Stingers—A Review of Current Understanding and Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology
4. Mechanism of Injury
5. Grading
6. Clinical Presentation
7. On-Field Evaluation and Management
7.1. Basic Support
7.2. Immobilization Protocols
7.3. Hospital Transfer Criteria and Further Care
8. Imaging
9. Electrodiagnostic Studies
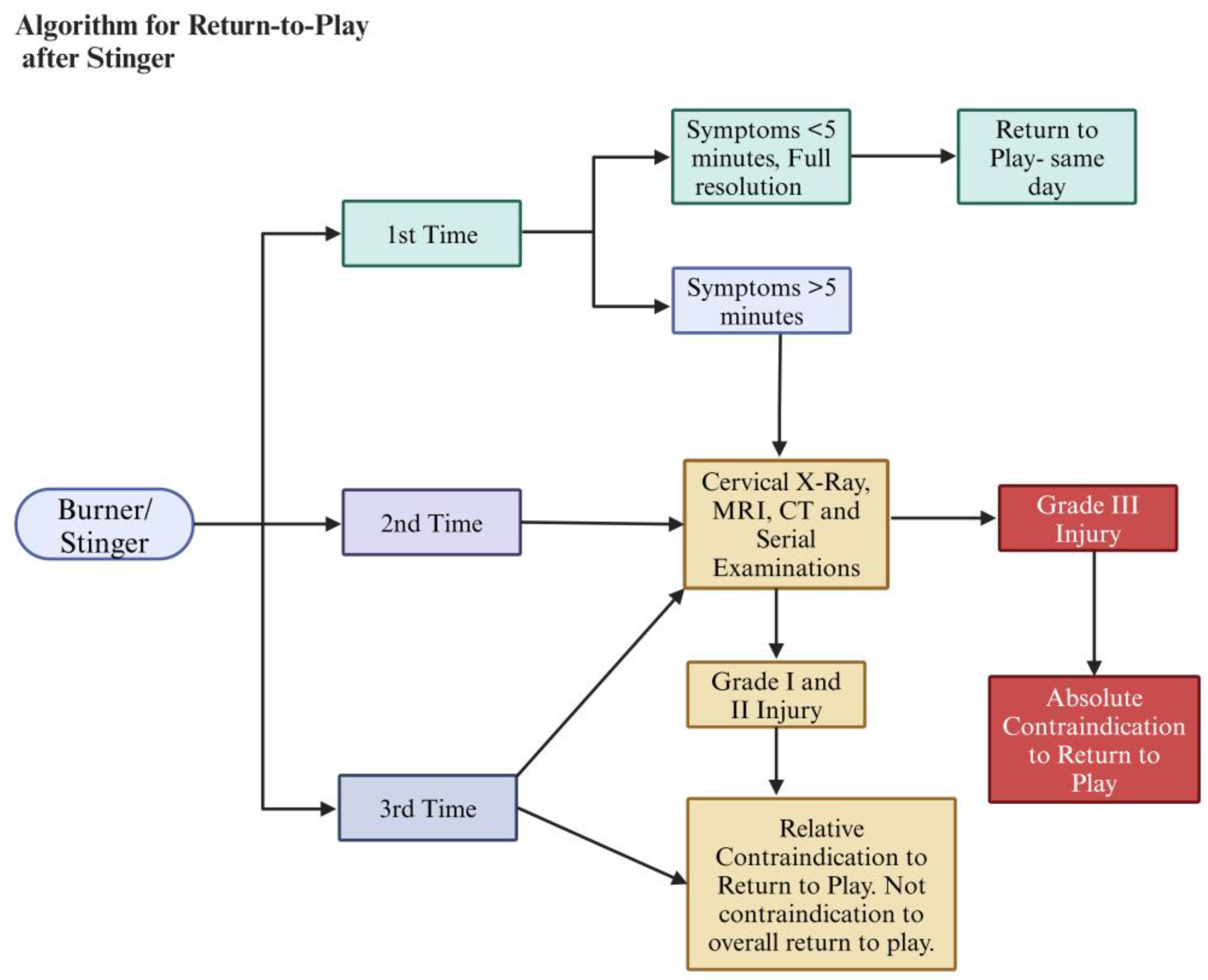
10. Return-to-Play Protocol
11. Treatment
12. Conclusions
13. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, J.; Zuckerman, S.L.; Dalton, S.L.; Djoko, A.; Folger, D.; Kerr, Z.Y. A 6-year surveillance study of “Stingers” in NCAA American Football. Res. Sports Med. 2017, 25, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Ota, C.; Yoneda, T.; Maki, N.; Urayama, S.; Nagao, M.; Nagayama, M.; Kaketa, T.; Takazawa, Y.; Kaneko, K. Incidence of Stingers in Young Rugby Players. Am. J. Sports Med. 2015, 43, 2809–2815. [Google Scholar] [CrossRef]
- Thomas, B.E.; McCullen, G.M.; Yuan, H.A. Cervical spine injuries in football players. J. Am. Acad. Orthop. Surg. 1999, 7, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.D.; Vaccaro, A.R. Cervical Spine Injuries in the Athlete. J. Am. Acad. Orthop. Surg. 2016, 24, e122–e133. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, B.M.; Starr, H.M.; Seiler, J.G. Traumatic Brachial Plexopathy in Athletes: Current Concepts for Diagnosis and Management of Stingers. J. Am. Acad. Orthop. Surg. 2019, 27, 677–684. [Google Scholar] [CrossRef]
- Bowles, D.R.; Canseco, J.A.; Alexander, T.D.; Schroeder, G.D.; Hecht, A.C.; Vaccaro, A.R. The Prevalence and Management of Stingers in College and Professional Collision Athletes. Curr. Rev. Musculoskelet. Med. 2020, 13, 651–662. [Google Scholar] [CrossRef]
- Torg, J.S. Cervical spine injuries and the return to football. Sports Health 2009, 1, 376–383. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Klein, G.R.; Ciccoti, M.; Pfaff, W.L.; Moulton, M.J.; Hilibrand, A.J.; Watkins, B. Return to play criteria for the athlete with cervical spine injuries resulting in stinger and transient quadriplegia/paresis. Spine J. 2002, 2, 351–356. [Google Scholar] [CrossRef]
- Kazarian, G.S.; Qureshi, S. Return to Play After Injuries to the Cervical Spine. Clin. Spine Surg. 2024, 37, 425–432. [Google Scholar] [CrossRef]
