Advances in Bio-Hydrogen Production: A Critical Review of Pyrolysis Gas Reforming
Abstract
:1. Introduction
2. Hydrogen Production by Using Pyrolysis Process
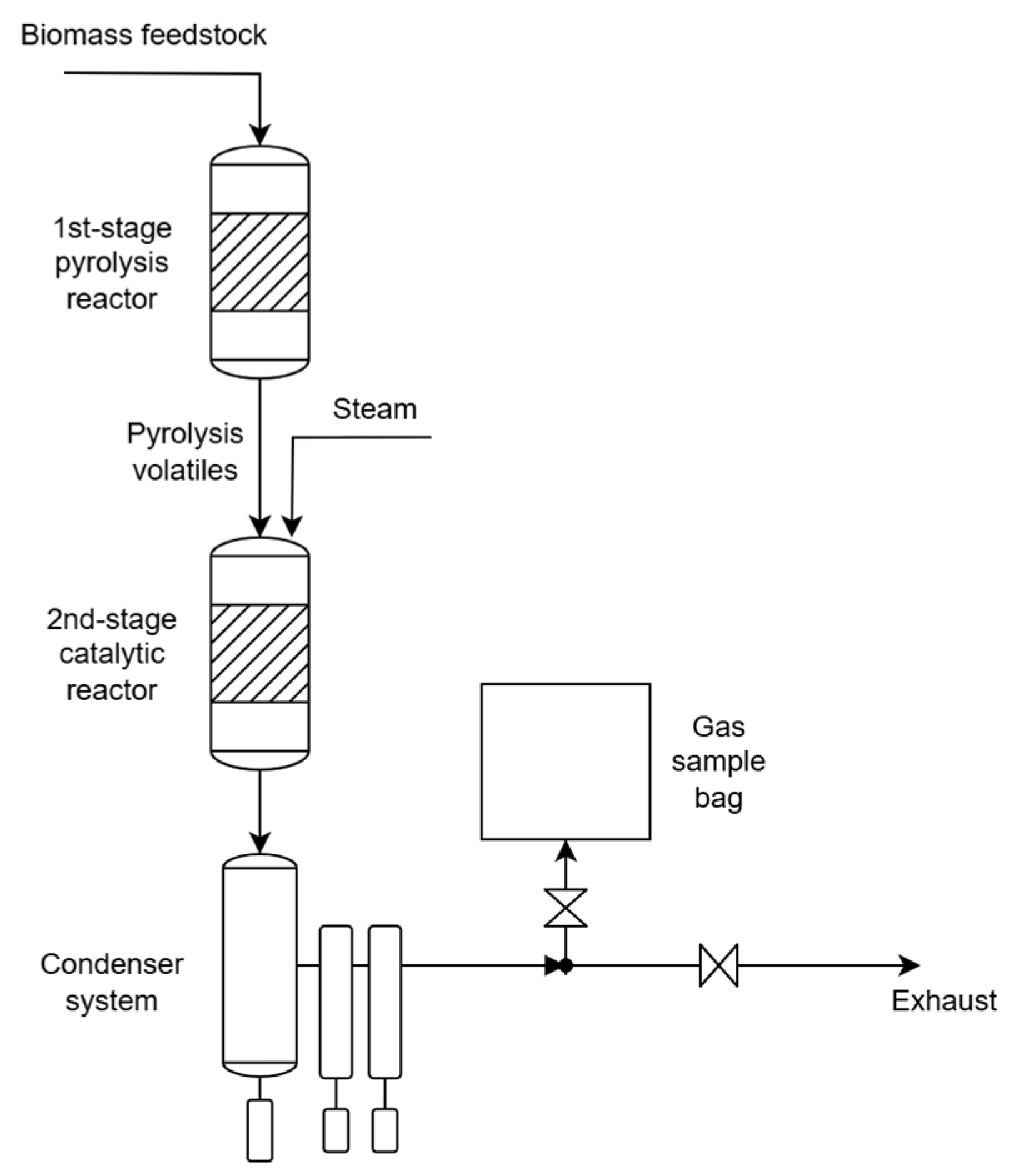
3. Hydrogen Production by Catalytic Reforming of Pyrolysis Volatiles
3.1. Slow Pyrolysis
3.1.1. Pyrolysis Temperature
3.1.2. Reforming Temperature
3.1.3. Steam-to-Biomass Ratio
3.1.4. Space Velocity
3.1.5. Catalyst Type
3.2. Intermediate and Fast Pyrolysis
4. Main Results
Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GHG | Greenhouse gas |
| PEM | Polymer electrolyte membrane |
| SOE | Solid oxide electrolyzer |
| TRL | Technology readiness level |
| HTL | Hydrothermal liquefaction |
| S/B | Steam to biomass |
| RDF | Refuse-derived fuel |
References
- BP. Energy Outlook 2020 Edition. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2020.pdf (accessed on 15 December 2024).
- Share of Primary Energy Consumption from Fossil Fuels. 2023. Available online: https://ourworldindata.org/grapher/fossil-fuels-share-energy (accessed on 3 September 2024).
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Ali, B.M.; Algburi, S.; Alzoubi, H.M.; Al-Jiboory, A.K.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. The Renewable Energy Role in the Global Energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Share of Primary Energy Consumption from Renewable Sources. 2023. Available online: https://ourworldindata.org/grapher/renewable-share-energy (accessed on 5 December 2024).
