Effectiveness of Photobiomodulation Therapy in the Management of Fibromyalgia Syndrome: A Systematic Review
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Selection of Studies
2.3. Data Extraction
2.4. Methodological Quality Assessment: PEDro Scale
2.5. Risk of Bias Assessment: ROB 2.0
2.6. Quality of Evidence: GRADE
3. Results
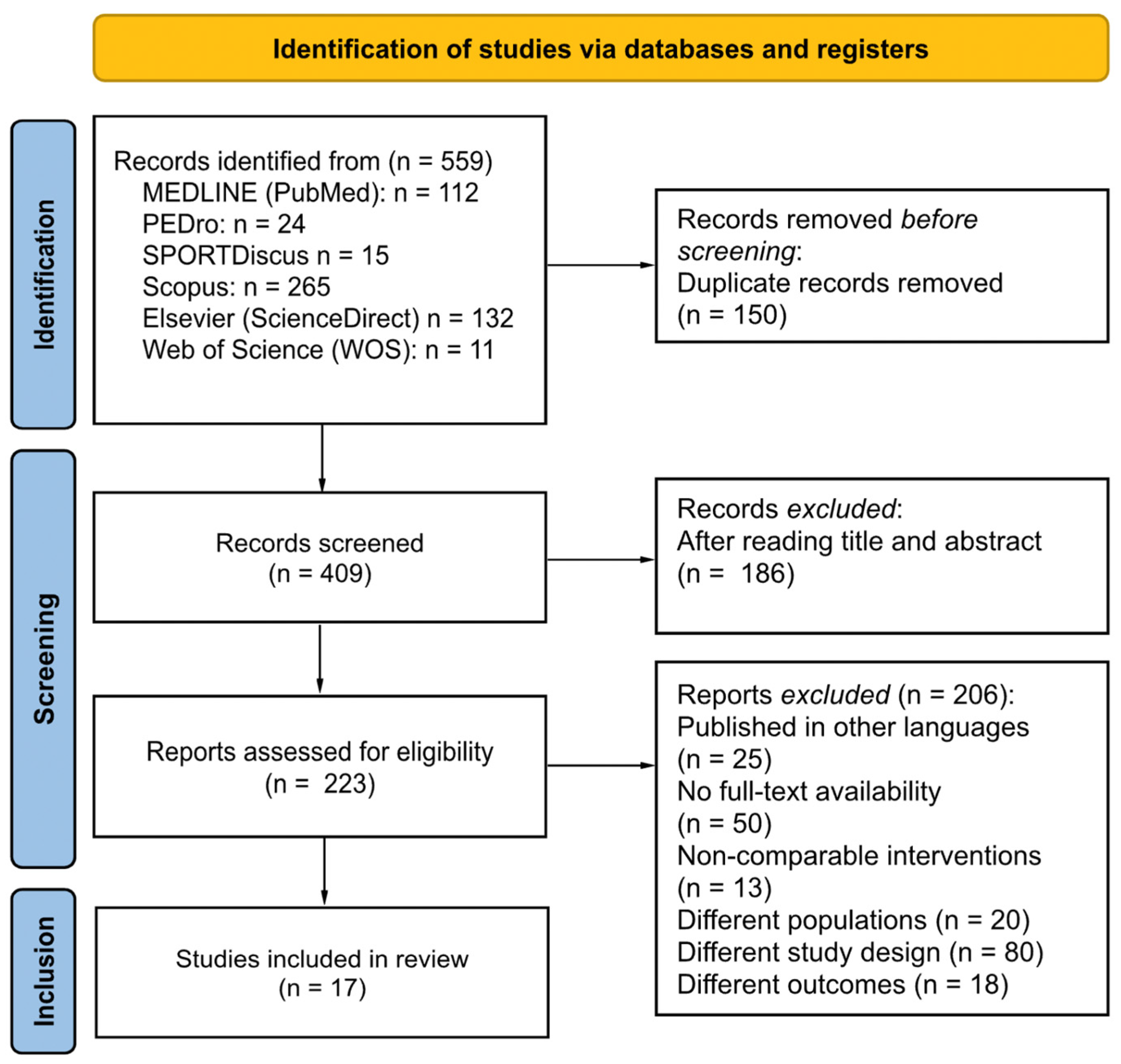
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Methodological Quality Assessment
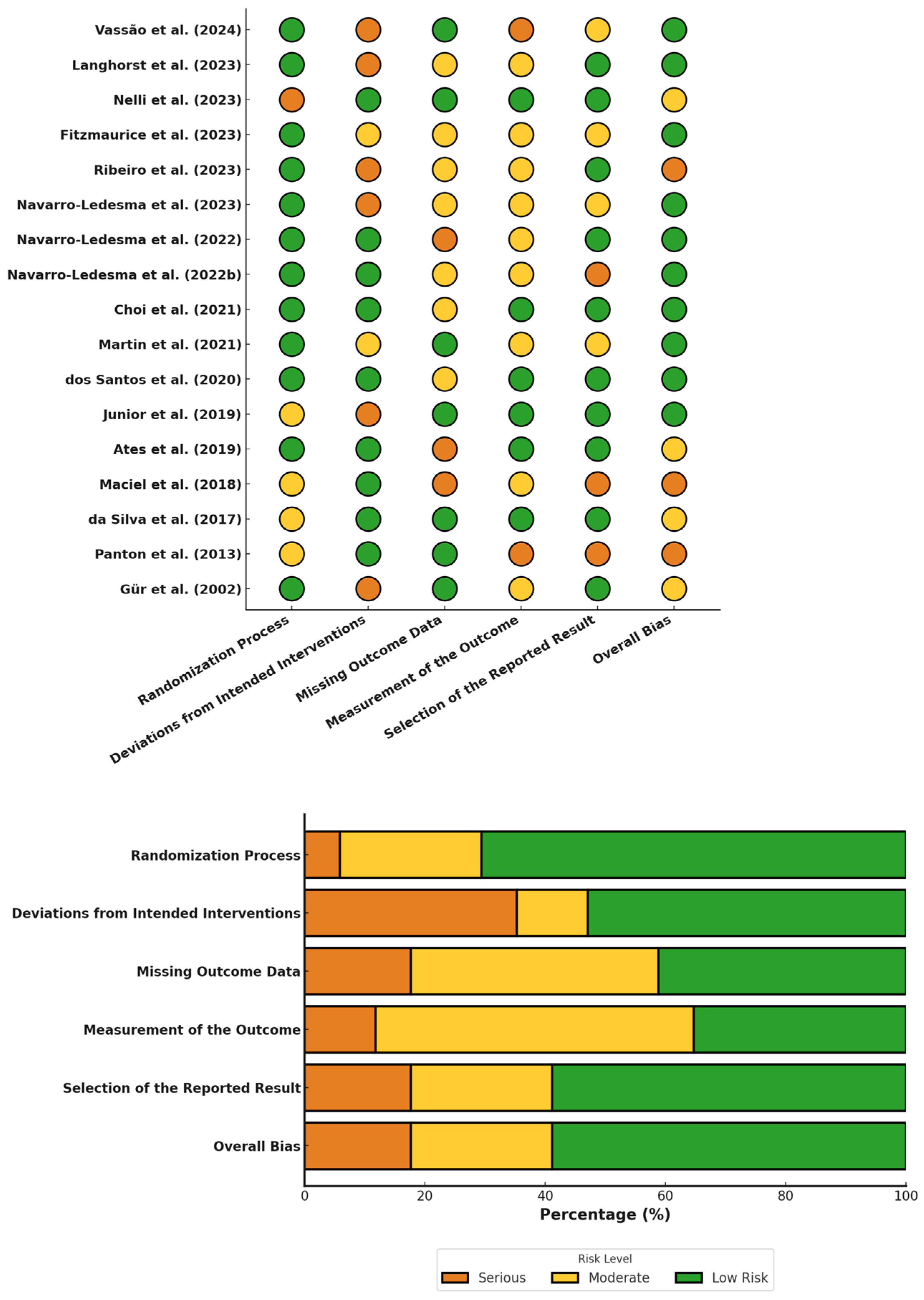
3.4. Risk of Bias Assessment
3.5. Grade of Recommendation
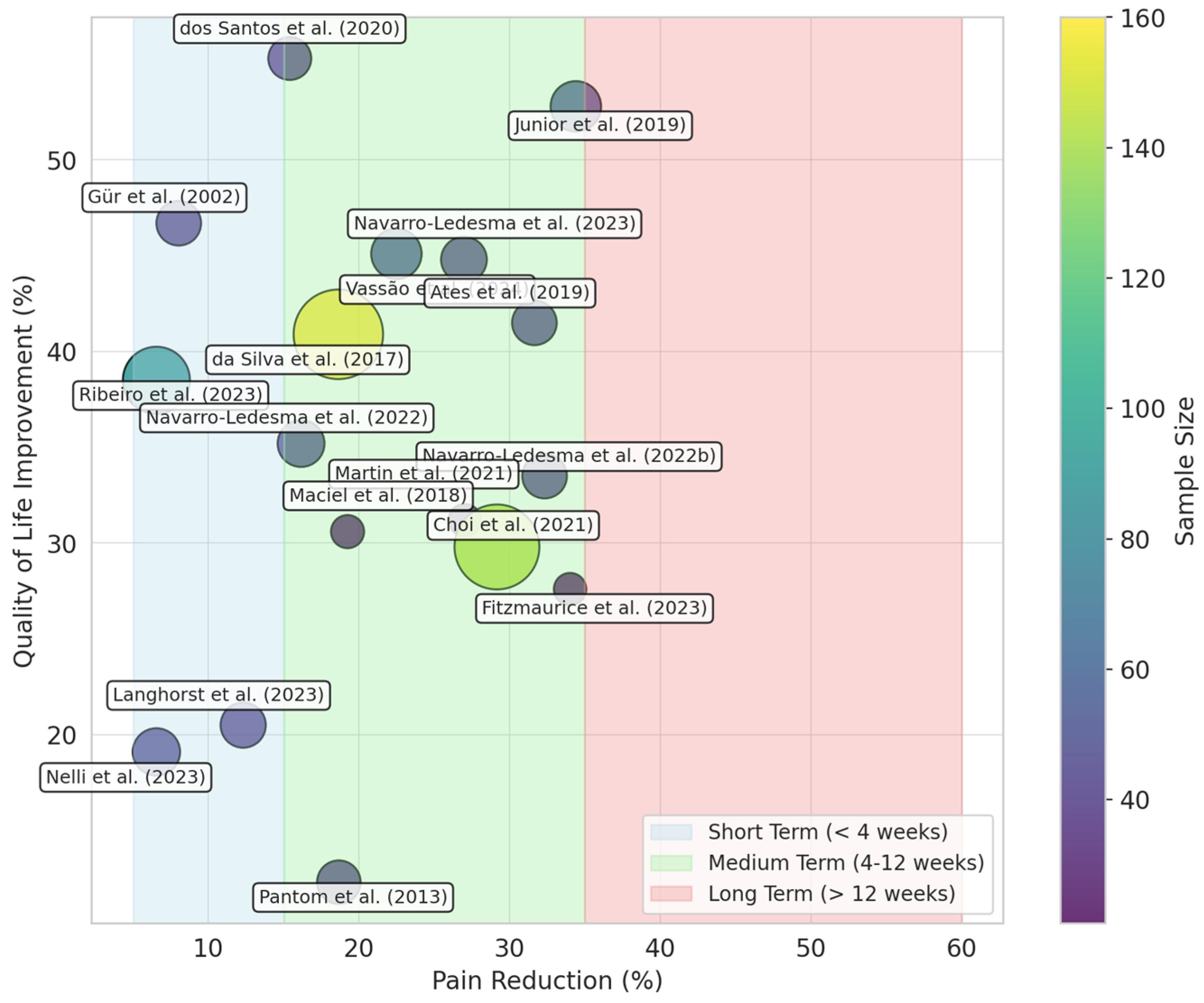
3.6. Data Synthesis
3.6.1. Photobiomodulation Therapy for Managing Pain Intensity in Fibromyalgia Patients
3.6.2. Photobiomodulation Therapy for Managing QoL in Fibromyalgia Patients
3.6.3. Photobiomodulation Therapy for Managing Fatigue in Fibromyalgia Patients
3.6.4. Photobiomodulation Therapy for Managing the Psychological Status in Fibromyalgia Patients
3.6.5. Photobiomodulation Therapy for Managing the Physiological Status in Fibromyalgia Patients
4. Discussion
4.1. Limitations
4.2. Recommendations for Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sluka, K.A.; Clauw, D.J. Neurobiology of Fibromyalgia and Chronic Widespread Pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Favretti, M.; Iannuccelli, C.; Di Franco, M. Pain Biomarkers in Fibromyalgia Syndrome: Current Understanding and Future Directions. Int. J. Mol. Sci. 2023, 24, 10443. [Google Scholar] [CrossRef]
- Jurado-Priego, L.N.; Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Fibromyalgia: A Review of the Pathophysiological Mechanisms and Multidisciplinary Treatment Strategies. Biomedicines 2024, 12, 1543. [Google Scholar] [CrossRef]
- Häuser, W.; Ablin, J.; Fitzcharles, M.A.; Littlejohn, G.; Luciano, J.V.; Usui, C.; Walitt, B. Fibromyalgia. Nat. Rev. Dis. Primers 2015, 1, 15022. [Google Scholar] [CrossRef]
- Vincent, A.; Benzo, R.P.; Whipple, M.O.; McAllister, S.J.; Erwin, P.J.; Saligan, L.N. Beyond Pain in Fibromyalgia: Insights into the Symptom of Fatigue. Arthritis Res. Ther. 2013, 15, 221. [Google Scholar] [CrossRef]
- Kosek, E. Disturbances of Pain Perception in Fibromyalgia. In Pathophysiology of Pain Perception; Plenum Series in Rehabilitation and Health; Lautenbacher, S., Fillingim, R.B., Eds.; Springer: Boston, MA, USA, 2004; pp. 93–108. [Google Scholar] [CrossRef]
- Martín Pérez, S.E.; Lucas Hernández, L.; Oliva de la Nuez, J.L.; Soussi El-Hammouti, A.; González Cobiella, T.; del Castillo Rodríguez, J.C.; Herrera Pérez, M.; Martín Pérez, I.M. Evaluation of Sleep Patterns and Chronotypes in Spanish Women with Fibromyalgia Syndrome: A Descriptive Cross-Sectional Study. J. Sleep Med. 2024, 21, 88–97. [Google Scholar] [CrossRef]
- Ibraheem, W.; Mckenzie, S.; Wilcox-Omubo, V.; Abdelaty, M.; Saji, S.E.; Siby, R.; Alalyani, W.; Mostafa, J.A. Pathophysiology and Clinical Implications of Cognitive Dysfunction in Fibromyalgia. Cureus 2021, 13, e19123. [Google Scholar] [CrossRef] [PubMed]
- Arout, C.A.; Sofuoglu, M.; Bastian, L.A.; Rosenheck, R.A. Gender Differences in the Prevalence of Fibromyalgia and in Concomitant Medical and Psychiatric Disorders: A National Veterans Health Administration Study. J. Womens Health 2018, 27, 1035–1044. [Google Scholar] [CrossRef]
- Ruschak, I.; Montesó-Curto, P.; Rosselló, L.; Aguilar Martín, C.; Sánchez-Montesó, L.; Toussaint, L. Fibromyalgia Syndrome Pain in Men and Women: A Scoping Review. Healthcare 2023, 11, 223. [Google Scholar] [CrossRef]
- Coles, M.L.; Weissmann, R.; Uziel, Y. Juvenile Primary Fibromyalgia Syndrome: Epidemiology, Etiology, Pathogenesis, Clinical Manifestations and Diagnosis. Pediatr. Rheumatol. 2021, 19, 22. [Google Scholar] [CrossRef]