- Schroeder, G.D.; Canseco, J.A.; Patel, P.D.; Hilibrand, A.S.; Kepler, C.K.; Mirkovic, S.M.; Watkins, R.G.I.; Dossett, A.; Hecht, A.C.; Vaccaro, A.R. Updated Return-to-Play Recommendations for Collision Athletes After Cervical Spine Injury: A Modified Delphi Consensus Study With the Cervical Spine Research Society. Neurosurgery 2020, 87, 647–654. [Google Scholar] [CrossRef]
- Papadopoulos, E. Brachial Plexus Anatomy A comprehensive Overview. Int. J. Anat. Var. 2024, 17, 669–670. [Google Scholar]
- Tosti, R.; Rossy, W.; Sanchez, A.; Lee, S.G. Burners, stingers, and other brachial plexus injuries in the contact athlete. Oper. Tech. Sports Med. 2016, 24, 273–277. [Google Scholar]
- Adele, M.; Christopher, M.; Scott, R.L.; Dustin, C.; Comstock, R.D. Epidemiology of Cervical Spine Injuries in High School Athletes Over a Ten-Year Period. Pm&R. 2018, 10, 365–372. [Google Scholar] [CrossRef]
- Deckey, D.G.; Makovicka, J.L.; Chung, A.S.; Hassebrock, J.D.; Patel, K.A.; Tummala, S.V.; Pena, A.; Asprey, W.; Chhabra, A. Neck and Cervical Spine Injuries in National College Athletic Association Athletes: A 5-Year Epidemiologic Study. Spine 2020, 45, 55–64. [Google Scholar] [CrossRef]
- DePasse, J.M.; Durand, W.; Palumbo, M.A.; Daniels, A.H. Sex- and Sport-Specific Epidemiology of Cervical Spine Injuries Sustained During Sporting Activities. World Neurosurg. 2019, 122, e540–e545. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu-Jones, B.R.; Rossy, W.H.; Sanchez, G.; Whalen, J.M.; Lavery, K.P.; McHale, K.J.; Vopat, B.G.; Van Allen, J.J.; Akamefula, R.A.; Provencher, M.T. Epidemiology of Injuries Identified at the NFL Scouting Combine and Their Impact on Performance in the National Football League: Evaluation of 2203 Athletes From 2009 to 2015. Orthop. J. Sports Med. 2017, 5, 2325967117708744. [Google Scholar] [CrossRef]
- Starr, H.M., Jr.; Anderson, B.; Courson, R.; Seiler, J.G. Brachial plexus injury: A descriptive study of American football. J. Surg. Orthop. Adv. 2014, 23, 90–97. [Google Scholar] [CrossRef]
- Standaert, C.J.; Herring, S.A. Expert opinion and controversies in musculoskeletal and sports medicine: Stingers. Arch. Phys. Med. Rehabil. 2009, 90, 402–406. [Google Scholar] [PubMed]