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Summary for Policymakers Edited by Science Officer Science Assistant Graphics Officer Working Group I Technical Support Unit; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Dahiru, A.R.; Vuokila, A.; Huuhtanen, M. Recent Development in Power-to-X: Part I—A Review on Techno-Economic Analysis. J. Energy Storage 2022, 56, 105861. [Google Scholar] [CrossRef]
- Superchi, F.; Mati, A.; Carcasci, C.; Bianchini, A. Techno-Economic Analysis of Wind-Powered Green Hydrogen Production to Facilitate the Decarbonization of Hard-to-Abate Sectors: A Case Study on Steelmaking. Appl. Energy 2023, 342, 121198. [Google Scholar] [CrossRef]
- Hydrogen. Available online: https://www.irena.org/Energy-Transition/Technology/Hydrogen (accessed on 6 September 2024).
- da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, Production and Characterization of the Main Process Worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Global Hydrogen Review 2024—Analysis—IEA. Available online: https://www.iea.org/reports/global-hydrogen-review-2024 (accessed on 6 January 2025).
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Gabriel, K.S.; El-Emam, R.S.; Zamfirescu, C. Technoeconomics of Large-Scale Clean Hydrogen Production—A Review. Int. J. Hydrogen Energy 2022, 47, 30788–30798. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Kuleshov, N.V.; Grigoriev, A.S.; Millet, P. Electrochemical Characterization of a High-Temperature Proton-Exchange Membrane Fuel Cell Using Doped-Poly Benzimidazole as Solid Polymer Electrolyte. J. Fuel Cell Sci. Technol. 2015, 12, 031004. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the Generation of Hydrogen, Oxygen and Synthesis Gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Ausfelder, F.; Bazzanella, A.M.; Wehrl, A. Roadmap Chemie 2050 Auf Dem Weg Zu Einer Treibhausgasneutralen Chemischen Industrie in Deutschland; FutureCamp Climate GmbH: Munich/Frankfurt, Germany, 2019; ISBN 9783897462236. [Google Scholar]
- Vidas, L.; Castro, R.; Cagno, E.; Soltani, M.; Pouresmaeil, E. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Buffi, M.; Prussi, M.; Scarlat, N. Energy and Environmental Assessment of Hydrogen from Biomass Sources: Challenges and Perspectives. Biomass Bioenergy 2022, 165, 106556. [Google Scholar] [CrossRef]
- Li, G.; Yang, Y.; Yu, Q.; Ma, Q.; Lam, S.S.; Chen, X.; He, Y.; Ge, S.; Sonne, C.; Peng, W. Application of Nanotechnology in Hydrogen Production from Biomass: A Critical Review. Adv. Compos. Hybrid. Mater. 2024, 7, 17. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an Energy Vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic Thermochemical Conversion of Biomass for Biofuel Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, L.; Guan, J.; Xiong, Q.; Zhang, S.; Jin, X. A Review on Biomass Gasification: Effect of Main Parameters on Char Generation and Reaction. Energy Fuels 2020, 34, 13438–13455. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of Thermochemical Routes for Hydrogen Production from Biomass: A Review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Guo, X.; Hu, Z.; Liu, S.; He, M. Hydrogen-Rich Gas from Catalytic Steam Gasification of Biomass in a Fixed Bed Reactor: Influence of Particle Size on Gasification Performance. Int. J. Hydrogen Energy 2009, 34, 1260–1264. [Google Scholar] [CrossRef]
- Fremaux, S.; Beheshti, S.M.; Ghassemi, H.; Shahsavan-Markadeh, R. An Experimental Study on Hydrogen-Rich Gas Production via Steam Gasification of Biomass in a Research-Scale Fluidized Bed. Energy Convers. Manag. 2015, 91, 427–432. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S. Experimental Investigation of Biomass Devolatilization in Steam Gasification in a Dual Fluidised Bed Gasifier. Fuel 2017, 188, 628–635. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Wang, S.; Luo, Z. Interactions of Biomass Components during Pyrolysis: A TG-FTIR Study. J. Anal. Appl. Pyrolysis 2011, 90, 213–218. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-Catalytic Steam Reforming of Agricultural Biomass Wastes and Biomass Components for Production of Hydrogen/Syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Z.; Ao, W.; Li, J.; Liu, G.; Fu, J.; Ran, C.; Mao, X.; Kang, Q.; Liu, Y.; et al. Microwave-Assisted Pyrolysis of Textile Dyeing Sludge Using Different Additives. J. Anal. Appl. Pyrolysis 2017, 127, 140–149. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Lotti, G.; Vaccari, F.P.; Sanei, H. Assessment of Long-Lived Carbon Permanence in Agricultural Soil: Unearthing 15 Years-Old Biochar from Long-Term Field Experiment in Vineyard. Biomass Bioenergy 2024, 191, 107484. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass Resource Facilities and Biomass Conversion Processing for Fuels and Chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Phan, A.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. Characterisation of Slow Pyrolysis Products from Segregated Wastes for Energy Production. J. Anal. Appl. Pyrolysis 2008, 81, 65–71. [Google Scholar] [CrossRef]
- Tinwala, F.; Mohanty, P.; Parmar, S.; Patel, A.; Pant, K.K. Intermediate Pyrolysis of Agro-Industrial Biomasses in Bench-Scale Pyrolyser: Product Yields and Its Characterization. Bioresour. Technol. 2015, 188, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Bartocci, P.; D’Amico, M.; Moriconi, N.; Bidini, G.; Fantozzi, F. Pyrolysis of Olive Stone for Energy Purposes. Energy Procedia 2015, 82, 374–380. [Google Scholar] [CrossRef]
- Soni, B.; Karmee, S.K. Towards a Continuous Pilot Scale Pyrolysis Based Biorefinery for Production of Bio oil and Biochar from Sawdust. Fuel 2020, 271, 117570. [Google Scholar] [CrossRef]
- Dai, X.; Wu, C.; Li, H.; Chen, Y. The Fast Pyrolysis of Biomass in CFB Reactor. Energy Fuels 2000, 14, 552–557. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Bade, S.O.; Tomomewo, O.S.; Meenakshisundaram, A.; Ferron, P.; Oni, B.A. Economic, Social, and Regulatory Challenges of Green Hydrogen Production and Utilization in the US: A Review. Int. J. Hydrogen Energy 2024, 49, 314–335. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Shah, J.; Valaki, J. Characterization of Bio-Oil, Bio-Char, and Pyro-Gas Derived from Cotton Stalk Slow Pyrolysis—As Sustainable Energy Sources. Indian J. Chem. Technol. 2022, 29, 380–389. Available online: http://blog.niscair.res.in/html/index.php/IJCT/article/view/60904 (accessed on 21 November 2024).
