The Impact of Pulsed Electric Field Treatment of Beetroots on the Physicochemical Properties of Juice, Dried Juice, and Dried Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.1.1. Materials
2.1.2. PEF Pretreatment
2.1.3. Juice Preparation
2.1.4. Spray Drying
2.1.5. Pomace Lyophilization
2.2. Analytical Methods
2.2.1. Dry Matter Content
2.2.2. Water Activity
2.2.3. Sugar Content
2.2.4. Color
2.2.5. Polyphenol Content
2.2.6. Betalain Content
2.2.7. Antioxidant Activity
2.2.8. Powder Morphology
2.2.9. Powder Particle Size
2.2.10. Powder Hygroscopicity
2.2.11. Powder Density and Cohesiveness
2.3. Statistical Analysis
3. Results and Discussion
3.1. Beetroots
3.2. Juice
3.2.1. Water Content in Juice
3.2.2. Sugar Content in Juice
3.2.3. Color in Juice
3.2.4. Phenolic Content in Juice
3.2.5. Content of Betalain Pigments in Juice
3.2.6. Antioxidant Activity in Juice
3.3. Dried Juice and Pomace
3.3.1. Powder Recovery of Dried Juice and Pomace
3.3.2. Morphology and Particle Size of Dried Juice and Pomace
3.3.3. Water Content and Water Activity of Dried Juice and Pomace
3.3.4. Hygroscopicity of Dried Juice and Pomace
3.3.5. Density and Cohesiveness of Dried Juice and Pomace
3.3.6. Color of Dried Juice and Pomace
3.3.7. Polyphenol Content in Dried Juice and Pomace
3.3.8. Content of Betalain Pigments in Dried Juice and Pomace
3.3.9. Antioxidant Activity in Dried Juice and Pomace
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raso, J.; Heinz, V.; Alvarez, I.; Toepfl, S. Pulsed Electric Fields Technology for the Food Industry; Springer International Publishing: Cham, Swizterland, 2022. [Google Scholar]
- Koubaa, M.; Roselló-Soto, E.; Žlabur, J.Š.; Jambrak, A.R.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Rosa, M.D. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar]
- Dellarosa, N.; Tappi, S.; Ragni, L.; Laghi, L.; Rocculi, P.; Dalla Rosa, M. Metabolic response of fresh-cut apples induced by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2016, 38, 356–364. [Google Scholar]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; Bekhit, A.E.-D.; Liu, Z.-W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar]
- Ranjha, M.M.A.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A critical review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Morotomi-Yano, K.; Akiyama, H.; Yano, K.I. Different involvement of extracellular calcium in two modes of cell death induced by nanosecond pulsed electric fields. Arch. Biochem. Biophys. 2014, 555, 47–54. [Google Scholar]