- Presciutti, S.M.; DeLuca, P.; Marchetto, P.; Wilsey, J.T.; Shaffrey, C.; Vaccaro, A.R. Mean subaxial space available for the cord index as a novel method of measuring cervical spine geometry to predict the chronic stinger syndrome in American football players. J. Neurosurg. Spine 2009, 11, 264–271. [Google Scholar] [CrossRef]
- Meyer, S.A.; Schulte, K.R.; Callaghan, J.J.; Albright, J.P.; Powell, J.W.; Crowley, E.T.; el-Khoury, G.Y. Cervical spinal stenosis and stingers in collegiate football players. Am. J. Sports Med. 1994, 22, 158–166. [Google Scholar] [CrossRef]
- Kelly, J.D.t.; Aliquo, D.; Sitler, M.R.; Odgers, C.; Moyer, R.A. Association of burners with cervical canal and foraminal stenosis. Am. J. Sports Med. 2000, 28, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.L.; Di Benedetto, M.; Curl, W.W. Upper trunk brachial plexopathy. The stinger syndrome. Am. J. Sports Med. 1993, 21, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Torg, J.S.; Vegso, J.J.; O’Neill, M.J.; Sennett, B. The epidemiologic, pathologic, biomechanical, and cinematographic analysis of football-induced cervical spine trauma. Am. J. Sports Med. 1990, 18, 50–57. [Google Scholar] [CrossRef]
- Levitz, C.L.; Reilly, P.J.; Torg, J.S. The pathomechanics of chronic, recurrent cervical nerve root neurapraxia. The chronic burner syndrome. Am. J. Sports Med. 1997, 25, 73–76. [Google Scholar] [CrossRef]
- Greenberg, J.; Leung, D.; Kendall, J. Predicting chronic stinger syndrome using the mean subaxial space available for the cord index. Sports Health 2011, 3, 264–267. [Google Scholar] [CrossRef]
- Hakkaku, T.; Nakazato, K.; Koyama, K.; Kouzaki, K.; Hiranuma, K. Cervical Intervertebral Disc Degeneration and Low Cervical Extension Independently Associated With a History of Stinger Syndrome. Orthop. J. Sports Med. 2017, 5, 2325967117735830. [Google Scholar] [CrossRef]
- Feinberg, J.H. Burners and stingers. Phys. Med. Rehabil. Clin. N. Am. 2000, 11, 771–784. [Google Scholar]
- Yang, L.J.; Chang, K.W.; Chung, K.C. A systematic review of nerve transfer and nerve repair for the treatment of adult upper brachial plexus injury. Neurosurgery 2012, 71, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Birch, R.; Dunkerton, M.; Bonney, G.; Jamieson, A.M. Experience with the free vascularized ulnar nerve graft in repair of supraclavicular lesions of the brachial plexus. Clin. Orthop. Relat. Res. 1988, 237, 96–104. [Google Scholar]