- Gheorghe, C.; Dinu, R.; Marculescu, C.; Badea, A.; Apostol, T. Two-Phase Pyrolysis Modelling Of Wooden Waste. WIT Trans. Ecol. Environ. 2008, 109, 31–38. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Aier, I.; Vijay, V.K.; Kaushal, P. Suitability of Rice Straw for Biochar Production through Slow Pyrolysis: Product Characterization and Thermodynamic Analysis. Bioresour. Technol. Rep. 2021, 15, 100818. [Google Scholar] [CrossRef]
- Varma, A.K.; Thakur, L.S.; Shankar, R.; Mondal, P. Pyrolysis of Wood Sawdust: Effects of Process Parameters on Products Yield and Characterization of Products. Waste Manag. 2019, 89, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Andries, J.; Spliethoff, H. Catalytic Pyrolysis of Biomass for Hydrogen Rich Fuel Gas Production. Energy Convers. Manag. 2003, 44, 2289–2296. [Google Scholar] [CrossRef]
- Alam, M.; Sarkar, I.; Chanda, N.; Ghosh, S.; Loha, C. Enhancing Syngas Production and Hydrogen Content in Syngas from Catalytic Slow Pyrolysis of Biomass in a Pilot Scale Fixed Bed Reactor. Biomass Convers. Biorefin 2025, 15, 6237–6249. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of Slow and Fast Pyrolysis for Converting Biomass into Fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Guizani, C.; Valin, S.; Billaud, J.; Peyrot, M.; Salvador, S. Biomass Fast Pyrolysis in a Drop Tube Reactor for Bio Oil Production: Experiments and Modeling. Fuel 2017, 207, 71–84. [Google Scholar] [CrossRef]
- Safdari, M.S.; Rahmati, M.; Amini, E.; Howarth, J.E.; Berryhill, J.P.; Dietenberger, M.; Weise, D.R.; Fletcher, T.H. Characterization of Pyrolysis Products from Fast Pyrolysis of Live and Dead Vegetation Native to the Southern United States. Fuel 2018, 229, 151–166. [Google Scholar] [CrossRef]
- Abou Rjeily, M.; Chaghouri, M.; Gennequin, C.; Abi Aad, E.; Randrianalisoa, J.H. Investigating Co-Production of Syngas, Biochar, and Bio-Oil from Flax Shives Biomass by Pyrolysis and in-Line Catalytic Hybrid Reforming. Biomass Convers. Biorefinery 2023, 14, 25599–25625. [Google Scholar] [CrossRef]
- Park, Y.; Namioka, T.; Sakamoto, S.; Min, T.J.; Roh, S.A.; Yoshikawa, K. Optimum Operating Conditions for a Two-Stage Gasification Process Fueled by Polypropylene by Means of Continuous Reactor over Ruthenium Catalyst. Fuel Process. Technol. 2010, 91, 951–957. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Pyrolysis–Gasification of Plastics, Mixed Plastics and Real-World Plastic Waste with and without Ni–Mg–Al Catalyst. Fuel 2010, 89, 3022–3032. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Artetxe, M.; Arregi, A.; Santamaria, L.; Bilbao, J.; Olazar, M. Pyrolysis and In-Line Catalytic Steam Reforming of Polystyrene through a Two-Step Reaction System. J. Anal. Appl. Pyrolysis 2016, 122, 502–510. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Alvarez, J.; Artetxe, M.; Arregi, A.; Bilbao, J.; Olazar, M. A Sequential Process for Hydrogen Production Based on Continuous HDPE Fast Pyrolysis and In-Line Steam Reforming. Chem. Eng. J. 2016, 296, 191–198. [Google Scholar] [CrossRef]