- Kim, Y.N.; Kwon, H.J.; Lee, D.U. Effects of pulsed electric field (PEF) treatment on physicochemical properties of Panax ginseng. Innovat Food Sci. Emerg. Tech. 2019, 58, 102232. [Google Scholar]
- Mok, J.H.; Choi, W.; Park, S.H.; Lee, S.H.; Jun, S. Emerging pulsed electric field (PEF) and static magnetic field (SMF) combination technology for food freezing. Int. J. Refrig. 2015, 50, 137–145. [Google Scholar]
- Ben Ammar, J.; Lanoisellé, J.L.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Effect of a Pulsed Electric Field and Osmotic Treatment on Freezing of Potato Tissue. Food Biophys. 2010, 5, 247–254. [Google Scholar]
- Liu, C.; Pirozzi, A.; Ferrari, G.; Vorobiev, E.; Grimim, N. Effects of Pulsed Electric Fields on Vacuum Drying and Quality Characteristics of Dried Carrot. Food Bioprocess. Tech. 2020, 13, 45–52. [Google Scholar]
- Giancaterino, M.; Werl, C.; Jaeger, H. Evaluation of the quality and stability of freeze-dried fruits and vegetables pre-treated by pulsed electric fields (PEF). LWT 2024, 191, 115651. [Google Scholar]
- Liu, C.; Grimi, N.; Lebovka, N.; Vorobiev, E. Effects of pulsed electric fields treatment on vacuum drying of potato tissue. LWT 2018, 95, 289–294. [Google Scholar] [CrossRef]
- Yang, N.; Huang, K.; Lyu, C.; Wang, J. Pulsed electric field technology in the manufacturing processes of wine, beer, and rice wine: A review. Food Control 2016, 61, 28–38. [Google Scholar]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. Effect of Pulsed Electric Field Pre-Treatment and the Freezing Methods on the Kinetics of the Freeze-Drying Process of Apple and Its Selected Physical Properties. Foods 2022, 11, 2407. [Google Scholar] [CrossRef]
- Samborska, K.; Barańska, A.; Szulc, K.; Jankowska, E.; Truszkowska, M.; Ostrowska-Ligęza, E.; Wołosiak, R.; Szymańska, E.; Jedlińska, A. Reformulation of spray-dried apple concentrate and honey for the enhancement of drying process performance and the physicochemical properties of powders. J. Sci. Food Agric. 2020, 100, 2224–2235. [Google Scholar]
- Ostrowska-Ligęza, E.; Szulc, K.; Jakubczyk, E.; Dolatowska-Żebrowska, K.; Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A. The Influence of a Chocolate Coating on the State Diagrams and Thermal Behaviour of Freeze-Dried Strawberries. Appl. Sci. 2022, 12, 1342. [Google Scholar] [CrossRef]
