Optimization of Ethanol Concentration and Wetting Time for Industrial-Scale Production of Ipomoea batatas L. Leaf Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Simplisia
2.2. Total Flavonoid Content (TFC) Determination
2.3. Flavonoid TLC Analysis
2.3.1. Fingerprint Detection
2.3.2. Alignment Chromatographic Data
Image Processing
Peak Alignment
2.4. Extract Preparation
2.5. Data Analysis
3. Results
3.1. Total Flavonoid Content
3.2. Flavonoid TLC Analysis
3.2.1. Fingerprint Detection
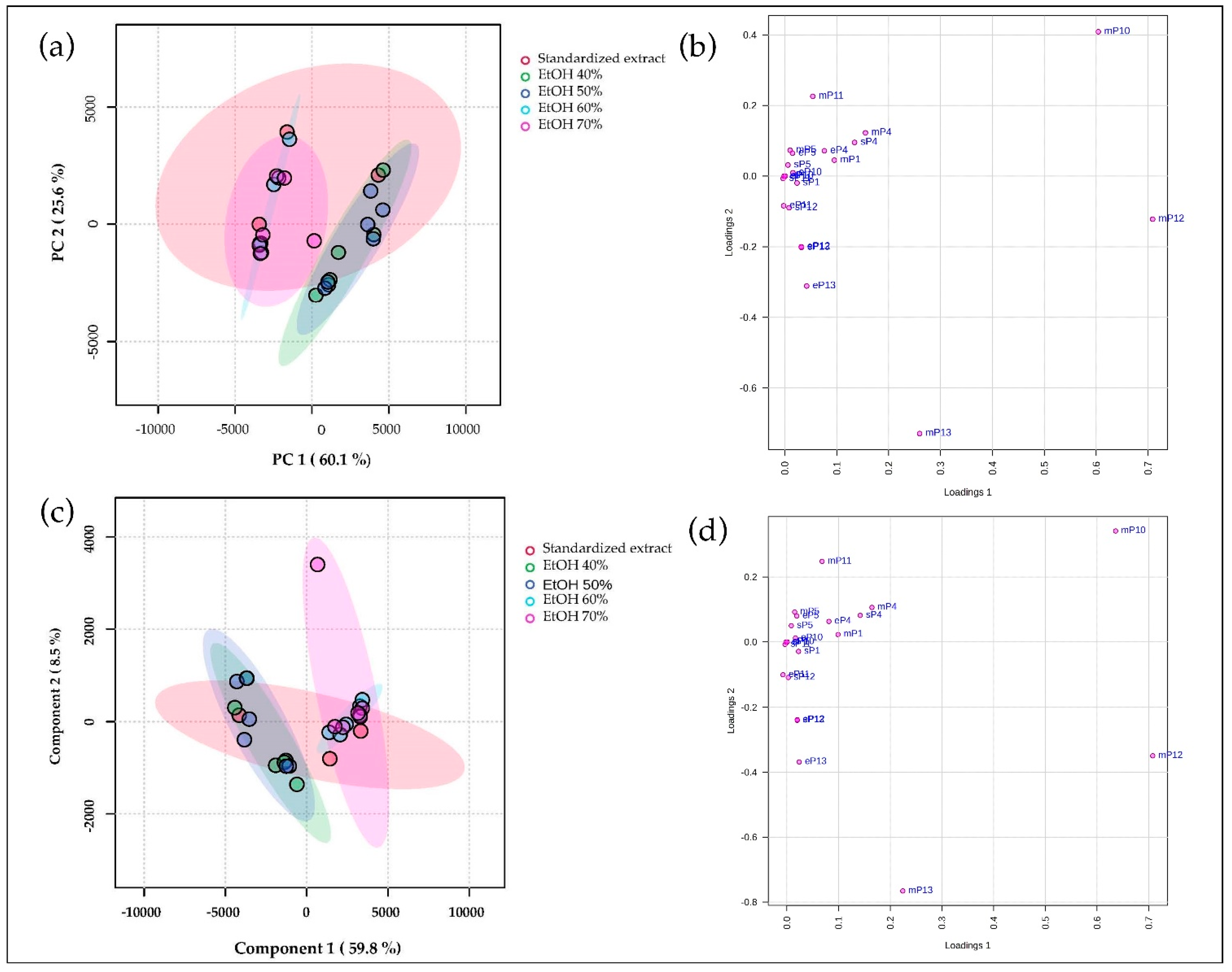
3.2.2. Peak Alignment
3.3. Application of TLC Fingerprint for Quality Control Extraction
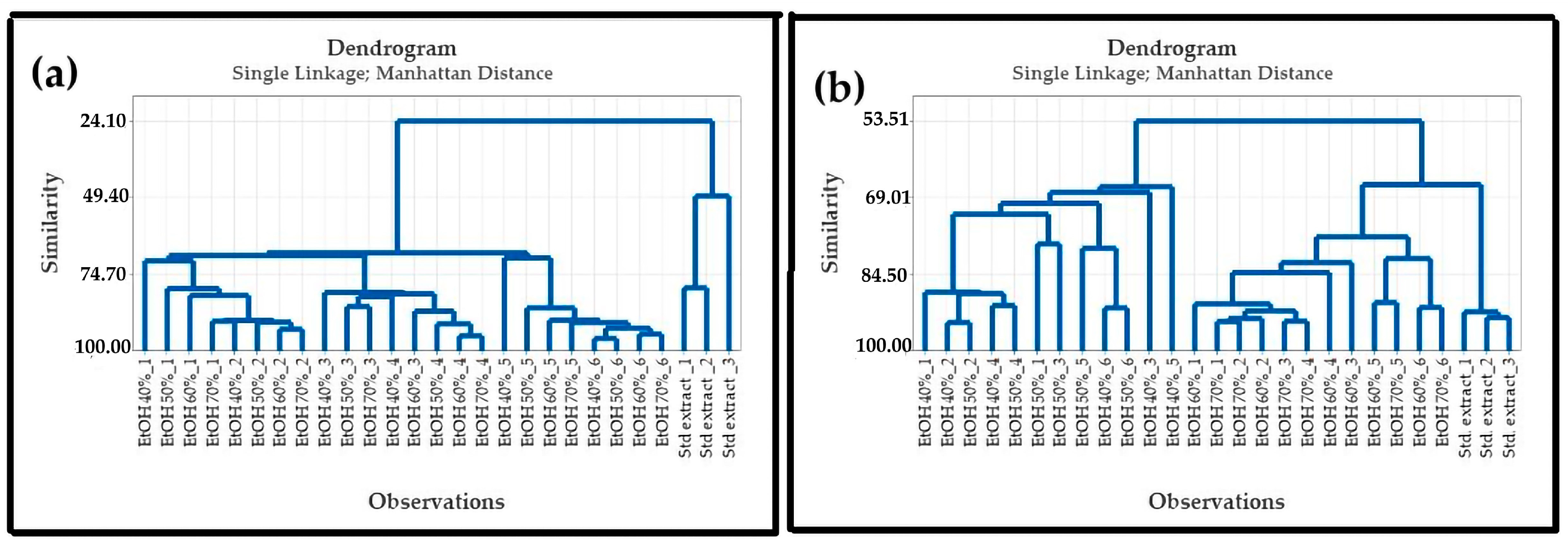
3.3.1. Effect of Ethanol Concentration on Fingerprint
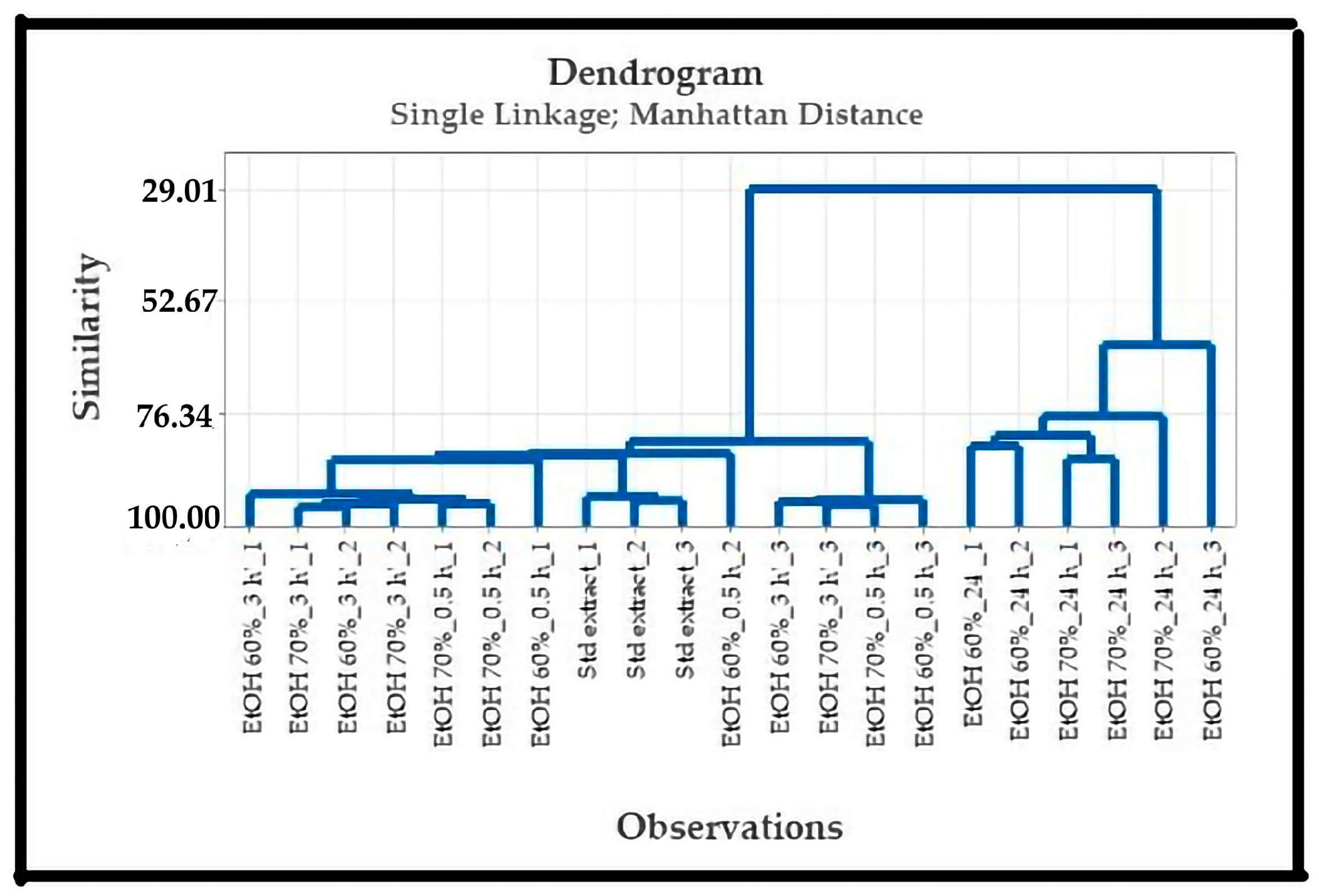
3.3.2. Effect of Wetting Time on Simplisia Fingerprint
4. Discussion
4.1. Total Flavonoid Content
4.2. Flavonoid TLC Analysis
4.2.1. Fingerprint Detection
4.2.2. Peak Alignment
4.3. Application of TLC Fingerprint for Extraction Quality Control
Effect of Ethanol Concentration and Wetting Time on Fingerprint
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cGMP | Current Good Manufacturing |
| EMEA | European Product Evaluation Agency |
| FDA | Food and Drug Administration |
| TLC | thin-layer chromatography |
| GLP-1 | glucagon-like peptide-1 |
| TFC | total flavonoid content |
| SME | start, middle, and end |
| PCA | principal component analysis |
| PLS-DA | partial least squares—discriminant analysis |
| HCA | hierarchical cluster analysis |
| EtOH | ethanol |
References
- Gao, M.; Lan, J.; Zhang, Y.; Yu, S.; Bao, B.; Yao, W.; Cao, Y.; Shan, M.; Cheng, F.; Zhang, L.; et al. Discovery of processing-associated Q-marker of carbonized traditional Chinese medicine: An integrated strategy of metabolomics, systems pharmacology and in vivo high-throughput screening model. Phytomedicine 2022, 102, 154152. [Google Scholar]
- Barthwal, R.; Saini, D.; Sharma, S.K.; Kumar, A. Current trends in extraction of plant bioactive molecules valuable for food use. J. Food Agric. Res. 2021, 1, 203–220. [Google Scholar]