- Minerbi, A.; Fitzcharles, M.A. Fibromyalgia in Older Individuals. Drugs Aging 2021, 38, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Ovrom, E.A.; Mostert, K.A.; Khakhkhar, S.; McKee, D.P.; Yang, P.; Her, Y.F. A Comprehensive Review of the Genetic and Epigenetic Contributions to the Development of Fibromyalgia. Biomedicines 2023, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Beiner, E.; Hermes, M.; Reichert, J.; Kleinke, K.; Vock, S.; Löffler, A.; Ader, L.; Sirazitdinov, A.; Keil, S.; Schmidt, T. Perceived and Endocrine Acute and Chronic Stress Indicators in Fibromyalgia Syndrome. Sci. Rep. 2024, 14, 30471. [Google Scholar] [CrossRef]
- Maes, M.; Almulla, A.F.; Zhou, B.; Algon, A.A.A.; Sodsai, P. In Major Dysmood Disorder, Physiosomatic, Chronic Fatigue and Fibromyalgia Symptoms are Driven by Immune Activation and Increased Immune-Associated Neurotoxicity. Sci. Rep. 2024, 14, 7344. [Google Scholar] [CrossRef]
- Minhas, D.; Murphy, A.; Clauw, D.J. Fibromyalgia and Centralized Pain in the Rheumatoid Arthritis Patient. Curr. Opin. Rheumatol. 2023, 35, 170–174. [Google Scholar] [CrossRef]
- Torrente-Segarra, V.; Carbonell-Abelló, J.; Castro-Oreiro, S.; Manresa Domínguez, J.M. Association Between Fibromyalgia and Psychiatric Disorders in Systemic Lupus Erythematosus. Clin. Exp. Rheumatol. 2010, 28, S22–S26. [Google Scholar] [PubMed]
- Ruiz-Pablos, M.; Paiva, B.; Montero-Mateo, R.; Garcia, N.; Zabaleta, A. Epstein-Barr Virus and the Origin of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Front. Immunol. 2021, 12, 656797. [Google Scholar] [CrossRef]
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia Prevalence, Somatic Symptom Reporting, and the Dimensionality of Polysymptomatic Distress: Results from a Survey of the General Population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef]
- Font Gayà, T.; Bordoy Ferrer, C.; Juan Mas, A.; Seoane-Mato, D.; Álvarez Reyes, F.; Delgado Sánchez, M.; Martínez Dubois, C.; Sánchez-Fernández, S.A.; Marena Rojas Vargas, L.; García Morales, P.V.; et al. Prevalence of Fibromyalgia and Associated Factors in Spain. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S123), 47–52. [Google Scholar]
- Salaffi, F.; Di Carlo, M.; Farah, S.; Mariani, C.; Fulginei, S.; Martino, G.P.; Sarzi-Puttini, P. Cross-Sectional Research on Female Workers Examining the Loss of Productivity Caused by Mild, Moderate, and Severe Fibromyalgia. Clin. Exp. Rheumatol. 2022, 40, 1151–1158. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluss, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR Revised Recommendations for the Management of Fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- El Miedany, Y.; Gadallah, N.; Mohasseb, D.; Nasr, A.; Sidhom, Y.; Mekkawy, D.; Kabil, N.; Fahmy, R.; Youssef, H.; Taha, S.; et al. Consensus Evidence-Based Clinical Practice Recommendations for the Management of Fibromyalgia. Egypt. Rheumatol. Rehabil. 2022, 49, 30. [Google Scholar] [CrossRef]
- Moore, R.A.; Fisher, E.; Häuser, W.; Bell, R.F.; Perrot, S.; Bidonde, J.; Makri, S.; Straube, S. Pharmacological Therapies for Fibromyalgia (Fibromyalgia Syndrome) in Adults—An Overview of Cochrane Reviews (Protocol). Cochrane Database Syst. Rev. 2021, 8, CD013151. [Google Scholar] [CrossRef]