- Bishop, A.T. Functioning free-muscle transfer for brachial plexus injury. Hand Clin. 2005, 21, 91–102. [Google Scholar] [CrossRef]
- Giuffre, J.L.; Kakar, S.; Bishop, A.T.; Spinner, R.J.; Shin, A.Y. Current concepts of the treatment of adult brachial plexus injuries. J. Hand Surg. Am. 2010, 35, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Htut, M.; Taggart, M.; Carlstedt, T.; Birch, R. The effects of operative delay on the relief of neuropathic pain after injury to the brachial plexus: A review of 148 cases. J. Bone Joint Surg. Br. 2006, 88, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.B.; Dines, J.S. Transient brachial plexopathy (stingers/burners). In Spinal Conditions in the Athlete: A Clinical Guide to Evaluation, Management and Controversies; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 109–121. [Google Scholar]
- Bateman, J.E. Nerve Injuries about the Shoulder in Sports. JBJS 1967, 49, 785–792. [Google Scholar]
- Weinstein, S.M. Assessment and rehabilitation of the athlete with a “stinger”: A model for the management of noncatastrophic athletic cervical spine injury. Clin. Sports Med. 1998, 17, 127–135. [Google Scholar]
- Cantu, R.C.; Li, Y.M.; Abdulhamid, M.; Chin, L.S. Return to play after cervical spine injury in sports. Curr. Sports Med. Rep. 2013, 12, 14–17. [Google Scholar] [CrossRef]
- Charbonneau, R.M.E.; McVeigh, S.A.; Thompson, K. Brachial Neuropraxia in Canadian Atlantic University Sport Football Players: What Is the Incidence of “Stingers”? Clin. J. Sport Med. 2012, 22, 472–477. [Google Scholar] [CrossRef]
- Ban, V.S.; Botros, J.A.; Madden, C.J.; Batjer, H.H. Neurosurgical Emergencies in Sports Neurology. Curr. Pain. Headache Rep. 2016, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Guterman, A.; Smith, R.W. Neurological sequelae of boxing. Sports Med. 1987, 4, 194–210. [Google Scholar] [CrossRef]
- Weinstein, S.M.; Cantu, R.C. Cerebral stroke in a semi-pro football player: A case report. Med. Sci. Sports Exerc. 1991, 23, 1119–1121. [Google Scholar]
