Abstract
In recent decades, heavy industrial discharges have caused severe soil and groundwater pollution. Many areas previously occupied by industries are now represented by lands contaminated by the accumulation of toxic metals, which pose serious risks to human health, plants, animals, and surrounding ecosystems. Among the various potential solutions, the solidification and stabilization (S/S) technique represents one of the most effective technologies for treating and disposing of a wide range of contaminated wastes. This study focuses on the theoretical definition of a green material mix, which will subsequently be used in the solidification process of contaminated industrial soils, optimizing the mix to ensure treatment effectiveness. The mix design was developed through a literature analysis, representing a preliminary theoretical study. This paper explores the application of the S/S process using various additives, including Portland cement, fly ash (FA), ground granulated blast furnace slag (GGBFS), and other industrial waste materials, to create an innovative mix design for the treatment of contaminated soils. The main objective is to reduce the permeability and solubility of contaminants while simultaneously improving the mechanical properties of the treated materials. The properties of the studied soils are described along with those of the green materials used, providing a comprehensive overview of the optimization of the resulting mixtures.
1. Introduction
The improper use of chemicals, combined with emissions from landfills, agriculture, and industries, contributes to surface soil pollution, which is estimated to affect about 50% of the land area in the EU [1]. The main sources of soil contamination by heavy metals and metalloids include atmospheric deposition due to traffic and other emissions, as well as emissions from landfills, agriculture, and industrial activities such as mining. These activities have significantly exacerbated soil pollution [2,3].
Soil pollution thus constitutes a significant environmental challenge, with substantial impacts on ecosystems, agricultural productivity, and human health [4].
Among the various technologies available for the treatment of contaminated soils, stabilization and solidification (S/S) is recognized as the most effective. The U.S. Environmental Protection Agency (EPA) has validated S/S as a proven treatment technology for 57 different types of waste [5]. S/S has been employed for the treatment of radioactive waste since the 1950s [6].
The methodology involves rendering the contaminating compounds inert by encapsulating them in a solid matrix (solidification) and inducing chemical reactions that reduce the mobility of contaminants in the material to be remediated (stabilization) [7]. The process mainly consists of mixing the contaminated soil with binders such as cement or cement–lime mixtures, sometimes integrated with specific additives [8,9].
In recent years, the use of more sustainable alternatives to traditional ordinary Portland cement (OPC) has been explored, such as calcium aluminate cement (CAC) and metakaolin (MK). These materials have demonstrated not only significant effectiveness in immobilizing contaminants within a monolithic matrix but also a remarkable reduction in greenhouse gas emissions, offering a more environmentally sustainable solution [10]. Therefore, this study focuses on the theoretical analysis of the definition of innovative material mixes, which can be used in the solidification and stabilization of contaminated soils. This research explores the possibility of combining sustainable additives by analyzing the results derived from sources of industrial waste materials to optimize material mixing. It should be noted that this study primarily focuses on the theoretical part of mix design, with the aim of developing a mix that can be validated in subsequent experimental studies. Solidification/stabilization (S/S) enhances the safety of contaminated soils for disposal or reuse, significantly mitigating risks to human health and the environment [11].
The treated sediments can be reused as filling material, blocks, aggregates for road construction, components for cement mortars, or raw materials for brick production [12,13]. Alternatively, they can be disposed of in landfills designated for non-hazardous waste. In this context, the present manuscript provides a detailed analysis of the contaminant content in the studied soils, the solidification/stabilization procedure, and the optimization of the mixes using industrial by-products. This study emphasizes the critical importance of adapting the mix design to address the specific contaminants in each case. It is important to note that this research complies with relevant legislation, including the Soil Framework Directive (SFD) and the Water Framework Directive (WFD), as well as European standards such as ISO 10381-1:2002 (Guidelines for the design of sampling programs) [14], ISO 17402:2008 (Soil Quality) [15], and Legislative Decree 152/2006 [16]. Key elements such as the selection of appropriate sampling techniques, sample management, and the calculation of field chemical parameters are addressed in ISO 10381-1:2002, ISO 17402:2008, and Legislative Decree 152/2006. Additionally, this study is aligned with ISO 23611-6:2013 [17], which provides comprehensive guidelines for designing soil invertebrate sampling programs. Although this section of ISO 23611 is intended for global application in all soils populated by invertebrates, most available data pertain to temperate regions. However, studies from tropical and boreal regions, supported by theoretical considerations, suggest that the principles outlined in ISO 23611 are broadly applicable. ISO 17402:2008 establishes requirements and guidelines for selecting and applying methods to assess the bioavailability of contaminants in soil and related materials. While it does not prescribe specific methods, it defines boundary conditions and principles for method application and development, ensuring minimum standards for methodological reliability. Results obtained using these methods can serve as estimates of bioavailability within a risk assessment framework. Contaminants addressed by ISO 17402:2008 include metals (and metalloids), organic pollutants, and organometallic compounds. The standard also applies to natural metals derived from geological and pedological processes (natural pedogeochemical content) and can be extended to sediment analysis.
The remainder of this manuscript is organized as follows: Section 2 reviews the state of the art, focusing on the traditional use of Portland cement. Section 3 outlines the characterization of contaminated soils and preparation of alternative materials and presents the results of geotechnical and chemical–physical analyses. Section 4 presents the results of the mixture used to stabilize contaminated soils, including the composition, advantages of selected materials, and the pelletization process, detailing factors influencing agglomeration, process conditions, and granule characteristics. Section 5 presents preliminary analyses of contaminated soils, focusing on soil characteristics, leaching tests, and the potential of industrial by-products for stabilization, before treatment processes. Finally, Section 6 concludes this study, offering reflections on the potential future use of green materials and perspectives for further research.
2. State of the Art
With industrialization, the increased production and disposal of industrial waste have resulted in significant soil pollution, primarily driven by industrial, agricultural, and urban activities [18], as illustrated in Figure 1.

Figure 1.
Causes of soil pollution (authors’ elaboration).
Major sources of contamination include industrial emissions into the atmosphere, effluents from industrial, urban, and agricultural sectors that pollute rivers and seas, landfills for both hazardous and non-hazardous waste, and insufficient treatment of industrial sludge [19,20]. Abandoned mines also play a significant role, causing persistent environmental damage [21]. Notably, areas of high environmental risk due to contaminated sites are often located near urban centers [22].
Since its inception, the stabilization and solidification (S/S) process has relied heavily on Portland cement as its primary material [6]. Over the years, numerous studies have highlighted the benefits of OPC, including its low cost, non-toxicity of chemical ingredients, long-term chemical and physical stability, high compressive and impact strength, resistance to biodegradation, low water permeability, and ease of processing [23].
Cement-based stabilization/solidification has been widely applied to treat various types of waste containing hazardous components, including inorganic, organic, or mixed contaminants, yielding excellent results [7,24]. This technique has also been successfully used for the treatment of marine sediments [25,26].
The use of mixed cement significantly reduces the leaching of contaminants into the soil by enhancing the properties of the granular material. This process improves soil compactness and decreases its water content, making it less permeable. Furthermore, the technique has proven particularly effective in reducing toxicity, demonstrating that contaminant immobilization can substantially mitigate environmental risks.
However, the production of Portland cement is an energy-intensive process that generates substantial greenhouse gas emissions, contributing approximately 8% of global anthropogenic CO2 emissions [27,28]. In this context, minimizing carbon emissions has become a critical priority [29].
This underscores the need to explore alternative materials for the S/S process, such as cement kiln dust (CKD), lime, lime kiln dust (LKD), limestone, fly ash (FA), bentonite [30], ground granulated blast furnace slag (GGBFS), gypsum, phosphate, and other industrial waste materials. Numerous studies have demonstrated the effectiveness of pozzolan-based stabilization/solidification in immobilizing heavy metals in contaminated soils and sludges [31]. One study compared the compressive, thermal, and morphological properties of cement mortar and mortar reinforced with BFS and fly ash, revealing that pozzolan-enhanced mortar exhibits lower thermal conductivity, higher porosity, and acceptable compressive strength [32]. These findings suggest that incorporating pozzolanic materials can improve the physical properties of treated materials, thereby enhancing the stabilization process for contaminated soils. Furthermore, research has shown that adding quicklime and fly ash to contaminated soils effectively reduces the leachability of heavy metals to levels well below regulatory limits. The addition of fly ash also broadens the pH range for heavy metal immobilization and significantly improves the stress–strain properties of treated solids [31]. However, a potential drawback of this treatment is the excessive formation of pozzolanic ettringite in the presence of sulfates. The use of ground granulated blast furnace slag has proven effective in immobilizing heavy metals such as Pb2⁺ and Cd2⁺. The excellent performance of geopolymer materials in immobilizing heavy metals is attributed to various solidification mechanisms, including the following:
- Chemical adsorption: The geopolymer structure, composed of silicon–oxygen and aluminum–oxygen tetrahedra, features negatively charged aluminum–oxygen tetrahedra that adsorb metal ions.
- Chemical precipitation: Lead reacts with the dissolved silicon phase to form Pb3SiO5 precipitates, which are encapsulated by geopolymer gels.
- Physical adsorption: Geopolymers, with their porous surfaces, adsorb molecules smaller than their pore size.
- Physical encapsulation: Under alkaline activation, the aluminosilicate source dissolves, initiates gelation, and forms a network structure, encapsulating heavy metals within the cementitious material [33].
GGBFS enhances conventional concrete by improving workability, rheological properties, and reducing carbon emissions. It also increases compressive strength, provides superior abrasion resistance, and offers better protection against chloride ion and gas penetration [34]. In green concrete, GGBFS further enhances performance by improving strength, reducing void ratios, and delivering excellent mechanical properties [35].
In comparison, cement kiln dust (CKD) and slag exhibit less pronounced cementitious properties but are often used for economic reasons. Lime and lime kiln dust (LKD) are employed to adjust pH and facilitate water removal due to their high heat of hydration. Green concrete, as an alternative to conventional concrete, incorporates industrial waste residues, significantly reducing energy requirements and CO2 emissions during production. By utilizing recycled materials and increasing recoverable resource use, green concrete decreases dependence on finite natural resources, contributing to a more sustainable production cycle [36].
Among the tested materials, Yin et al. demonstrated that mixing ordinary Portland cement with rice husk ash (RHA) effectively remediated Pb-contaminated soil. The incorporation of RHA into the binder system significantly reduced Pb leachability, highlighting its potential in heavy metal immobilization [37].
A key concern is the long-term durability of soils treated with this process. Studies have shown that even after 17 years, stabilized soil continues to meet performance criteria [38]. For this reason, specific tests will be conducted in the future to further analyze this phenomenon.
This research specifically aims to explore new mixtures and solidification and stabilization technologies to develop materials that not only meet regulatory and performance requirements but can also be reused in civil and industrial applications. This approach reduces the need for new resources and contributes to the transition towards a circular economy.
3. Materials and Methods
The remediation of contaminated areas involves various technologies designed to contain pollutants and reduce their mobility within natural environments.
