371 |
| V′O2p | 0.859 |
| PImax | 0.769 |
| V′E-V′CO2 slope | 0.386 |
| 6 MWT | 0.731 |
| OUES | 0.976 |
| Circulatory power | 0.511 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, W.A.; Sá Filho, A.S.; Ramos, P.S.; Chiappa, A.M.G.; Aprigliano, V.; Oliveira-Silva, I.; Cunha, R.M.; Fajemiroye, J.O.; Vieira, R.P.; Ferrari, G.; et al. Exploring the Clinical Utility of Cardiorespiratory Optimal Point in Heart Failure Patients: Creating a New Research Gap. Appl. Sci. 2025, 15, 3495. https://doi.org/10.3390/app15073495
Silva WA, Sá Filho AS, Ramos PS, Chiappa AMG, Aprigliano V, Oliveira-Silva I, Cunha RM, Fajemiroye JO, Vieira RP, Ferrari G, et al. Exploring the Clinical Utility of Cardiorespiratory Optimal Point in Heart Failure Patients: Creating a New Research Gap. Applied Sciences. 2025; 15(7):3495. https://doi.org/10.3390/app15073495
Chicago/Turabian StyleSilva, Weder A., Alberto Souza Sá Filho, Plinio S. Ramos, Adriana M. Güntzel Chiappa, Vicente Aprigliano, Iransé Oliveira-Silva, Raphael Martins Cunha, James Oluwagbamigbe Fajemiroye, Rodolfo P. Vieira, Gerson Ferrari, and et al. 2025. "Exploring the Clinical Utility of Cardiorespiratory Optimal Point in Heart Failure Patients: Creating a New Research Gap" Applied Sciences 15, no. 7: 3495. https://doi.org/10.3390/app15073495
APA StyleSilva, W. A., Sá Filho, A. S., Ramos, P. S., Chiappa, A. M. G., Aprigliano, V., Oliveira-Silva, I., Cunha, R. M., Fajemiroye, J. O., Vieira, R. P., Ferrari, G., & Chiappa, G. R. (2025). Exploring the Clinical Utility of Cardiorespiratory Optimal Point in Heart Failure Patients: Creating a New Research Gap. Applied Sciences, 15(7), 3495. https://doi.org/10.3390/app15073495