- Pietraszek, A.; Koubaissy, B.; Roger, A.C.; Kiennemann, A. The Influence of the Support Modification over Ni-Based Catalysts for Dry Reforming of Methane Reaction. Catal. Today 2011, 176, 267–271. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Waste Ashes as Catalysts for the Pyrolysis–Catalytic Steam Reforming of Biomass for Hydrogen-Rich Gas Production. J. Mater. Cycles Waste Manag. 2019, 21, 1224–1231. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Synthetic Natural Gas Production from the Three Stage (i) Pyrolysis (Ii) Catalytic Steam Reforming (Iii) Catalytic Hydrogenation of Waste Biomass. Fuel Process. Technol. 2020, 208, 106515. [Google Scholar] [CrossRef]
- Waheed, Q.M.K.; Williams, P.T. Hydrogen Production from High Temperature Pyrolysis/Steam Reforming of Waste Biomass: Rice Husk, Sugar Cane Bagasse, and Wheat Straw. Energy Fuels 2013, 27, 6695–6704. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Dong, L.; Vassallo, A.; Williams, P.T.; Huang, J. Characteristics and Catalytic Properties of Ni/CaAlOx Catalyst for Hydrogen-Enriched Syngas Production from Pyrolysis-Steam Reforming of Biomass Sawdust. Appl. Catal. B 2016, 183, 168–175. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Dong, L.; Jin, F.; Williams, P.T.; Huang, J. Catalytic Steam Reforming of Volatiles Released via Pyrolysis of Wood Sawdust for Hydrogen-Rich Gas Production on Fe–Zn/Al2O3 Nanocatalysts. Fuel 2015, 158, 999–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Zhang, T.; Wang, Q. Hydrogen-Rich Syngas Production from Biomass Pyrolysis and Catalytic Reforming Using Biochar-Based Catalysts. Fuel 2022, 313, 123006. [Google Scholar] [CrossRef]
- Olaleye, A.K.; Adedayo, K.J.; Wu, C.; Nahil, M.A.; Wang, M.; Williams, P.T. Experimental Study, Dynamic Modelling, Validation and Analysis of Hydrogen Production from Biomass Pyrolysis/Gasification of Biomass in a Two-Stage Fixed Bed Reaction System. Fuel 2014, 137, 364–374. [Google Scholar] [CrossRef]
- Dong, L.; Wu, C.; Ling, H.; Shi, J.; Williams, P.T.; Huang, J. Promoting Hydrogen Production and Minimizing Catalyst Deactivation from the Pyrolysis-Catalytic Steam Reforming of Biomass on Nanosized NiZnAlOx Catalysts. Fuel 2017, 188, 610–620. [Google Scholar] [CrossRef]
- Jin, F.; Sun, H.; Wu, C.; Ling, H.; Jiang, Y.; Williams, P.T.; Huang, J. Effect of Calcium Addition on Mg-AlOx Supported Ni Catalysts for Hydrogen Production from Pyrolysis-Gasification of Biomass. Catal. Today 2018, 309, 2–10. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Hydrogen-Rich Syngas Production and Tar Removal from Biomass Gasification Using Sacrificial Tyre Pyrolysis Char. Appl. Energy 2017, 190, 501–509. [Google Scholar] [CrossRef]
- Abou Rjeily, M.; Chaghouri, M.; Gennequin, C.; Abi Aad, E.; Pron, H.; Randrianalisoa, J.H. Biomass Pyrolysis Followed by Catalytic Hybrid Reforming for Syngas Production. Waste Biomass Valorization 2023, 14, 2715–2743. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ren, J.; Cao, J.P.; Wei, F.; Zhu, C.; Fan, X.; Zhao, Y.P.; Wei, X.Y. Catalytic Reforming of Volatiles from Biomass Pyrolysis for Hydrogen-Rich Gas Production over Limonite Ore. Energy Fuels 2017, 31, 4054–4060. [Google Scholar] [CrossRef]