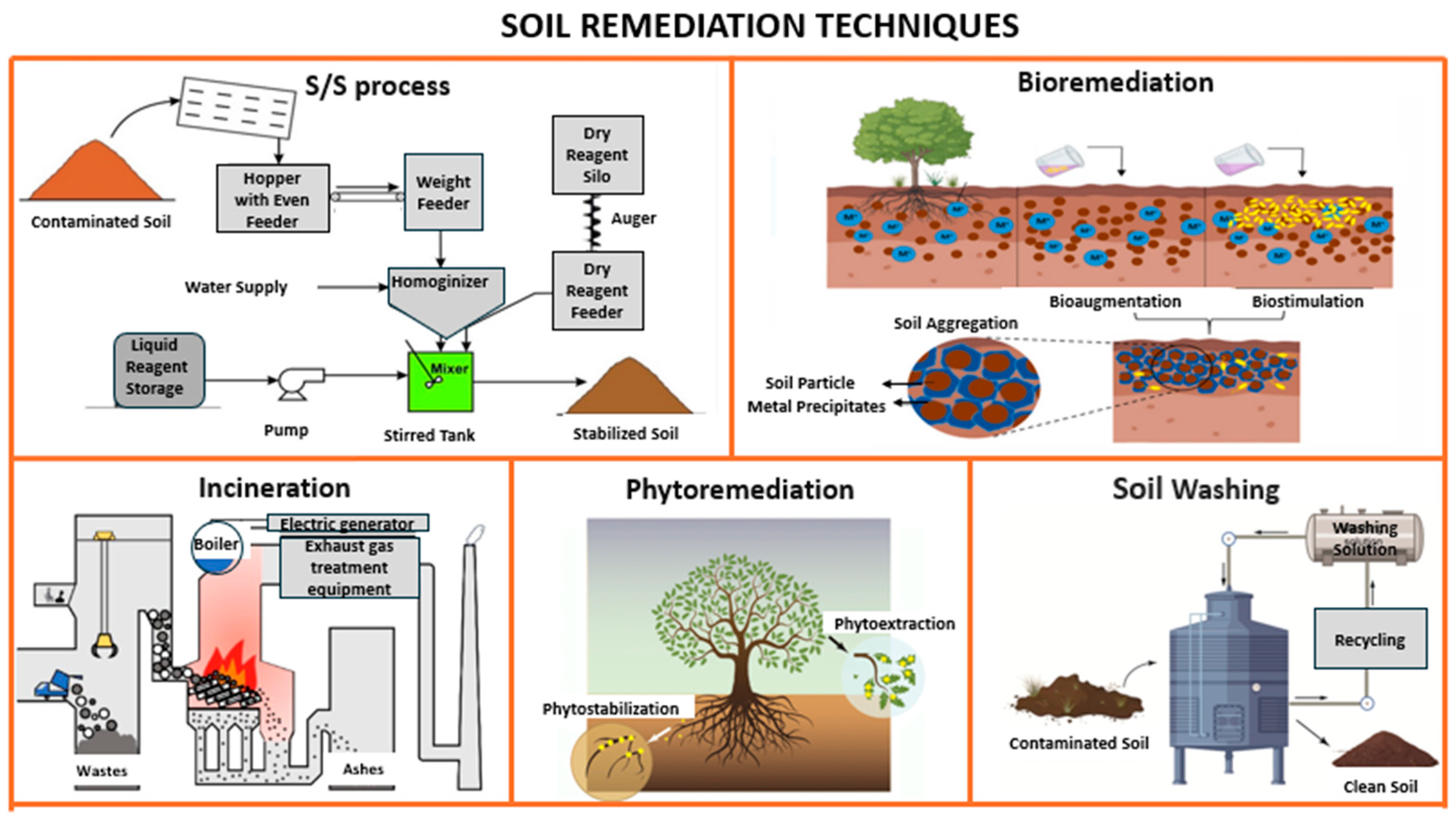
Commonly employed techniques (see Table 1) for soil remediation include incineration, phytoremediation, bioremediation, solidification and stabilization processes, as well as soil washing [9,39,40].
Among these techniques, stabilization/solidification (S/S) is one of the most used methods, involving the use of reagents to immobilize contaminants [41].
Solidification and stabilization technologies are widely applied to reduce the mobility and leachability of contaminants in soils. Solidification physically encapsulates contaminants within a solid matrix using binders, thereby reducing soil permeability and limiting the spread of contaminants to the surrounding environment. Stabilization, in contrast, chemically interacts with contaminants to reduce their solubility and minimize their mobility, thus preventing water resource contamination [4] (see Figure 2).
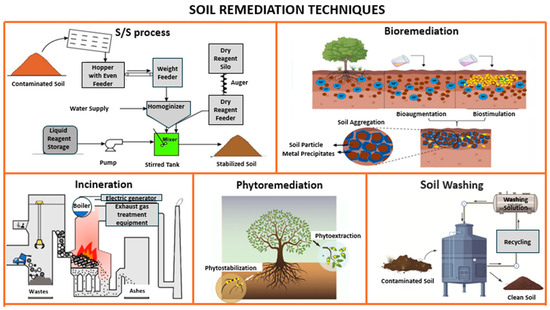
Figure 2.
Various techniques for soil contamination remediation (source: authors’ elaboration).
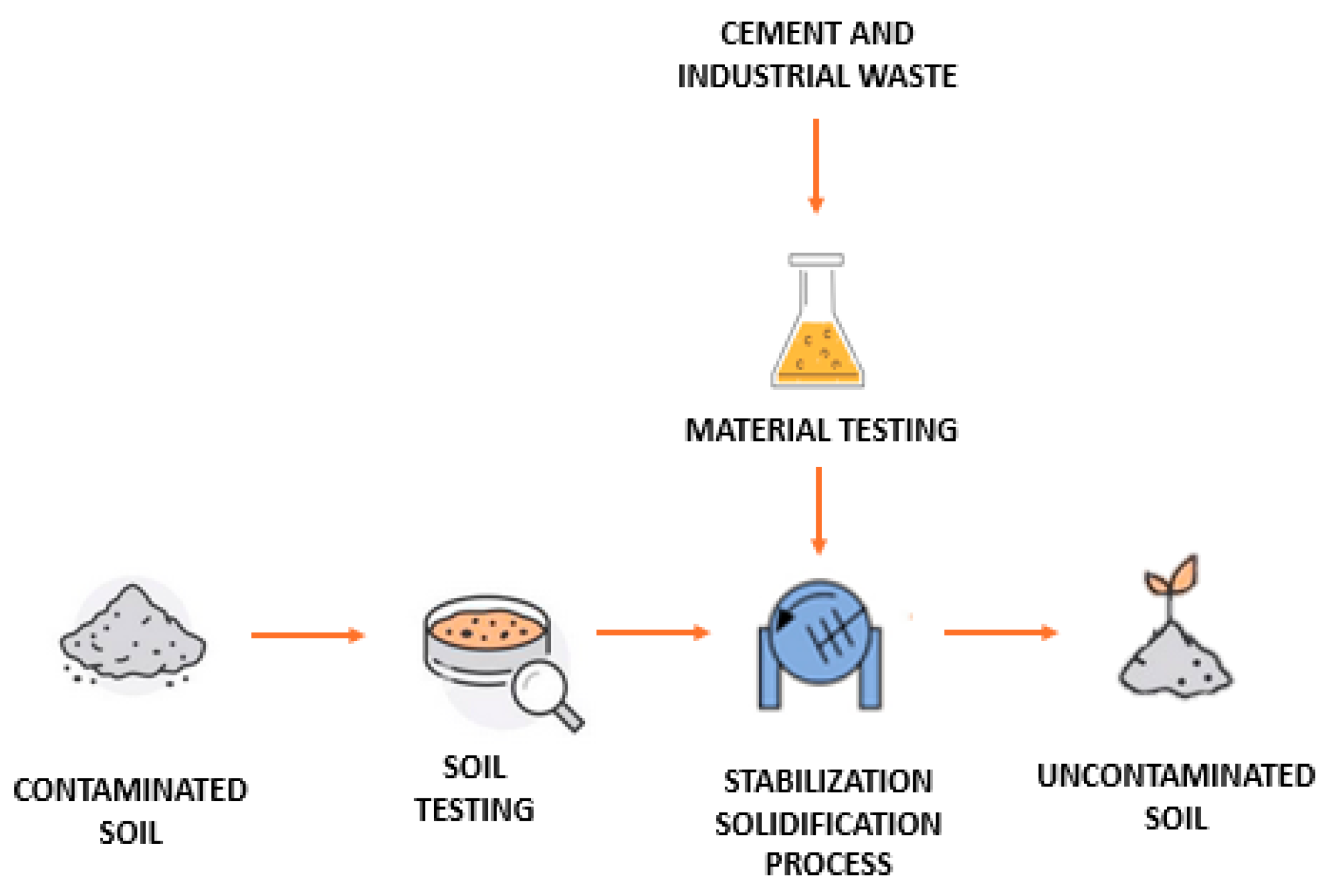
As illustrated in Figure 2, contaminated soils were treated using a combined solidification and stabilization process. This approach aimed to reduce both the permeability and solubility of contaminants by utilizing binding materials such as cement in combination with industrial waste. The process is shown in Figure 3 in schematic form. As seen in the image, there are phases for testing the raw materials, both the contaminated soil and the waste materials used. These are then mixed in the mixer to be processed together, ultimately resulting in non-contaminated and stabilized soil. This phase will be explained in detail in the following sections.
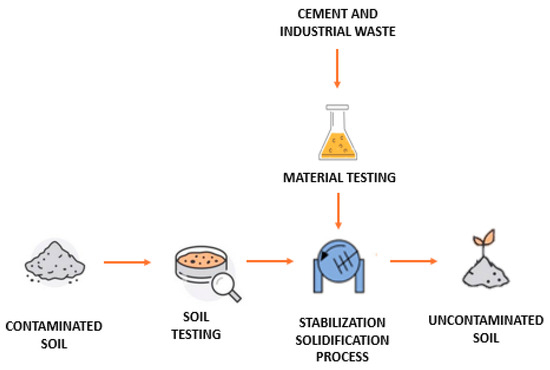
Figure 3.
Schematic representation of the solidification/stabilization process for soils from industrial sites (source: authors’ elaboration).
The process involved the following main phases:
- Collection and preparation of representative samples.
- Chemical, physical, and geotechnical characterization of the samples.
- Selection and preparation of green materials.
- Characterization of green materials.
- Mix design procedures and optimization of the mixtures.
The S/S process begins with the collection of representative samples, which are then chemically, physically, and geotechnically characterized. This characterization provides information on the nature and extent of the contamination, as well as on the mechanical properties of the soil, such as permeability and strength [42].
Simultaneously, “green” materials derived from industrial waste, such as fly ash or blast furnace slag, are selected and prepared. These materials are chosen for their ability to bind with contaminants and are characterized to ensure their compatibility with the soil and their effectiveness in stabilizing the contaminants [43]. Since this study focuses on mix design, solidification/stabilization tests are not yet performed.
After selecting the materials, tests are conducted to simulate their integration into the soil, assessing their ability to reduce the permeability and solubility of the contaminants. These tests ensure that the mixes improve environmental safety [44]. Subsequently, a mix design process is carried out to optimize the proportions of materials, with the aim of creating a stable and effective mixture. The resulting mix immobilizes contaminants over the long term and minimizes the risk of dispersion. The design aims to determine the optimal proportions of materials to achieve a stable mix that immobilizes contaminants in the long term and minimizes the risk of dispersion [16].

Table 1.
Overview of various techniques for remediating contaminated soil.
Table 1.
Overview of various techniques for remediating contaminated soil.
| Technique | Benefits | Disadvantages | Fields of Application | Source |
|---|---|---|---|---|
| Incineration | Highly effective in treating organic contaminants. Reduces waste volume. | High costs and energy consumption. Can produce polluting emissions if not managed properly. | Highly contaminated soils with heavy metals or organic substances. | [45] |
| Phytoremediation | Ecological and cost-effective solution. Uses plants to absorb or degrade contaminants. Non-invasive. | Slow process. Effective only for specific contaminants and for shallow soils. | Agricultural land or small areas with superficial contamination. | [46] |
| Bioremediation | Natural and low-cost approach. Uses microorganisms to degrade contaminants. | Not always effective for highly persistent contaminants or heavily contaminated soils. | Agricultural land, industrial areas, controlled landfills. | [47] |
| Solidification/Stabilization (S/S) | Immobilizes contaminants, reducing the risk of dispersion over time. Reduces costs compared to other technologies. | Does not eliminate contaminants but makes them less mobile. Limited effect on contaminants that are hard to immobilize. | Industrial areas, soils contaminated with heavy metals or solvents. | [24] |
| Soil Washing | Efficient removal of soluble contaminants. Suitable for contamination spread over large areas. | High water consumption and potential contamination of surface waters. | Soils contaminated with soluble substances like heavy metals or hydrocarbons. | [48] |
Each of these stages is crucial for developing a treatment that effectively remediates the contaminated site while ensuring the protection of the surrounding environment.
