Enhanced Catalytic Surfaces for Catechol Sensing: Combining Grafted Aryldiazonium Derivative with Cross-Linking Dopamine or Coupling Tyrosinase Immobilizations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solution
2.2. Instrumentation
2.3. Electrochemical Measurements
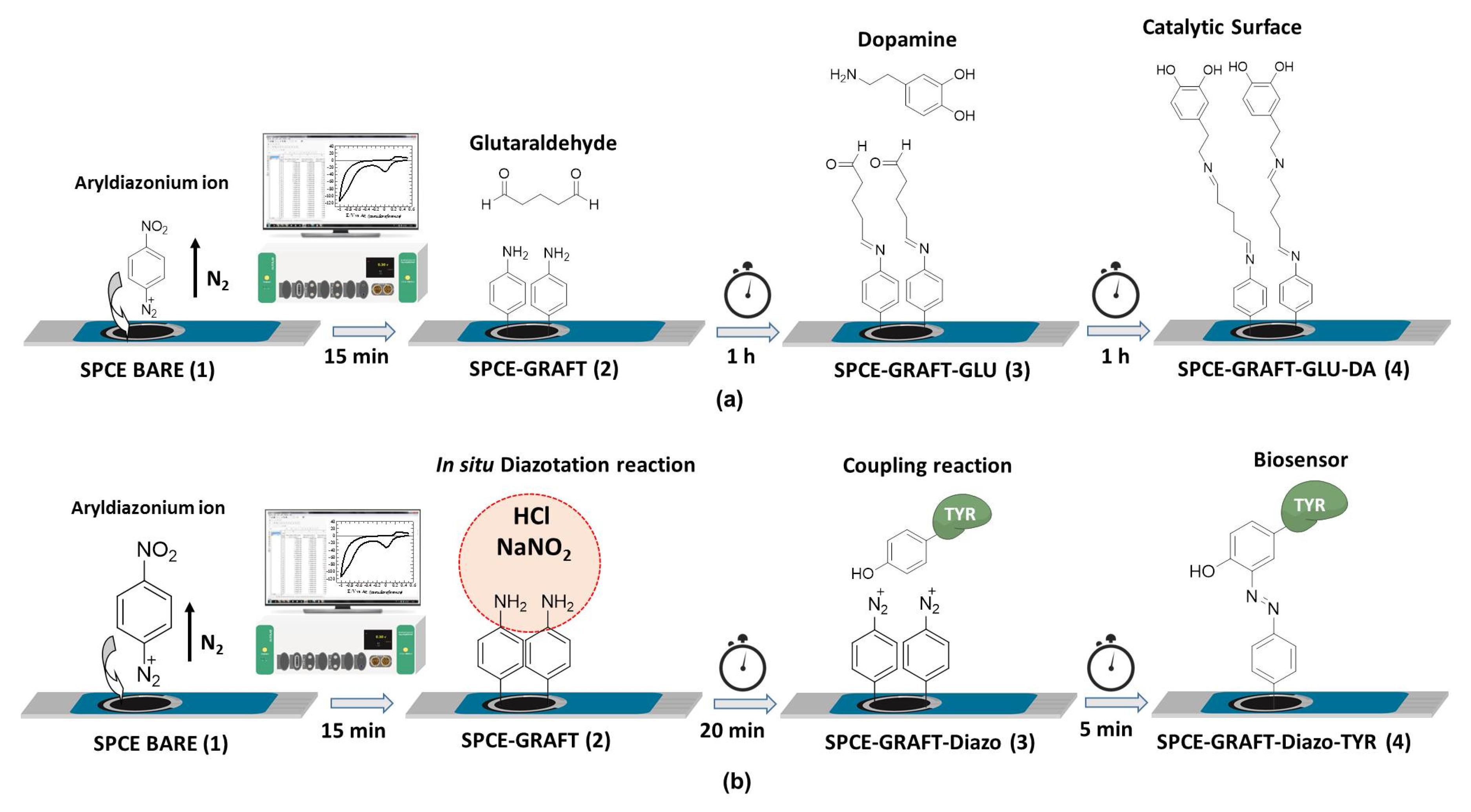
2.4. Procedures
2.4.1. Electrografting of 4-NBD+ on SPE
2.4.2. Dopamine Immobilization
2.4.3. Tyrosinase Enzyme Immobilization
3. Results and Discussion
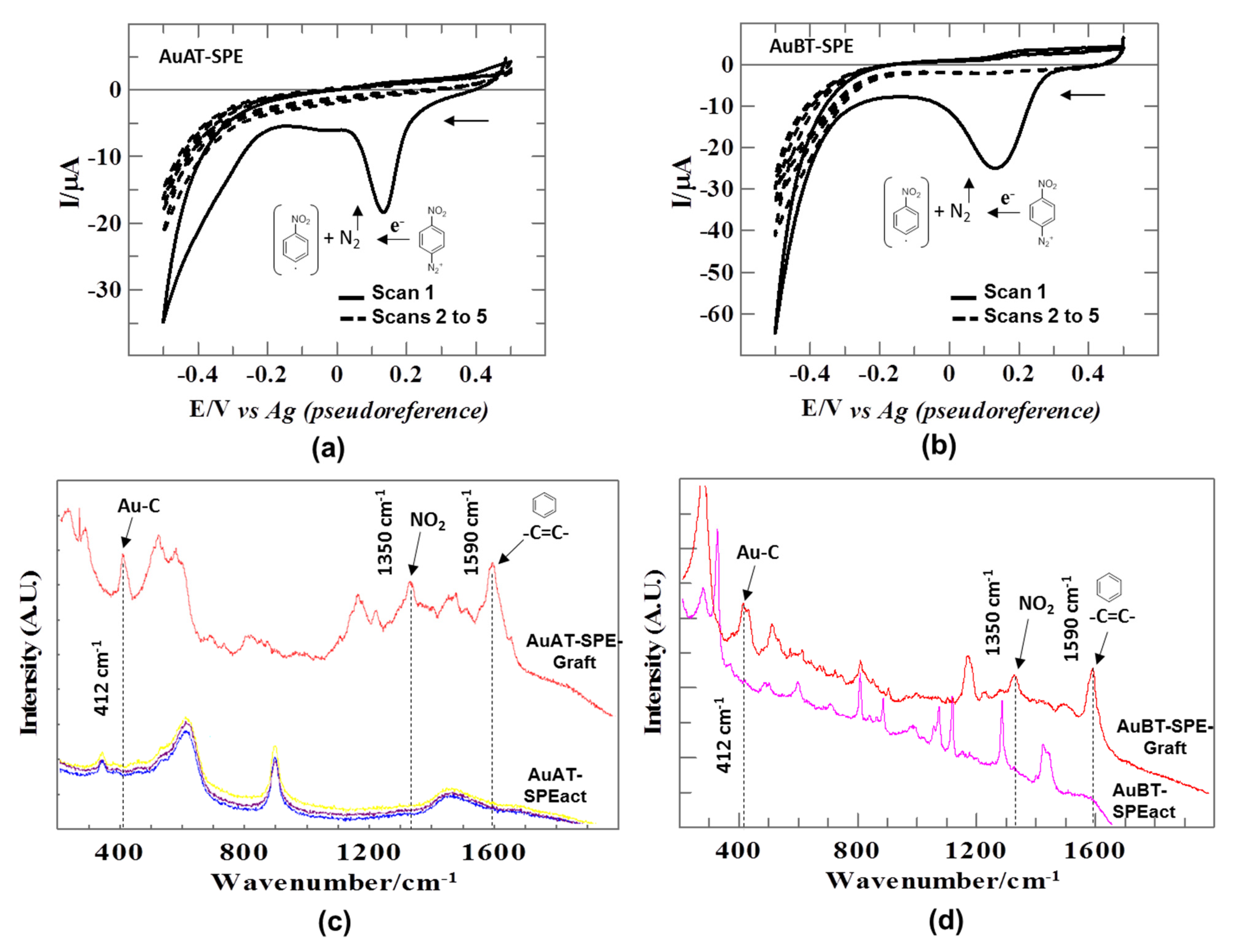
3.1. Electrografting of Gold Electrodes with 4-NBD+: Spectroscopic Characterization
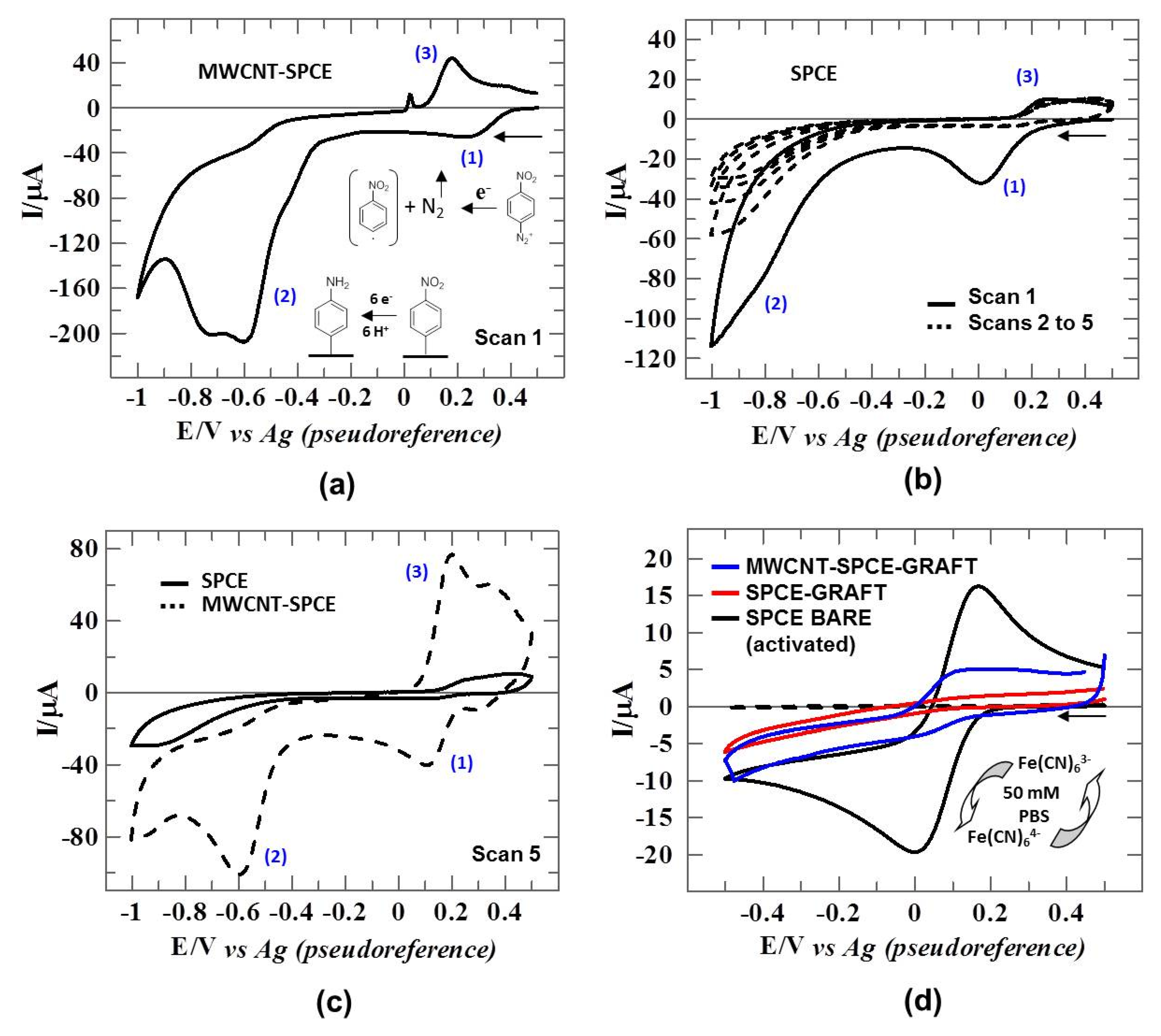
3.2. Electrografting of Carbon Electrodes with 4-NBD+: Electrochemical Characterization
3.3. Effect of Scan Rate on Electrografting of 4-NBD+
3.4. Effect of Concentration of 4-NBD+ on Surface Coverage
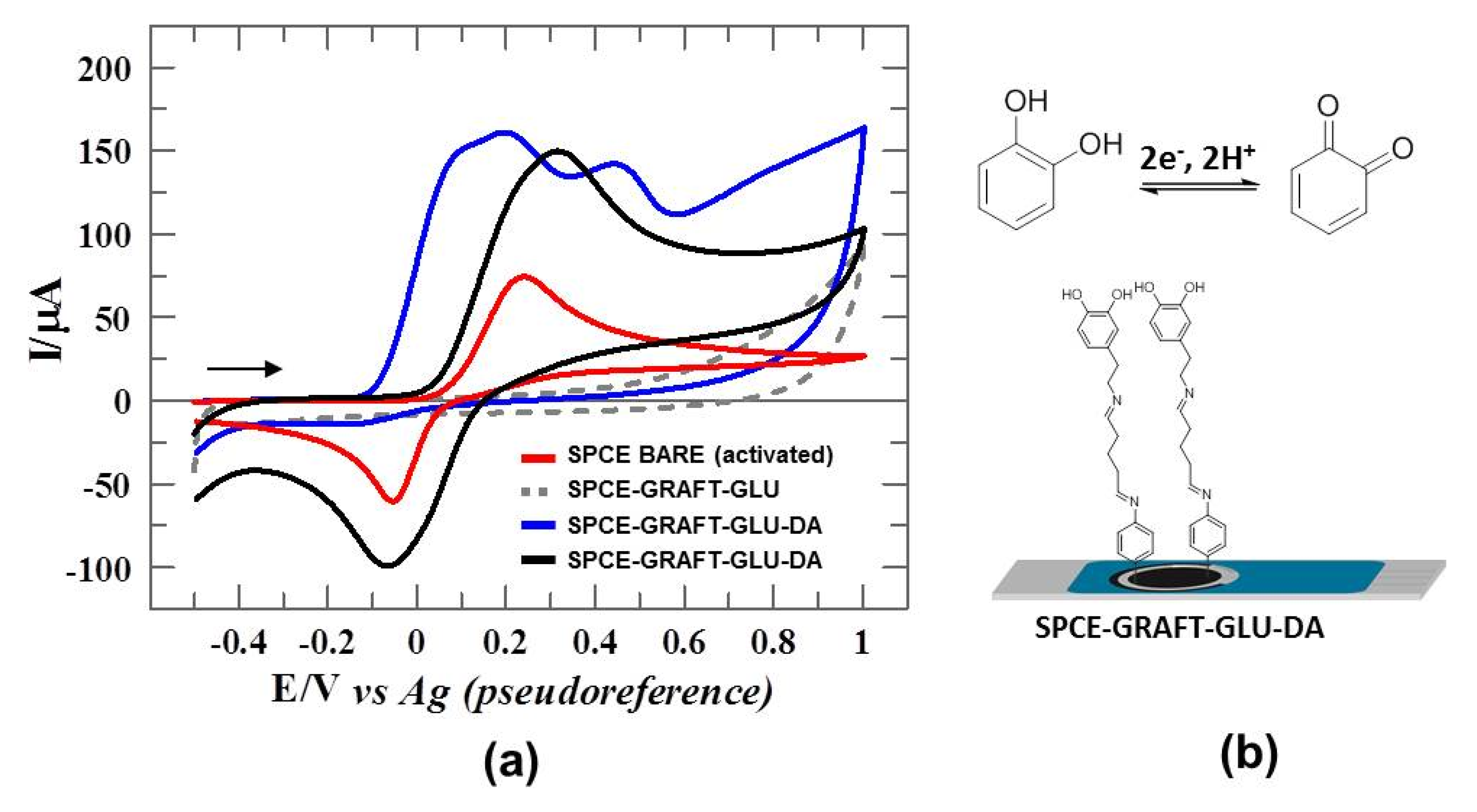
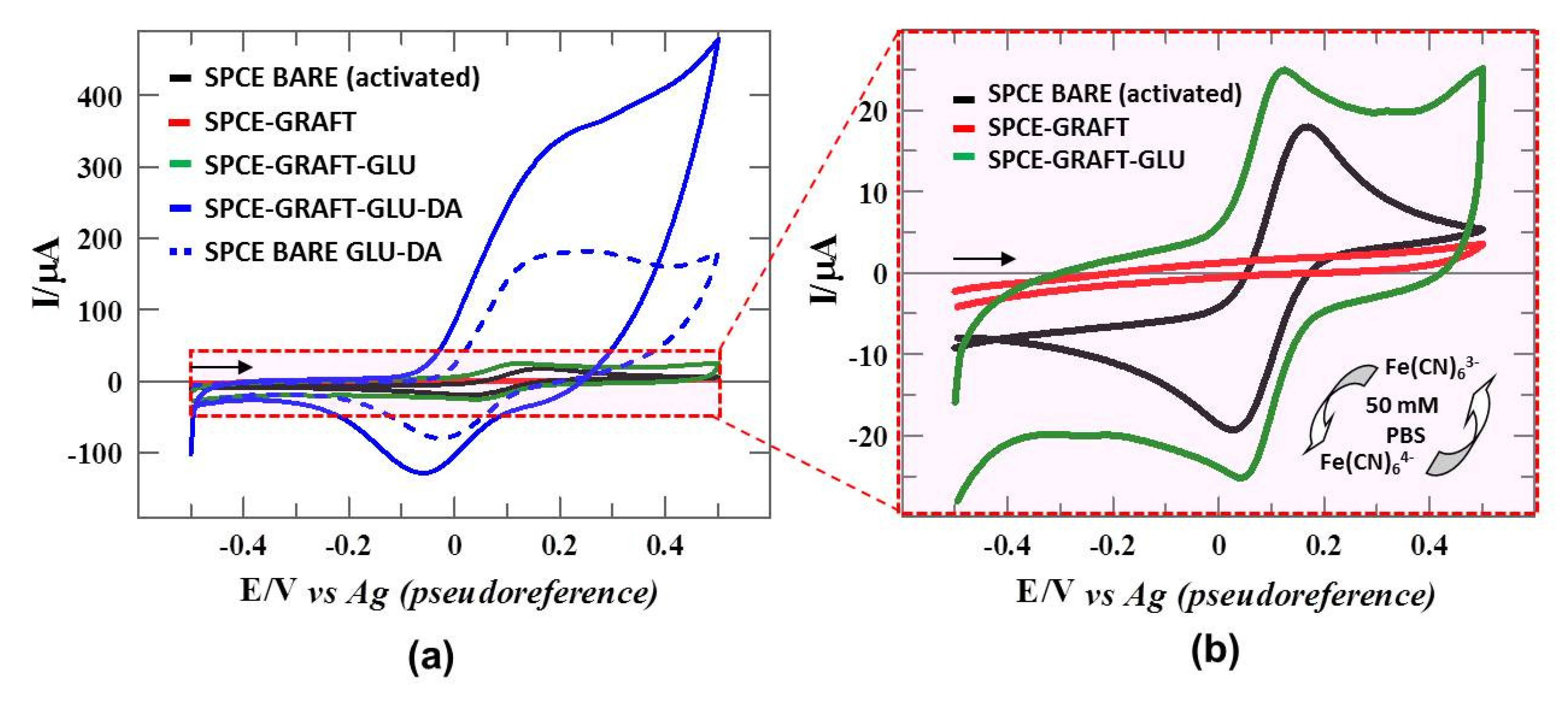
3.5. Electrochemical Characterization of Catalytic Surface: DA-Sensor
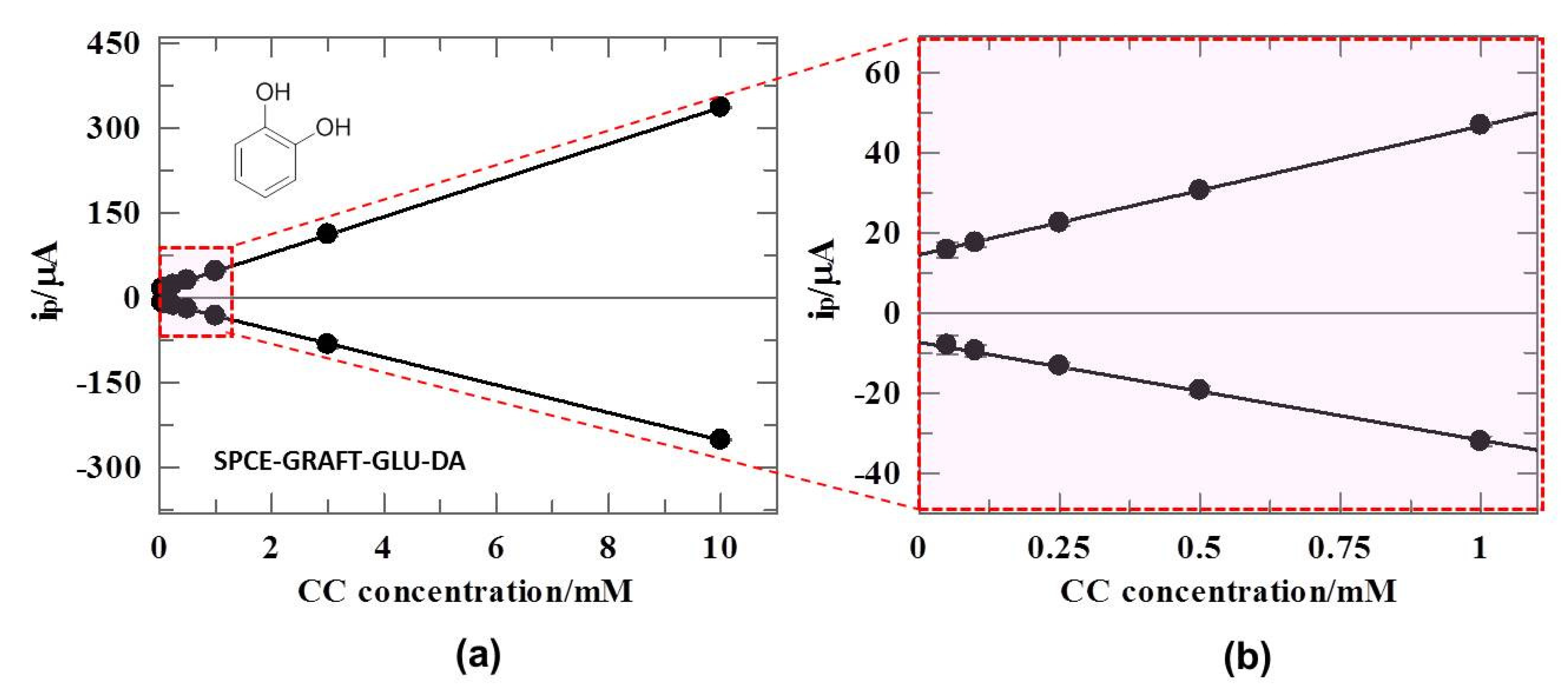
3.6. DA-Sensor for CC Sensing
3.7. Electrochemical Characterization of TYR-Biosensor for CC Sensing
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-NBD+ | 4-Nitrobenzenediazonium ions |
| Amp | Amperometry |
| AO | Acridine Orange |
| AuAT-SPE | Screen-Printed Gold Electrode (High temperature cured ink) |
| AuBT-SPE | Screen-Printed Gold Electrode (Low temperature cured ink) |
| AuNPs | Gold nanoparticles |
| BSA | Bovine Serum Albumin |
| BDD | Boron-Doped Diamond |
| CC | Catechol |
| CA | Chronoamperometry |
| CB4 | Carbon Black Ink |
| CBPE | Carbon Black Paste Electrode |
| CdTe-QDs | Cadmium Telluride Quantum Dots |
| CGGE | Chitosan Biopolymer, Graphite Powder and Glycerol Mixture Electrode |
| CMS | Carboxymethyl Starch |
| Cu-CMCS2 | Enzyme-less-Cubic Mesoporous Carbon Stage 2 (Tyrosinase-mimicking) |
| CV | Cyclic Voltammetry/Cyclic voltammogram |
| CVs | Cyclic voltammograms |
| DA | Dopamine |
| Diazo | Surface modified by diazotization reaction |
| DHP | Dihexadecylphosphate |
| DPV | Differential Pulse Voltammetry |
| Epa | Anodic peak potential |
| Epc | Cathodic peak potential |
| GCE | Glassy Carbon Electrode |
| GLU | Glutaraldehyde |
| GNPs | Graphene Nanoplatelets |
| GR | Graphene |