- Xiao, X.; Cao, J.; Meng, X.; Le, D.D.; Li, L.; Ogawa, Y.; Sato, K.; Takarada, T. Synthesis Gas Production from Catalytic Gasification of Waste Biomass Using Nickel-Loaded Brown Coal Char. Fuel 2013, 103, 135–140. [Google Scholar] [CrossRef]
- García, L.; Salvador, M.L.; Arauzo, J.; Bilbao, R. Catalytic Steam Gasification of Pine Sawdust. Effect of Catalyst Weight/Biomass Flow Rate and Steam/Biomass Ratios on Gas Production and Composition. Energy Fuels 1999, 13, 851–859. [Google Scholar] [CrossRef]
- Cao, J.P.; Shi, P.; Zhao, X.Y.; Wei, X.Y.; Takarada, T. Catalytic Reforming of Volatiles and Nitrogen Compounds from Sewage Sludge Pyrolysis to Clean Hydrogen and Synthetic Gas over a Nickel Catalyst. Fuel Process. Technol. 2014, 123, 34–40. [Google Scholar] [CrossRef]
- Wang, B.S.; Cao, J.P.; Zhao, X.Y.; Bian, Y.; Song, C.; Zhao, Y.P.; Fan, X.; Wei, X.Y.; Takarada, T. Preparation of Nickel-Loaded on Lignite Char for Catalytic Gasification of Biomass. Fuel Process. Technol. 2015, 136, 17–24. [Google Scholar] [CrossRef]
- Cao, J.P.; Liu, T.L.; Ren, J.; Zhao, X.Y.; Wu, Y.; Wang, J.X.; Ren, X.Y.; Wei, X.Y. Preparation and Characterization of Nickel Loaded on Resin Char as Tar Reforming Catalyst for Biomass Gasification. J. Anal. Appl. Pyrolysis 2017, 127, 82–90. [Google Scholar] [CrossRef]
- Azhar Uddin, M.; Tsuda, H.; Wu, S.; Sasaoka, E. Catalytic Decomposition of Biomass Tars with Iron Oxide Catalysts. Fuel 2008, 87, 451–459. [Google Scholar] [CrossRef]
- Efika, C.E.; Wu, C.; Williams, P.T. Syngas Production from Pyrolysis–Catalytic Steam Reforming of Waste Biomass in a Continuous Screw Kiln Reactor. J. Anal. Appl. Pyrolysis 2012, 95, 87–94. [Google Scholar] [CrossRef]
- Koike, M.; Ishikawa, C.; Li, D.; Wang, L.; Nakagawa, Y.; Tomishige, K. Catalytic Performance of Manganese-Promoted Nickel Catalysts for the Steam Reforming of Tar from Biomass Pyrolysis to Synthesis Gas. Fuel 2013, 103, 122–129. [Google Scholar] [CrossRef]
- Yang, H.; Nurdiawati, A.; Gond, R.; Chen, S.; Wang, S.; Tang, B.; Jin, Y.; Zaini, I.N.; Shi, Z.; Wang, W.; et al. Carbon-Negative Valorization of Biomass Waste into Affordable Green Hydrogen and Battery Anodes. Int. J. Hydrogen Energy 2024, 49, 459–471. [Google Scholar] [CrossRef]
- Zaini, I.N.; Sophonrat, N.; Sjöblom, K.; Yang, W. Creating Values from Biomass Pyrolysis in Sweden: Co-Production of H2, Biocarbon and Bio-Oil. Processes 2021, 9, 415. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Hydrogen Production from Biomass by Continuous Fast Pyrolysis and In-Line Steam Reforming. RSC Adv. 2016, 6, 25975–25985. [Google Scholar] [CrossRef]
- Arregi, A.; Santamaria, L.; Lopez, G.; Olazar, M.; Bilbao, J.; Artetxe, M.; Amutio, M. Appraisal of Agroforestry Biomass Wastes for Hydrogen Production by an Integrated Process of Fast Pyrolysis and in Line Steam Reforming. J. Environ. Manage 2023, 347, 119071. [Google Scholar] [CrossRef]
- Soria, J.; Li, R.; Flamant, G.; Mazza, G.D. Influence of Pellet Size on Product Yields and Syngas Composition during Solar-Driven High Temperature Fast Pyrolysis of Biomass. J. Anal. Appl. Pyrolysis 2019, 140, 299–311. [Google Scholar] [CrossRef]