- Glass, G.E. Photobiomodulation: The clinical applications of low-level light therapy. Aesthetic Surg. J. 2021, 41, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Capon, W.; Pang, V.; Vila, D.; Bicknell, B.; McLachlan, C.S.; Kiat, H. Photophysical mechanisms of photobiomodulation therapy as precision medicine. Biomedicines 2023, 11, 237. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Markoulli, M.; Chandramohan, N.; Papas, E.B. Photobiomodulation (low-level light therapy) and dry eye disease. Clin. Exp. Optom. 2021, 104, 561–566. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Lesniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Álvarez-Martínez, M.; Borden, G. A Systematic Review on Whole-Body Photobiomodulation for Exercise Performance and Recovery. Lasers Med. Sci. 2025, 40, 55. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Vassão, P.G.; Silva, M.F.; Maciel, M.J.; Ribeiro, A.L.; Navarro-Ledesma, S. Whole-body photobiomodulation therapy in Fibromyalgia patients: A randomized controlled trial. J. Clin. Med. 2024, 13, 1234. [Google Scholar] [CrossRef]
- Fitzmaurice, B.C.; Heneghan, N.R.; Rayen, A.T.A.; Grenfell, R.L.; Soundy, A.A. Whole-Body Photobiomodulation Therapy for Fibromyalgia: A Feasibility Trial. Behav. Sci. 2023, 13, 717. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Koch, A.K.; Kehm, C.; Öznur, Ö.; Engler, H.; Häuser, W. Mild Water-Filtered Infrared-A Whole-Body Hyperthermia Reduces Pain in Patients with Fibromyalgia Syndrome—A Randomized Sham-Controlled Trial. J Clin Med. 2023, 12, 2945. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S.; Vassão, P.G.; Ribeiro, A.L.; Maciel, M.J.; da Silva, M.F. Photobiomodulation therapy improves mitochondrial function and reduces oxidative stress in Fibromyalgia patients: A randomized controlled trial. Antioxidants 2022, 11, 987. [Google Scholar] [CrossRef]
- Nelli, A.H.; Wright, M.C.; Gulur, P. Green light-based analgesia-novel nonpharmacological approach to fibromyalgia pain: A pilot study. Pain Physician 2023, 26, 403–410. [Google Scholar]
- Ribeiro, N.F.; Leal-Junior, E.C.P.; Johnson, D.S.; Demchak, T.; Machado, C.M.; Dias, L.B.; De Oliveira, M.F.; Lino, M.F.; Lino, M.M.; Rodrigues, W.D.; et al. Photobiomodulation Therapy Combined with Static Magnetic Field Is Better than Placebo in Patients with Fibromyalgia: A Randomized Placebo-Controlled Trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 754–762. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S.; Gonzalez-Muñoz, A.; Carroll, J.; Burton, P. Short- and Long-Term Effects of Whole-Body Photobiomodulation on Pain, Functionality, Tissue Quality, Central Sensitisation and Psychological Factors in a Population Suffering from Fibromyalgia: Protocol for a Triple-Blinded Randomised Clinical Trial. Ther. Adv. Chronic Dis. 2022, 13, 20406223221078095. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S.; Carroll, J.; González-Muñoz, A.; Pruimboom, L.; Burton, P. Changes in Circadian Variations in Blood Pressure, Pain Pressure Threshold and the Elasticity of Tissue after a Whole-Body Photobiomodulation Treatment in Patients with Fibromyalgia: A Tripled-Blinded Randomized Clinical Trial. Biomedicines 2022, 10, 2678. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Porreca, F.; Mata, E.I.; Salloum, M.; Goel, V.; Gunnala, P.; Killgore, W.D.S.; Jain, S.; Jones-MacFarland, F.N.; Khanna, R.; et al. Green light exposure improves pain and quality of life in fibromyalgia patients: A preliminary one-way crossover clinical trial. Pain Med. 2021, 22, 118–130. [Google Scholar] [CrossRef]
- Choi, S.; dos Santos, L.; Junior, M.; Ates, B.; Maciel, M.J. Photobiomodulation therapy improves sleep quality and circadian rhythm in Fibromyalgia patients: A randomized controlled trial. Sleep Med. 2021, 87, 245–252. [Google Scholar] [CrossRef]
- dos Santos, R.C.; Souza Guedes, K.W.H.S.; de Sousa Pinto, J.M.; Oliveira, M.F. Acute low-level laser therapy effects on peripheral muscle strength and resistance in patients with fibromyalgia. Lasers Med. Sci. 2020, 35, 505–510. [Google Scholar] [CrossRef]
- Junior, A.E.A.; Carbinatto, F.M.; Fernandes, A.C.; Franco, D.M.; de Lara, A.A.B.; Bagnato, V.S. The Combined Fotobiomodulation and Therapeutic Ultrasound: How does the Efficient Treatment of Fibromyalgia by the Palms Promote a Prolonged Effect? J. Nov. Physiother. 2021, 11, 472. [Google Scholar]