A key factor driving the adoption of S/S is its proven effectiveness, which is largely due to the well-established chemistry for treating metal contaminants. Stabilization techniques, which transform contaminants into a less hazardous solid form, are already widely applied across various industrial and environmental fields. Additionally, advancements in treatment technologies have made it possible to reach greater depths and address deeper contaminations, thereby enhancing the overall effectiveness of the S/S method and ensuring greater safety for both public health and the environment.
While S/S was initially used primarily for metals, its capability to treat inorganic compounds and many organic contaminants is expanding its range of applications [9].
3.1. Solidification and Stabilization Process
Treatment procedures for the solidification and stabilization of contaminated soils involve the use of specific materials, which are either combined with or injected into the soil to reduce the mobility and toxicity of pollutants. These treatments aim to improve the physical and chemical properties of the soil, stabilize the contaminants, and limit their leaching potential [49]. The addition of waste to specific agents is intended to reduce the number and mobility of the contaminants. These contaminants could otherwise infiltrate through the soil’s physical and chemical properties, potentially contaminating the surrounding environment and spreading towards landfills or other dispersion routes [10].
Stabilization/solidification processes can be applied both in situ and ex situ, depending on the nature and extent of the contamination [24].
The procedure is carried out in several phases. First, the soil sample intended for treatment is analyzed and classified. Next, it is mixed with the binder selected for the treatment [50].
The maturation conditions can be adjusted to meet the specific needs of the project, considering the desired characteristics for the final intervention.
These conditions are selected based on the performance objectives and application methods of the treatment, optimizing results for the future operational phases of the project [51].
The benefits of this methodology include the following:
- Improvement in mechanical properties: Enhances the soil’s ability to support loads and reduces the risk of subsidence.
- Permeability and moisture control: Prevents the formation of puddles and facilitates drainage.
- Durability and wear resistance: Contributes to prolonging the lifespan of the road surface, reducing the need for frequent maintenance.
Stabilization/solidification treatments are primarily chosen for their ability to reduce the mobility and toxicity of contaminants in soils, particularly those contaminated by heavy metals such as arsenic (As), zinc (Zn), cadmium (Cd), chromium (Cr), antimony (Sb), and mercury (Hg). If left untreated, these metals can infiltrate the soil and contaminate the surrounding environment, posing serious risks to public health and ecosystems. S/S treatment is especially effective in this context, as it transforms metals into more stable and less mobile forms, significantly reducing their hazardousness.
S/S technology is not limited to heavy metals. It can also be applied to other inorganic compounds and a wide range of organic contaminants [9].
This makes stabilization/solidification a versatile technique, effectively addressing the needs of environmental safety and soil remediation.
3.2. Contaminated Soils
The soil analyzed in this study originates from the province of Naples (Italy), a region historically marked by industrial activity and currently affected by contamination.
Figure 4 shows piles of the soil analyzed in this study.

Figure 4.
Soil samples analyzed in this study: (a) some representative samples; (b) an example of the consistency of a soil sample.
The sampling was carried out using excavators, and four tons of each type of soil were used to create representative samples. All soil samples were thoroughly mixed to ensure that each sample was as representative as possible. The mixing method employed was the quartering method, which was performed as follows:
- Each soil sample was divided into four quarters, and each quarter was mixed individually.
- Two quarters were combined to form one half.
- The two halves were then mixed to create a homogeneous matrix.
This procedure was repeated several times until the sample was adequately mixed.
Subsequently, 3 kg of each sample was collected in boxes and labeled as SOIL1, SOIL2, SOIL3, SOIL4, SOIL5, and SOIL6.
3.3. Green Materials
“Green materials” refer to materials that are environmentally friendly in terms of low environmental impact, low carbon emissions, and, above all, recycled materials such as fly ash, granulated and ground blast furnace slag, and marble sludge (MS), which, despite being waste products, are reused.
The materials considered in this study include Portland cement CEM II/A-LL 42.5 R (CEM) (UNI EN 197-1:2011) [52], fly ash, granulated and ground blast furnace slag, and marble sludge (MS).
Fly ash is a waste product obtained from coal-based power plants. It is collected in electrostatic precipitators and transferred to silos. The fly ash considered in this study comes from the incineration of municipal solid waste (MSWI-FA), specifically from an incineration plant located in Italy. According to the European Waste Catalogue, this waste is assigned the code 19.01.13* and is classified as hazardous. Ground granulated blast-furnace slag, a by-product of the cast iron production process, results from the iron production process and was provided by RILEM (Research Institute of Liaisons of European Construction). Marble sludge, classified as inert waste according to the current legislative framework with the CER code 10413, is produced by the processing of ornamental stone and was supplied by a plant located in Potenza, Italy. The fly ash, GGBFS, and MS were used as partial substitutes for cement in this study. The use of residual waste from various domestic and industrial sectors leads to significantly lower energy consumption during production, thereby reducing carbon dioxide emissions [53].
The fly ash improves the strength of the treated material, especially in terms of compressive strength, due to its chemical reactivity that promotes the formation of stable compounds during solidification. Fly ash increases the resistance of the material to aggressive chemicals such as acids (H2SO4, HCl) and salts (Na2SO4, MgSO4), enhancing long-term stability, particularly in soils contaminated by chemical agents [54,55]. In the case of soils contaminated with heavy metals, fly ash, in addition to improving the strength of the treated material, stabilizes the metals through adsorption and complexation, reducing their solubility and availability to plants, particularly for metals such as lead, cadmium, and nickel [40].
GGBFS was chosen to improve the rheological properties of the mix, facilitating the mixing and application process. Its ability to increase the stability of the treated material is crucial for achieving uniform solidification. GGBFS enhances the compressive strength of the stabilized material, improving its long-term durability [34]. Its ability to reduce the permeability of the treated material helps limit damage from external chemical attacks, such as water ingress and corrosive substances [35]. GGBFS, with its ability to precipitate metals, promotes their transformation into insoluble forms, stabilizing them over the long term, particularly for iron (Fe), chromium (Cr), and manganese (Mn) [56].
Although there are few studies focusing on marble sludge, the use of this material helps reduce the volume of industrial waste and improve waste material management, making the process an environmentally friendly option [57]. Marble sludge contributes to improving the cohesion and stability of the treated material, reducing permeability, and enhancing both physical and chemical resistance. Additionally, due to its calcium carbonate (CaCO3) content, it increases the soil pH, promoting the adsorption of metals such as lead (Pb) and cadmium (Cd), making it particularly useful in acidic soils [58].
3.4. Chemical–Physical Tests and Non-Destructive Analytical Technique
The upcoming sections outline the results from the chemical and physical tests. We discuss the results of the geotechnical analyses on soil samples and the characterization of the green materials used. To determine the chemical composition of the green materials, energy-dispersive X-ray spectroscopy (EDS) was employed using a Phenom Pro X microscope (Boston, MA, USA). This equipment enabled both Scanning Electron Microscopy (SEM) for morphological analysis and energy-dispersive spectroscopy (EDS) for compositional analysis. The powder samples were first dried at 105 °C until a constant weight was achieved, then ground in a ball mill to sizes smaller than 100 µm. Each component was pressed into a 1 cm diameter disk, selecting an area of approximately 1 mm2.
For the mineralogical analysis, a Rigaku Powder Miniflex 600 diffractometer (Tokyo, Japan) was used, equipped with Cu Kα radiation and a 2θ range from 5° to 80°, scanning at 0.05° 2θ/s. Mineral phase identification was performed using Rigaku PDXL2 software version 2.0.
Thermal analysis was carried out with a Mettler Toledo TGA/DSC 2 STAR system, capable of performing thermogravimetric analysis (TGA) in the 20 °C to 1200 °C temperature range, with a heating rate of 10 °C/min under an air flow. To assess particle size distribution, a Malvern Instruments Mastersizer 2000 laser scattering analyzer (Worcestershire, UK) was employed, covering a particle sizes range of 0.02 to 2000 µm. The pH of MSWI-FA and washed fly ash (W-FA) was measured using a Thermo Orion 420A+ pH meter (Boston, MA, USA), with a mixture of MSWI-FA and deionized water in a 1:2.5 (m/v) ratio.
Heavy metal content was analyzed using atomic absorption spectroscopy (AAS) with a Varian SpectrAA 55B spectrometer, equipped with a flame (acetylene/air) and a deuterium lamp for background correction.
Finally, the chloride and sulfate content in MSWI-FA and W-FA samples was determined through a leaching test in deionized water for 24 h, followed by ion chromatography to analyze the leachate solution.
3.5. Chemical, Physical, and Geotechnical Characterization of Contaminated Soil
Geotechnical investigations and leaching tests were conducted according to European and Italian standards [59] to obtain information on the physical properties of the soil samples and the levels of contaminants present.
Three types of analyses were performed for the chemical–physical characterization of the samples: a geotechnical analysis, a particle size distribution analysis, and leaching tests. A percolation test for soil, along with two batch leaching methods (one with a low liquid/solid (L/S) ratio and one with a high L/S ratio), was used.
Geotechnical analyses of the various soil samples revealed that the soil is primarily composed of sand with gravel. Additionally, a slightly condensed thickening was observed, along with moderate humidity and an absence of plasticity. The results of the geotechnical analysis are presented below (see Table 2).

Table 2.
Geotechnical analysis results of soil samples.
The results (Table 2) show significant variability in moisture content between the different samples, with Soil 5 having the highest moisture, followed by Soil 4, both exhibiting relatively high water content. Soil 6, despite having a lower moisture content, shows a higher wet weight than the other samples, suggesting a higher density and a more compact soil composition. In contrast, soils with lower moisture content, such as Soil 3, display a more balanced wet-to-dry weight ratio, indicating lower water content and potentially a more permeable structure. These differences suggest varying levels of compaction, porosity, and water retention capacity across the samples.