- Pattanotai, T.; Watanabe, H.; Okazaki, K. Experimental Investigation of Intraparticle Secondary Reactions of Tar during Wood Pyrolysis. Fuel 2013, 104, 468–475. [Google Scholar] [CrossRef]
- Weldekidan, H.; Strezov, V.; Town, G.; Kan, T. Production and Analysis of Fuels and Chemicals Obtained from Rice Husk Pyrolysis with Concentrated Solar Radiation. Fuel 2018, 233, 396–403. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Abakr, Y.A.; Musa, M.; Yusup, S.; Singh, A.; Kazi, F.K. Valorization of Bambara Groundnut Shell via Intermediate Pyrolysis: Products Distribution and Characterization. J. Clean. Prod. 2016, 139, 717–728. [Google Scholar] [CrossRef]
- Mahmood, A.S.N.; Brammer, J.G.; Hornung, A.; Steele, A.; Poulston, S. The Intermediate Pyrolysis and Catalytic Steam Reforming of Brewers Spent Grain. J. Anal. Appl. Pyrolysis 2013, 103, 328–342. [Google Scholar] [CrossRef]
- Wang, X.; Kersten, S.R.A.; Prins, W.; Van Swaaij, W.P.M. Biomass Pyrolysis in a Fluidized Bed Reactor. Part 2: Experimental Validation of Model Results. Ind. Eng. Chem. Res. 2005, 44, 8786–8795. [Google Scholar] [CrossRef]
- Fernandez-Akarregi, A.R.; Makibar, J.; Lopez, G.; Amutio, M.; Olazar, M. Design and Operation of a Conical Spouted Bed Reactor Pilot Plant (25 Kg/h) for Biomass Fast Pyrolysis. Fuel Process. Technol. 2013, 112, 48–56. [Google Scholar] [CrossRef]
- Duanguppama, K.; Suwapaet, N.; Pattiya, A. Fast Pyrolysis of Contaminated Sawdust in a Circulating Fluidised Bed Reactor. J. Anal. Appl. Pyrolysis 2016, 118, 63–74. [Google Scholar] [CrossRef]
- Maliutina, K.; Tahmasebi, A.; Yu, J.; Saltykov, S.N. Comparative Study on Flash Pyrolysis Characteristics of Microalgal and Lignocellulosic Biomass in Entrained-Flow Reactor. Energy Convers. Manag. 2017, 151, 426–438. [Google Scholar] [CrossRef]

| Pyrolysis Process Type (Temperature) | Feedstock | H2 (vol%) | CO (vol%) | CO2 (vol%) | CH4 (vol%) | C2+ (vol%) | N2 (Vol%) | O2 (vol%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Slow (650) | Waste wood | 7 | 30.5 | 44.7 | 14.7 | 3.1 | [38] | ||
| Cardboard | 13.4 | 36.6 | 32.4 | 15.3 | 2.3 | ||||
| Textile | 5.4 | 34.7 | 35.6 | 19.2 | 5.1 | ||||
| Slow (500) | Neem seed | 6.89 ± 1 | 0.46 ± 0.3 | 7.16 ± 1 | 15.87 ± 2 | 18.46 ± 2 | [39] | ||
| Pigeon pea | 4.66 ± 1 | 1.60 ± 1 | 8.93 ± 1 | 21.57 ± 2 | 14.58 ± 1 | ||||
| Yellow pea | 0.15± 0.1 | 1.30 ± 1 | 11.23 ± 1 | 24.77 ± 2 | 15.56 ± 2 | ||||
| Ground nut shell | 5.02 ± 1 | 10.16 ± 1 | 6.97 ± 1 | 13.21 ± 1 | 10.93 ± 1 | ||||
| Channa straw cicer | 4.36 ± 1 | 14.02 ± 1 | 6.94± 1 | 19.93 ± 2 | 10.58 ± 1 | ||||
| Soyabean Straw Glycine | 8.29 ± 1 | 13.85 ± 1 | 7.80± 1 | 18.53 ± 2 | 10.60 ± 1 | ||||
| Wheat straw | 7.05 ± 1 | 7.51 ± 1 | 5.62 ± 1 | 18.70 ± 2 | 13.38 ± 1 | ||||
| Sawdust | 7.11 ± 1 | 6.26 ± 1 | 4.93± 0.5 | 22.25 ± 2 | 15.40 ± 2 | ||||
| Slow (600) | Olive stone | 29.47 | 31.48 | 14.1 | 20.5 | 3.65 | 0.8 | [40] | |
| Fast (550) | Sawdust | 4.54 | 34.49 | 16.8 | 4.41 | 35.55 | 4.25 | [41] | |
| Fast (550) | Woody biomass | 7.01 | 24.13 | 36.35 | 4.41 | 28.1 | [42] |