- Fonseca, M.T.; Vital, A.C.; Silva, M.B.; Monteiro, S.S.; Nascimento, A.; Trindade, A.P.; Lisboa, H.M.; Pasquali, M.B. Improving the stability of spray-dried probiotic acerola juice: A study on hydrocolloids’ efficacy and process variables. Food Bioprod. Process. 2024, 147, 209–218. [Google Scholar]
- Fouskaki, M.; Karametsi, K.; Chaniotakis, N.A. Method for the determination of water content in sultana raisins using a water activity probe. Food Chem. 2003, 82, 133–137. [Google Scholar]
- Ignaczak, A.; Woźniak, Ł.; Salamon, A.; Szczepańska-Stolarczyk, J.; Trych, U.; Chobot, M.; Kowalska, J.; Kowalska, H. Shaping the Physicochemical and Health-Promoting Properties of Carrot Snacks Produced by Microwave-Vacuum Drying with Preliminary Thermal and Enriching Treatment. Molecules 2024, 29, 5100. [Google Scholar] [CrossRef] [PubMed]
- Peñaranda, I.; Garrido, M.D. Viability of fructooligosaccharides as substitutes for methylcellulose reduction in plant-based burgers. Food Hydrocoll. 2024, 154, 110104. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: New York, NY, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Arslan, D.; Özcan, M.M. Dehydration of red bell-pepper (Capsicum annuum L.): Change in drying behavior, colour and antioxidant content. Food Bioprod. Process 2011, 89, 504–513. [Google Scholar]
- Čakarević, J.; Šeregelj, V.; Šaponjac, V.T.; Ćetković, G.; Brunet, J.Č.; Popović, S.; Kostić, M.H.; Popović, L. Encapsulation of beetroot juice: A study on the application of pumpkin oil cake protein as new carrier agent. J. Microencapsul. 2020, 37, 121–133. [Google Scholar]
- Niemira, J.; Galus, S. Valorization of Red Beetroot (Beta vulgaris L.) Pomace Combined with Golden Linseed (Lini semen) for the Development of Vegetable Crispbreads as Gluten-Free Snacks Rich in Bioactive Compounds. Molecules 2024, 29, 2105. [Google Scholar] [CrossRef]
- Khatun, M.; Khatun, Z.; Karim, M.R.; Habib, M.R.; Rahman, M.H.; Aziz, M.A. Green synthesis of silver nanoparticles using extracts of Mikania cordata leaves and evaluation of their antioxidant, antimicrobial and cytotoxic properties. Food Chem. Adv. 2023, 3, 100386. [Google Scholar]
- Middleton, R.; Tunstad, S.A.; Knapp, A.; Winters, S.; Mccallum, S.; Whitney, H. Self-assembled, disordered structural color from fruit wax bloom. Sci. Adv. 2024, 10, eadk4219. [Google Scholar]