- WHO. Guidelines on Good Herbal Processing, Annex 1 WHO Guidelines on Good Herbal Processing Practices for Herbal Medicines; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Pudziuvelyte, L.; Jakštas, V.; Ivanauskas, L.; Laukevičienė, A.; Ibe, C.F.D.; Kursvietiene, L.; Bernatoniene, J. Different extraction methods for phenolic and volatile compounds recovery from Elsholtzia ciliata fresh and dried herbal materials. Ind. Crops Prod. 2018, 120, 286–294. [Google Scholar]
- Wilson, J.; Simpson, T.; Spelman, K. Total cannabidiol (CBD) concentrations and yields from traditional extraction methods: Percolation vs. maceration. Front. Pharmacol. 2022, 13, 886993. [Google Scholar] [CrossRef]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar]
- Uhlenbrock, L.; Sixt, M.; Tegtmeier, M.; Schulz, H.; Hagels, H.; Ditz, R.; Strube, J. Natural products extraction of the future-sustainable manufacturing solutions for societal needs. Processes 2018, 6, 177. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res. Int. 2001, 34, 223–227. [Google Scholar]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar]
- Chanda, S.V.; Kaneria, M.J. Optimization of conditions for the extraction of antioxidants from leaves of Syzygium cumini L. using different solvents. Food Anal. Methods 2012, 5, 332–338. [Google Scholar]
- Widiputri, D.I.; Julisantika, I.; Kartawiria, I.S.; Gunawan-Puteri, M.D.P.T.; Ignatia, F. Upscaling the Cymbopogon citratus (lemongrass) extraction process to obtain optimum alpha-glucosidase inhibitor (AGI) levels. Int. J. Technol. 2020, 11, 532–543. [Google Scholar]
- European Medicines Agency (EMA). Specifications: Test Procedures and Acceptance Criteria for Herbal Substances, Herbal Preparations and Herbal Medicinal Products/Traditional Herbal Medicinal Products. 2022, Volume 31, pp. 1–32. Available online: https://www.ema.europa.eu/en/specifications-test-procedures-acceptance-criteria-herbal-substances-herbal-preparations-herbal (accessed on 13 September 2024).
- Food and Drug Administration. Botanical Drug Development—Guidance for Industry; U.S. Department of Health and Human Services: Washington, DC, USA, 2016; pp. 1–30. Available online: https://www.fda.gov/downloads/Drugs/Guidances/UCM458484.pdf (accessed on 15 October 2024).