| GRAFT | Surface modified with aminophenyl groups |
| IL | Ionic Liquid |
| ipa | Anodic peak current |
| ipc | Cathodic peak current |
| ITO | Indium Tin Oxide |
| LDR | Linear Dynamic Range |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| LSV | Linear Sweep Voltammetry |
| MLN | Natural Molybdenite |
| MNP | Magnetic Nanoparticles |
| MWCNTs | Multiwalled Carbon Nanotubes |
| NDs | Carbon Nanodiamonds |
| PANI | Polyaniline |
| PBS | Phosphate-Buffered Solution |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PET | Polyethylene Terephthalate |
| PTA | Poly-3-Thiophenemalonic Acid |
| PS | Potato Starch |
| pTN | Poly-Thionine |
| RGO | Reduced Graphene Oxide |
| RSD | Relative standard deviation |
| SERS | Surface-Enhanced Raman Spectroscopy |
| SPE | Screen-Printed Electrode |
| SPCE | Screen-Printed Carbon Electrode |
| SWV | Square Wave Voltammetry |
| TRIS | 2-Amino-2-(hidroxymethyl)-1,3-propanediol |
| TYR | Tyrosinase |
References
- Hetemi, D.; Noël, V.; Pinson, J. Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, M.M. Aryl Diazonium Salts: New Coupling Agents in Polymer and Surface Science, 1st ed.; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-3-527-32998-4. [Google Scholar]
- Toupin, M.; Bélanger, D. Thermal Stability Study of Aryl Modified Carbon Black by in Situ Generated Diazonium Salt. J. Phys. Chem. C 2007, 111, 5394–5401. [Google Scholar] [CrossRef]
- Liu, G.; Böcking, T.; Gooding, J.J. Diazonium Salts: Stable Monolayers on Gold Electrodes for Sensing Applications. J. Electroanal. Chem. 2007, 600, 335–344. [Google Scholar] [CrossRef]
- Haque, A.-M.J.; Kim, K. Reusable Bio-Functionalized Surfaces Based on Electrochemical Desorption of Benzenediazonium-Grafted Organic Layers. Chem. Commun. 2011, 47, 6855. [Google Scholar] [CrossRef]
- Randriamahazaka, H.; Ghilane, J. Electrografting and Controlled Surface Functionalization of Carbon Based Surfaces for Electroanalysis. Electroanalysis 2016, 28, 13–26. [Google Scholar] [CrossRef]
- Matrab, T.; Chancolon, J.; L’hermite, M.M.; Rouzaud, J.-N.; Deniau, G.; Boudou, J.-P.; Chehimi, M.M.; Delamar, M. Atom Transfer Radical Polymerization (ATRP) Initiated by Aryl Diazonium Salts: A New Route for Surface Modification of Multiwalled Carbon Nanotubes by Tethered Polymer Chains. Colloids Surf. A Physicochem. Eng. Asp. 2006, 287, 217–221. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Iruthayaraj, J.; Chernyy, S.; Lillethorup, M.; Ceccato, M.; Røn, T.; Hinge, M.; Kingshott, P.; Besenbacher, F.; Pedersen, S.U.; Daasbjerg, K. On Surface-Initiated Atom Transfer Radical Polymerization Using Diazonium Chemistry To Introduce the Initiator Layer. Langmuir 2011, 27, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Laforgue, A.; Addou, T.; Bélanger, D. Characterization of the Deposition of Organic Molecules at the Surface of Gold by the Electrochemical Reduction of Aryldiazonium Cations. Langmuir 2005, 21, 6855–6865. [Google Scholar] [CrossRef]
- Lee, L.; Brooksby, P.A.; Hapiot, P.; Downard, A.J. Electrografting of 4-Nitrobenzenediazonium Ion at Carbon Electrodes: Catalyzed and Uncatalyzed Reduction Processes. Langmuir 2016, 32, 468–476. [Google Scholar] [CrossRef]
- Pilan, L. Tailoring the Performance of Electrochemical Biosensors Based on Carbon Nanomaterials via Aryldiazonium Electrografting. Bioelectrochemistry 2021, 138, 107697. [Google Scholar] [CrossRef] [PubMed]
- Combellas, C.; Jiang, D.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Steric Effects in the Reaction of Aryl Radicals on Surfaces. Langmuir 2009, 25, 286–293. [Google Scholar] [CrossRef]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Ivasenko, O.; Vanderlinden, W.; Van Gorp, H.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent Modification of Graphene and Graphite Using Diazonium Chemistry: Tunable Grafting and Nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Firestone, M.A.; Auciello, O.; Carlisle, J.A. Surface Functionalization of Ultrananocrystalline Diamond Films by Electrochemical Reduction of Aryldiazonium Salts. Langmuir 2004, 20, 11450–11456. [Google Scholar] [CrossRef]
- Haghshenas, E.; Madrakian, T.; Afkhami, A.; Saify Nabiabad, H. An Electrochemical Ceruloplasmin Aptasensor Using a Glassy Carbon Electrode Modified by Diazonium-Functionalized Multiwalled Carbon Nanotubes. J. Iran. Chem. Soc. 2019, 16, 593–602. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, S.; Lee, K.; Shin, I.-S.; Kim, Y.-R. Tunable Electrochemical Grafting of Diazonium for Highly Sensitive Impedimetric DNA Sensor. J. Electrochem. Soc. 2020, 167, 087504. [Google Scholar] [CrossRef]
- Mooste, M.; Kibena, E.; Matisen, L.; Tammeveski, K. Blocking Properties of Nickel Electrodes Modified with Aryldiazonium Compounds. Int. J. Electrochem. Sci. 2015, 10, 3803–3819. [Google Scholar] [CrossRef]
- Combellas, C.; Delamar, M.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Spontaneous Grafting of Iron Surfaces by Reduction of Aryldiazonium Salts in Acidic or Neutral Aqueous Solution. Application to the Protection of Iron against Corrosion. Chem. Mater. 2005, 17, 3968–3975. [Google Scholar] [CrossRef]
- İsbir-Turan, A.A.; Üstündağ, Z.; Solak, A.O.; Kılıç, E.; Avseven, A. Electrochemical and Spectroscopic Characterization of a Benzo[c]Cinnoline Electrografted Platinum Surface. Thin Solid Films 2009, 517, 2871–2877. [Google Scholar] [CrossRef]
- Ullien, D.; Thüne, P.C.; Jager, W.F.; Sudhölter, E.J.R.; De Smet, L.C.P.M. Controlled Amino-Functionalization by Electrochemical Reduction of Bromo and Nitro Azobenzene Layers Bound to Si(111) Surfaces. Phys. Chem. Chem. Phys. 2014, 16, 19258–19265. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.D.; Dowsett, M.R.; Bentley, P.; Dickenson-Fogg, J.A.; Pratt, A.; Blanford, C.F.; Fascione, M.A.; Parkin, A. Aldehyde-Mediated Protein-to-Surface Tethering via Controlled Diazonium Electrode Functionalization Using Protected Hydroxylamines. Langmuir 2020, 36, 5654–5664. [Google Scholar] [CrossRef] [PubMed]
- Richard, W.; Evrard, D.; Gros, P. A Novel Electrochemical Sensor Based on a Mixed Diazonium/PEDOT Surface Functionalization for the Simultaneous Assay of Ascorbic and Uric Acids. Towards an Improvement in Amperometric Response Stability. Electroanalysis 2014, 26, 1390–1399. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. Tracking Metal Ions with Polypyrrole Thin Films Adhesively Bonded to Diazonium-Modified Flexible ITO Electrodes. Environ. Sci. Pollut. Res. 2018, 25, 20012–20022. [Google Scholar] [CrossRef]
- Hayat, A.; Barthelmebs, L.; Sassolas, A.; Marty, J.-L. An Electrochemical Immunosensor Based on Covalent Immobilization of Okadaic Acid onto Screen Printed Carbon Electrode via Diazotization-Coupling Reaction. Talanta 2011, 85, 513–518. [Google Scholar] [CrossRef]
- Revenga-Parra, M.; García-Mendiola, T.; González-Costas, J.; González-Romero, E.; Marín, A.G.; Pau, J.L.; Pariente, F.; Lorenzo, E. Simple Diazonium Chemistry to Develop Specific Gene Sensing Platforms. Anal. Chim. Acta 2014, 813, 41–47. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Tyrosinase Immobilization Strategies for the Development of Electrochemical Biosensors—A Review. Nanomaterials 2023, 13, 760. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Ribovski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino, L.H.; Banks, C.E.; Oliveira, O.N.; et al. Polyphenol Oxidase-Based Electrochemical Biosensors: A Review. Anal. Chim. Acta 2020, 1139, 198–221. [Google Scholar] [CrossRef] [PubMed]
- Vlamidis, Y.; Gualandi, I.; Tonelli, D. Amperometric Biosensors Based on Reduced GO and MWCNTs Composite for Polyphenols Detection in Fruit Juices. J. Electroanal. Chem. 2017, 799, 285–292. [Google Scholar] [CrossRef]
- Campanhã Vicentini, F.; Garcia, L.L.C.; Figueiredo-Filho, L.C.S.; Janegitz, B.C.; Fatibello-Filho, O. A Biosensor Based on Gold Nanoparticles, Dihexadecylphosphate, and Tyrosinase for the Determination of Catechol in Natural Water. Enzym. Microb. Technol. 2016, 84, 17–23. [Google Scholar] [CrossRef]
- Camargo, J.R.; Baccarin, M.; Raymundo-Pereira, P.A.; Campos, A.M.; Oliveira, G.G.; Fatibello-Filho, O.; Oliveira, O.N.; Janegitz, B.C. Electrochemical Biosensor Made with Tyrosinase Immobilized in a Matrix of Nanodiamonds and Potato Starch for Detecting Phenolic Compounds. Anal. Chim. Acta 2018, 1034, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Rajarathinam, T.; Chakravarthi Nagarajan, D.; Kandasamy, P.; Jeon, S.; Kim, C.-S.; Won Hong, S.; Paik, H.; Chang, S.-C. A Smartphone-Based Tunable Tyrosinase Functional Mimic Modulated Portable Amperometric Sensor for the Rapid and Real-Time Monitoring of Catechol. Chem. Eng. J. 2024, 497, 154811. [Google Scholar] [CrossRef]
- Baluta, S.; Meloni, F.; Halicka, K.; Szyszka, A.; Zucca, A.; Pilo, M.I.; Cabaj, J. Differential Pulse Voltammetry and Chronoamperometry as Analytical Tools for Epinephrine Detection Using a Tyrosinase-Based Electrochemical Biosensor. RSC Adv. 2022, 12, 25342–25353. [Google Scholar] [CrossRef] [PubMed]
- Nurul Karim, M.D.; Lee, H.J. Amperometric Phenol Biosensor Based on Covalent Immobilization of Tyrosinase on Au Nanoparticle Modified Screen Printed Carbon Electrodes. Talanta 2013, 116, 991–996. [Google Scholar] [CrossRef]
- Rita Montereali, M.; Vastarella, W.; Della Seta, L.; Pilloton, R. Tyrosinase Biosensor Based on Modified Screen Printed Electrodes: Measurements of Total Phenol Content. Int. J. Environ. Anal. Chem. 2005, 85, 795–806. [Google Scholar] [CrossRef]
- Vulcano, F.; Kovtun, A.; Bettini, C.; Xia, Z.; Liscio, A.; Terzi, F.; Heras, A.; Colina, A.; Zanfrognini, B.; Melucci, M.; et al. Dopamine-Functionalized Graphene Oxide as a High-Performance Material for Biosensing. 2D Materials 2020, 7, 024007. [Google Scholar] [CrossRef]
- Mollamohammadi, F.; Faridnouri, H.; Zare, E.N. Electrochemical Biosensing of L-DOPA Using Tyrosinase Immobilized on Carboxymethyl Starch-Graft-Polyaniline@MWCNTs Nanocomposite. Biosensors 2023, 13, 562. [Google Scholar] [CrossRef]
- Goodarzi, M.; Tavakoli, H.; Khalaj, M.A.; Tavakoli, H. A Novel Approach to Tyrosinase-Based Biosensors: Electrode Reactions and Biological Measurement. Biosens. Bioelectron. X 2024, 21, 100550. [Google Scholar] [CrossRef]
- Hosseinzadeh, L.; Khoshroo, A.; Adib, K.; Rahimi-Nasrabadi, M.; Ahmadi, F. Determination of Homocysteine Using a Dopamine-Functionalized Graphene Composite. Microchem. J. 2021, 165, 106124. [Google Scholar] [CrossRef]
- Gorle, D.B.; Kulandainathan, M.A. Electrochemical Sensing of Dopamine at the Surface of a Dopamine Grafted Graphene Oxide/Poly(Methylene Blue) Composite Modified Electrode. RSC Adv. 2016, 6, 19982–19991. [Google Scholar] [CrossRef]
- Ghilane, J.; Hauquier, F.; Lacroix, J.-C. Oxidative and Stepwise Grafting of Dopamine Inner-Sphere Redox Couple onto Electrode Material: Electron Transfer Activation of Dopamine. Anal. Chem. 2013, 85, 11593–11601. [Google Scholar] [CrossRef]
- Rubianes, M.D.; Strumia, M.C. Polyethylenimine Functionalized with Dopamine: Characterization and Electrocatalytic Properties. Electroanalysis 2010, 22, 1200–1206. [Google Scholar] [CrossRef]
- González-Romero, E.; González-Costas, J.; González-Veiga, M.C.; Carro-Pérez, I. Superficie Catalítica. Patent No. ES 2 389 936 B2, 17 January 2014. Available online: https://consultas2.oepm.es/pdf//ES/0000/000/02/38/99/ES-2389936_B2.pdf (accessed on 15 January 2025).
- Zollinger, H. Diazo Chemistry; VCH: Weinheim, Germany; New York, NY, USA, 1994; ISBN 978-3-527-29213-4. [Google Scholar]
- García-González, R.; Fernández-Abedul, M.T.; Pernía, A.; Costa-García, A. Electrochemical Characterization of Different Screen-Printed Gold Electrodes. Electrochim. Acta 2008, 53, 3242–3249. [Google Scholar] [CrossRef]
- Laurentius, L.; Stoyanov, S.R.; Gusarov, S.; Kovalenko, A.; Du, R.; Lopinski, G.P.; McDermott, M.T. Diazonium-Derived Aryl Films on Gold Nanoparticles: Evidence for a Carbon–Gold Covalent Bond. ACS Nano 2011, 5, 4219–4227. [Google Scholar] [CrossRef]
- Berisha, A.; Combellas, C.; Kanoufi, F.; Médard, J.; Decorse, P.; Mangeney, C.; Kherbouche, I.; Seydou, M.; Maurel, F.; Pinson, J. Alkyl-Modified Gold Surfaces: Characterization of the Au–C Bond. Langmuir 2018, 34, 11264–11271. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, S.; Abraham John, S. Spontaneous Grafting: A Novel Approach to Graft Diazonium Cations on Gold Nanoparticles in Aqueous Medium and Their Self-Assembly on Electrodes. J. Colloid Interface Sci. 2014, 428, 84–94. [Google Scholar] [CrossRef]
- Blacha-Grzechnik, A.; Turczyn, R.; Burek, M.; Zak, J. In Situ Raman Spectroscopic Studies on Potential-Induced Structural Changes in Polyaniline Thin Films Synthesized via Surface-Initiated Electropolymerization on Covalently Modified Gold Surface. Vib. Spectrosc. 2014, 71, 30–36. [Google Scholar] [CrossRef]
- Wang, J.; Carlisle, J.A. Covalent Immobilization of Glucose Oxidase on Conducting Ultrananocrystalline Diamond Thin Films. Diam. Relat. Mater. 2006, 15, 279–284. [Google Scholar] [CrossRef]
- Gui, A.L.; Liu, G.; Chockalingam, M.; Le Saux, G.; Luais, E.; Harper, J.B.; Gooding, J.J. A Comparative Study of Electrochemical Reduction of 4-Nitrophenyl Covalently Grafted on Gold and Carbon. Electroanalysis 2010, 22, 1824–1830. [Google Scholar] [CrossRef]
- Cullen, R.J.; Jayasundara, D.R.; Soldi, L.; Cheng, J.J.; Dufaure, G.; Colavita, P.E. Spontaneous Grafting of Nitrophenyl Groups on Amorphous Carbon Thin Films: A Structure–Reactivity Investigation. Chem. Mater. 2012, 24, 1031–1040. [Google Scholar] [CrossRef]
- Ortiz, B.; Saby, C.; Champagne, G.Y.; Bélanger, D. Electrochemical Modification of a Carbon Electrode Using Aromatic Diazonium Salts. 2. Electrochemistry of 4-Nitrophenyl Modified Glassy Carbon Electrodes in Aqueous Media. J. Electroanal. Chem. 1998, 455, 75–81. [Google Scholar] [CrossRef]
- Phal, S.; Shimizu, K.; Mwanza, D.; Mashazi, P.; Shchukarev, A.; Tesfalidet, S. Electrografting of 4-Carboxybenzenediazonium on Glassy Carbon Electrode: The Effect of Concentration on the Formation of Mono and Multilayers. Molecules 2020, 25, 4575. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A. Electrochemical Characterization of Screen-Printed and Conventional Carbon Paste Electrodes. Electrochim. Acta 2008, 53, 3635–3642. [Google Scholar] [CrossRef]
- Pinson, J.; Podvorica, F. Attachment of Organic Layers to Conductive or Semiconductive Surfaces by Reduction of Diazonium Salts. Chem. Soc. Rev. 2005, 34, 429. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analytical Electrochemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-67879-3. [Google Scholar]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Saleh, G.A.; Askal, H.F.; Refaat, I.H.; Naggar, A.H.; Abdel-aal, F.A.M. Adsorptive Square Wave Voltammetric Determination of the Antiviral Drug Valacyclovir on a Novel Sensor of Copper Microparticles–Modified Pencil Graphite Electrode. Arab. J. Chem. 2016, 9, 143–151. [Google Scholar] [CrossRef]
- Sui, L.; Wu, T.; Liu, L.; Wang, H.; Wang, Q.; Hou, H.; Guo, Q. A Sensitive Pyrimethanil Sensor Based on Electrospun TiC/C Film. Sensors 2019, 19, 1531. [Google Scholar] [CrossRef] [PubMed]
- Vilà, N.; Bélanger, D. Mixtures of Functionalized Aromatic Groups Generated from Diazonium Chemistry as Templates towards Bimetallic Species Supported on Carbon Electrode Surfaces. Electrochim. Acta 2012, 85, 538–547. [Google Scholar] [CrossRef]
- Bouden, S.; Bellakhal, N.; Chaussé, A.; Dachraoui, M.; Vautrin-Ul, C. Correlations between the Grafting Conditions and the Copper Detection by Diazonium Functionalized Carbon Screen-Printed Electrodes. Electrochim. Acta 2014, 125, 149–155. [Google Scholar] [CrossRef]
- Brooksby, P.A.; Downard, A.J. Electrochemical and Atomic Force Microscopy Study of Carbon Surface Modification via Diazonium Reduction in Aqueous and Acetonitrile Solutions. Langmuir 2004, 20, 5038–5045. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Sornambikai, S.; Gayathri, P.; Zen, J.-M. Selective Covalent Immobilization of Catechol on Activated Carbon Electrodes. J. Electroanal. Chem. 2010, 641, 131–135. [Google Scholar] [CrossRef]
- Smanhotto Malvessi, G.; Dannehl Hoppe, T.; Zapp, E.; Brondani, D. Lab-Made Screen-Printed Electrode with Conductive Ink Based on Carbon Nanomaterials for Simultaneous Electrochemical Analysis of Bisphenol A, Catechol, and 4-Nitrophenol. Measurement 2024, 231, 114601. [Google Scholar] [CrossRef]
- De Oliveira, S.P.; De Oliveira Cândido, T.C.; Pereira, A.C.; Da Silva, D.N. Electrochemical Determination of Catechol Using a Disposable Printed Electrode with Conductive Ink Based on Graphite and Carbon Black. Analytica 2024, 5, 250–262. [Google Scholar] [CrossRef]
- Manoj, D.; Gnanasekaran, L.; Rajendran, S.; Jalil, A.A.; Siddiqui, M.N.; Gracia, F.; Soto-Moscoso, M. A Mechanothermal Approach for the Synthesis of Fe3O4 Nanoparticles as Dopant on Mesoporous TiO2 for Electrochemical Determination of Catechol. Environ. Res. 2023, 222, 115358. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, J.; Yue, S.; Zhang, L.; Wang, Z.; Guo, P.; Liu, Q. Nickel Oxide/Carbon Nanotube Nanocomposites Prepared by Atomic Layer Deposition for Electrochemical Sensing of Hydroquinone and Catechol. J. Electroanal. Chem. 2018, 808, 245–251. [Google Scholar] [CrossRef]
- Gao, Z.-Y.; Gao, Y.-L.; Wang, E.; Xu, S.; Chen, W. Electrochemical Determination of Catechol Based on Cadmium Telluride Quantum Dots/Graphene Composite Film Modified Electrode. J. Electrochem. Soc. 2016, 163, H528–H533. [Google Scholar] [CrossRef]
- Xu, G.; Tang, B.; Jing, S.; Tao, J. Simultaneous Determination of Hydroquinone, Catechol and Resorcinol at Poly(3-Thiophenemalonic Acid) Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2015, 10, 10659–10667. [Google Scholar] [CrossRef]
- Hu, F.; Chen, S.; Wang, C.; Yuan, R.; Yuan, D.; Wang, C. Study on the Application of Reduced Graphene Oxide and Multiwall Carbon Nanotubes Hybrid Materials for Simultaneous Determination of Catechol, Hydroquinone, p-Cresol and Nitrite. Anal. Chim. Acta 2012, 724, 40–46. [Google Scholar] [CrossRef]
- Pérez-López, B.; Merkoçi, A. Magnetic Nanoparticles Modified with Carbon Nanotubes for Electrocatalytic Magnetoswitchable Biosensing Applications. Adv. Funct. Mater. 2011, 21, 255–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, Z.; Sobhy, A.; Sato, S.; Uchida, M.; Hasebe, Y. Natural Molybdenite- and Tyrosinase-Based Amperometric Catechol Biosensor Using Acridine Orange as a Glue, Anchor, and Stabilizer for the Adsorbed Tyrosinase. ACS Omega 2021, 6, 13719–13727. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, B.; Zhu, G.; Wu, Y.; Liu, S.; Xu, C. Electron Transfer Properties and Electrocatalytic Behavior of Tyrosinase on ZnO Nanorod. J. Electroanal. Chem. 2008, 617, 7–13. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fatibello-Filho, O.; Sotomayor, M.D.P.T. Amperometric Tyrosinase Biosensor Based on Carbon Black Paste Electrode for Sensitive Detection of Catechol in Environmental Samples. Electroanalysis 2021, 33, 431–437. [Google Scholar] [CrossRef]
- Camargo, J.R.; Silva, T.A.; Rivas, G.A.; Janegitz, B.C. Novel Eco-Friendly Water-Based Conductive Ink for the Preparation of Disposable Screen-Printed Electrodes for Sensing and Biosensing Applications. Electrochim. Acta 2022, 409, 139968. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Janegitz, B.C.; Brett, C.M.A.; Fatibello-Filho, O. Tyrosinase Biosensor Based on a Glassy Carbon Electrode Modified with Multi-Walled Carbon Nanotubes and 1-Butyl-3-Methylimidazolium Chloride within a Dihexadecylphosphate Film. Sens. Actuators B Chem. 2013, 188, 1101–1108. [Google Scholar] [CrossRef]
- Cagnani, G.R.; Ibáñez-Redín, G.; Tirich, B.; Gonçalves, D.; Balogh, D.T.; Oliveira, O.N. Fully-Printed Electrochemical Sensors Made with Flexible Screen-Printed Electrodes Modified by Roll-to-Roll Slot-Die Coating. Biosens. Bioelectron. 2020, 165, 112428. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Y.; Zhang, Z.; Shen, Y.; Li, M. Tyrosinase Modified Poly(Thionine) Electrodeposited Glassy Carbon Electrode for Amperometric Determination of Catechol. Electrochemistry 2017, 85, 17–22. [Google Scholar] [CrossRef]



| Electrode * | Technique ** | LDR *** (μM) | LOD (µM) | Sensitivity (µA/mM) | Ref. |
|---|---|---|---|---|---|
| SPCE/GNPs | DPV | 23–200 | 6.9 | 150 | [67] |
| SPCE | SWV | 20–220 | 5.9 | 31.0 | [68] |
| GCE/Fe3O4-TiO2 | CV | 150–500 | 45 | 5.6 | [69] |
| GCE/MWCNT/NiO | DPV | 10–400 | 2.5 | 196 | [70] |
| GCE/GR/CdTe-QDs | DPV | 80–1000 | 18.3 | 6.4 | [71] |
| GCE/PTA | DPV | 26–500 | 7.8 | 120 | [72] |
| GCE/RGO-MWNTs | DPV | 6–540 | 1.8 | 70 | [73] |
| SPCE/Cu-CMCS2 | CA | 7.3–40 | 7.3 | 10.0 | [33] |
| SPCE/TYR | CA | 29–40 | 29 | 3.3 | [33] |
| GCE/CMS-g-PANI@MWCNTs/TYR | DPV | 83–100 | 25 | 78.5 | [38] |
| SPCE/MNP/MWCNTs/TYR | DPV | 10–80 | 7.6 | 4.8 | [74] |
| GCE-DHP/AuNPs/TYR | Amp. | 2.5–95 | 0.7 | 115 | [31] |
| GCE-MLN/AO/TYR | Amp. | 1.7–80 | 0.5 | 31.5 | [75] |
| GCE/PS-ND/TYR | DPV | 5–740 | 0.9 | 23.0 | [32] |
| GCE/ZnO/TYR/Nafion | Amp. | 13–400 | 6 | 2.14 | [76] |
| CBPE/GLU/TYR | Amp. | 0.05–8.5 | 0.015 | 460 | [77] |
| CGGE-PET/MWCNT/TYR | CA | 0.5–50 | 0.3 | 231 | [78] |
| GCE/MWCNT-IL-TYR | LSV | 4.9–1100 | 0.6 | 32.8 | [79] |
| SPCE/CB4/TYR | CV | 6.3–100 | 1.9 | 130 | [80] |
| SPCE/BSA-GLU/TYR | DPV | 19–103 | 5.6 | 6.2 | [36] |
| GCE/pTN-GLU/TYR | CV | 20–300 | 6.0 | 5.04 | [81] |
| SPCE-GRAFT-GLU-DA | CV | 62–10,000 | 18.6 | 32.2 | This work |
| SPCE-GRAFT-Diazo-TYR | CV | 14.9–400 | 4.5 | 317 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Costas, J.M.; Caruncho-Pérez, S.; González-Romero, E. Enhanced Catalytic Surfaces for Catechol Sensing: Combining Grafted Aryldiazonium Derivative with Cross-Linking Dopamine or Coupling Tyrosinase Immobilizations. Appl. Sci. 2025, 15, 4250. https://doi.org/10.3390/app15084250
González-Costas JM, Caruncho-Pérez S, González-Romero E. Enhanced Catalytic Surfaces for Catechol Sensing: Combining Grafted Aryldiazonium Derivative with Cross-Linking Dopamine or Coupling Tyrosinase Immobilizations. Applied Sciences. 2025; 15(8):4250. https://doi.org/10.3390/app15084250
Chicago/Turabian StyleGonzález-Costas, Javier M., Sara Caruncho-Pérez, and Elisa González-Romero. 2025. "Enhanced Catalytic Surfaces for Catechol Sensing: Combining Grafted Aryldiazonium Derivative with Cross-Linking Dopamine or Coupling Tyrosinase Immobilizations" Applied Sciences 15, no. 8: 4250. https://doi.org/10.3390/app15084250
APA StyleGonzález-Costas, J. M., Caruncho-Pérez, S., & González-Romero, E. (2025). Enhanced Catalytic Surfaces for Catechol Sensing: Combining Grafted Aryldiazonium Derivative with Cross-Linking Dopamine or Coupling Tyrosinase Immobilizations. Applied Sciences, 15(8), 4250. https://doi.org/10.3390/app15084250