| Process | Advantages | Disadvantages | Efficiency (%) |
|---|---|---|---|
| Steam methane reforming | Mature technology, low production cost | GHG emissions, fossil fuel resource depletion | 74–85 |
| Partial oxidation | Proven technology | GHG emissions, fossil fuel resource depletion | 60–75 |
| Electrolysis | Mature technology, no emissions, cheap and available feedstock, O2 byproduct | Low overall efficiency, high capital cost, corrosion challenges | 40–60 |
| Biomass pyrolysis | Cheap feedstock, CO2-neutral | Tar formation, seasonal availability of and impurities in feedstock | 35–50 |
| Biomass gasification | Cheap feedstock, CO2-neutral | Tar formation, seasonal availability of and impurities in feedstock | 35 |
| Reaction | Description |
|---|---|
| Biomass pyrolysis | |
| Catalytic tar cracking | |
| Tar steam reforming | |
| Hydrocarbon volatile steam reforming | |
| Tar dry reforming | |
| Hydrocarbon volatile dry reforming | |
| Water gas shift | |
| Char steam gasification | |
| Char CO2 gasification |
| Feedstock | Catalyst | H2 Yield (mmol/g) | Ref. |
|---|---|---|---|
| Corncob | Ni/Al2O3 | 51 | [72] |
| Sewage sludge | Ni/Al2O3 | 53 | [75] |
| Wood sawdust | Ni/Al2O3 | 11.01 | [67] |
| Corncob | Ni/Al2O3 | 21.2 | [76] |
| Rice husk | Ni–dolomite | 23.71 | [63] |
| Sugar cane | 21.18 | ||
| Wheat straw | 21.59 | ||
| Corncob | Ni–exchanged resin char | 25 | [77] |
| Poplar wood | Poplar char | 14.4 | [66] |
| Catalpa wood | Catalpa char | 17.1 | |
| Elm wood | Elm char | 15.7 | |
| Pine wood | Pine char | 14.4 | |
| Poplar wood | 5% Ni/poplar char | 21.4 | |
| Poplar wood | 10% Ni/poplar char | 27.2 | |
| Poplar wood | 15% Ni/poplar char | 18.9 | |
| Corncob | Natural limonite ore | 50.2 | [72] |
| Feedstock | Pyrolysis Temperature °C (Heating Rate °C/min) | Scale | Total Gas Yield (wt.%) | H2 Yield (vol%) | Ref. |
|---|---|---|---|---|---|
| Slow pyrolysis | |||||
| Pine | 1600 (50) | Lab scale | 55 | 41.7 | [85] |
| Cypress wood | 600 (30) | Lab scale | 11 | 14.4 | [86] |
| Sugar cane bagasse | 480 (20) | Lab scale | 25 | 9.6 | [52] |
| 780 (20) | 36 | 28.8 | |||
| Waste wood | 500 (30) | Lab scale | 63 | 40.6 | [47] |
| 550 (30) | 63.4 | 47.8 | |||
| 600 (30) | 65.6 | 52.5 | |||
| 650 (30) | 67.8 | 54.2 | |||
| 700 (30) | 71.3 | 53.6 | |||
| 750 (30) | 74.3 | 53.4 | |||
| 800 (30) | 76.5 | 52.5 | |||
| Rice husk | 800 (2) | Lab scale | 25.5 | 8.6 | [87] |
| Olive stone | 600 | Lab scale | 44.17 | 29.47 | [40] |
| Intermediate pyrolysis | |||||
| Agricultural residue | 500 (50) | Lab scale | 32 | 2.5 | [88] |
| Brewers spent grain | 450 (100) | Lab scale | 21 | 1.6 | [89] |
| Fast pyrolysis | |||||
| Sugar cane bagasse | 480 (120) | Lab scale | 14.12 | 8.7 | [52] |
| 580 (120) | 15.46 | 15.2 | |||
| 680 (120) | 17.94 | 45.3 | |||
| Beach wood | 350 (1000) | Lab scale | 10 | 0.9 | [90] |
| 400 (1000) | 18.4 | 1.4 | |||
| 450 (1000) | 10.1 | 0.8 | |||
| 500 (1000) | 17.7 | 2.3 | |||
| 550 (1000) | 20.5 | 3.4 | |||
| 800 (1000) | 56.9 | 13.8 | |||
| Pine | 550 (1000) | 17.9 | 6.9 | ||
| Bamboo | 500 (1000) | 19.1 | 1.5 | ||