- Cichońska, P.; Domian, E.; Ziarno, M. Application of Optical and Rheological Techniques in Quality and Storage Assessment of the Newly Developed Colloidal-Suspension Products: Yogurt-Type Bean-Based Beverages. Sensors 2022, 22, 838. [Google Scholar] [CrossRef]
- Tan, S.L.; Sulaiman, R.; Rukayadi, Y.; Ramli, N.S. Physical, chemical, microbiological properties and shelf life kinetic of spray-dried cantaloupe juice powder during storage. LWT 2021, 140, 110597. [Google Scholar]
- Marinopoulou, A.; Zoumaki, M.; Raphaelides, S.; Karageorgiou, V.; Goulas, A. Characterization of Spray Dried Starch Systems of Natural Antioxidant Compounds. Starch-Stärke 2024, 76, 2300069. [Google Scholar]
- Kannan, V. Extraction of Bioactive Compounds from Whole Red Cabbage and Beetroot Using Pulsed Electric Fields and Evaluation of Their Functionality; University of Nebraska—Lincoln: Lincoln, NE, USA, 2011. [Google Scholar]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.V.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical-chemical characteristics of blended orange and carrot juice. LWT 2006, 39, 1163–1170. [Google Scholar]
- Bi, X.; Liu, F.; Rao, L.; Li, J.; Liu, B.; Liao, X.; Wu, J. Effects of electric field strength and pulse rise time on physicochemical and sensory properties of apple juice by pulsed electric field. Innov. Food Sci. Emerg. Techno. 2013, 17, 85–92. [Google Scholar]
- Vasconcellos, J.; Conte-Junior, C.; Silva, D.; Pierucci, A.P.; Paschoalin, V.; Alvares, T.S. Comparison of total antioxidant potential, and total phenolic, nitrate, sugar, and organic acid contents in beetroot juice, chips, powder, and cooked beetroot. Food Sci. Biotechnol. 2016, 25, 79–84. [Google Scholar]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Bulski, K.; Oziembłowski, M. Effect of pulsed electric field treatment on shelf life and nutritional value of apple juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar]
- Mannozzi, C.; Rompoonpol, K.; Fauster, T.; Tylewicz, U.; Romani, S.; Rosa, M.D.; Jaeger, H. Influence of pulsed electric field and ohmic heating pretreatments on enzyme and antioxidant activity of fruit and vegetable juices. Foods 2019, 8, 247. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems associated with spray drying of sugar-rich foods. Dry. Technol. 1997, 15, 671–684. [Google Scholar]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar]