- Ates, Z.; Cogalgil, S. Comparing efficiencies of local anesthetic injection and photobiomodulation in the treatment of fibromyalgia syndrome. Aegean J. Med. Sci. 2019, 3, 112–118. [Google Scholar] [CrossRef]
- Maciel, D.G.; da Silva, M.T.; Rodrigues, J.A.; Viana Neto, J.B.; de França, I.M.; Melo, A.B.M.; Barros da Silva, T.Y.P.; Vieira, W.H.d.B. Low-level laser therapy combined to functional exercise on treatment of fibromyalgia: A double-blind randomized clinical trial. Lasers Med. Sci. 2018, 33, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.M.; Albertini, R.; de Carvalho, P.T.C.; Leal-Junior, E.C.P.; Bussadori, S.K.; Vieira, S.S.; Bocalini, D.S.; de Oliveira, L.V.F.; Grandinetti, V.; Silva, J.A., Jr.; et al. Randomized, Blinded, Controlled Trial on Effectiveness of Photobiomodulation Therapy and Exercise Training in the Fibromyalgia Treatment. Lasers Med. Sci. 2017, 33, 343–351. [Google Scholar] [CrossRef]
- Panton, L.B.; Simonavice, E.; Williams, K.; Mojock, C.; Kim, J.S.; Kingsley, J.D.; McMillan, V.; Mathis, R. Effects of Class IV Laser Therapy on Fibromyalgia Impact and Function in Women with Fibromyalgia. J. Altern. Complement. Med. 2013, 19, 445–452. [Google Scholar] [CrossRef]
- Gür, A.; Karakoç, M.; Nas, K.; Cevik, R.; Sarac, J.; Demir, E. Efficacy of Low Power Laser Therapy in Fibromyalgia: A Single-Blind, Placebo-Controlled Trial. Lasers Med. Sci. 2002, 17, 57–61. [Google Scholar] [CrossRef]
- Martin, L.F.; Cheng, K.; Washington, S.M.; Denton, M.; Goel, V.; Khandekar, M.; Largent-Milnes, T.M.; Patwardhan, A.; Ibrahim, M.M. Green Light Exposure Elicits Anti-Inflammation, Endogenous Opioid Release and Dampens Synaptic Potentiation to Relieve Post-Surgical Pain. J. Pain 2023, 24, 509–529. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, A.; Cuevas-Cervera, M.; Pérez-Montilla, J.J.; Aguilar-Núñez, D.; Hamed-Hamed, D.; Aguilar-García, M.; Pruimboom, L.; Navarro-Ledesma, S. Efficacy of Photobiomodulation Therapy in the Treatment of Pain and Inflammation: A Literature Review. Healthcare 2023, 11, 938. [Google Scholar] [CrossRef]
- Fitzmaurice, S.; Martin, J.; Choi, S.; dos Santos, L.; Junior, M. Comparative efficacy of whole-body versus localized photobiomodulation therapy in Fibromyalgia: A systematic review and meta-analysis. Pain Med. 2023, 24, 567–580. [Google Scholar] [CrossRef]
- Buzza, A.S.; Cousins, H.; Tapas, K.E.; Lewis, S.J.; Jenkins, M.W.; Moffitt, M.A. Direct Photobiomodulation Therapy on the Sciatic Nerve Significantly Attenuates Acute Nociceptive Sensitivity Without Affecting Motor Output. Basic Sci. 2024, 27, 1338–1346. [Google Scholar] [CrossRef]
- Khan, M.A.; Fatima, G.; Ashiquzzaman, A.; Kim, S.S.; Kwon, H.; Kim, Y.R.; Chung, E. Evaluating the Preclinical Efficacy of Photobiomodulation in Alleviating Neuropathic Corneal Pain: A Behavioral Study. Ophthalmol. Sci. 2024, 100680, in press. [Google Scholar] [CrossRef]
- Desiderá, A.C.; Nascimento, G.C.; Gerlach, R.F.; Leite-Panissi, C.R. Laser Therapy Reduces Gelatinolytic Activity in the Rat Trigeminal Ganglion during Temporomandibular Joint Inflammation. Oral Dis. 2015, 21, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Prindeze, N.J.; Ardanuy, J.G.; Carney, B.C.; Moffatt, L.T.; Shupp, J.W. Photobiomodulation Elicits a Differential Cytokine Response in a Cultured Analogue of Human Skin. Eplasty 2019, 19, e3. [Google Scholar]
- Shamloo, S.; Defensor, E.; Ciari, P.; Ogawa, G.; Vidano, L.; Lin, J.S.; Fortkort, J.A.; Shamloo, M.; Barron, A.E. The Anti-Inflammatory Effects of Photobiomodulation Are Mediated by Cytokines: Evidence from a Mouse Model of Inflammation. Front. Neurosci. 2023, 17, 1150156. [Google Scholar] [CrossRef]