| Pine wood | 440 (N.A) | Pilot scale | 61.5 | 4.2 | [91] |
| 460 (N.A) | 62.6 | 4.9 | |||
| 480 (N.A) | 65.9 | 6.1 | |||
| 510 (N.A) | 64 | 7 | |||
| 525 (N.A) | 63.4 | 9.1 | |||
| 565 (N.A) | 61.5 | 15.1 | |||
| Sawdust | 400 (N.A) | Lab scale | 23.7 | 10 | [92] |
| 450 (N.A) | 22.1 | 20 | |||
| 500 (N.A) | 15.2 | 24 | |||
| 550 (N.A) | 22.7 | 21 | |||
| 600 (N.A) | 26.7 | 19 | |||
| Flash pyrolysis | |||||
| Palm kernel shell | 600 (N.A) | Lab scale | 5 | 18.5 | [93] |
| 900 (N.A) | Lab scale | 32 | 26.6 | ||
| Feedstock | Pyrolysis Temperature °C (Heating Rate °C/min) | Reforming Temperature °C | Catalyst | H2 Concentration (vol%) | H2 Yield (mmol/g) | Ref. |
|---|---|---|---|---|---|---|
| Rice husk | 950 (20) | 950 | 10 wt % Ni–dolomite | 59.32 | 25.44 | [63] |
| Sugar cane bagasse | 57.4 | 25.41 | ||||
| Wheat straw | 58.3 | 24.47 | ||||
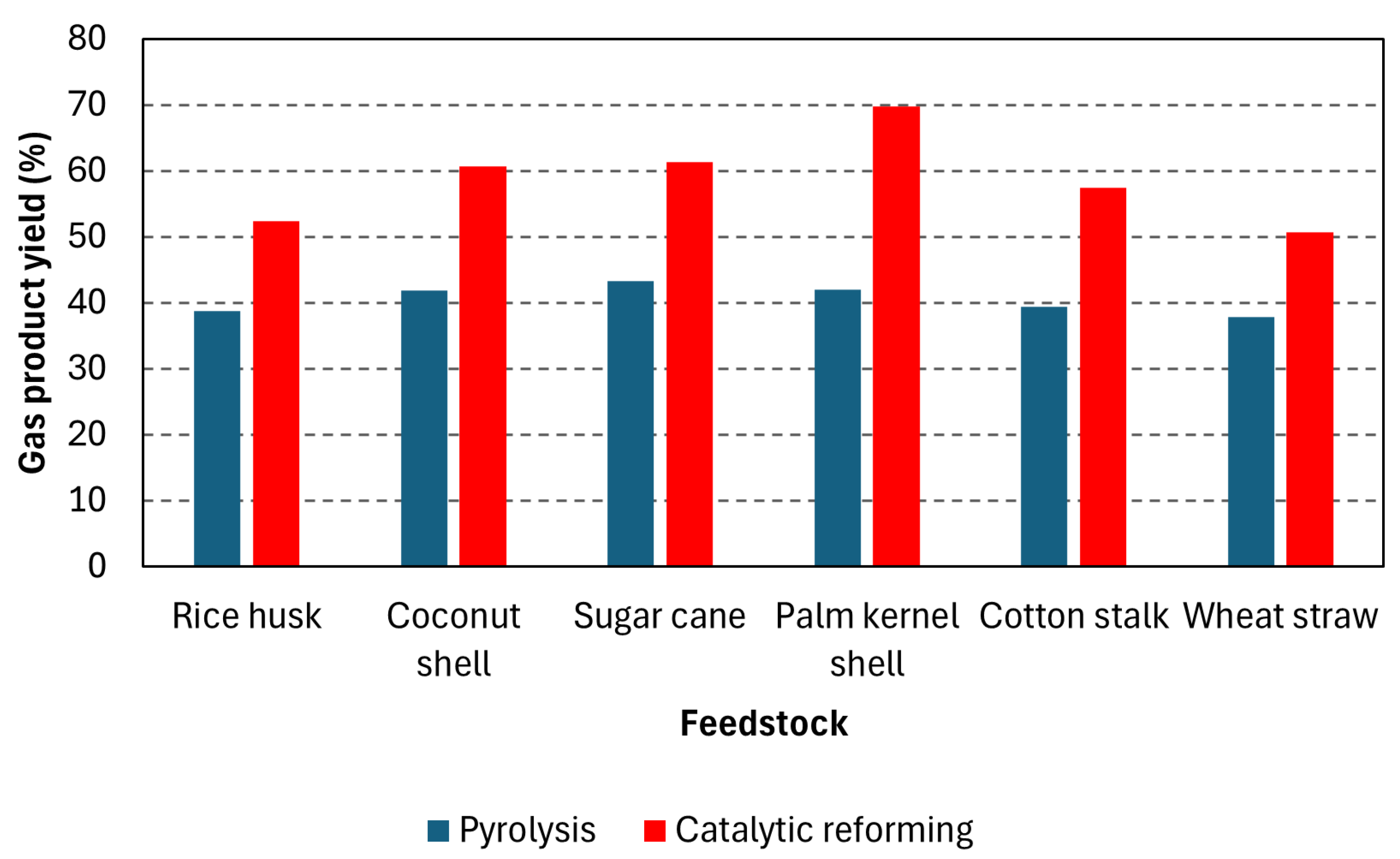
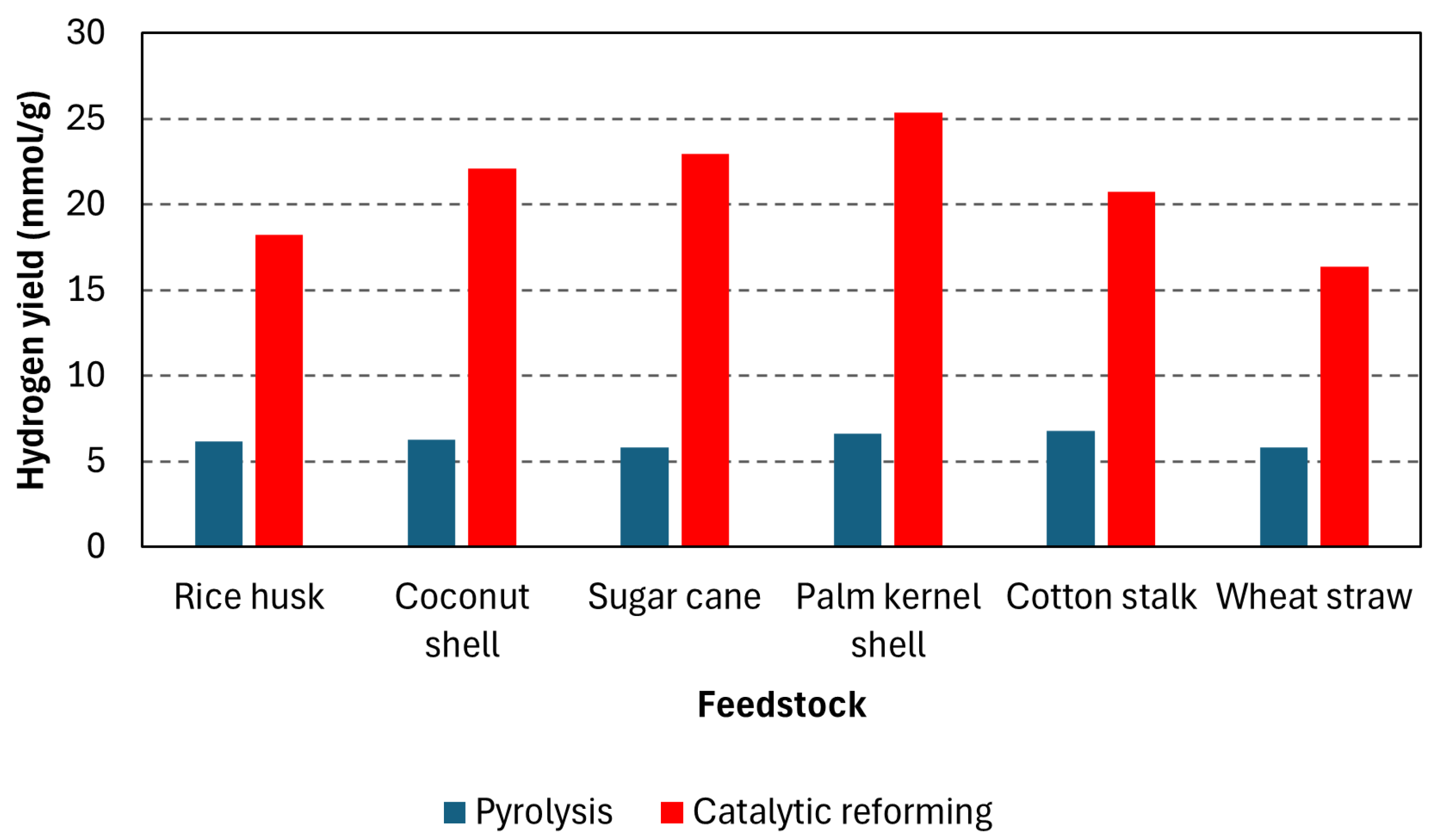
| Rice husk | 550 (20) | 750 | 10 wt% NiAl2O3 | 57.63 | 18.22 | [33] |
| Coconut shell | 58.21 | 22.11 | ||||
| Sugar cane | 59.23 | 22.96 | ||||
| Palm kernel shell | 57.36 | 25.35 | ||||
| Cotton stalk | 57.95 | 20.74 | ||||
| Wheat straw | 54.06 | 16.38 | ||||
| Lignin | 64.02 | 25.25 | ||||
| Cellulose | 56.43 | 19.72 | ||||
| Xylan | 58.77 | 20.54 | ||||
| Corncob | 900 (10) | 650 | Ni–exchanged resin char (Ni/RC) | 72.5 | 61.2 | [77] |
| Wood pellets | 500 (40) | 700 | Tire char | 34.6 | 10.2 | [70] |
| 800 | Tire char | 38.2 | 15.1 | |||
| 900 | Tire char | 51.8 | 47.6 | |||
| 700 | Acid-treated tire char | 14.3 | 3.11 | |||
| 800 | Acid-treated tire char | 28.3 | 10.2 | |||
| 900 | Acid-treated tire char | 47.1 | 36.7 | |||
| Wood sawdust | 550 (40) | 800 | Blank | 23.2 | 6 | [69] |
| Ni-Ca-Mg-Al (1:1:1:1) | 52.3 | 18.2 | ||||
| Ni-Mg-Al | 35.1 | 10.4 | ||||
| Wood sawdust | 500 (40) | 800 | Sand | 17.5 | 2.4 | [65] |
| Fe-Zn/Al2O3 (1:1) | 40.6 | 9.65 | ||||
| Fe-Zn/Al2O3 (1:2) | 35.1 | 7.25 | ||||
| Fe-Zn/Al2O3 (1:3) | 35.6 | 6.79 | ||||
| Fe-Zn/Al2O3 (1:4) | 35.1 | 6.59 | ||||
| Waste wood | 500 (2400) | 760 | Sand | 18.2 | N.A | [80] |
| NiO/Al2O3 | 44.4 | N.A | ||||
| NiO/SiO2 | 38.7 | N.A | ||||
| NiO/CeO2/Al2O3 | 43.1 | N.A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayer Kabeh, K.; Prussi, M.; Chiaramonti, D. Advances in Bio-Hydrogen Production: A Critical Review of Pyrolysis Gas Reforming. Appl. Sci. 2025, 15, 3995. https://doi.org/10.3390/app15073995
Zayer Kabeh K, Prussi M, Chiaramonti D. Advances in Bio-Hydrogen Production: A Critical Review of Pyrolysis Gas Reforming. Applied Sciences. 2025; 15(7):3995. https://doi.org/10.3390/app15073995
Chicago/Turabian StyleZayer Kabeh, Kaveh, Matteo Prussi, and David Chiaramonti. 2025. "Advances in Bio-Hydrogen Production: A Critical Review of Pyrolysis Gas Reforming" Applied Sciences 15, no. 7: 3995. https://doi.org/10.3390/app15073995
APA StyleZayer Kabeh, K., Prussi, M., & Chiaramonti, D. (2025). Advances in Bio-Hydrogen Production: A Critical Review of Pyrolysis Gas Reforming. Applied Sciences, 15(7), 3995. https://doi.org/10.3390/app15073995