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal drugs: Standards and regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Spriano, D. Modern HPTLC—A perfect tool for quality control of herbals and their preparations. J. AOAC Int. 2010, 93, 1399–1409. [Google Scholar] [CrossRef]
- Lebot, V.; Michalet, S.; Legendre, L. Identification and quantification of phenolic compounds responsible for the antioxidant activity of sweet potatoes with different flesh colours using high performance thin layer chromatography (HPTLC). J. Food Compos. Anal. 2016, 49, 94–101. [Google Scholar] [CrossRef]
- Yustiantara, P.S.; Warditiani, N.K.; Sari, P.M.N.A.; Dewi, N.L.K.A.A.; Ramona, Y.; Jawi, I.M.; Wirasuta, I.M.A.G. Determination of TLC fingerprint biomarker of Ipomoea batatas (L.) Lam leaves extracted with ethanol and its potential as antihyperglycemic agent. Pharmacia 2021, 68, 907–917. [Google Scholar] [CrossRef]
- Bingbing, L.; Qian, W.; Caixia, L.; Wenjing, H.; Guoliang, C.; Yongxia, G.; Ishaq, M.; Xue, X.; Shikai, Y. Study on GC-MS fingerprint of petroleum ether fraction of Shenqi Jiangtang Granules. Digit. Chin. Med. 2021, 4, 32–41. [Google Scholar] [CrossRef]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2016, 53, 1071–1083. [Google Scholar] [CrossRef]
- Gao, S.; Liu, J.-S.; Wang, M.; Cao, T.-T.; Qi, Y.-D.; Zhang, B.-G.; Liu, H.-T.; Sun, X.-B.; Xiao, P.-G. Quantitative and HPLC fingerprint analysis combined with chemometrics for quality evaluation of Codonopsis Radix processed with different methods. Chin. Herb. Med. 2019, 11, 160–168. [Google Scholar] [CrossRef]
- CIP. Discovery to Impact; International Potato Center: Lima, Peru, 2019; p. 38. [Google Scholar]
- Fu, Z.F.; Tu, Z.C.; Zhang, L.; Wang, H.; Wen, Q.H.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Zurek, N. The content of phenolic acids and flavonols in the leaves of nine varieties of sweet potatoes (Ipomoea batatas L.) depending on their development, grown in central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef]