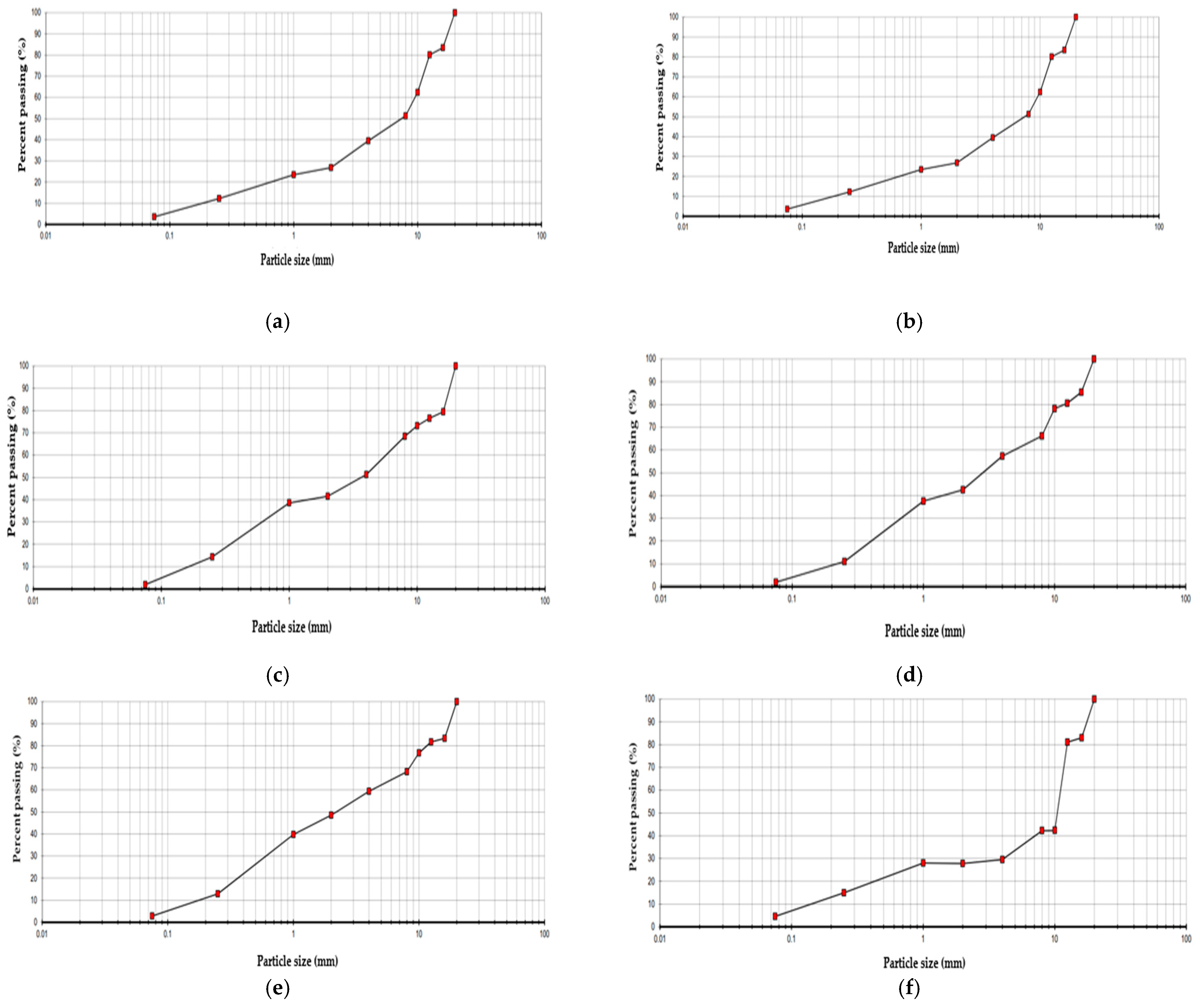
In Figure 5, the particle size distribution of the six analyzed soil samples is presented.
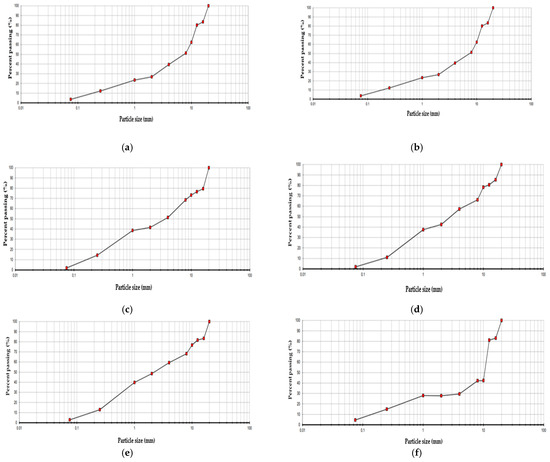
Figure 5.
Particle size distribution curve: (a) SOIL1, (b) SOIL2, (c) SOIL3, (d) SOIL4, (e) SOIL5, (f) SOIL6.
The curves of all six samples (Figure 5) are very similar. The intermediate slope of the curves suggests a well-distributed particle size, without a dominant presence of any specific interval. Generally, the soils are coarse-grained, with a predominant sandy component (ranging from 0.1 to 2 mm) and a significant proportion of gravel (greater than 2 mm).
Specifically, SOIL2 exhibits a smoother and more continuous curve without interruptions, indicating that the sample is more homogeneous. In contrast, the curves for SOIL4 and SOIL5 show a higher percentage of gravel compared to the other samples.
To quantitatively analyze the differences in soil structure, the uniformity and curvature coefficients were calculated. The results are shown in the Table 3 below.

Table 3.
Uniformity coefficient and coefficient of curvature of the analyzed soil.
Regarding the uniformity coefficient, all the soils show high values, indicating a very wide distribution. In fact, the soils are well graded, as is evident from the curves. In particular, Soils 1, 2, and 6 exhibit this characteristic. As for the curvature coefficient, there are greater differences between the soils analyzed. Soils 1, 2, and 6 again have high values, exceeding 2, which further confirms the high gradation of these soils. The others, however, have values below 1, indicating finer grain sizes. Overall, all samples display similar curves, suggesting a uniform baseline composition and evenly distributed contamination. However, subtle differences between the curves may indicate localized variations in soil conditions.
Table 4 below shows the quantities of contaminants present in the various soil samples analyzed, specifically hexavalent chromium and arsenic, in relation to the acceptable concentration limit values in soil and subsoil, as established by Legislative Decree 152/2006. The decree sets two different limits: one for the contamination threshold concentration in soil, subsoil, and groundwater for sites intended for public green spaces, private use, and residential areas (indicated by letter a in the table), and another for sites designated for commercial and industrial use (indicated by letter b in the table).

Table 4.
Contaminant quantities above limit in soil analysis.
For SOIL1, the values of arsenic and hexavalent chromium are below the threshold limits, for both designated purposes, indicating relatively low contamination. However, soil samples SOIL3, SOIL4, SOIL5, and SOIL6 show concerning levels of arsenic and hexavalent chromium, with values exceeding the established limits, indicating significant contamination. These results highlight the need for remediation and monitoring interventions to mitigate environmental and health risks. In particular, arsenic intake can cause gastrointestinal issues, severe skin damage, and lung cancer. Its mechanism of action is typical of metals, as it binds to sulfur-containing enzymes, inhibiting their proper function. Since arsenic forms stable covalent bonds with carbon, it undergoes partial methylation in the liver, reducing its toxicity. In this methylated form, it can no longer bind to enzymes and is excreted through urine. Instead, chromium is known to be associated with sensitizing effects, such as allergic contact dermatitis.
The evaluation of the leachability of the samples was carried out using the following methods, in accordance with the UNI EN 12457-2 standard [60]:
- The pH-dependent leaching test, which determines the mobilization of elements as a function of pH.
- A percolation leaching test, which simulates the behavior of percolation.
- A dynamic leaching test for monolithic materials or materials that behave like monoliths in their application. This method addresses the surface phenomena relevant to these materials.
To quantify the potential release of chloride, sulfate, and heavy metals (HMs) from the stabilized aggregates, the leaching tests were performed with a single-washing step of 24 h and a liquid-to-solid ratio of 10. After the tests, the aggregates were separated from the leaching solution and dried at 105 °C for weight determination to assess any mass loss. To ensure the reliability of the results and compliance with internationally standardized methods, the leaching tests followed recognized industry protocols. Specifically, methods outlined by APAT CNR IRSA were followed for parameters such as nitrates, chlorides, and chemical oxygen demand (COD). The same APAT CNR IRSA procedure was adopted for the analysis of fluorides, sulfates, and zinc.
For the determination of metals and metalloids like barium, copper, zinc, arsenic, and cadmium, the international method UNI EN ISO 17294-2:2016 [61] was applied. Cyanides were analyzed following EPA methods 9010C and 9213, widely recognized in environmental studies. Asbestos analysis was carried out according to the Italian Ministerial Decree (M.D. 06/09/94), ensuring adherence to national regulations.
Finally, dissolved organic carbon (DOC) and total dissolved solids (TDS) were quantified using the UNI EN 1484:1999 [62] and 15216:2008 standards [63], respectively.
These methods ensure a robust and reliable foundation for leaching tests, providing accurate and comparable results consistent with international standards.
Table 5 presents the results of the leaching tests on the different soil samples collected.

Table 5.
Leaching test results for soil samples.
The analysis of soil samples shows that most parameters do not exceed the reference limits. However, some parameters exhibit significant exceedances. Chlorides, copper, zinc, arsenic, cadmium, cobalt, total chromium, lead, and vanadium have concentrations above the reference limits in one or more of the analyzed samples.
Although chlorides do not exceed the limit of 2500 mg/L, their concentrations are close to the maximum value in all the soils. Copper, zinc, and arsenic exceed the more restrictive limits, with values above 0.05 mg/L or 0.1 mg/L in some samples. Additionally, cobalt and vanadium exceed the limits in certain areas, while total chromium and lead are above the limits of 0.05 mg/L and 0.1 mg/L, respectively.
These exceedances suggest the presence of contamination in some of the areas analyzed and highlight the need for further investigation to assess the extent of the risk.
3.6. Green Material Characterization
The materials listed above, MSWI-FA, GGBFS, MS, and CEM, were subjected to detailed morphological and chemical–physical characterization.
The following techniques were used:
- EDS/SEM analysis;
- X-ray diffraction (XRD);
- TGA analysis;
- Particle size distribution analysis;
- Leaching and pH assessment of MSWI-FA.
3.6.1. EDS/SEM Analysis
The EDS analysis was chosen for its ability to provide detailed information on the mineral composition and the distribution of chemical elements during heat treatment. This technique is essential for identifying both active and inert mineral phases, enabling the optimization of production conditions and improving cementitious properties such as hydration activity and reactivity [64].
The chemical composition of the selected materials, determined through EDS testing, is presented in Table 6.

Table 6.
Chemical composition in terms of equivalent oxides (wt%) of MSWI-FA, W-FA, GGBFS, MS and CEM.
The results of the EDS analysis reflected the differing compositions of the industrial waste precursors. The average composition of MSWI-FA consisted of approximately 24.31 wt% calcium oxide, 21.20 wt% chloride, 16.35 wt% carbon monoxide, and 8.57 wt% sulfate. Calcium oxide was present in higher quantities than other elements, particularly in comparison to the silica and alumina content. The chemical characterization of W-FA revealed significant differences between the two ash samples. Specifically, W-FA showed lower environmental risks in terms of chlorides. A significant reduction in chloride content was observed after the washing pretreatment of W-FA, resulting in a residual percentage of 8.77 wt%. Nearly 90% by weight of GGBFS consisted of iron, aluminum, magnesium, and silicon oxides, which can be attributed to the oxidation of metal components during the steelmaking process. Marble sludge contained 50% by weight of calcium oxide, 22.74% by weight of carbon oxide, and nearly 30% by weight of silica, alumina, and magnesium oxide. The chemical composition of the cement matched typical characteristics related to the raw materials and cement manufacturing process.