- Ovadia-Blechman, Z.; Hauptman, Y.; Rabin, N.; Wiezman, G.; Hoffer, O.; Gertz, S.D.; Gavish, B.; Gavish, L. Morphological Features of the Photoplethysmographic Signal: A New Approach to Characterize the Microcirculatory Response to Photobiomodulation. Front. Physiol. 2023, 14, 1175470. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Lee, M.Y. Photobiomodulation of Neurogenesis through the Enhancement of Stem Cell and Neural Progenitor Differentiation in the Central and Peripheral Nervous Systems. Int. J. Mol. Sci. 2023, 24, 15427. [Google Scholar] [CrossRef]
- Cardoso, F.S.; Salehpour, F.; Coimbra, N.C.; Gonzalez-Lima, F.; Gomes da Silva, S. Photobiomodulation for the Treatment of Neuroinflammation: A Systematic Review of Controlled Laboratory Animal Studies. Front. Neurosci. 2022, 16, 1006031. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.C.; Hang, N.L.; Colley, M.M.S.; Chang, J.; Hsiao, Y.C.; Lu, L.S.; Li, B.S.; Chang, C.J.; Yang, T.S. Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells. Cells 2022, 11, 972. [Google Scholar] [CrossRef]
- Trajano, L.A.D.S.N.; Siqueira, P.B.; Rodrigues, M.M.S.; Pires, B.R.B.; da Fonseca, A.S.; Mencalha, A.L. Does Photobiomodulation Alter Mitochondrial Dynamics? Photochem. Photobiol. 2025, 101, 21–37. [Google Scholar] [CrossRef]
- Cardoso, F.D.S.; Gonzalez-Lima, F.; Coimbra, N.C. Mitochondrial Photobiomodulation as a Neurotherapeutic Strategy for Epilepsy. Front. Neurol. 2022, 13, 873496. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.D.S.; Barrett, D.W.; Wade, Z.; Gomes da Silva, S.; Gonzalez-Lima, F. Photobiomodulation of Cytochrome c Oxidase by Chronic Transcranial Laser in Young and Aged Brains. Front Neurosci. 2022, 16, 818005. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Litscher, G. Molecular and Cellular Mechanisms of Arthritis in Children and Adults: New Perspectives on Applied Photobiomodulation. Int. J. Mol. Sci. 2020, 21, 6565. [Google Scholar] [CrossRef] [PubMed]
- Felician, M.C.P.; Belotto, R.; Tardivo, J.P.; Baptista, M.S.; Martins, W.K. Photobiomodulation: Cellular, Molecular, and Clinical Aspects. J. Photochem. Photobiol. 2023, 17, 100197. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Choi, J.E. Proposed Mechanisms of Photobiomodulation (PBM) Mediated via the Stimulation of Mitochondrial Activity in Peripheral Nerve Injuries. Med. Lasers 2021, 10, 195–200. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A. Therapeutic Photobiomodulation: Nitric Oxide and a Novel Function of Mitochondrial Cytochrome c Oxidase. Discov. Med. 2011, 11, 154–159. [Google Scholar]
- Baldassarro, V.A.; Alastra, G.; Lorenzini, L.; Giardino, L.; Calzà, L. Photobiomodulation at Defined Wavelengths Regulates Mitochondrial Membrane Potential and Redox Balance in Skin Fibroblasts. Oxid. Med. Cell. Longev. 2023, 2023, 7638223. [Google Scholar] [CrossRef] [PubMed]
- Gavish, L.; Hoffer, O.; Rabin, N.; Halak, M.; Shkilevich, S.; Shayovitz, Y.; Weizman, G.; Haim, O.; Gavish, B.; Gertz, S.D.; et al. Microcirculatory Response to Photobiomodulation—Why Some Respond and Others Do Not: A Randomized Controlled Study. Lasers Surg. Med. 2020, 52, 863–872. [Google Scholar] [CrossRef]
- Su, C.T.; Huang, Y.T. Assessment of the Microcirculation in Superficial and Deep Biotissue with Photobiomodulation. J. Emerg. Med. OA 2023, 1, 53–62. [Google Scholar]