- Barańska, A.; Świeca, M.; Samborska, K. Sour cherry juice concentrate powdered by high and low temperature spray drying with pea protein as a carrier—Physical properties, antioxidant activity and in vitro bioaccessibility. Dry. Technol. 2023, 41, 444–459. [Google Scholar]
- Samborska, K.; Edris, A.; Jedlińska, A.; Barańska, A. The production of white mulberry molasses powders with prebiotic carrier by dehumidified air-assisted spray drying. J. Food Process Eng. 2021, 45, e13928. [Google Scholar]
- Saguy, I.; Cohen, E. Effect of Water Activity and Moisture Content on the Stability of Beet Powder Pigments. J. Food Sci. 1983, 48, 703–707. [Google Scholar]
- Ng, M.L.; Sulaiman, R. Development of beetroot (Beta vulgaris) powder using foam mat drying. LWT 2018, 88, 80–86. [Google Scholar] [CrossRef]
- Sadowska, A.; Dybkowska, E.; Rakowska, R.; Hallmann, E.; Świderski, F. Ocena zawartości składników bioaktywnych i właściwości przeciwutleniających proszków wyprodukowanych metodą liofilizacji z wybranych surowców roślinnych. Żywn. Nauka Technol. Ja./Food. Sci. Technol. Nutr. 2017, 24, 59–75. [Google Scholar] [CrossRef]
- Gawałek, J.; Bartczak, P. Wpływ warunków suszenia rozpyłowego soku buraka cwikłowego na wybrane właściwości otrzymywanego proszku. Żywn. Nauka Technol. Ja./Food. Sci. Technol. Nutr. 2014, 21, 164–174. [Google Scholar]
- Bazaria, B.; Kumar, P. Effect of whey protein concentrate as drying aid and drying parameters on physicochemical and functional properties of spray dried beetroot juice concentrate. Food Biosci. 2016, 14, 21–27. [Google Scholar] [CrossRef]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The impact of pulsed electric field pretreatment of bell pepper on the selected properties of spray dried juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Mykhailyk, V.; Wiktor, A.; Toepfl, S.; Nowacka, M.; Bialik, M.; Czyżewski, J.; Witrowa-Rajchert, D.; Parniakov, O. Impact of pulsed electric fields on physical properties of freeze-dried apple tissue. Innov. Food Sci. Emerg. Technol. 2019, 57, 102211. [Google Scholar] [CrossRef]
- Janiszewska, E.; Wlodarczyk, J. Influence of spray drying conditions on beetroot pigments retention after microencapsulation process. Acta Agrophys. 2013, 20, 343–356. [Google Scholar]