- Blatz, D.; Ross, B.; Dadabo, J. Cervical spine trauma evaluation. Handb. Clin. Neurol. 2018, 158, 345–351. [Google Scholar]
- Bailes, J.E.; Petschauer, M.; Guskiewicz, K.M.; Marano, G. Management of cervical spine injuries in athletes. J. Athl. Train. 2007, 42, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Palumbo, M.A.; Fadale, P.D. Catastrophic cervical spine injuries in the collision sport athlete, part 1: Epidemiology, functional anatomy, and diagnosis. Am. J. Sports Med. 2004, 32, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D.; Divi, S.N.; Canseco, J.A.; Donnally, C.J., 3rd; Galetta, M.; Vaccaro, A., Jr.; Schroeder, G.D.; Hsu, W.K.; Hecht, A.C.; Dossett, A.B.; et al. Management of Acute Subaxial Trauma and Spinal Cord Injury in Professional Collision Athletes. Clin. Spine Surg. 2022, 35, 241–248. [Google Scholar] [CrossRef]
- Swartz, E.E.; Boden, B.P.; Courson, R.W.; Decoster, L.C.; Horodyski, M.B.; Norkus, S.A.; Rehberg, R.S.; Waninger, K.N. National Athletic Trainers’ Association position statement: Acute management of the cervical spine–injured athlete. J. Athl. Train. 2009, 44, 306–331. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Chaplin, T.; Zelt, D. Management of Major Vascular Injuries: Neck, Extremities, and Other Things that Bleed. Emerg. Med. Clin. N. Am. 2018, 36, 181–202. [Google Scholar] [CrossRef]
- Torg, J.S.; Naranja, R.J., Jr.; Pavlov, H.; Galinat, B.J.; Warren, R.; Stine, R.A. The relationship of developmental narrowing of the cervical spinal canal to reversible and irreversible injury of the cervical spinal cord in football players. J. Bone Joint Surg. Am. 1996, 78, 1308–1314. [Google Scholar] [CrossRef]
- Zaremski, J.L.; Horodyski, M.; Herman, D.C. Recurrent stingers in an adolescent American football player: Dilemmas of return to play. A case report and review of the literature. Res. Sports Med. 2017, 25, 384–390. [Google Scholar] [CrossRef]
- Herzog, R.J.; Wiens, J.J.; Dillingham, M.F.; Sontag, M.J. Normal Cervical Spine Morphometry and Cervical Spinal Stenosis in Asymptomatic Professional Football Players: Plain Film Radiography, Multiplanar Computed Tomography, and Magnetic Resonance Imaging. Spine 1991, 16, S178–S186. [Google Scholar] [CrossRef]