- Dos Santos Malavazzi, T.C.; Fernandes, K.P.S.; Lopez, T.C.C.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of the invasive and non-invasive systemic photobiomodulation using low-level laser in experimental models: A systematic review. Lasers Med. Sci. 2023, 38, 137. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Du, Y.; Xie, D.; Wei, Z.Z.; Pan, Y.; Zhang, Y. Recent Advances in Light Energy Biotherapeutic Strategies with Photobiomodulation on Central Nervous System Disorders. Brain Res. 2024, 1822, 148615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Liu, P.; Xue, X.; Zhang, C.; Peng, L.; Shen, W.; Yang, S.; Wang, F. The Role of Photobiomodulation to Modulate Ion Channels in the Nervous System: A Systematic Review. Cell Mol. Neurobiol. 2024, 44, 79. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Z.; Wang, P.; Sun, Z. Current Application and Future Directions of Photobiomodulation in Central Nervous Diseases. Neural. Regen. Res. 2021, 16, 1177–1185. [Google Scholar] [CrossRef]
- Moro, C.; Valverde, A.; Dole, M.; Hoh Kam, J.; Hamilton, C.; Liebert, A.; Bicknell, B.; Benabid, A.L.; Magistretti, P.; Mitrofanis, J. The Effect of Photobiomodulation on the Brain During Wakefulness and Sleep. Front. Neurosci. 2022, 16, 942536. [Google Scholar] [CrossRef]
- Valverde, A.; Hamilton, C.; Moro, C.; Billeres, M.; Magistretti, P.; Mitrofanis, J. Lights at Night: Does Photobiomodulation Improve Sleep? Neural. Regen. Res. 2023, 18, 474–477. [Google Scholar] [CrossRef]
- Park, J.O.; Hong, N.; Lee, M.Y.; Ahn, J.C. Photobiomodulation Regulates Astrocyte Activity and Ameliorates Scopolamine-Induced Cognitive Behavioral Decline. Front. Cell. Neurosci. 2024, 18, 1448005. [Google Scholar] [CrossRef]
- Monteiro, F.; Carvalho, Ó.; Sousa, N.; Silva, F.S.; Sotiropoulos, I. Photobiomodulation and Visual Stimulation Against Cognitive Decline and Alzheimer’s Disease Pathology: A Systematic Review. Alzheimers Dement. 2022, 8, e12249. [Google Scholar] [CrossRef] [PubMed]
- Bathini, M.; Raghushaker, C.R.; Mahato, K.K. The Molecular Mechanisms of Action of Photobiomodulation Against Neurodegenerative Diseases: A Systematic Review. Cell Mol. Neurobiol. 2022, 42, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.T.; Liu, P.M.; Ma, D.; Yang, J.J. Advances in Photobiomodulation for Cognitive Improvement by Near-Infrared Derived Multiple Strategies. J. Transl. Med. 2023, 21, 135. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.; Tolentino, M.; Lyons, J.A.; Ng, A.V. Effects of Photobiomodulation Therapy on Muscle Function in Individuals with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2024, 86, 105598. [Google Scholar] [CrossRef]
- Kumar, P.; Umakanth, S.; N, G. Photobiomodulation Therapy as an Adjunct to Resistance Exercises on Muscle Metrics, Functional Balance, Functional Capacity, and Physical Performance Among Older Adults: A Systematic Scoping Review. Lasers Med. Sci. 2024, 39, 232. [Google Scholar] [CrossRef]
- Baik, J.S.; Lee, T.Y.; Kim, N.G.; Pak, K.; Ko, S.H.; Min, J.H.; Shin, Y.I. Effects of Photobiomodulation on Changes in Cognitive Function and Regional Cerebral Blood Flow in Patients with Mild Cognitive Impairment: A Pilot Uncontrolled Trial. J. Alzheimers Dis. 2021, 83, 1513–1519. [Google Scholar] [CrossRef]
- Hipskind, S.G.; Grover, F.L.; Fort, T.R.; Helffenstein, D.; Burke, T.J.; Quint, S.A.; Bussiere, G.; Stone, M.; Hurtado, T. Pulsed Transcranial Red/Near-Infrared Light Therapy Using Light-Emitting Diodes Improves Cerebral Blood Flow and Cognitive Function in Veterans with Chronic Traumatic Brain Injury: A Case Series. Photomed. Laser Surg. 2018, 1–8. [Google Scholar] [CrossRef]
- Hamblin, M.R. Shining Light on the Head: Photobiomodulation for Brain Disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, D.; Zhu, J.; Liu, S.; Li, J.; Yu, T.; Tuchin, V.V.; Semyachkina-Glushkovskaya, O.; Zhu, D. Transcranial Photobiomodulation for Brain Diseases: Review of Animal and Human Studies Including Mechanisms and Emerging Trends. Neurophotonics 2024, 11, 010601. [Google Scholar] [CrossRef]