- Arisanti, C.I.S.; Wirasuta, I.M.A.G.; Musfiroh, I.; Ikram, E.H.K.; Muchtaridi, M. Mechanism of anti-diabetic activity from sweet potato (Ipomoea batatas): A systematic review. Foods 2023, 12, 2810. [Google Scholar] [CrossRef]
- Dewi, N.K.S.M.; Ramona, Y.; Saraswati, M.R.; Wihandani, D.M.; Wirasuta, I.M.A.G. The Potential of the flavonoid content of Ipomoea batatas L. as an alternative analog GLP-1 for diabetes type 2 treatment—Systematic Review. Metabolites 2024, 14, 29. [Google Scholar]
- Kemenkes, R.I. Farmakope Herbal Indonesia Edisi II; Kementrian Kesehatan Republik Indonesia: Jakarta, Indonesia, 2017.
- Wirasuta, I.M.A.G.; Srinadi, I.G.A.M.; Dwidasmara, I.B.G.; Ardiyanti, N.L.P.P.; Trisnadewi, I.G.A.A.; Paramita, N.L.P.V. Authentication of Piper betle L. folium and quantification of their antifungal-activity. J. Tradit. Complement. Med. 2017, 7, 288–295. [Google Scholar]
- Schibli, A.; Reich, E. Modern TLC: A key technique for identification and quality control of botanicals and dietary supplements. J. Planar Chromatogr. 2007, 18, 34–38. [Google Scholar]
- Wirasuta, I.M.A.G.; Arisanti, C.I.S. Metoda Pembuatan Ekstrak Terstandar Daun Ubi Jalar (Ipomoea batatas) Sebagai Anti. Diabetes. Patent IDS000008593, 26 July 2024. [Google Scholar]
- Diem, Q.; Elisa, A.; Tran-nguyen, P.L. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2013, 22, 296–302. [Google Scholar]
- Carmagnani, H.J.; Mansano, G.B.; Sobreira, F. Optimization of the extraction process of Phyllanthus niruri L. Mundo Saude 2020, 44, 134–143. [Google Scholar]
- Li, F.; Li, Q.; Gao, D.; Peng, Y. The optimal extraction parameters and anti-diabetic activity of flavonoids from Ipomoea batatas leaf. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 195–202. [Google Scholar]
- Nagamine, R.; Ueno, S.; Tsubata, M.; Yamaguchi, K.; Takagaki, K.; Hira, T.; Hara, H.; Tsuda, T. Dietary sweet potato (Ipomoea batatas L.) leaf extract attenuates hyperglycaemia by enhancing the secretion of glucagon-like peptide-1 (GLP-1). Food Funct. 2014, 5, 2309–2316. [Google Scholar]
- Echeverry, S.M.; Medina, H.I.; Costa, G.M.; Aragón, D.M. Optimization of flavonoid extraction from Passiflora quadrangularis leaves with sedative activity and evaluation of its stability under stress conditions. Rev. Bras. Farmacogn. 2018, 28, 610–617. [Google Scholar]
