Transcranial Direct Current Stimulation Improves Bilateral Ankle-Dorsiflexion Force Control in Healthy Young Adults
Abstract
1. Introduction
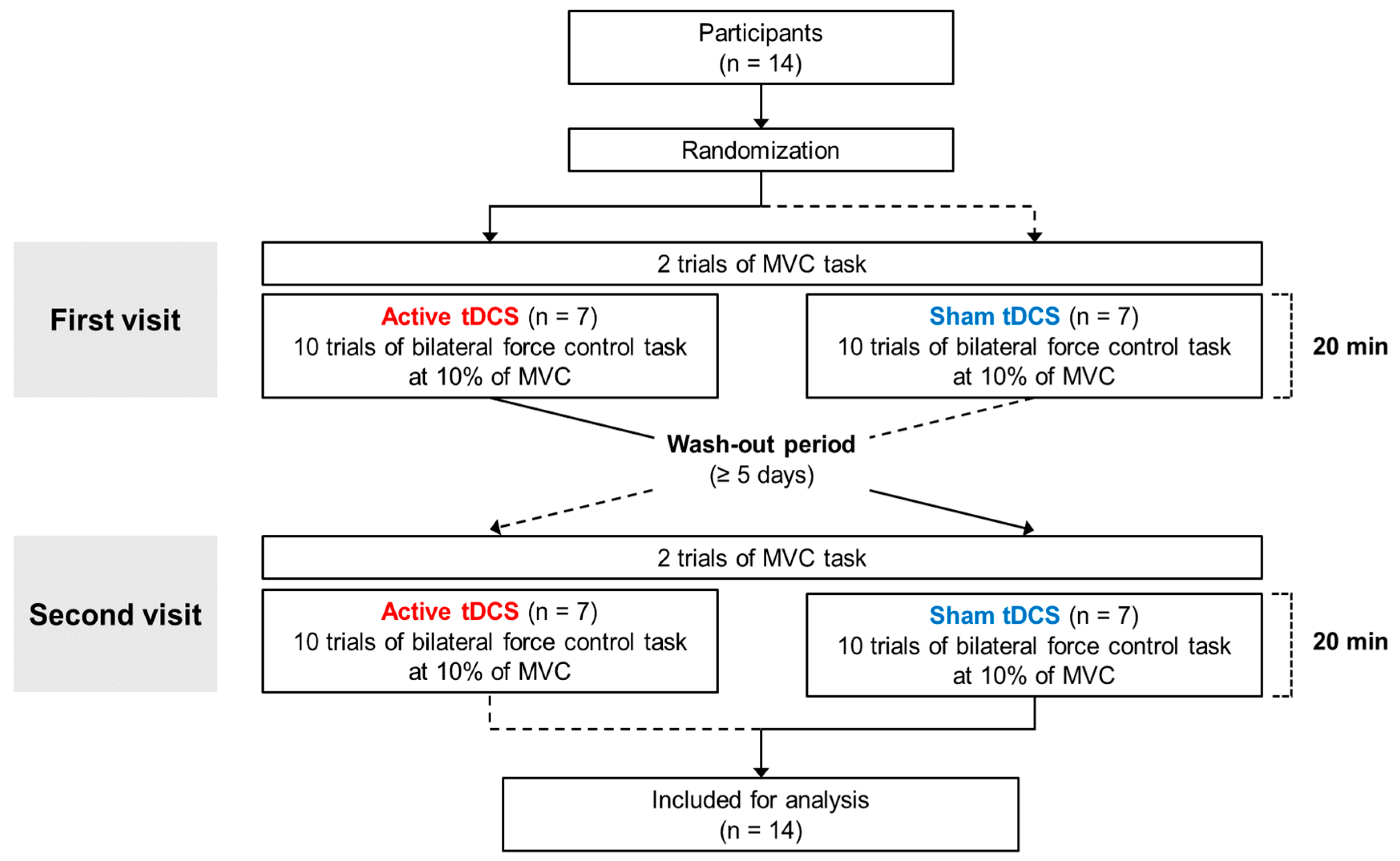
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
2.3. Bilateral Ankle-Dorsiflexion Force Control Tasks
2.4. tDCS Protocols
2.5. Data Analyses
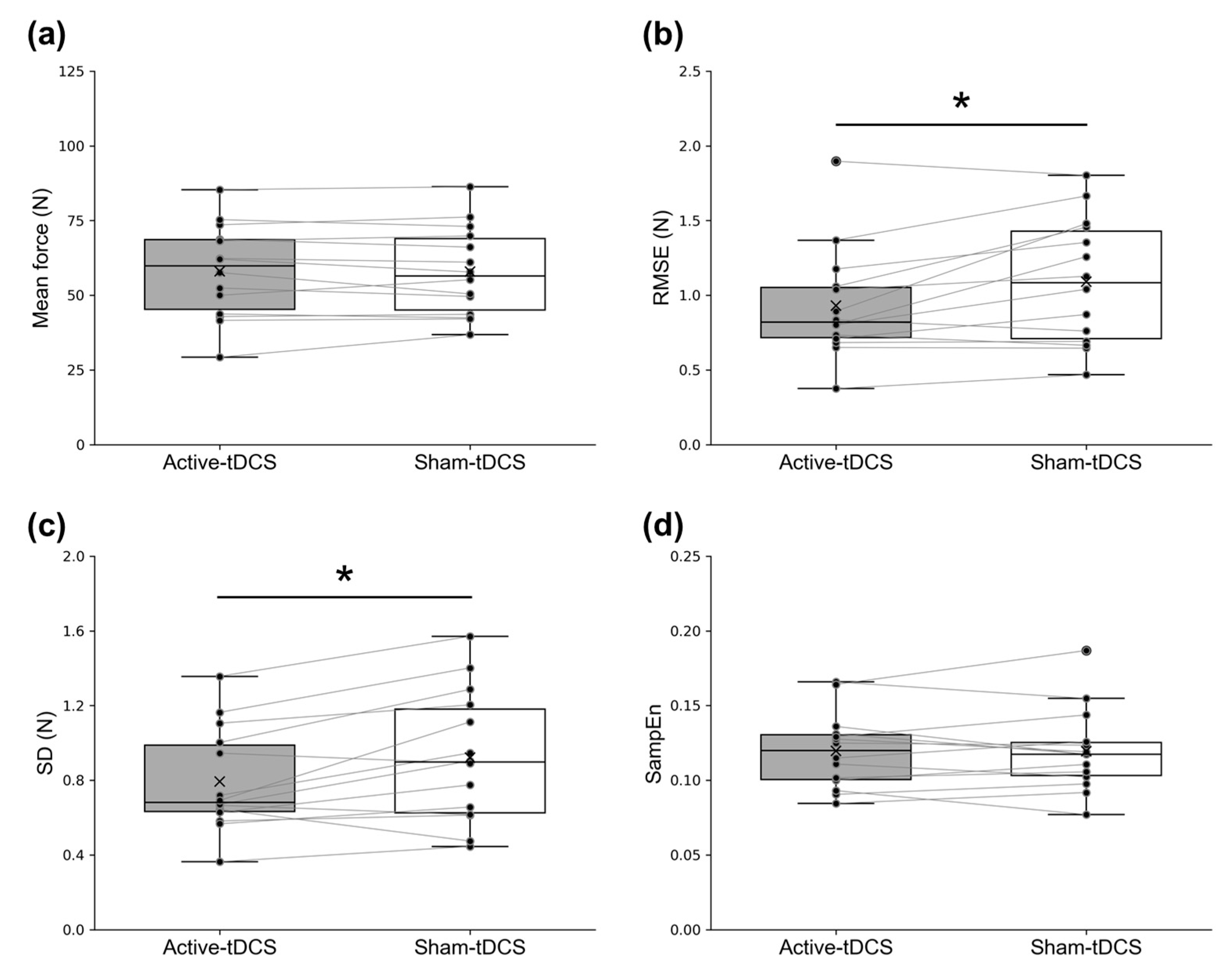
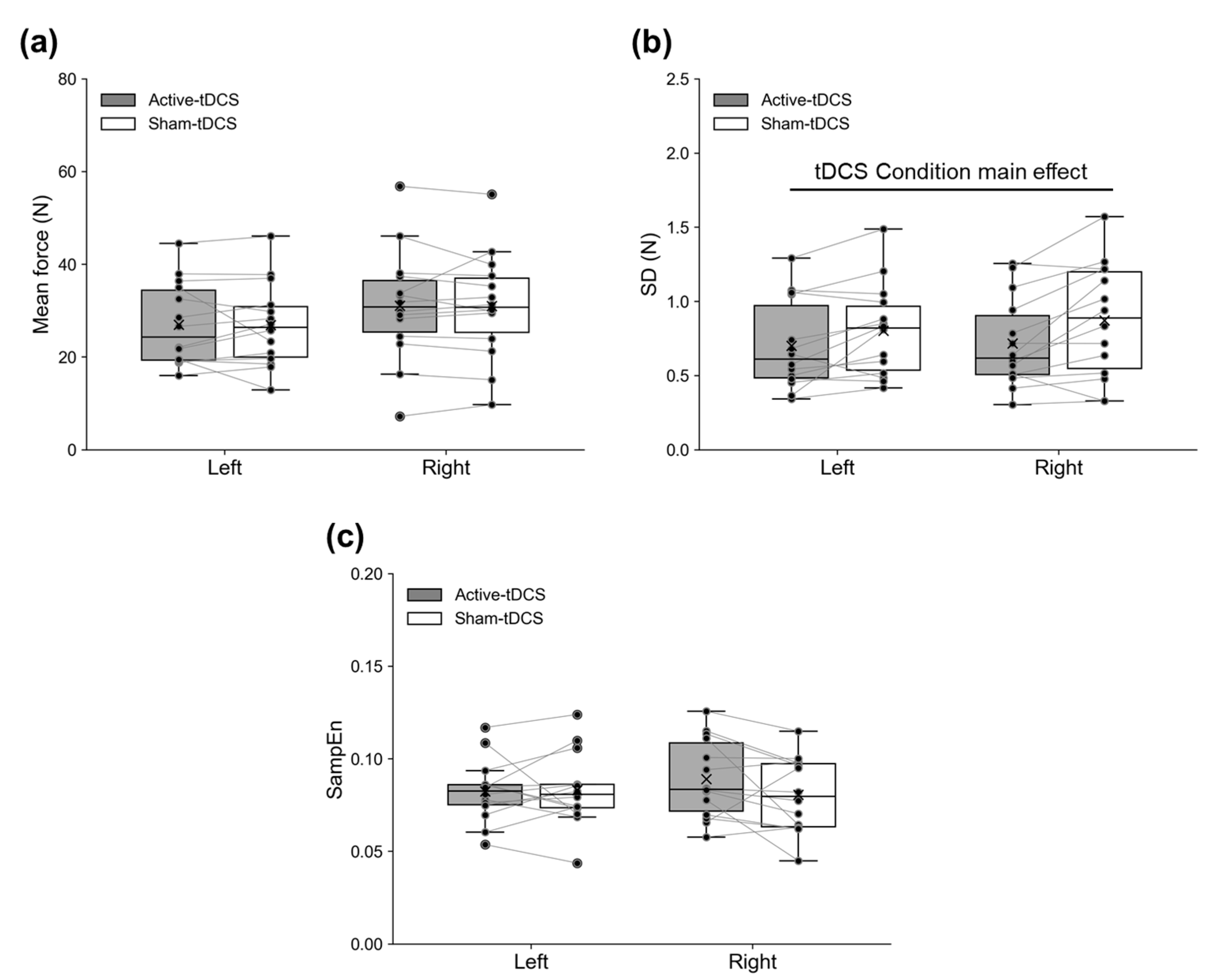
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harjpal, P.; Kovela, R.K.; Jain, M. Efficacy of Bilateral Lower-Limb Training Over Unilateral Lower-Limb Training To Reeducate Balance and Walking in Post-Stroke Survivors: A Randomized Clinical Trial. Cureus 2022, 14, e30748. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kurematsu, R.; Shigetome, S.; Matsumoto, T.; Tsuruda, K.; Tokuyasu, T. Motor control mechanism underlying pedaling skills: An analysis of bilateral coordination in the lower extremities. Artif. Life Robot 2020, 25, 308–315. [Google Scholar] [CrossRef]
- Promsri, A. Modulation of bilateral lower-limb muscle coordination when performing increasingly challenging balance exercises. Neurosci. Lett. 2022, 767, 136299. [Google Scholar] [CrossRef]
- Noble, J.W.; Eng, J.J.; Boyd, L.A. Bilateral motor tasks involve more brain regions and higher neural activation than unilateral tasks: An fMRI study. Exp. Brain Res. 2014, 232, 2785–2795. [Google Scholar] [CrossRef]
- Kumai, K.; Ikeda, Y.; Sakai, K.; Goto, K.; Morikawa, K.; Shibata, K. Brain and muscle activation patterns during postural control affect static postural control. Gait Posture 2022, 96, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Ishii, K.; Ishiwata, K.; Oda, K.; Suzukawa, M.; Makizako, H.; Doi, T.; Suzuki, T. Gait adaptability and brain activity during unaccustomed treadmill walking in healthy elderly females. Gait Posture 2013, 38, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.F.; de Souza, I.C.C.; Vidor, L.P.; de Souza, A.; Deitos, A.; Volz, M.S.; Fregni, F.; Caumo, W.; Torres, I.L. Neurobiological effects of transcranial direct current stimulation: A review. Front. Psychiatry 2012, 3, 110. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Wendling, F. Mechanisms of action of tDCS: A brief and practical overview. Neurophysiol. Clin. 2019, 49, 269–275. [Google Scholar] [CrossRef]
