Physical and Respiratory Rehabilitation in Spinal Muscular Atrophy: A Critical Narrative Review
Abstract
1. Introduction
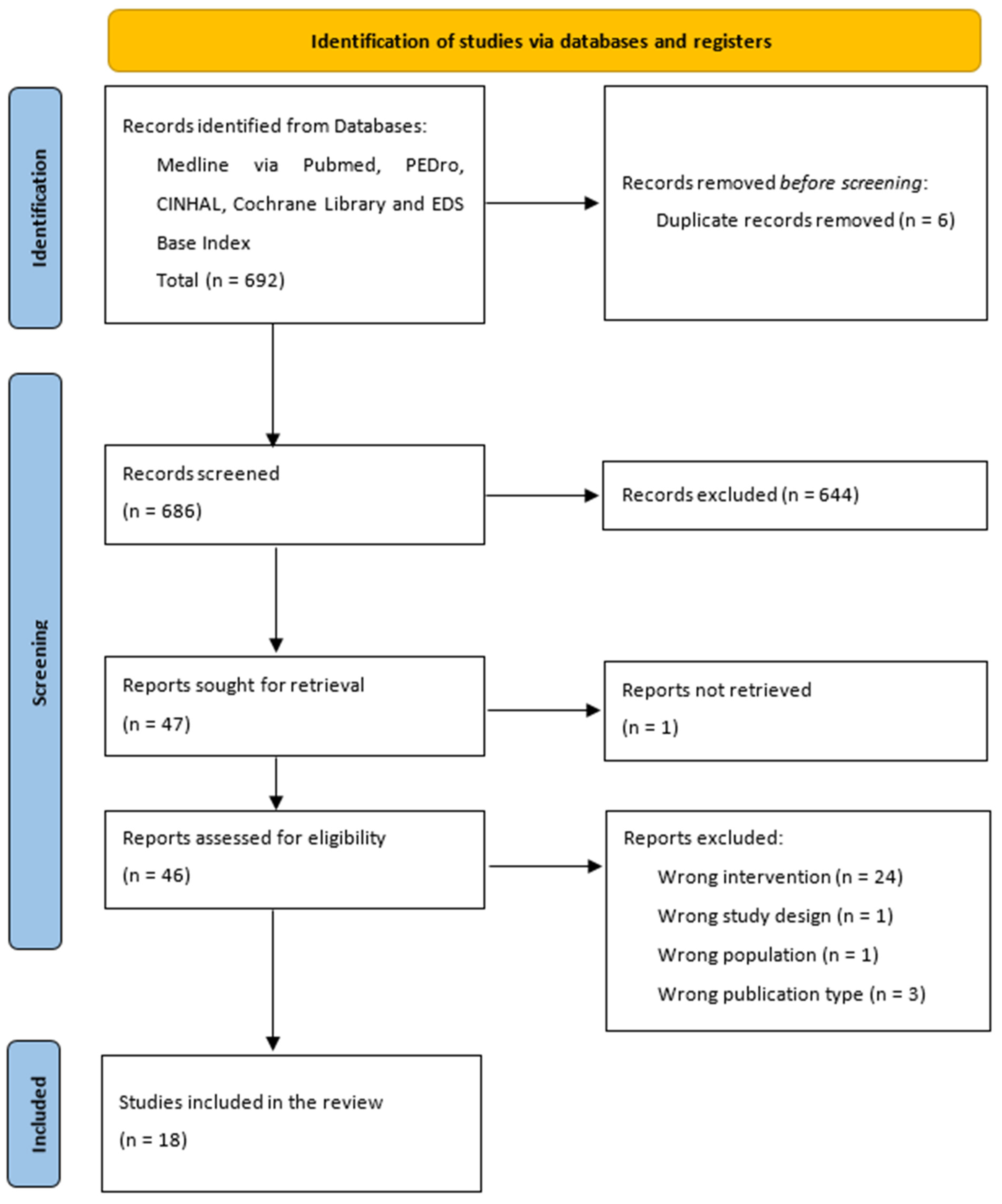
2. Materials and Methods
3. Results
3.1. Motor Rehabilitation
3.1.1. Hydrotherapy
3.1.2. Aerobic Training
3.1.3. Electrical Stimulation
3.1.4. Whole Body Vibration
3.2. Respiratory Interventions
3.2.1. NIV vs. Tracheostomy/Supportive Care
3.2.2. NIV to Improve Respiratory and Sleep Parameters
4. Discussion
4.1. Physiotherapy Interventions
4.2. Respiratory Interventions
4.3. Limits and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Ojala, K.S.; Reedich, E.J.; Didonato, C.J.; Meriney, S.D. In Search of a Cure: The Development of Therapeutics to Alter the Progression of Spinal Muscular Atrophy. Brain Sci. 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Arkblad, E.; Tulinius, M.; Kroksmark, A.K.; Henricsson, M.; Darin, N. A population-based study of genotypic and phenotypic variability in children with spinal muscular atrophy. Acta Paediatr. 2009, 98, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Norwood, F.L.M.; Harling, C.; Chinnery, P.F.; Eagle, M.; Bushby, K.; Straub, V. Prevalence of genetic muscle disease in Northern England: In-depth analysis of a muscle clinic population. Brain 2009, 132 Pt 11, 3175–3186. [Google Scholar] [CrossRef]
- Barbo, M.; Glavač, D.; Jezernik, G.; Ravnik-Glavač, M. MicroRNAs as Biomarkers in Spinal Muscular Atrophy. Biomedicines 2024, 12, 2428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mercuri, E.; Finkel, R.; Montes, J.; Mazzone, E.S.; Sormani, M.P.; Main, M.; Ramsey, D.; Mayhew, A.; Glanzman, A.M.; Dunaway, S.; et al. Patterns of disease progression in type 2 and 3 SMA: Implications for clinical trials. Neuromuscul. Disord. 2016, 26, 126–131. [Google Scholar] [CrossRef]
- Kanner, C.H.; Rodriguez-Torres, R.; Wallach, R.; Bakarania, P.; Montes, J. Therapeutic Scoliosis-Specific Exercises for a Child with Spinal Muscular Atrophy: A Case Report. Pediatr. Phys. