Machine Learning Introduces Electrophysiology Assessment as the Best Predictor for the Recovery Prognosis of Spinal Cord Injury Patients for Personalized Rehabilitation Approaches
Abstract
1. Introduction
1.1. Pathophysiology and Diagnosis of SCI
1.2. Classification and Assessment of SCI
1.3. Emergency and Acute Treatment of SCI
1.4. Rehabilitation and Prognosis in SCI
1.5. Electrophysiology and Artificial Intelligence in SCI
2. Materials and Methods
- MS: Motor score;
- DST: Distance from the motor level of injury;
- LT: Light touch sensation;
- PP: Pin prick sensation;
- SSEP_Amp diff_uln: Somatosensory evoked potential amplitude difference ulnar nerve;
- Hupp_score_SEP: Somatosensory evoked potential score;
- MEP_Amplitude_abd: Motor evoked potential amplitude recorded from abductor muscle;
- Hupp_score_MEP: Motor evoked potential score;
- F-wave persistance uln: F-wave persistence ulnar nerve;
- Hupp score-NCS: Nerve conduction studies score;
- REC: muscle strength final recovery {recovery class, no recovery class};
- AIS: ASIA score {A, B, C, D, E}.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witiw, C.D.; Fehlings, M.G. Acute Spinal Cord Injury. J. Spinal Disord. Tech. 2015, 28, 202–210. [Google Scholar] [CrossRef]
- Ditunno, J.F.; Young, W.; Donovan, W.H.; Creasey, G. The international standards booklet for neurological and functional classification of spinal cord injury. Spinal Cord 1994, 32, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Rupp, R.; Biering-Sørensen, F.; Burns, S.P.; Graves, D.E.; Guest, J.; Jones, L.; Read, M.S.; Rodriguez, G.M.; Schuld, C.; Tansey-MD, K.E. International standards for neurological classification of spinal cord injury revised 2019. Top. Spinal Cord Inj. Rehabil. 2021, 27, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Daud, M.F.; Idris, J.; Ng, A.M.H.; Naicker, A.S.; Ismail, O.H.R.; Kumar, R.K.A.; Lokanathan, Y. Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Freund, P.; Seif, M.; Weiskopf, N.; Friston, K.; Fehlings, M.G.; Thompson, A.J.; Curt, A. MRI in traumatic spinal cord injury: From clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019, 18, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.L.; Kershah, S.M. Advances in Imaging of Vertebral and Spinal Cord Injury. J. Spinal Cord Med. 2010, 33, 105–116. [Google Scholar] [CrossRef]
- Pinchi, E.; Frati, A.; Cantatore, S.; D’errico, S.; La Russa, R.; Maiese, A.; Palmieri, M.; Pesce, A.; Viola, R.V.; Frati, P.; et al. Acute spinal cord injury: A systematic review investigating miRNA families involved. Int. J. Mol. Sci. 2019, 20, 1841. [Google Scholar] [CrossRef]
- Nas, K.; Yazmalar, L.; Şah, V.; Aydın, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sørensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef]
- Kirshblum, S.; Waring, W. Updates for the international standards for neurological classification of spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 505–517. [Google Scholar] [CrossRef]
- Krawetz, P.; Nance, P. Gait analysis of spinal cord injured subjects: Effects of injury level and spasticity. Arch. Phys. Med. Rehabil. 1996, 77, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Rekand, T.; Hagen, E.; Grønning, M. Spastisitet etter ryggmargsskade. Tidsskr. Den Nor. Legeforening 2012, 132, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.L.; Adkins, R.H.; Yakura, J.S. International Standards for Neurological Classifications of Spinal Cord Injury; American Spinal Injury Association: Richmond, VA, USA, 2000; pp. 1–23. [Google Scholar]
- Waters, R.L.; Adkins, R.; Yakura, J.; Vigil, D. Prediction of ambulatory performance based on motor scores derived from standards of the American Spinal Injury Association. Arch. Phys. Med. Rehabil. 1994, 75, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.L.; Nixon-Cave, K. A tool for clinical reasoning and reflection using the international classification of functioning, disability and health (ICF) framework and patient management model. Phys. Ther. 2011, 91, 416–430. [Google Scholar] [CrossRef]
- Ballert, C.S.; Stucki, G.; Biering-Sørensen, F.; Cieza, A. Towards the development of clinical measures for spinal cord injury based on the international classification of functioning, disability and health with rasch analyses. Arch. Phys. Med. Rehabil. 2014, 95, 1685–1694. [Google Scholar] [CrossRef]
- Joseph, C.; Phillips, J.; Wahman, K.; Wikmar, L.N. Mapping two measures to the International classification of functioning, disability and health and the brief ICF core set for spinal cord injury in the post-acute context. Disabil. Rehabil. 2016, 38, 1730–1738. [Google Scholar] [CrossRef]
- Eli, I.; Lerner, D.P.; Ghogawala, Z. Acute traumatic spinal cord injury. Neurol. Clin. 2021, 39, 471–488. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. Immune response following traumatic spinal cord injury: Pathophysiology and therapies. Front. Immunol. 2023, 13, 1084101. [Google Scholar] [CrossRef]
- Safdarian, M.; Trinka, E.; Rahimi-Movaghar, V.; Thomschewski, A.; Aali, A.; Abady, G.G.; Abate, S.M.; Abd-Allah, F.; Abedi, A.; Adane, D.E.; et al. Global, regional, and national burden of spinal cord injury, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2023, 22, 1026–1047. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 1–28. [Google Scholar] [CrossRef]
- Cadotte, D.W.; Fehlings, M.G. Traumatic spinal cord injury: Acute spinal cord injury and prognosis. In Quantitative MRI of the Spinal Cord; Academic Press: Cambridge, MA, USA, 2014; pp. 39–48. [Google Scholar] [CrossRef]
- Hubli, M.; Kramer, J.L.K.; Jutzeler, C.R.; Rosner, J.; Furlan, J.C.; Tansey, K.E.; Schubert, M. Application of electrophysiological measures in spinal cord injury clinical trials: A narrative review. Spinal Cord 2019, 57, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Pikija, S.; Mutzenbach, J.S.; Seidl, M.; Leis, S.; Trinka, E.; Sellner, J. Current and emerging treatment options for spinal cord ischemia. Drug Discov. Today 2016, 21, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Korupolu, R.; Stampas, A.; Singh, M.; Zhou, P.; Francisco, G. Electrophysiological outcome measures in spinal cord injury clinical trials: A systematic review. Top. Spinal Cord Inj. Rehabil. 2019, 25, 340–354. [Google Scholar] [CrossRef]
- Curt, A.; Ellaway, P.H. Clinical neurophysiology in the prognosis and monitoring of traumatic spinal cord injury. Handb. Clin. Neurol. 2012, 109, 63–75. [Google Scholar] [CrossRef]
- Kakulas, B.A. Neuropathology: The foundation for new treatments in spinal cord injury. Spinal Cord 2004, 42, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Fustes, O.J.H.; Kay, C.S.K.; Lorenzoni, P.J.; Ducci, R.D.-P.; Werneck, L.C.; Scola, R.H. Somatosensory evoked potentials in clinical practice: A review. Arq. Neuro Psiquiatr. 2021, 79, 824–831. [Google Scholar] [CrossRef]
- Zhu, Q.A.; Hu, Y.; Li, R.; Huang, Z.C.; Cui, H.Y.; Huang, Z.P.; Liu, J.H. Utility of somatosensory and motor-evoked potentials in reflecting gross and fine motor functions after unilateral cervical spinal cord contusion injury. Neural Regen. Res. 2021, 16, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Wadhwani, J.; Meena, V.S.; Sharma, P.; Kaur, K.; Svareen. Electrophysiological study in acute spinal cord injury patients: Its correlation to neurological deficit and subsequent recovery assessment by ASIA score. Indian J. Orthop. 2020, 54, 678–686. [Google Scholar] [CrossRef]
- Lawal, A.I.; Kwon, S. Application of artificial intelligence to rock mechanics: An overview. J. Rock Mech. Geotech. Eng. 2021, 13, 248–266. [Google Scholar] [CrossRef]
- Yan, H.; Jiang, Y.; Zheng, J.; Peng, C.; Li, Q. A multilayer perceptron-based medical decision support system for heart disease diagnosis. Expert Syst. Appl. 2006, 30, 272–281. [Google Scholar] [CrossRef]
- Balbinot, G.; Li, G.; Kalsi-Ryan, S.; Abel, R.; Maier, D.; Kalke, Y.B.; Weidner, N.; Rupp, R.; Schubert, M.; Curt, A.; et al. Segmental motor recovery after cervical spinal cord injury relates to density and integrity of corticospinal tract projections. Nat. Commun. 2023, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A.; Smith, L.A. Practical feature subset selection for machine learning. In Computer Science ’98, Proceedings of the 21st Australasian Computer Science Conference ACSC’98, Perth, Australia, 4–6 February 1998; Springer: Berlin/Heidelberg, Germany, 1998; pp. 181–191. [Google Scholar]
- Zhou, Z.H. Machine Learning; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Zhou, Z.H. Ensemble Methods: Foundations and Algorithms; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Holmes, G.; Donkin, A.; Witten, I. WEKA: A machine learning workbench. In Proceedings of the ANZIIS ’94—Australian New Zeland Intelligent Information Systems Conference, Brisbane, QLD, Australia, 29 November–2 December 1994; pp. 357–361. [Google Scholar] [CrossRef]
- Huang, Y.N.; Meftah, E.-M.; Pion, C.H.; Mac-Thiong, J.-M.; Cohen-Adad, J.; Barthélemy, D. Quantitative electrophysiological assessments as predictive markers of lower limb motor recovery after spinal cord injury: A pilot study with an adaptive trial design. Spinal Cord Ser. Cases 2022, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Margaritella, N.; Mendozzi, L.; Garegnani, M.; Nemni, R.; Colicino, E.; Gilardi, E.; Pugnetti, L. Exploring the predictive value of the evoked potentials score in MS within an appropriate patient population: A hint for an early identification of benign MS? BMC Neurol. 2012, 12, 80. [Google Scholar] [CrossRef]
- Chrysanthakopoulou, D.C.; Koutsojannis, C. Machine learning algorithms introduce evoked potentials as alternative biomarkers for the expanded disability status scale prognosis of multiple sclerosis patients. Cureus 2025, 17, e80335. [Google Scholar] [CrossRef] [PubMed]


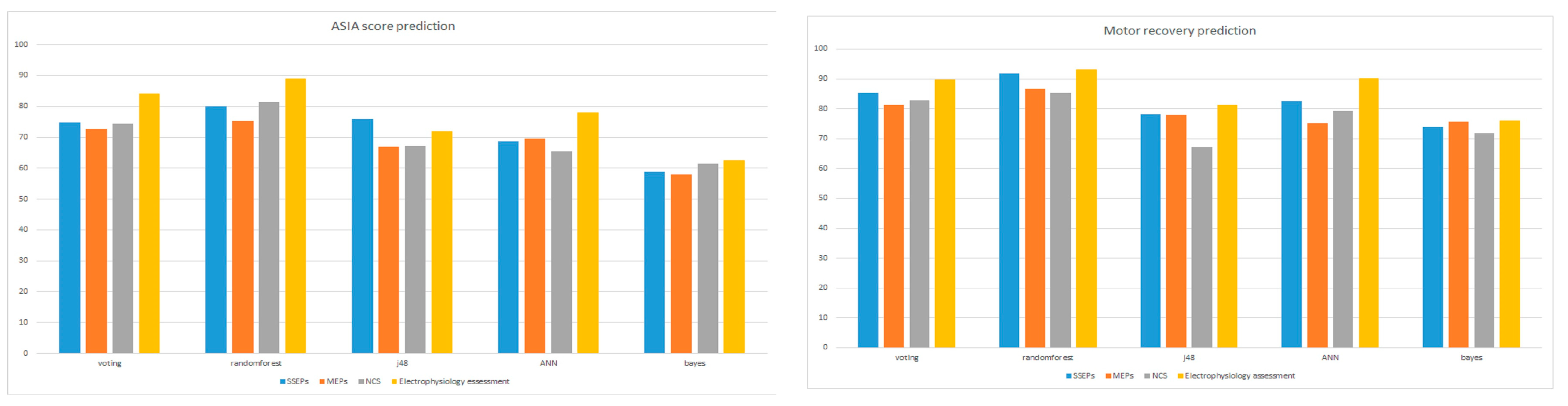
| (a) Ensemble Algorithm (Vote) | |||||
| Biomarker Accuracy (%) | All Together | SSEPs | MEPs | NCS | Clinical Assessment |
| Motor Recovery | 89.8 | 85.4 | 81.3 | 82.9 | 75.6 |
| AIS index | 84.1 | 74.9 | 72.7 | 74.4 | 63.4 |
| (b) Randomforest | |||||
| Biomarker Accuracy (%) | All Together | SSEPs | MEPs | NCS | Clinical Assessment |
| Motor Recovery | 93.1 | 91.9 | 86.6 | 85.4 | 75.6 |
| AIS index | 89.0 | 80.1 | 75.2 | 81.3 | 66.3 |
| (c) Decision Trees (J48) | |||||
| Biomarker Accuracy (%) | All Together | SSEPs | MEPs | NCS | Clinical Assessment |
| Recovery | 81.3 | 79.2 | 77.9 | 67.1 | 73.5 |
| AIS index | 71.9 | 76.0 | 67.0 | 67.1 | 59.8 |
| (d) Neural Networks (Multilayer Perceptron) | |||||
| Biomarker Accuracy (%) | All Together | SSEPs | MEPs | NCS | Clinical Assessment |
| Motor Recovery | 90.2 | 82.5 | 75.2 | 79.2 | 71.5 |
| AIS index | 78.0 | 68.7 | 69.5 | 65.4 | 57.7 |
| (e) Bayes (Naive Bayes) | |||||
| Biomarker Accuracy (%) | All Together | SSEPs | MEPs | NCS | Clinical Assessment |
| Motor Recovery | 76.0 | 73.9 | 75.6 | 71.9 | 69.5 |
| AIS index | 62.6 | 58.9 | 58.1 | 61.4 | 56.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysanthakopoulou, D.; Matzaroglou, C.; Trachani, E.; Koutsojannis, C. Machine Learning Introduces Electrophysiology Assessment as the Best Predictor for the Recovery Prognosis of Spinal Cord Injury Patients for Personalized Rehabilitation Approaches. Appl. Sci. 2025, 15, 4578. https://doi.org/10.3390/app15084578
Chrysanthakopoulou D, Matzaroglou C, Trachani E, Koutsojannis C. Machine Learning Introduces Electrophysiology Assessment as the Best Predictor for the Recovery Prognosis of Spinal Cord Injury Patients for Personalized Rehabilitation Approaches. Applied Sciences. 2025; 15(8):4578. https://doi.org/10.3390/app15084578
Chicago/Turabian StyleChrysanthakopoulou, Dionysia, Charalampos Matzaroglou, Eftychia Trachani, and Constantinos Koutsojannis. 2025. "Machine Learning Introduces Electrophysiology Assessment as the Best Predictor for the Recovery Prognosis of Spinal Cord Injury Patients for Personalized Rehabilitation Approaches" Applied Sciences 15, no. 8: 4578. https://doi.org/10.3390/app15084578
APA StyleChrysanthakopoulou, D., Matzaroglou, C., Trachani, E., & Koutsojannis, C. (2025). Machine Learning Introduces Electrophysiology Assessment as the Best Predictor for the Recovery Prognosis of Spinal Cord Injury Patients for Personalized Rehabilitation Approaches. Applied Sciences, 15(8), 4578. https://doi.org/10.3390/app15084578