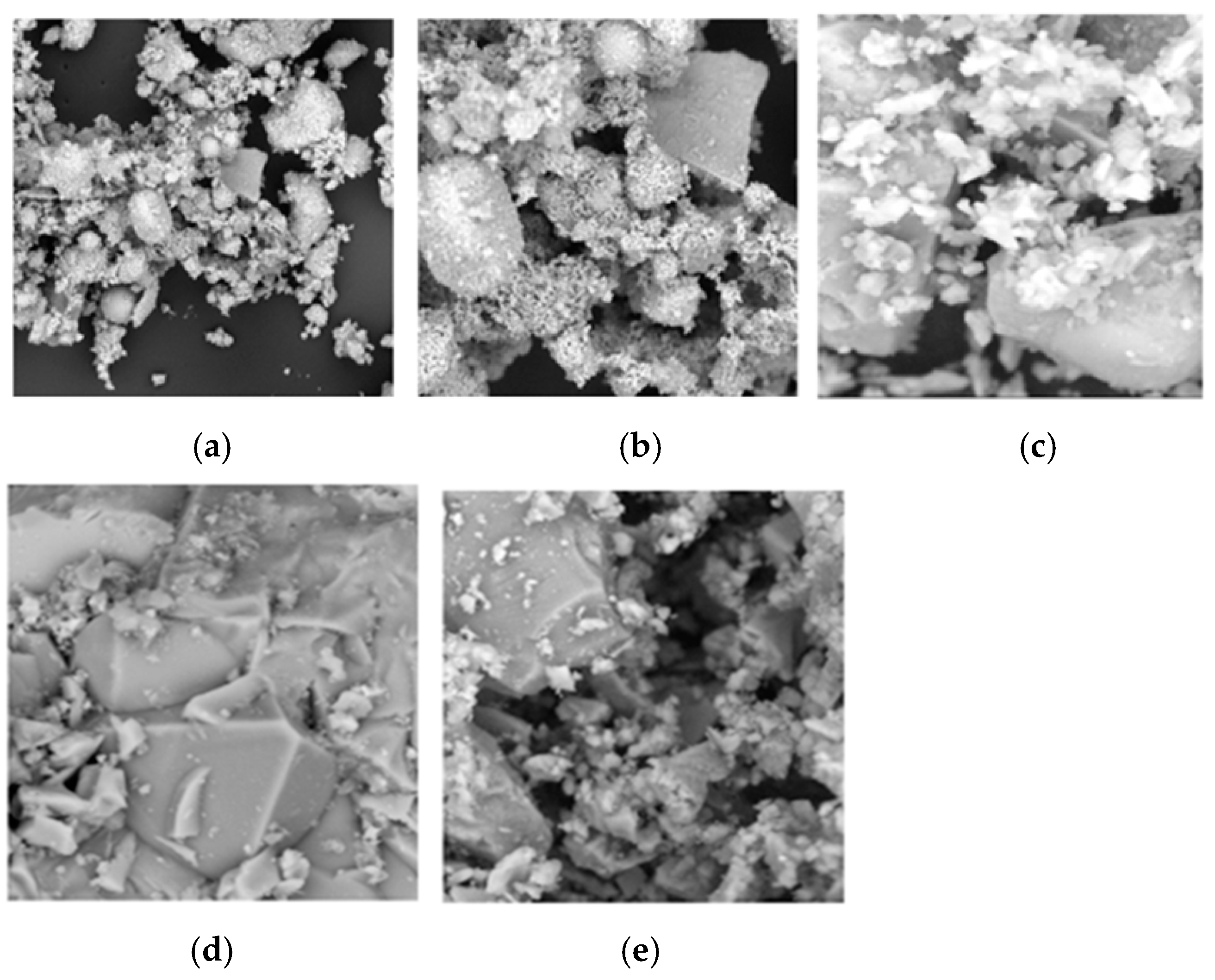
Scanning electron micrographs of MSWI-FA, W-FA, GGBFS, and MS are shown in Figure 6.
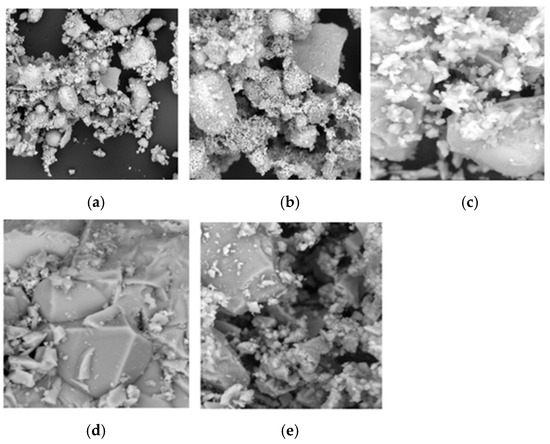
Figure 6.
Scanning electron micrographs of (a) MSWI-FA, (b) W-FA, (c) CEM, (d) GGBFS, and (e) MS.
Figure 6a presents the results of the SEM analysis on MSWI-FA and Figure 6b on W-FA, with micrographs at low magnification (500×) for MSWI-FA and at high magnification (3000×) for W-FA. Figure 6c–e reveal the microstructure of CEM, GGBFS, and MS at 3000×.
The micrographs of MSWI-FA reveal a rough and irregular surface. MSWI-FA exhibits a medium-fine particle distribution, with the fine particle fraction being predominant. However, when comparing the MSWI-FA micrographs to those of W-FA, it is evident that the particles in W-FA are larger than those in MSWI-FA.
SEM analyses were conducted to characterize the morphology of GGBFS, marble sludge (MS), and cement.
The SEM results indicated that both cement and MS have irregular surfaces and contain a higher presence of crystalline structures compared to GGBFS. In contrast, GGBFS has a rough, porous surface with a largely amorphous matrix and fewer crystalline phases. For marble sludge, the micrographs reveal a distribution of fine particles, and at higher magnifications, the presence of a rough and irregular surface along with crystalline phases is noticeable.
3.6.2. XRD Analysis
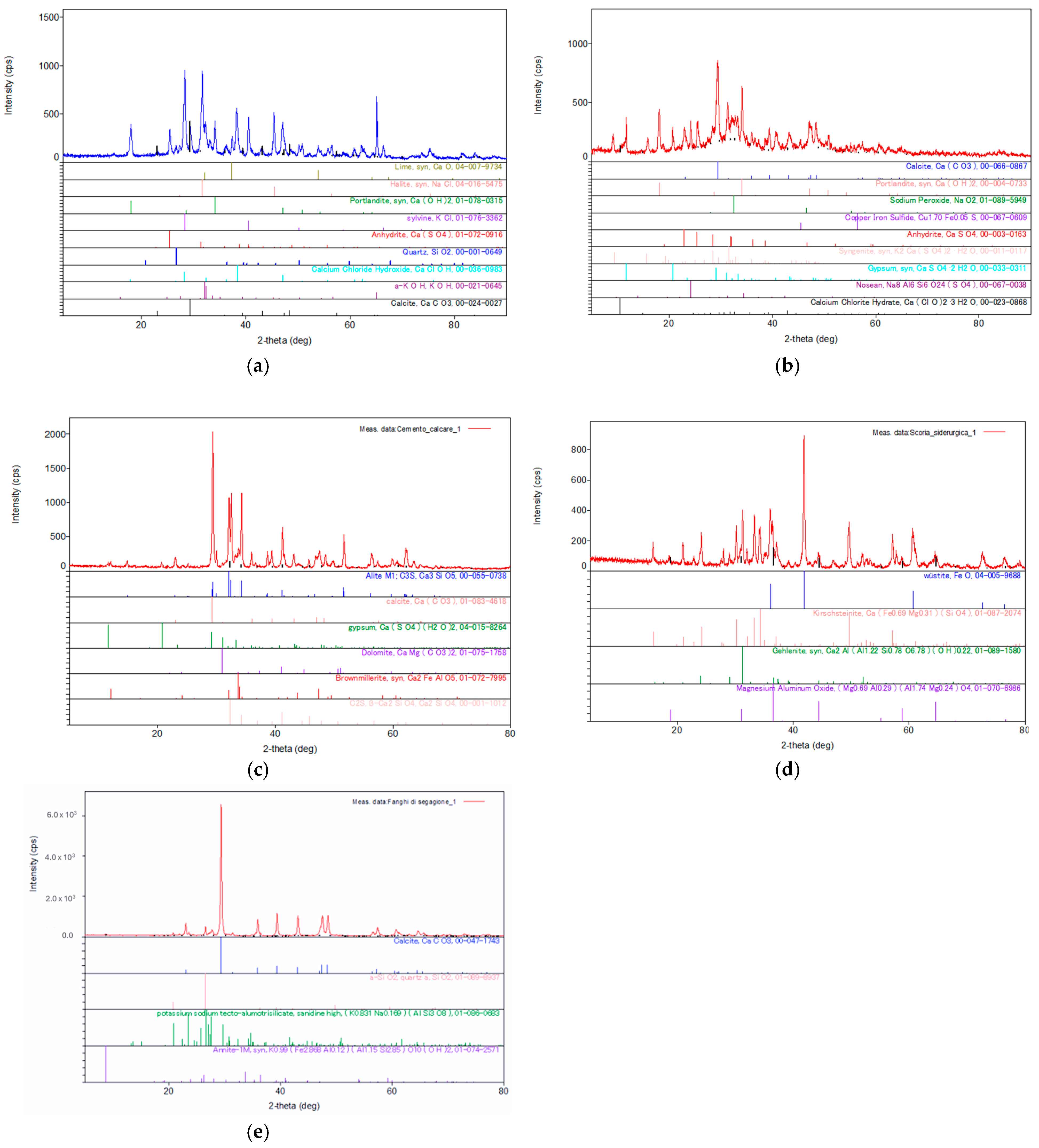
To evaluate the changes in the mineralogical characteristics of the contaminated soils, selected samples were analyzed by X-ray diffraction (XRD) using the Rigaku Miniflex 600 X-ray diffractometer (Tokyo, Japan). The results of the analysis, including the diffractogram and the mineralogical components detected in MSWI-FA, W-FA, GGBFS, MS, and CEM, are presented in Figure 7 below.
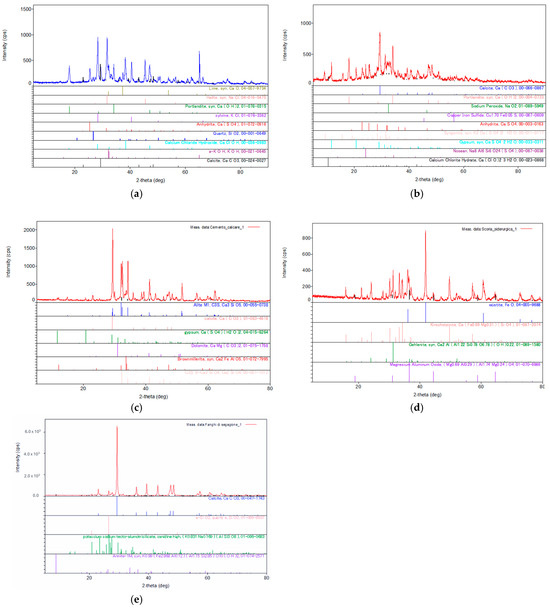
Figure 7.
XRD analysis results, diffractogram and mineralogical components detected in (a) MSWI-FA, reprinted from [65], (b) W-FA, reprinted from [65], (c) GGBFS, (d) MS, and (e) CEM.
The XRD spectra of MSWI-FA show the presence of crystalline species containing calcium, such as lime (CaO), calcite (CaCO3), portlandite (Ca(OH)2), and calcium sulfate (CaSO4), and phases containing chlorine, such as sodium chloride (NaCl), potassium chloride (KCl), and calcium chloride hydroxide (CaClOH). The identification of these crystalline species reinforces the EDS chemical analysis, where a high percentage of calcium and chlorine relative to other elements is found. Another crystalline species easily distinguishable in the diffraction spectrum is SiO2.
The XRD data of W-FA showed the presence of crystalline phases similar to those of MSWI-FA, as well as additional hydrated crystalline species containing calcium, such as singenite (K2Ca(SO4)2·H2O), gypsum (CaSO4·2H2O), and calcium chloride dehydrate (Ca(ClO)2·3H2O). The presence of these latter mineralogical phases is justifiable given the pretreatment of the ashes, carried out through water washing. Another noticeable difference from the mineralogical analysis of untreated fly ashes and treated ones is that the latter contain fewer crystalline phases containing chlorine (NaCl). The XRD results are consistent with the EDS analysis, as after washing pretreatment, a significant decrease in chloride content was observed in W-FA. The results of the XRD analysis of CEM show that the main crystalline phases are alite (Ca3SiO5) and belite (Ca2SiO4). There are additional crystalline phases containing aluminum, such as brownmillerite, sodium aluminum, and calcium aluminum. In particular, alite (Ca3SiO5) gives the material early strength development, followed by belite (Ca2SiO4), which performs a similar function to alite but acts over the long term. Moreover, these calcium silicates, reacting with water, form portlandite, a crystalline phase (Ca(OH)2), and an amorphous calcium silicate hydrate phase, commonly known as CSH, responsible for hardening.