- Garg, R.; Merrell, G.A.; Hillstrom, H.J.; Wolfe, S.W. Comparison of nerve transfers and nerve grafting for traumatic upper plexus palsy: A systematic review and analysis. JBJS 2011, 93, 819–829. [Google Scholar] [CrossRef]
- Epstein, N.E. Major risks and complications of cervical epidural steroid injections: An updated review. Surg. Neurol. Int. 2018, 9, 86. [Google Scholar] [CrossRef]
- Gorden, J.A.; Straub, S.J.; Swanik, C.B.; Swanik, K.A. Effects of Football Collars on Cervical Hyperextension and Lateral Flexion. J. Athl. Train. 2003, 38, 209–215. [Google Scholar] [PubMed]

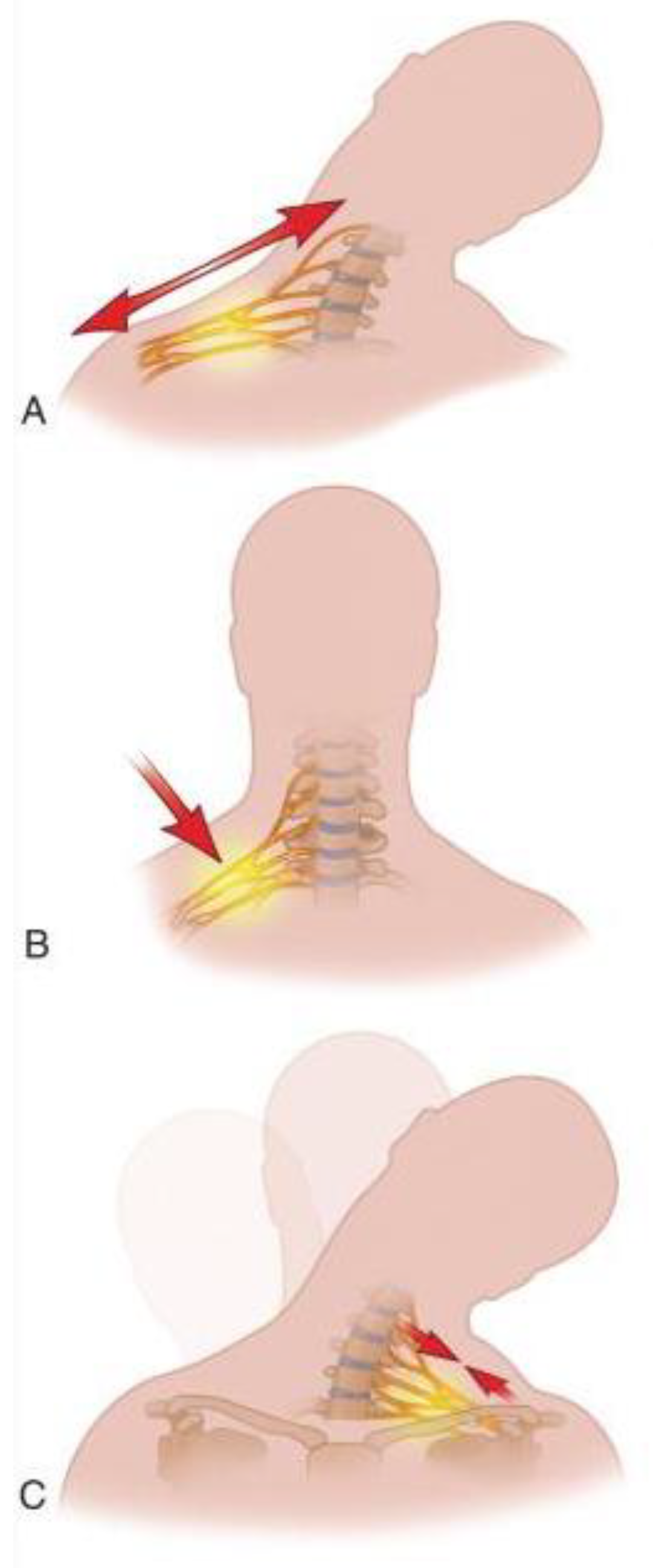


| Grade | Nerve Injury | Mode of Treatment |
| I | Neurapraxia | Usually transient with no need for any intervention. Pain control, rest, and physiotherapy as needed if symptoms persist. Consider MRI and other imaging with serial examinations if symptoms persist for >5 min. |
| II | Axonotmesis | Pain control, rest and physiotherapy, serial monitoring with electrodiagnostic studies, and imaging (MRI, radiographs). |
| IIIA | Neurotmesis | Imaging, electrodiagnostic studies, nerve repair ± nerve transfer [28,29,30,31], pain control, and physiotherapy. |
| IIIB | Nerve root avulsion | Imaging, electrodiagnostic studies, nerve transfer [28,29,30,31], pain control, and physiotherapy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebeyehu, T.F.; Harrop, J.S.; Dian, J.A.; Matsoukas, S.; Vaccaro, A.R. Stingers—A Review of Current Understanding and Management. Appl. Sci. 2025, 15, 3510. https://doi.org/10.3390/app15073510
Gebeyehu TF, Harrop JS, Dian JA, Matsoukas S, Vaccaro AR. Stingers—A Review of Current Understanding and Management. Applied Sciences. 2025; 15(7):3510. https://doi.org/10.3390/app15073510
Chicago/Turabian StyleGebeyehu, Teleale F., James S. Harrop, Joshua A. Dian, Stavros Matsoukas, and Alexander R. Vaccaro. 2025. "Stingers—A Review of Current Understanding and Management" Applied Sciences 15, no. 7: 3510. https://doi.org/10.3390/app15073510
APA StyleGebeyehu, T. F., Harrop, J. S., Dian, J. A., Matsoukas, S., & Vaccaro, A. R. (2025). Stingers—A Review of Current Understanding and Management. Applied Sciences, 15(7), 3510. https://doi.org/10.3390/app15073510