- Muhammad, U.; Zhu, X.; Lu, Z.; Han, J.; Sun, J.; Tayyaba, S.; Abbasi, B.; Siyal, F.A.; Dhama, K.; Saqib, J. Effects of extraction variables on pharmacological activities of vine tea extract (Ampelopsis grossedentata). Int. J. Pharmacol. 2018, 14, 495–505. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef]
- Ristivojević, P.; Andrić, F.L.; Trifković, J.Đ.; Vovk, I.; Stanisavljević, L.Ž.; Tešić, Ž.L.; Milojković-Opsenica, D.M. Pattern recognition methods and multivariate image analysis in HPTLC fingerprinting of propolis extracts. J. Chemom. 2014, 28, 301–310. [Google Scholar]
- Marby, M.B.; Markham, T.J.; Thomas, K.R. The Systematic Identification of Flavonoids, 1st ed.; Springer: New York, NY, USA, 1970. [Google Scholar]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Biosci. 2021, 39, 100801. [Google Scholar]
- Gad, H.A.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Al-Azizi, M.M. Application of chemometrics in authentication of herbal medicines: A review. Phytochem. Anal. 2013, 24, 1–24. [Google Scholar]
- Milojković Opsenica, D.; Ristivojević, P.; Trifković, J.; Vovk, I.; Lušić, D.; Tešić, Ž. TLC fingerprinting and pattern recognition methods in the assessment of authenticity of poplar-type propolis. J. Chromatogr. Sci. 2016, 54, 1077–1083. [Google Scholar]
- Korifi, R.; Le Dréau, Y.; Dupuy, N. Comparative study of the alignment method on experimental and simulated chromatographic data. J. Sep. Sci. 2014, 37, 3276–3291. [Google Scholar]
- Bloemberg, T.G.; Gerretzen, J.; Lunshof, A.; Wehrens, R.; Buydens, L.M.C. Warping methods for spectroscopic and chromatographic signal alignment: A tutorial. Anal. Chim. Acta 2013, 781, 14–32. [Google Scholar]
- Patel, M.; Bansal, K. Development and application of HPTLC method for estimation of Rivaroxaban and Aspirin in bulk drug and in-house tablet form. J. Chem. Metrol. 2022, 16, 125–134. [Google Scholar]
- Senapati, M.R.; Behera, P.C.; Bisoi, P.C.; Maity, A.; Parija, S.C. HPTLC finger print analysis of phytophenols of Paederia foetida under different extraction regimen. Bioscan 2013, 8, 603–609. [Google Scholar]
- Buckow, C.; Kastell, R.; Terefe, A.; Versteeg, N.S. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J. Agric. Food Chem. 2010, 58, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gullón, P.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Optimization of solvent extraction of antioxidants from Eucalyptus globulus leaves by response surface methodology: Characterization and assessment of their bioactive properties. Ind. Crops Prod. 2017, 108, 649–659. [Google Scholar]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in Pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Mitho, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar]
- Ayeleso, T.; Ramachela, K.; Mukwevho, E. Aqueous-methanol extracts of orange-fleshed sweet potato (Ipomoea batatas) ameliorate oxidative stress and modulate type 2 diabetes associated genes in insulin resistant C2C12 cells. Molecules 2018, 23, 2058. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arisanti, C.I.S.; Musfiroh, I.; Wirasuta, I.M.A.G.; Ikram, N.K.K.; Muchtaridi, M. Optimization of Ethanol Concentration and Wetting Time for Industrial-Scale Production of Ipomoea batatas L. Leaf Extract. Appl. Sci. 2025, 15, 4299. https://doi.org/10.3390/app15084299
Arisanti CIS, Musfiroh I, Wirasuta IMAG, Ikram NKK, Muchtaridi M. Optimization of Ethanol Concentration and Wetting Time for Industrial-Scale Production of Ipomoea batatas L. Leaf Extract. Applied Sciences. 2025; 15(8):4299. https://doi.org/10.3390/app15084299
Chicago/Turabian StyleArisanti, Cokorda Istri Sri, Ida Musfiroh, I Made Agus Gelgel Wirasuta, Nur Kusaira Khairul Ikram, and Muchtaridi Muchtaridi. 2025. "Optimization of Ethanol Concentration and Wetting Time for Industrial-Scale Production of Ipomoea batatas L. Leaf Extract" Applied Sciences 15, no. 8: 4299. https://doi.org/10.3390/app15084299
APA StyleArisanti, C. I. S., Musfiroh, I., Wirasuta, I. M. A. G., Ikram, N. K. K., & Muchtaridi, M. (2025). Optimization of Ethanol Concentration and Wetting Time for Industrial-Scale Production of Ipomoea batatas L. Leaf Extract. Applied Sciences, 15(8), 4299. https://doi.org/10.3390/app15084299