To ensure the principle of identity for the mineralogical components, granulated blast furnace slag (GGBFS), a by-product of the iron industry, was also analyzed using the X-ray diffraction (XRD) method. The mineral phases present in GGBFS are wustite (FeO), monticellite, and gehlenite. Finally, the crystalline phases present in marble sludge are quartz, calcite, and annite.
3.6.3. TGA Analysis
The TGA results for MSWI-FA and W-FA showed three distinct mass losses at approximately 100, 700, and 900 °C. The mass loss at around 100 °C is associated with the removal of moisture from the samples. The only difference between the thermogravimetric analyses of MSWI-FA and W-FA is that W-FA exhibits an additional weight loss at around 290 °C. This is linked to the weakly bound water content in the cementitious hydrated calcium phases, which tend to bond more readily with water molecules. A significant weight loss in the temperature range of 600–800 °C, with an inflection point around 700 °C, is typical of calcination. The mass loss observed at 900 °C is likely due to the degradation of CaSO4, as indicated by the XRD data, where both crystalline phases were observed in MSWI-FA and W-FA. The TGA analysis of cement revealed three distinct weight losses at 120, 400, and 700 °C. These correspond to the loss of moisture from the sample, the decomposition of Ca(OH)2 into calcium oxide, and the calcination of calcite (CaCO3), resulting in the production of calcium oxide and carbon dioxide. In the TGA analysis of GGBFS and MS, only one weight loss was detected at approximately 800 °C, which is also attributable to the calcination of calcite (CaCO3).
3.6.4. Particle Size Distribution Analysis
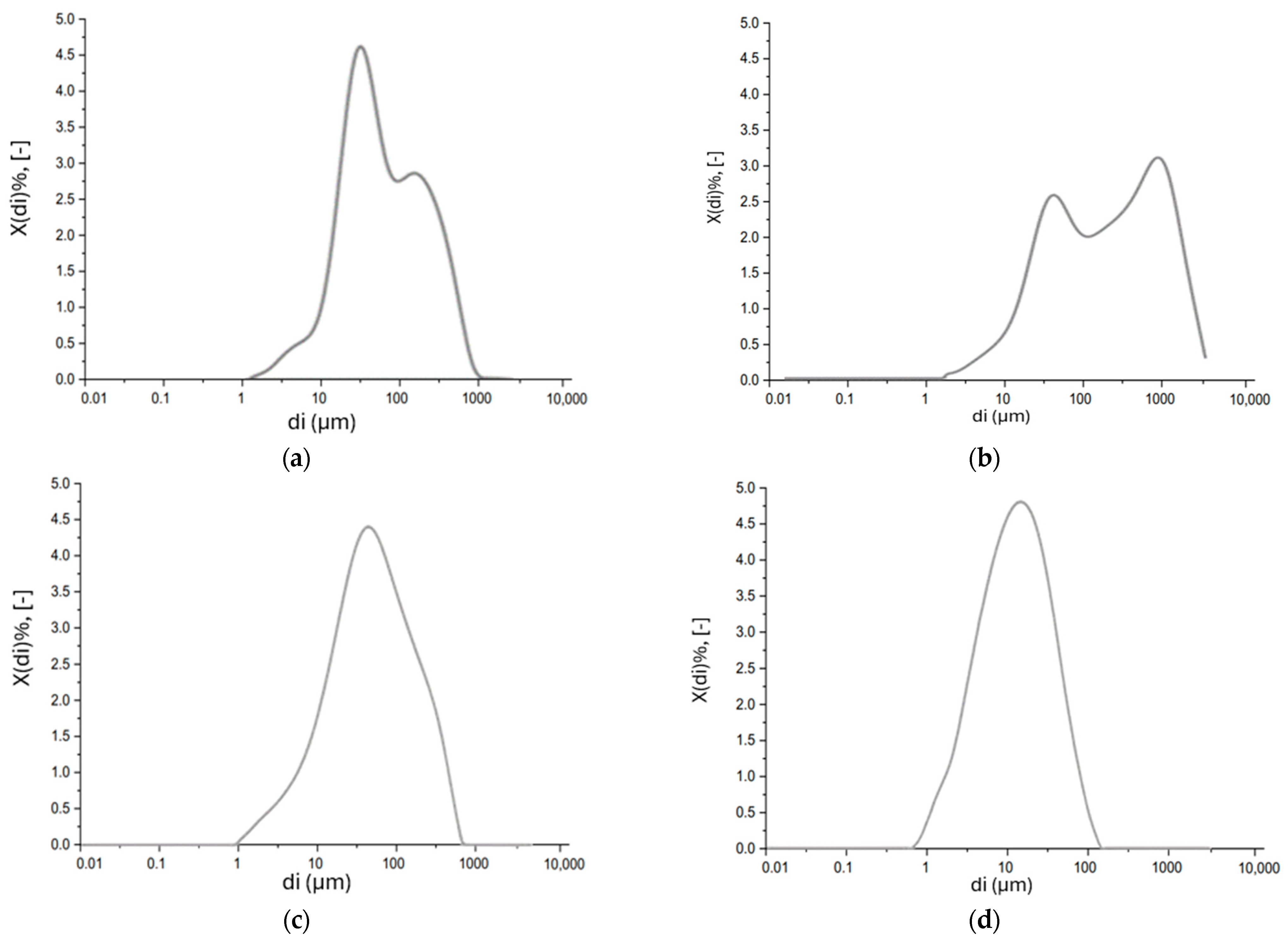
The particle size distribution was determined using a Malvern Instruments Mastersizer 2000 laser scattering analyzer (particle size range: 0.02 to 2000 μm). The absolute particle size distribution of MSWI-FA and W-FA is presented in Table 7 below.

Table 7.
Particle size distribution of MSWI-FA and W-FA.
Bimodal particle size distribution is observed for both MSWI-FA and W-FA, with the main modal values equal to 66.9 μm and 454 μm for MSWI-FA and 42.8 μm and 916 μm for W-FA (Figure 8a,b below). In particular, for MSWI-FA the D10 is 14.8 μm, the D50 is 63.7 μm, and the D90 is 131 μm, while for W-FA the D10 is 19.6 μm, the D50 is 198 μm, and the D90 is 1320 μm.
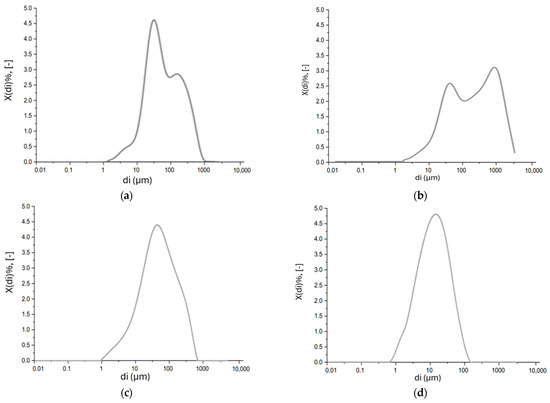
Figure 8.
Particle size distribution analysis of (a) MSWI-FA, (b) W-FA, (c) GGBFS, and (d) MS.
The absolute particle size distribution of GGBFS and MS is reported (Figure 8c,d below).
GGBFS exhibits a monomodal absolute particle size distribution, and the representative diameter of the particle population is 33.2 μm. The marble sludge appears to have a monomodal particle size distribution, and the representative diameter of the particle population is 15.5 μm.
3.6.5. Leaching and pH Assessment of MSWI-FA
Table 8 summarizes the results regarding pH, chloride and sulphate concentrations of untreated MSWI-FA and W-FA, as well as the same values regarding the water resulting from the washing procedure.

Table 8.
Leaching test results for FA, washed FA, and washing water.
MSWI-FA shows a high chloride content and low sulfate content. The residual chloride content in the W-FA was not significantly lower than the initial levels observed in untreated FA. In contrast, the sulfate content in W-FA was even higher compared to MSWI-FA. A notable increase in calcium content in the washed FA can be attributed to the concentration of this element, resulting from mass loss during the washing procedure due to the solubilization of salts.
4. Results
The mixture used to stabilize the soils consist of a combination of Portland cement, granulated blast furnace slag, fly ash from municipal solid waste incineration, marble sludge, and the contaminated soil matrix. These materials are selected for their chemical and physical properties, which help enhance the final characteristics of the product. The soil, its grain size, plasticity, and composition will naturally influence the final behavior of the stabilized material.
Table 9 presents the weight percentages of the various components used in the formulation of the mixture for the double granulation process.

Table 9.
Weight percentages of the components in the produced mixture.
To create an environmentally sustainable mix, using different materials instead of traditional cement alone, their quantities were selected based on various factors through multiple trials. Soil was chosen as the base of the mix, as it is the material to be treated and therefore constitutes the largest proportion. As a result, only 30% of the mix remains available for the other four components, which were selected for their role in the soil inertization process to achieve the highest possible resistance. Among these components, ground granulated blast furnace slag was chosen as the predominant one due to its ability to enhance long-term mechanical resistance and act as a hydraulic binder [66]. Its activation is facilitated by the presence of cement, which is included in smaller quantities. Cement slurries contribute to the mix by increasing fineness, improving cohesion, and enhancing initial strength through their calcium carbonate (CaCO3) content. Additionally, they help activate GGBFS and MSWI-FA. Lastly, a small amount of MSWI-FA is included, as it reacts with cement and water to form pozzolanic compounds, improving durability and chemical resistance.
Process of Material Agglomeration
Several methods are used to stabilize contaminated soils, one of which is pelletization, the method employed in our process [67,68]. Thanks to this process, the finer moistened particles transform into larger solid material. The strength of the pellets is achieved through collisions in the pelletizer disc, which requires minimal operating space and allows for precise control over pellet size [69,70]. Performance depends on factors such as moisture, particle shape, size, and the adjustments of the pelletizer’s speed and angle [71].
A correct balance of these parameters is essential to avoid problems in pellet formation and optimize efficiency [68]. Additionally, the speed and angle of the pelletizer, as well as the process duration, significantly influence the outcome. At low speeds, gravitational force dominates, whereas at high speeds, centrifugal force prevails. Achieving a proper balance of these parameters is crucial to avoid loose pellet structures or interruptions in the process. Optimally adjusting the angle and speed of the disc improves production efficiency. This technique transforms fine, humidified particles into solid pellets.
In this study, a water content of 10% was used in the mixture. This percentage was selected to ensure proper cohesion of the materials during the granulation process. Additionally, it was determined that a working time of 15 min is ideal for producing well-formed and uniform granules.
The pelletization process was carried out using a granulator equipped with a rotating plate with a diameter of 80 cm. In the experimental tests conducted, the plate’s rotation speed was varied between 15 and 45 rpm, and the inclination angle was adjusted within a range of 30° to 45°.
Laboratory investigations revealed that a 45° inclination angle combined with a speed of 45 rpm produced the best results in terms of the quality and consistency of the granules. Below these conditions, difficulties in mixing were observed, with low efficiency in the agglomeration process.
Table 10 provides a clear summary of the tested parameters, with the indication of the optimal value that produced the best results during the granulation process.

Table 10.
Characteristics of pelletization process.