- Ammelt, D.; Lammerskitten, A.; Wiktor, A.; Barba, F.J.; Toepfl, S.; Parniakov, O. The impact of pulsed electric fields on quality parameters of freeze-dried red beets and pineapples. Int. J. Food Sci. Technol. 2021, 56, 1777–1787. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Kidon, M.; Czapski, J. Wplyw obrobki termicznej na zawartosc barwnikow betalainowych i zdolnosc przeciwutleniajaca buraka cwiklowego. Żywn. Nauka Technol. Ja./Food. Sci. Technol. Nutr. 2007, 14, 124–131. [Google Scholar]

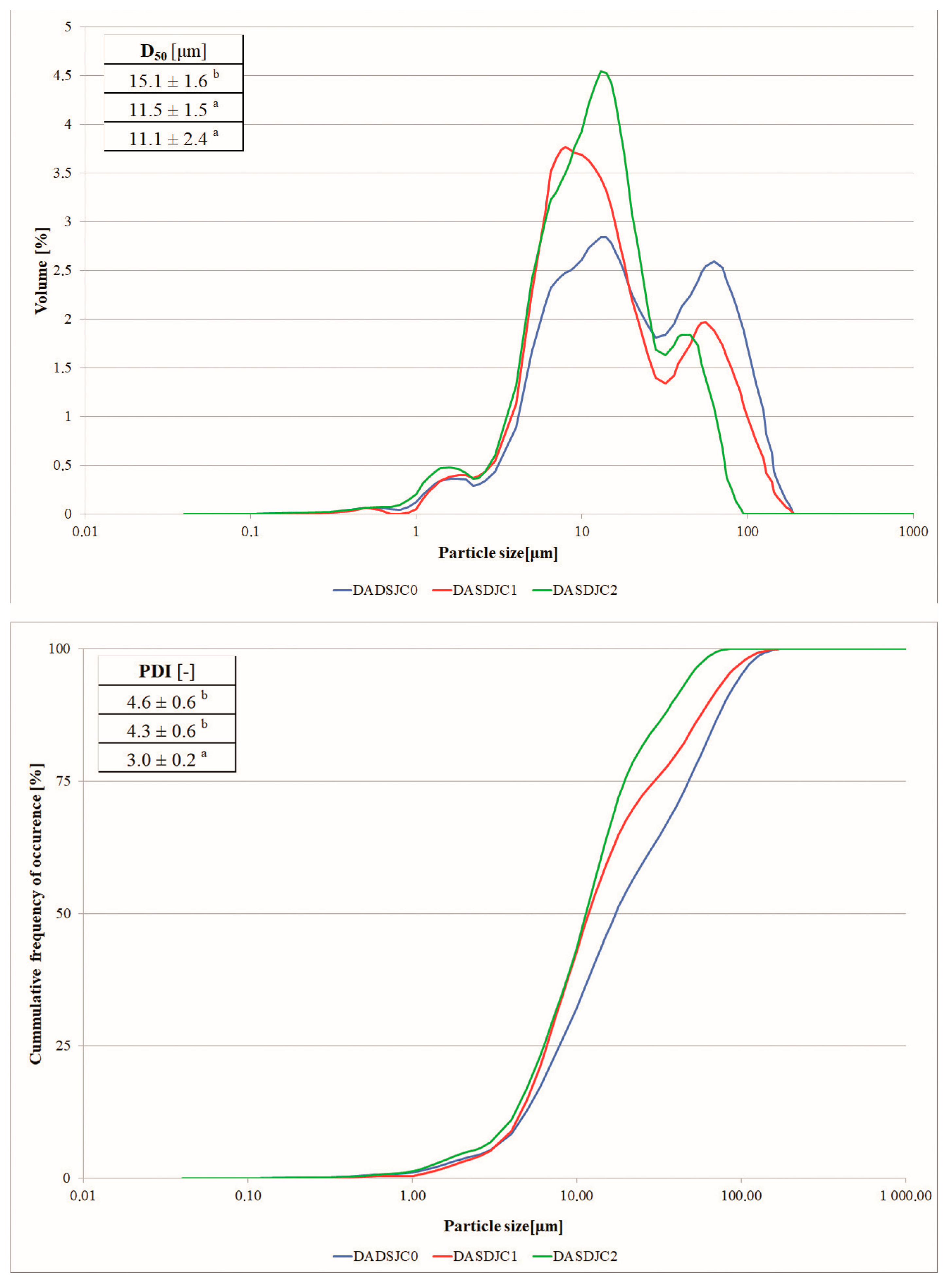
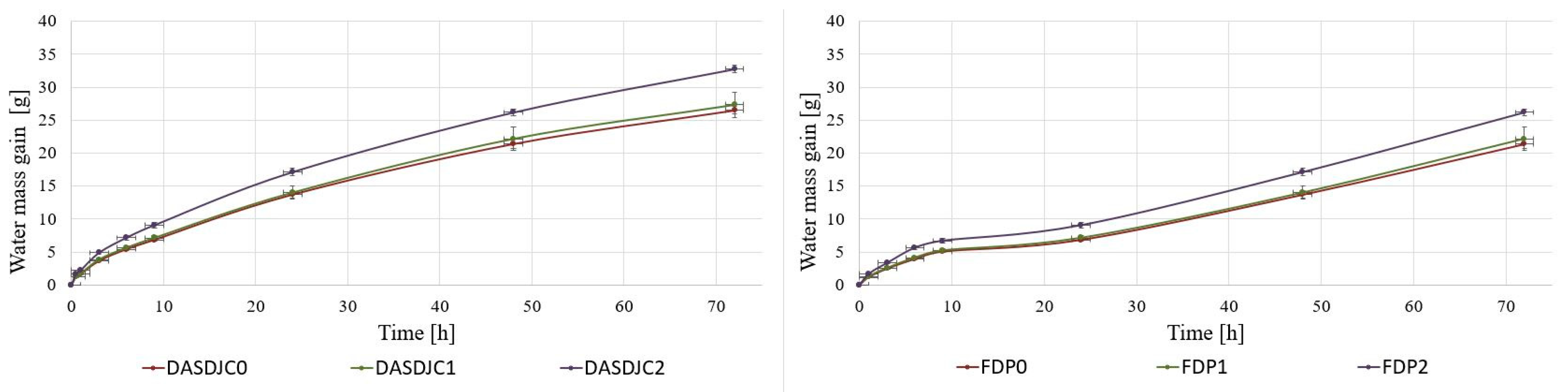
| Sample Name | Material | Number of Pulses | Electric Field Strength [kV/cm] | Total Energy [kJ/kg] | NUTRIOSE (Carrier Agent) |
|---|---|---|---|---|---|
| B0 | Beetroots | 0 | 0 | 0 | - |
| B1 | 3 | 1.07 | 0.5 | - | |
| B2 | 44 | 4 | - | ||
| J0 | Beetroot juice | 0 | 0 | 0 | - |
| J1 | 3 | 1.07 | 0.5 | - | |
| J2 | 44 | 4 | - | ||
| JC0 | Beetroot juice with a carrier | 0 | 0 | 0 | + |
| JC1 | 3 | 1.07 | 0.5 | + | |
| JC2 | 44 | 4 | + | ||
| DASDJC0 | Dehumidified air spray-dried beetroot juice with a carrier | 0 | 0 | 0 | + |
| DASDJC1 | 3 | 1.07 | 0.5 | + | |
| DASDJC2 | 44 | 4 | + | ||
| FDP0 | Freeze-dried pomace | 0 | 0 | 0 | - |
| FDP1 | 3 | 1.07 | 0.5 | - | |
| FDP2 | 44 | 4 | - |
| Sample Code | TPC [mg Chlorogenic Acid/100 g Solids] | BC [mg Betanin/100 g Solids | VC [mg Vulgaxanthin/100 g solids] | ABTS [mg Solids/100 mL] |
|---|---|---|---|---|
| B0 | 2547 ± 50 a | 778 ± 9 a | 261 ± 4 a | 14.1 ± 0.8 b |
| B1 | 2751 ± 12 b | 898 ± 7 b | 311 ± 3 b | 10.7 ± 0.4 a |
| B2 | 2645 ± 11 a,b | 848 ± 23 b | 361 ± 13 c | 12.1 ± 0.4 a,b |
| J0 | 958 ± 19 a | 2615 ± 83 a | 743 ± 21 a | 8.7 ± 0.6 c |
| J1 | 1030 ± 11 a | 2867 ± 37 b | 848 ± 20 b | 2.1 ± 0.3 b |
| J2 | 978 ± 21 a | 2783 ± 17 a,b | 878 ± 5 b | 5.2 ± 0.8 a |
| DASDJC0 | 2070 ± 43 b (288%) | 496 ± 3 b (25%) | 390 ± 6 b (70%) | 36.0 ± 18.0 a |
| DASDJC1 | 1479 ± 73 a (191%) | 418 ± 5 a (19%) | 292 ± 3 a (46%) | 36.1 ± 18.1 a |
| DASDJC2 | 1992 ± 82 b (272%) | 491 ± 5 b (24%) | 400 ± 8 b (61%) | 36.0 ± 16.7 a |
| FDP0 | 1532 ± 69 a | 542 ± 42 a | 241 ± 10 a | 26.2 ± 1.6 b |
| FDP1 | 1523 ± 42 a | 619 ± 12 a | 282 ± 5 b | 17.4 ± 1.3 a |
| FDP2 | 1705 ± 47 a | 576 ± 10 a | 245 ± 11 a,b | 21.6 ± 0.6 a,b |
| Sample Code | Water Content [%] | S [g/100 g Solids] | G [g/100 g Solids] | F [g/100 g Solids] | L* | a* | b* |
|---|---|---|---|---|---|---|---|
| J0 | 91.3 ± 0.5 a | 2.22 ± 0.04 b | 1.74 ± 0.02 b | 1.42 ± 0.02 a | 4.48 ± 0.12 c | 15.46 ± 0.18 c | 3.04 ± 0.12 b |