- Filmer, H.L.; Dux, P.E.; Mattingley, J.B. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014, 37, 742–753. [Google Scholar] [CrossRef]
- Lattari, E.; Campos, C.; Lamego, M.K.; Legey, S.; Neto, G.M.; Rocha, N.B.; Oliveira, A.J.; Carpenter, C.S.; Machado, S. Can transcranial direct current stimulation improve muscle power in individuals with advanced weight-training experience? J. Strength Cond. Res. 2020, 34, 97–103. [Google Scholar] [CrossRef]
- Zhang, N.; Nitsche, M.A.; Miao, Y.; Xiong, Z.; Vicario, C.M.; Qi, F. Transcranial Direct-Current Stimulation Over the Primary Motor Cortex and Cerebellum Improves Balance and Shooting Accuracy in Elite Ice Hockey Players. Int. J. Sport Physiol. Perform. 2024, 1, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhiqiang, Z.; Wei, W.; Yunqi, T.; Yu, L. Effects of bilateral extracephalic transcranial direct current stimulation on lower limb kinetics in countermovement jumps. Int. J. Environ. Res. Public Health 2023, 20, 2241. [Google Scholar] [CrossRef]
- Wang, I.-L.; Gu, C.-Y.; Lei, T.-H.; Chen, C.-H.; Chiu, C.-H.; Su, Y. The effect of transcranial direct current stimulation on bilateral asymmetry and joint angles of the lower limb for females when crossing obstacles. BMC Sports Sci. Med. Rehabil. 2023, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, E.; Steele, C.J.; Hoff, M.; Gundlach, C.; Rjosk, V.; Sehm, B.; Villringer, A.; Ragert, P. Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin. Neurophysiol. 2016, 127, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Shen, B.; Zhang, C.; Zhang, X.; Yang, S.; Zhou, J.; Fu, W. Anodal transcranial direct current stimulation enhances ankle force control and modulates the beta-band activity of the sensorimotor cortex. Cereb. Cortex 2023, 33, 7670–7677. [Google Scholar] [CrossRef]
- Hou, J.; Nitsche, M.A.; Yi, L.; Kong, Z.; Qi, F. Effects of transcranial direct current stimulation over the primary motor cortex in improving postural stability in healthy young adults. Biology 2022, 11, 1370. [Google Scholar] [CrossRef]
- Nasseri, P.; Nitsche, M.A.; Ekhtiari, H. A Framework for Categorizing Electrode Montages in Transcranial Direct Current Stimulation. Front. Hum. Neurosci. 2015, 9, 54. [Google Scholar] [CrossRef]
- Bikson, M.; Datta, A.; Rahman, A.; Scaturro, J. Electrode montages for tDCS and weak transcranial electrical stimulation: Role of “return” electrode’s position and size. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2010, 121, 1976. [Google Scholar] [CrossRef]
- Moliadze, V.; Antal, A.; Paulus, W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin. Neurophysiol. 2010, 121, 2165–2171. [Google Scholar] [CrossRef]
- Hamajima, H.; Gomez-Tames, J.; Uehara, S.; Otaka, Y.; Tanaka, S.; Hirata, A. Computation of group-level electric field in lower limb motor area for different tDCS montages. Clin. Neurophysiol. 2023, 150, 69–78. [Google Scholar] [CrossRef]
- Shen, B.; Xiao, S.; Yu, C.; Zhang, C.; Zhan, J.; Liu, Y.; Fu, W. Cerebral hemodynamics underlying ankle force sense modulated by high-definition transcranial direct current stimulation. Cereb. Cortex 2024, 34, bhae226. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Nguyen, T.T.; Madhavan, S. Polarity independent effects of cerebellar tDCS on short term ankle visuomotor learning. Brain Stimul. 2013, 6, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, A.; Oishi, T.; Madhavan, S. Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res. 2014, 1581, 23–29. [Google Scholar] [CrossRef]
- Sehm, B.; Kipping, J.; Schäfer, A.; Villringer, A.; Ragert, P. A comparison between uni-and bilateral tDCS effects on functional connectivity of the human motor cortex. Front. Hum. Neurosci. 2013, 7, 183. [Google Scholar] [CrossRef]
- Tazoe, T.; Endoh, T.; Kitamura, T.; Ogata, T. Polarity specific effects of transcranial direct current stimulation on interhemispheric inhibition. PLoS ONE 2014, 9, e114244. [Google Scholar] [CrossRef]
- Lindenberg, R.; Nachtigall, L.; Meinzer, M.; Sieg, M.M.; Flöel, A. Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J. Neurosci. 2013, 33, 9176–9183. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Pascual-Leone, A.; Fregni, F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys. Ther. 2010, 90, 398–410. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, B.; Zhang, X.; Zhou, J.; Fu, W. Systematic Review of the Impact of Transcranial Direct Current Stimulation on the Neuromechanical Management of Foot and Ankle Physical Performance in Healthy Adults. Front. Bioeng. Biotechnol. 2020, 8, 587680. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, J.; Oh, S.; Celeste Flores Gimenez, M.; Yoon, B. Noninvasive brain stimulation over the M1 enhances bimanual force control ability: A randomized double-blind sham-controlled study. J. Mot. Behav. 2019, 51, 521–531. [Google Scholar] [CrossRef]
- Kang, Y.; NA, D.-L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Urbaniak & Plous. Research Randomizer (Version 4.0). Available online: https://www.randomizer.org/ (accessed on 10 April 2025).
- Lee, J.H.; Kang, N. Transcranial direct current stimulation influences repetitive bimanual force control and interlimb force coordination. Exp. Brain Res. 2023, 241, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophys. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef]
- Lee, T.L.; Ko, D.-K.; Kang, N. Advanced Force Coordination of Lower Extremities During Visuomotor Control Task in Soccer Players. Res. Q. Exerc. Sport 2023, 95, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, T.L.; Kang, N. Effects of visual feedback and force level on bilateral ankle-dorsiflexion force control. Neurosci. Lett. 2024, 824, 137671. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.A.; McNair, P.J.; Lewis, G.N.; Mannion, J. Experimental knee pain impairs submaximal force steadiness in isometric, eccentric, and concentric muscle actions. Arthritis Res. Ther. 2015, 17, 259. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.H.; Lee, T.L.; Ko, D.-K.; Kang, N. Dual-hemisphere anodal transcranial direct current stimulation improves bilateral motor synergies. Front. Psychol. 2023, 14, 1211034. [Google Scholar] [CrossRef]
- Fonteneau, C.; Mondino, M.; Arns, M.; Baeken, C.; Bikson, M.; Brunoni, A.R.; Burke, M.J.; Neuvonen, T.; Padberg, F.; Pascual-Leone, A. Sham tDCS: A hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 2019, 12, 668–673. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circul. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Ho, K.-A.; Taylor, J.L.; Chew, T.; Gálvez, V.; Alonzo, A.; Bai, S.; Dokos, S.; Loo, C.K. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: Evidence from single and repeated sessions. Brain Stimul. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Christova, M.; Rafolt, D.; Gallasch, E. Cumulative effects of anodal and priming cathodal tDCS on pegboard test performance and motor cortical excitability. Behav. Brain Res. 2015, 287, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lo, O.-Y.; Lipsitz, L.A.; Zhang, J.; Fang, J.; Manor, B. Transcranial direct current stimulation enhances foot sole somatosensation when standing in older adults. Exp. Brain Res. 2018, 236, 795–802. [Google Scholar] [CrossRef]
- Waters, S.; Wiestler, T.; Diedrichsen, J. Cooperation not competition: Bihemispheric tDCS and fMRI show role for ipsilateral hemisphere in motor learning. J. Neurosci. 2017, 37, 7500–7512. [Google Scholar] [CrossRef]
- Edwards, L.L.; King, E.M.; Buetefisch, C.M.; Borich, M.R. Putting the “sensory” into sensorimotor control: The role of sensorimotor integration in goal-directed hand movements after stroke. Front. Integr. Neurosci. 2019, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Seidel, O.; Ragert, P. Effects of transcranial direct current stimulation of primary motor cortex on reaction time and tapping performance: A comparison between athletes and non-athletes. Front. Hum. Neurosci. 2019, 13, 103. [Google Scholar] [CrossRef]
- Vollmann, H.; Conde, V.; Sewerin, S.; Taubert, M.; Sehm, B.; Witte, O.W.; Villringer, A.; Ragert, P. Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul. 2013, 6, 101–107. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, N.; Suganuma, S.; Laakso, I.; Tanabe, S.; Osu, R.; Tanaka, S.; Yamaguchi, T. Simulating tDCS electrode placement to stimulate both M1 and SMA enhances motor performance and modulates cortical excitability depending on current flow direction. Front. Neurosci. 2024, 18, 1362607. [Google Scholar] [CrossRef]
- Hubers, A.; Orekhov, Y.; Ziemann, U. Interhemispheric motor inhibition: Its role in controlling electromyographic mirror activity. Eur. J. Neurosci. 2008, 28, 364–371. [Google Scholar] [CrossRef]
- Ghasemian-Shirvan, E.; Farnad, L.; Mosayebi-Samani, M.; Verstraelen, S.; Meesen, R.L.; Kuo, M.-F.; Nitsche, M.A. Age-related differences of motor cortex plasticity in adults: A transcranial direct current stimulation study. Brain Stimul. 2020, 13, 1588–1599. [Google Scholar] [CrossRef]
- Jung, H.; Yamasaki, M. Association of lower extremity range of motion and muscle strength with physical performance of community-dwelling older women. J. Physiol. Anthropol. 2016, 35, 30. [Google Scholar] [CrossRef] [PubMed]
- Godinho, I.; Pinheiro, B.N.; Júnior, L.D.S.; Lucas, G.C.; Cavalcante, J.F.; Monteiro, G.M.; Uchoa, P.A.G. Effect of reduced ankle mobility on jumping performance in young athletes. Motricidade 2019, 15, 46–51. [Google Scholar]
- Lodha, N.; Naik, S.K.; Coombes, S.A.; Cauraugh, J.H. Force control and degree of motor impairments in chronic stroke. Clin. Neurophysiol. 2010, 121, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Seynnes, O.; Hue, O.A.; Garrandes, F.; Colson, S.S.; Bernard, P.L.; Legros, P.; Singh, M.A.F. Force steadiness in the lower extremities as an independent predictor of functional performance in older women. J. Aging Phys. Act. 2005, 13, 395–408. [Google Scholar] [CrossRef]
- Patel, P.; Kaingade, S.R.; Wilcox, A.; Lodha, N. Force control predicts fine motor dexterity in high-functioning stroke survivors. Neurosci. Lett. 2020, 729, 135015. [Google Scholar] [CrossRef]

| Characteristics | Healthy Young Adults |
|---|---|
| Sample size | 14 |
| Gender (female/male) | 6/8 |
| Dominant leg (left/right) | 1/13 |
| Age (years) | 24.9 ± 2.5 (22–30) |
| Height (cm) | 168.8 ± 8.6 (155.6–183.0) |
| Weight (kg) | 67.8 ± 13.4 (46.6–85.7) |
| Skeletal muscle mass (kg) | 30.4 ± 8.4 (17.6–42.6) |
| Body fat mass (kg) | 13.4 ± 2.0 (11.1–18.4) |
| BMI (%) | 23.3 ± 2.6 (18.5–26.5) |
| K-MMSE | 29.1 ± 1.6 (25–30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Choi, B.J.; Kang, N. Transcranial Direct Current Stimulation Improves Bilateral Ankle-Dorsiflexion Force Control in Healthy Young Adults. Appl. Sci. 2025, 15, 4391. https://doi.org/10.3390/app15084391
Lee H, Choi BJ, Kang N. Transcranial Direct Current Stimulation Improves Bilateral Ankle-Dorsiflexion Force Control in Healthy Young Adults. Applied Sciences. 2025; 15(8):4391. https://doi.org/10.3390/app15084391
Chicago/Turabian StyleLee, Hajun, Beom Jin Choi, and Nyeonju Kang. 2025. "Transcranial Direct Current Stimulation Improves Bilateral Ankle-Dorsiflexion Force Control in Healthy Young Adults" Applied Sciences 15, no. 8: 4391. https://doi.org/10.3390/app15084391
APA StyleLee, H., Choi, B. J., & Kang, N. (2025). Transcranial Direct Current Stimulation Improves Bilateral Ankle-Dorsiflexion Force Control in Healthy Young Adults. Applied Sciences, 15(8), 4391. https://doi.org/10.3390/app15084391