Regarding the granule size, the granules formed during the process typically have a diameter ranging from 5 to 8.5 mm. Specifically, the resulting samples were cast in cylindrical steel molds with a radius of 10 cm and a height of 20 cm and left to cure for 28 days in a room at ambient temperature.
5. Discussion
The results of the chemical–physical and geotechnical analyses of the contaminated soil samples, along with the characterization of green materials and pelletization tests, provide valuable insights into the potential for soil remediation and the use of industrial by-products as sustainable alternatives in the production of stabilized soils.
The geotechnical analyses revealed that the variability in the physical properties of the soils—such as compaction and moisture content—plays a crucial role in determining their suitability for practical applications. Samples with higher moisture content, such as Soil 5, tend to be more compact and stable, while those with lower moisture content, like Soil 3, are more permeable. These findings suggest that, before using contaminated soils in the stabilization process, a pretreatment phase to regulate moisture content and improve material cohesion would be beneficial, ensuring the quality and stability of the final product. The interaction between the soil’s physical structure and the presence of contaminants could significantly affect the treatment’s effectiveness, thus requiring careful management of process variables like moisture and soil compaction.
Leaching tests, which identified contamination primarily from heavy metals (As, Cr, and Cd), are particularly relevant, suggesting that while the stabilization process may reduce the accessibility of these contaminants, long-term stability must still be monitored. The pH-dependent, percolation, and dynamic leaching tests demonstrated how contaminant mobilization varies under different environmental conditions. Therefore, it is crucial to design the stabilization process with consideration for the materials’ ability to bind contaminants and prevent their release into the environment. Further analysis of the contaminants’ behavior over the product’s lifecycle could provide valuable insights for safer and more sustainable applications.
The characterization of green materials highlighted the potential of several industrial by-products, such as municipal solid waste fly ash, ground granulated blast furnace slag, and marble sludge, all of which, despite their differing chemical compositions, share characteristics that make them suitable for the stabilization of contaminated soils. The significant presence of calcium in many of these materials suggests that they could improve the mechanical properties and chemical stability of the resulting pellets. However, the variability in composition—such as the difference in chloride content between MSWI-FA and W-FA—emphasizes the importance of preliminary treatments, like washing, to reduce corrosion risks and enhance environmental stability. Washing appears to be a crucial step in lowering chloride and sulfate content, which would otherwise compromise the durability of the final product. The XRD analysis results, showing the presence of crystalline phases such as calcite and portlandite, support the possibility of improving the rheological properties and thermal stability of the finished products. Among other advantages, as also highlighted in previous work [32], the presence of GGBFS reduces pore volume and has been recognized increases the immobilization ratio of heavy metals.
Additionally, MS for improving the cohesion of the treated material and reducing the risks of contaminant dispersion. Compared to similar studies, our approach stands out for the integration of these materials into an innovative matrix that not only preserves immobilization properties but also offers significant advantages in terms of environmental sustainability and a reduction in greenhouse gas emissions.
From the stabilization perspective, the results from laboratory trials are crucial for understanding the optimal conditions needed to produce pellets with superior mechanical and chemical properties. Precise control over parameters like the speed and inclination of the pelletizing disk is essential for producing compact and uniform samples. Optimizing these parameters not only impacts the quality of the final product but also improves process efficiency, thereby reducing operational costs and enhancing the overall sustainability of production.
6. Conclusions
This study explores the application of MSWI-FA, GGBFS, and MS, combined with cement, for the stabilization of industrial soils contaminated by heavy metals. This treatment not only makes the soil safer but also provides the opportunity to valorize industrial waste, offering significant economic and environmental benefits. Some conclusions can be drawn, as follows:
- Fly ash and GGBFS, rich in silicon (Si) and aluminum, act as pozzolanic agents, enhancing the reactivity of the treated soil and promoting the formation of stable bonds with the cement. These chemical bonds create a robust structure that reduces soil permeability and improves its load-bearing capacity.
- The use of marble sludge as an additional material not only enables the valorization of industrial by-products but also enhances the cohesion of the treated soil, reducing the risk of concentration and improving its long-term stability. When combined with cement and pozzolanic materials, marble sludge contributes to improving the soil’s overall quality, making it more stable and resistant to mechanical stress.
- Using materials such as fly ash, blast furnace slag, and marble sludge helps reduce environmental impact compared to traditional methods, particularly when compared to cement, which produces higher greenhouse gas emissions.
Stabilized soils are commonly used in road infrastructure as subbases or bases for roads, highways, and bike paths. In the construction sector, they serve as foundations for buildings and civil engineering works, improving soil bearing capacity and reducing the need for additional aggregates. They are also employed in industrial and logistics areas to create durable surfaces for yards, ports, and loading/unloading zones. In environmental rehabilitation projects, stabilized soils contribute to the redevelopment of degraded areas, such as former quarries or contaminated sites. Additionally, they are used in hydraulic works for the construction of embankments, canals, and containment basins. In the transportation sector, they enhance the stability of railway embankments and airport infrastructure.
Several aspects of this work open avenues for further research and broader applications. Future studies should focus on the rheological characterization of the inert products produced by the new mixtures. Additionally, these products will need to be classified based on their characterization and designated for new uses. Another valuable line of investigation would involve testing other materials to fine-tune the mix design based on the specific contaminants present in the soil, optimizing the process for various types of contamination. A key aspect concerns the long-term durability of the treated soils. This can be studied, for example, through expansion tests to evaluate the possible swelling of the soil when exposed to humidity or variable climatic conditions, or pH tests to assess chemical changes over time. Specifically, the material’s resistance to freeze–thaw cycles and humidity fluctuations is crucial for assessing the sustainability and safety of the treatment under varying environmental conditions such as the two mentioned. Currently, this work aims to refine the stabilization process, but an evaluation of long-term durability under variable environmental conditions is a critical goal for future research directions in terms of material’s resistance to freeze–thaw cycles and humidity changes to better understand its ability to maintain stabilization over time.
Future research will also include comparisons between newly developed inert products, evaluating not only their mechanical and environmental performance but also their long-term durability under different environmental conditions, to ensure more efficient and sustainable solutions for the remediation of contaminated industrial soils.
Author Contributions
Conceptualization: A.P. and F.F.; Methodology: A.A. and G.D.C.; Investigation: G.D.C., I.F. and F.C.; Data Curation: A.P., A.A. and G.D.C.; Writing—Original Draft Preparation: A.P., G.D.C. and A.A.; Writing—Review and Editing: F.F., I.F. and A.P.; Supervision: A.P., F.F. and I.F. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support under the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.1, Call for tender No. 1409 published on 14 September 2022 by the Italian Ministry of University and Research (MUR), funded by the European Union–NextGenerationEU–Project Title: “STABilization of contaminated SOILs (STABSOIL)”. CUP I53D23006430001, Grant Assignment Decree No. 1385 adopted on 1 September 2023 by the Italian Ministry of Ministry of University and Research (MUR). It has also been supported by the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.1, Call for tender No. 104 published on 2.2.2022 by the Italian Ministry of University and Research (MUR), funded by the European Union—NextGenerationEU—Project Title ‘Innovative tensegrity lattices and architectured metamaterials’ (ILAM)—20224LBXMZ—CUP D53D23003020006 (F.F. PI). F.F. also acknowledges support by the Italian Ministry of Foreign Affairs and International Cooperation within the Italy-USA Science and Technology Cooperation Program 2023–2025, Project ‘Next-generation green structures for natural disaster-proof buildings’, grant number US23GR15. G.D.C. thanks the Italian Dottorato di Interesse Nazionale ‘Defense against Natural Risks and Ecological Transition of Built Environment’.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the great support received by Gerardo Carpentieri (University of Salerno) in the development of the present work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jarsjö, J.; Andersson-Sköld, Y.; Fröberg, M.; Pietroń, J.; Borgström, R.; Löv, Å.; Kleja, D.B. Projecting impacts of climate change on metal mobilization at contaminated sites: Controls by the groundwater level. Sci. Total Environ. 2020, 712, 135560. [Google Scholar] [PubMed]
- Tóth, G.; Hermann, T.; Da Silva, M.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [PubMed]
- Li, Z.Y.; Ma, Z.W.; van der Kuijp, T.J.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. An evaluation of technologies for the heavy metal remediation of dredged sediments. J. Hazard. Mater. 2001, 85, 145–163. [Google Scholar]
- Lewis, N.M.; Parker, R.A. Awma update of the u.s. environmental protection agency’s site emerging technology program. J. Air Waste Manag. Assoc. 1993, 44, 195–203. [Google Scholar] [CrossRef]
- Conner, J.R. Guide to Improving the Effectiveness of Cement-Based Stabilization/Solidification; Portland Cement Association: Washington, DC, USA, 1997. [Google Scholar]
- Wilk, C.M. Solidification/Stabilization Treatment and Examples of Use at Port Facilities; Portland Cement Association: Washington, DC, USA, 2004. [Google Scholar]
- Youcai, Z. Solidification/Stabilization Process of Fly Ash. In Pollution Control and Resource Recovery; Municipal Solid Wastes Incineration: Oxford, UK, 2017. [Google Scholar]
- Reza, A.; Anzum, S.; Saha, R.C.; Chakraborty, S. Implementation of solidification/stabilization process to reduce hazardous impurities and stabilize soil matrices. E3S Web Conf. 2018, 96, 01003. [Google Scholar]
- Jama, N.S.; Saeed, K.A. Pozzolanic materials for stabilization /solidification of soil contaminated by heavy metals—A review. J. Eng. Sustain. Dev. 2023, 27, 487–498. [Google Scholar]
- Bates, E.R.; Dean, P.V.; Klich, I. Chemical Stabilization of Mixed Organic and Metal Compounds: EPA SITE Program Demonstration of the Silicate Technology Corporation Process. J. Air Waste Manag. Assoc. 1992, 42, 724–728. [Google Scholar]
- Wang, L.; Tsang, D.; Poon, C.S. Green remediation and recycling of contaminated sediment by waste-incorporated stabilization/solidification. Chemosphere 2014, 122, 257–264. [Google Scholar]
- Kasmi, A.; Abriak, N.E.; Benzerzour, M.; Azrar, H. Environmental impact and mechanical behavior study of experimental road made with river sediments: Recycling of river sediments in road construction. J. Mater. Cycles Waste Manag. 2017, 19, 1405–1414. [Google Scholar]
- Hildebrandt, T.; Pick, D.; Einax, J.W. Improvement of sampling strategies for randomly distributed hotspots in soil applying a computerized simulation considering the concept of uncertainty. Environ. Sci. Pollut. Res. 2012, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.; Adrover, M.; Frontera, A.; Ortega-Castro, J.; Miró, M. In-vitro prediction of the membranotropic action of emerging organic contaminants using a liposome-based multidisciplinary approach. Sci. Total Environ. 2020, 738, 140096. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, S.; Salata, S.; Arcidiacono, A.; Piroli, E.; Montanarella, L. Policy instruments for soil protection among the EU member states: A comparative analysis. Land Use Policy 2019, 82, 763–780. [Google Scholar] [CrossRef]
- ISO 23611-6; Soil Quality Sampling of Soil Invertebrates. Part 6: Guidance for the Design of Sampling Programmes with Soil Invertebrates. International Organization for Standardization: Geneva, Switzerland, 2013.