| J1 | 90.9 ± 0.3 a | 1.81 ± 0.03 a | 1.49 ± 0.05 a | 1.12 ± 0.16 a | 3.74 ± 0.07 b | 10.53 ± 0.42 b | 1.63 ± 0.19 a |
| J2 | 91.0 ± 0.4 a | 1.69 ± 0.04 a | 1.39 ± 0.03 a | 1.13 ± 0.01 a | 2.71 ± 0.08 a | 9.13 ± 0.28 a | 1.65 ± 0.16 a |
| Sample Code | MC [%] | L* | a* | b* | aw [-] | ρL [g/cm3] |
|---|---|---|---|---|---|---|
| DASDJC0 | 9.9 ± 0.4 a | 23.88 ± 1.09 b | 34.48 ± 1.29 a | 7.62 ± 0.11 a | 0.247 ± 0.017 a | 0.68 ± 0.04 a |
| DASDJC1 | 10.9 ± 0.7 a,b | 22.62 ± 0.75 a | 40.86 ± 0.79 b | 8.67 ± 0.12 b | 0.265 ± 0.003 b | 0.74 ± 0.01 b |
| DASDJC2 | 11.3 ± 0.8 b | 23.58 ± 0.39 a,b | 42.00 ± 0.19 c | 11.52 ± 0.21 c | 0.238 ± 0.002 a | 0.65 ± 0.02 a |
| FDP0 | 7.4 ± 1.6 a | 42.26 ± 0.48 a | 26.70 ± 0.18 a | 0.13 ± 0.03 b | 0.030 ± 0.002 a | 0.32 ± 0.01 b |
| FDP1 | 6.5 ± 0.9 a | 42.92 ± 0.59 a | 27.16 ± 0.21 b | −0.92 ± 0.20 a | 0.080 ± 0.002 c | 0.29 ± 0.00 a |
| FDP2 | 5.8 ± 0.7 a | 42.85 ± 0.67 a | 26.91 ± 0.19 a,b | 0.05 ± 0.11 b | 0.040 ± 0.002 b | 0.36 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedlińska, A.; Barańska-Dołomisiewicz, A.; Samborska, K.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. The Impact of Pulsed Electric Field Treatment of Beetroots on the Physicochemical Properties of Juice, Dried Juice, and Dried Pomace. Appl. Sci. 2025, 15, 3834. https://doi.org/10.3390/app15073834
Jedlińska A, Barańska-Dołomisiewicz A, Samborska K, Rybak K, Wiktor A, Witrowa-Rajchert D, Nowacka M. The Impact of Pulsed Electric Field Treatment of Beetroots on the Physicochemical Properties of Juice, Dried Juice, and Dried Pomace. Applied Sciences. 2025; 15(7):3834. https://doi.org/10.3390/app15073834
Chicago/Turabian StyleJedlińska, Aleksandra, Alicja Barańska-Dołomisiewicz, Katarzyna Samborska, Katarzyna Rybak, Artur Wiktor, Dorota Witrowa-Rajchert, and Małgorzata Nowacka. 2025. "The Impact of Pulsed Electric Field Treatment of Beetroots on the Physicochemical Properties of Juice, Dried Juice, and Dried Pomace" Applied Sciences 15, no. 7: 3834. https://doi.org/10.3390/app15073834
APA StyleJedlińska, A., Barańska-Dołomisiewicz, A., Samborska, K., Rybak, K., Wiktor, A., Witrowa-Rajchert, D., & Nowacka, M. (2025). The Impact of Pulsed Electric Field Treatment of Beetroots on the Physicochemical Properties of Juice, Dried Juice, and Dried Pomace. Applied Sciences, 15(7), 3834. https://doi.org/10.3390/app15073834