- Fekri, A.; Jahan, A.; Moghadam Salimi, M.; Oskouei, A.E. Short-Term Effects of Transcranial Near-Infrared Photobiomodulation on Motor Performance in Healthy Human Subjects: An Experimental Single-Blind Randomized Clinical Trial. J. Lasers Med. Sci. 2019, 10, 317–323. [Google Scholar] [CrossRef]
- Nairuz, T.; Cho, S.; Lee, J.-H. Photobiomodulation Therapy on Brain: Pioneering an Innovative Approach to Revolutionize Cognitive Dynamics. Cells 2024, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, L.; Antal, A.; Paulus, W. Photobiomodulation of the Human Cortex Using Infrared Laser Stimulation. Klin. Neurophysiol. 2012, 43, P147. [Google Scholar] [CrossRef]
- Jung, J.; Kim, T. Photobiomodulation and Its Therapeutic Potential in Sleep Disturbances. Sleep Med. Res. 2024, 15, 218–227. [Google Scholar] [CrossRef]
- Ferraresi, C.; Huang, Y.-Y.; Hamblin, M.R. Photobiomodulation in Human Muscle Tissue: An Advantage in Sports Performance? J. Biophotonics 2016, 9, 1273–1299. [Google Scholar] [CrossRef]
- das Neves, M.F.; Pinto, A.P.; Maegima, L.T.; Lima, F.P.S.; Lopes-Martins, R.Á.B.; Lo Schiavo Arisawa, E.A.; Lima, M.O. Effects of Photobiomodulation on Pain, Lactate, and Muscle Performance (ROM, Torque, and EMG Parameters) of Paretic Upper Limb in Patients with Post-Stroke Spastic Hemiparesis—A Randomized Controlled Clinical Trial. Lasers Med. Sci. 2024, 39, 88. [Google Scholar] [CrossRef]
- Almeida, J.N.; Prado, W.L.; Terra, C.M.; Oliveira, M.G.; Garcia, R.A.; Pinfildi, C.E.; Botero, J.P. Effects of Photobiomodulation on Muscle Strength in Post-Menopausal Women Submitted to a Resistance Training Program. Lasers Med. Sci. 2020, 35, 355–363. [Google Scholar] [CrossRef]
- Santana-Blank, L.; Rodríguez-Santana, E. Photobiomodulation in Light of Our Biological Clock’s Inner Workings. Photomed. Laser Surg. 2018, 36, 119–121. [Google Scholar] [CrossRef]
- Cheng, K.; Martin, L.F.; Slepian, M.J.; Patwardhan, A.M.; Ibrahim, M.M. Mechanisms and Pathways of Pain Photobiomodulation: A Narrative Review. J. Pain 2021, 22, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Farazi, N.; Salehi-Pourmehr, H.; Farajdokht, F.; Mahmoudi, J.; Sadigh-Eteghad, S. Photobiomodulation Combination Therapy as a New Insight in Neurological Disorders: A Comprehensive Systematic Review. BMC Neurol. 2024, 24, 101. [Google Scholar] [CrossRef]
- Xie, K.; El Khoury, H.; Mitrofanis, J.; Austin, P.J. A systematic review of the effect of photobiomodulation on the neuroinflammatory response in animal models of neurodegenerative diseases. Rev. Neurosci. 2022, 34, 459–481. [Google Scholar] [CrossRef]
- Almeida, P.; Lopes-Martins, R.Á.; Tomazoni, S.S.; Albuquerque-Pontes, G.M.; Santos, L.A.; Vanin, A.A.; Frigo, L.; Vieira, R.P.; Albertini, R.; de Tarso Camillo de Carvalho, P.; et al. Low-Level Laser Therapy and Sodium Diclofenac in Acute Inflammatory Response Induced by Skeletal Muscle Trauma: Effects in Muscle Morphology and mRNA Gene Expression of Inflammatory Markers. Photochem. Photobiol. 2013, 89, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Pasternak-Mnich, K.; Kujawa, J.; Agier, J.; Kozłowska, E. Impact of Photobiomodulation Therapy on Pro-Inflammation Functionality of Human Peripheral Blood Mononuclear Cells—A Preliminary Study. Sci. Rep. 2024, 14, 23111. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhou, J.; Yan, F.; Gao, M.; Tang, J.; Huang, L.; Luo, Y. Unlocking the Potential of Photobiomodulation Therapy for Brain Neurovascular Coupling: The Biological Effects and Medical Applications. J. Cereb. Blood Flow Metab. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martinez, N.; Chabardes, S.; Mitrofanis, J. Lights for Epilepsy: Can Photobiomodulation Reduce Seizures and Offer Neuroprotection? Neural Regen. Res. 2023, 18, 1423–1426. [Google Scholar] [CrossRef]