- Wang, L.K.; Sung Wang, M.H. Ecologically Sustainable Industrial Development, Hazardous Wastes Management, and Heavy Metals Removal from Landfill Leachate, Groundwater and Soil. In Control of Heavy Metals in the Environment Advanced Methods and Strategies for Heavy Metal Remediation and Environmental Protection; CRC Press: Boca Raton, FL, USA, 2025; pp. 269–286. [Google Scholar]
- Lasheen, M.R.; Ashmawy, A.M.; Ibrahim, H.S.; Moniem, S.M.A. Pozzolanic-based materials for stabilization/solidification of contaminated sludge with hazardous heavy metal: Case study. Desalination Water Treat. 2013, 51, 2644–2655. [Google Scholar] [CrossRef]
- Todaro, F.; Barjoveanu, G.; De Gisi, S.; Teodosiu, C.; Notarnicola, M. Sustainability assessment of reactive capping alternatives for the remediation of contaminated marine sediments. J. Clean. Prod. 2021, 286, 124946. [Google Scholar] [CrossRef]
- Bini, C. Environmental Impact of Abandoned Mine Waste: A Review; Nova Science Publishers, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Marsili, D. A cross-disciplinary approach to global environmental health: The case of contaminated sites. Ann. Ist. Super. Sanità 2016, 52, 516–523. [Google Scholar]
- Shi, C.; Spence, R. Designing of Cement-Based Formula for Solidification/Stabilization of Hazardous, Radioactive, and Mixed Wastes. Crit. Rev. Environ. Sci. Technol. 2004, 34, 391–417. [Google Scholar] [CrossRef]
- Paria, S.; Yuet, P. Solidification-stabilization of organic and inorganic contaminants using Portland cement: A literature review. Environ. Rev. 2006, 14, 217–255. [Google Scholar] [CrossRef]
- Todaro, F.; Colangelo, F.; De Gisi, S.; Farina, I.; Ferone, C.; Labianca, C.; Petrella, A.; Cioffi, R.; Notarnicola, M. Recycling of Contaminated Marine Sediment and Industrial By-Products through Combined Stabilization/Solidification and Granulation Treatment. Materials 2023, 16, 2399. [Google Scholar] [CrossRef]
- Couvidat, J.; Benzaazoua, M.; Chatain, V.; Bouamrane, A.; Bouzahzah, H. Feasibility of the reuse of total and processed contaminated marine sediments as fine aggregates in cemented mortars. Constr. Build. Mater. 2016, 112, 892–902. [Google Scholar] [CrossRef]
- Mohamad, N.; Muthusamy, K.; Embong, R.; Kusbiantoro, A. Environmental impact of cement production and Solutions: A review. Mater. Today Proc. 2022, 48, 741–746. [Google Scholar] [CrossRef]
- Shen, W.; Cao, L.; Li, Q.; Zhang, W.; Wang, G.; Li, C. Quantifying CO2 emissions from China’s cement industry. Renew. Sustain. Energy Rev. 2015, 50, 1004–1012. [Google Scholar]
- Singh, N.; Farina, I.; Petrillo, A.; Colangelo, F.; De Felice, F. Carbon capture, sequestration, and usage for clean and green environment: Challenges and opportunities. Int. J. Sustain. Eng. 2023, 16, 248–268. [Google Scholar]
- Mao, Y.; Muhammad, F.; Yu, L.; Xia, M.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. The Solidification of Lead-Zinc Smelting Slag through Bentonite Supported Alkali-Activated Slag Cementitious Material. Int. J. Environ. Res. Public Health 2019, 16, 1121. [Google Scholar] [CrossRef] [PubMed]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Farina, I.; Singh, R.; Singh, M.; Preet, P.; Kumar, R.; Fraternali, F.; Colangelo, F. Thermomechanical and morphological properties of sustainable mortars employing blast furnace slag and fly ash reinforced cement. Mater. Sci. Eng. A 2020, 999, 012009. [Google Scholar]
- Wang, H.; Zhu, Z.; Pu, S.; Song, W. Solidification/Stabilization of Pb2+ and Cd2+ Contaminated Soil Using Fly Ash and GGBS Based Geopolymer. Arab. J. Sci. Eng. 2022, 47, 4385–4400. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar]
- Li, Y.; Qiao, C.; Ni, W. Green concrete with ground granulated blast-furnace slag activated by desulfurization gypsum and electric arc furnace reducing slag. J. Clean. Prod. 2020, 269, 122212. [Google Scholar]
- Sivakrishna, A.; Adesina, A.; Awoyera, P.; Kumar, K.R. Green concrete: A review of recent developments. Mater. Today Proc. 2020, 27, 54–58. [Google Scholar]
- Yin, C.Y.; Mahmud, H.; Shaaban, M. Stabilization/solidification of lead-contaminated soil using cement and rice husk ash. J. Hazard. Mater. 2006, 137, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Al-Tabba, A. Leachability and heavy metal speciation of 17-year old stabilised/solidified contaminated site soils. J. Hazard. Mater. 2014, 278, 144–151. [Google Scholar]
- Hu, Y.; Wang, J.; Yang, Y.; Li, S.; Pate, Q.; Nepovimova, E.; Zhang, X.; Kuca, K. Revolutionizing soil heavy metal remediation: Cutting-edge innovations in plant disposal technology. Sci. Total Environ. 2024, 918, 170577. [Google Scholar] [CrossRef]
- Zhanga, Y.; Hua, X.; Wang, Y.; Jianga, N. A critical review of biomineralization in environmental geotechnics: Applications, trends, and perspectives. Biogeotechnics 2023, 1, 100003. [Google Scholar] [CrossRef]
- De Gisi, S.; Labianca, C.; Todaro, F.; Notarnicola, M. Stabilization/solidification of contaminated marine sediment. In Low Carbon Stabilization and Solidification of Hazardous Wastes; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Khan, M.I.; Irfan, M.; Aziz, M.; Khan, A.H. Geotechnical characteristics of effluent contaminated cohesive soils. J. Environ. Eng. Landsc. Manag. 2016, 25, 75–82. [Google Scholar]
- El-Mahllawy, M.S.; Mohsen, S.A. Characterization and utilization capabilities of industrial wastes for green bricks production. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 57. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Jelínek, N.; Petrlík, J.; Bremmer, J.; Kuepouo, G.; Ochieng, G.; Ožanová, S.; Bell, L. Waste Incineration and the Environment; Elsevier: Amsterdam, Netherlands, 2024. [Google Scholar]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar]
- Akamad, D.K.; Sravani, G.; Nagendrasai, K. Advancements in Bioremediation Techniques: A comprehensive Review with Implications for Aquaculture Sustainability. Acta Sci. Vet. Sci. 2024, 6, 40–51. [Google Scholar]
- Souza, L.; Pomarolli, L.; Veiga, M. From classic methodologies to application of nanomaterials for soil remediation: An integrated view of methods for decontamination of toxic metal(oid)s. Environ. Sci. Pollut. Res. 2020, 27, 10205–10227. [Google Scholar] [CrossRef]
- Tantawy, M.; El-Roudi, A.; Salem, A. Immobilization of Cr (VI) in bagasse ash blended cement pastes. Constr. Build. Mater. 2012, 30, 218–223. [Google Scholar]
- Curti, M.; Napoleoni, Q.; Pardini, A.; Scarapazzi, M. Il consolidamento delle torbe con Stabilizzazione di massa. Strade Autostrade 2008, 70, 108–114. [Google Scholar]
- Ahnberg, H. Strength of Stabilized Soils—A Laboratory Study on Clays and Organic Soils Stabilized with Different Types of Binder; Swedish Deep Stabilization Research Cente: Linköping, Sweden, 2006. [Google Scholar]
- EN 197-1; Cement, Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- Hiremath, P.; Parray, A.B.; Hassan, A.; Kankeri, P. Durability of Green Concrete Composed of Fly Ash, GGBS, Quarry Dust, and RCA after Acid Exposure. Pract. Period. Struct. Des. Constr. 2024, 29, 04024047. [Google Scholar]
- Chindaprasirt, P.; Kanchanda, P.; Sathonsaowaphak, A.; Cao, H.T. Sulfate resistance of blended cements containing fly ash and rice husk ash. Constr. Build. Mater. 2007, 21, 1356–1361. [Google Scholar]
- Roy, D.; Arjunan, P.; Silsbee, M. Effect of silica fume, metakaolin, and low-calcium fly ash on chemical resistance of con-crete. Cem. Concr. Res. 2001, 31, 1809–1813. [Google Scholar]
- Wu, Y.; Zhang, X.; Li, X.; Zhang, L.; Li, Y. Ground granulated blast furnace slag for stabilization of heavy metals in contaminated soils. J. Hazard. Mater. 2019, 366, 123–131. [Google Scholar]
- Khan, A.; Khan, M.T.; Ahad, M.Z.; Adil, M.; Shah, M.A.; Shah, S.K. Strength assessment of green concrete for structural use. J. Mech. Continua Math. Sci. 2020, 15, 294–305. [Google Scholar]
- Tozsin, G.; Arol, A.İ.İ.; Öztaş, T.; Kalkan, E. Using marble wastes as a soil amendment for acidic soil neutralization. J. Environ. Manage 2014, 133, 374–377. [Google Scholar]
- Volante, M.; Marmai, S.; Attuati, S.; Rinaldi, L.; Guarneri, R.; Amico, F.; Calvisi, F. La macinazione sotto 4 mm dei materiali granulari per il test di cessione secondo le norme UNI EN 12457-2 e UNI 10802. Una questione ancora aperta. BEA boLLettino 2013, 4, 31–37. [Google Scholar]
- UNI EN 12457-2; Characterization of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One-Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 4 mm (Without or With Size Reduction). European Committee for Standardization: Brussels, Belgium, 2002.
- UNI EN ISO 17294-2; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. International Organization for Standardization: Geneva, Switzerland, 2016.
- UNI EN 1484:1999; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). European Committee for Standardization: Brussels, Belgium, 1999.
- UNI EN 15216:2008; Environmental Solid Matrices—Determination of Total Dissolved Solids (TDS) in Water and Eluates. European Committee for Standardization: Brussels, Belgium, 2008.
- Yuan, D.; Liang, X.; Gao, Y.; Ping, H.; Wang, C.; Ma, J.; Zheng, Y.; Jing, J.; Qi, Y.; Zhai, Y.; et al. High-temperature modification of steel slag using composite modifier containing silicon calcium slag, fly ash, and reservoir sediment. Front. Earth Sci. 2023, 11, 1214182. [Google Scholar]
- Ferraro, A.; Ducman, V.; Colangelo, F.; Korat, L.; Spasiano, S.; Farina, F. Production and characterization of lightweight aggregates from municipal solid waste incineration fly-ash through single- and double-step pelletization process. J. Clean. Prod. 2023, 383, 135275. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; Wang, Y.; Liang, S. Experimental study on solidification/stabilization of high-salt sludge by alkali-activated GGBS and MSWI bottom ash cementitious materials. Case Stud. Constr. Mater. 2023, 19, e02417. [Google Scholar]
- Bekkeri, G.B.; Shetty, K.K.; Nayak, G. Synthesis of artifcial aggregates and their impact on performance of concrete: A review. J. Mater. Cycles Waste Manag. 2023, 25, 1988–2011. [Google Scholar] [CrossRef]
- Colangelo, F.; Farina, I.; Travaglioni, M.; Salzano, C.; Cioffi, R.; Petrillo, A. Innovative Materials in Italy for Eco-Friendly and Sustainable Buildings. Materials 2021, 14, 2048. [Google Scholar] [CrossRef]
- Manikandan, R.; Ramamurthy, K. Influence of fineness of fly ash on the aggregate pelletization process. Cem. Concr. Compos. 2007, 29, 456–464. [Google Scholar] [CrossRef]
- Harikrishnan, K.I.; Ramamurthy, K. Influence of pelletization process on the properties of fly ash aggregates. Waste Manag. 2006, 26, 846–852. [Google Scholar] [CrossRef]
- Tajra, F.; Elrahman, M.A.; Stephan, D. The production and properties of cold-bonded aggregate and its applications in concrete: A review. Constr. Build. Mater. 2019, 225, 29–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).