| Author, Year | Score | Quality | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vassão et al. (2024) [35] | 9 | Excellent | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Fitzmaurice et al. (2023) [36] | 8 | Good | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Langhorst et al. (2023) [37] | 8 | Good | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Navarro-Ledesma et al. (2023) [38] | 9 | Excellent | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Nelli et al. (2023) [39] | 7 | Good | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes |
| Ribeiro et al. (2023) [40] | 9 | Excellent | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Navarro-Ledesma et al. (2022) [41] | 8 | Good | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Navarro-Ledesma et al. (2022b) [42] | 9 | Excellent | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Martin et al. (2021) [43] | 8 | Good | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Choi et al. (2021) [44] | 8 | Good | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| dos Santos et al. (2020) [45] | 9 | Excellent | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Junior et al. (2021) [46] | 9 | Excellent | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Ates et al. (2019) [47] | 8 | Good | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Maciel et al. (2018) [48] | 8 | Good | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| da Silva et al. (2017) [49] | 9 | Excellent | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Panton et al. (2013) [50] | 8 | Good | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Gür et al. (2002) [51] | 7 | Good | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Outcome | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Recommendation |
|---|---|---|---|---|---|---|---|---|
| Pain | 6 (n = 250) | Moderate | Low | Moderate | Moderate | Not serious | Moderate | Moderate in favor |
| Quality of Life | 5 (n = 210) | Low | Moderate | Moderate | Moderate | Not serious | Moderate | Strong in favor |
| Fatigue | 3 (n = 150) | Moderate | Moderate | Moderate | Moderate | Not serious | Moderate | Moderate in favor |
| Tender Points | 4 (n = 180) | Moderate | Low | Moderate | Moderate | Not serious | Moderate | Moderate in favor |
| Self-efficacy | 2 (n = 80) | Low | Low | Moderate | Moderate | Not serious | Moderate | Moderate in favor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín Pérez, S.E.; Rodríguez Niebla, J.; Giraud Pérez, L.; Campo León, R.; López Mejías, A.; Morales Tejera, D.; Martín Pérez, I.M. Effectiveness of Photobiomodulation Therapy in the Management of Fibromyalgia Syndrome: A Systematic Review. Appl. Sci. 2025, 15, 4161. https://doi.org/10.3390/app15084161
Martín Pérez SE, Rodríguez Niebla J, Giraud Pérez L, Campo León R, López Mejías A, Morales Tejera D, Martín Pérez IM. Effectiveness of Photobiomodulation Therapy in the Management of Fibromyalgia Syndrome: A Systematic Review. Applied Sciences. 2025; 15(8):4161. https://doi.org/10.3390/app15084161
Chicago/Turabian StyleMartín Pérez, Sebastián Eustaquio, Joel Rodríguez Niebla, Loanne Giraud Pérez, Raquel Campo León, Alejandro López Mejías, David Morales Tejera, and Isidro Miguel Martín Pérez. 2025. "Effectiveness of Photobiomodulation Therapy in the Management of Fibromyalgia Syndrome: A Systematic Review" Applied Sciences 15, no. 8: 4161. https://doi.org/10.3390/app15084161
APA StyleMartín Pérez, S. E., Rodríguez Niebla, J., Giraud Pérez, L., Campo León, R., López Mejías, A., Morales Tejera, D., & Martín Pérez, I. M. (2025). Effectiveness of Photobiomodulation Therapy in the Management of Fibromyalgia Syndrome: A Systematic Review. Applied Sciences, 15(8), 4161. https://doi.org/10.3390/app15084161






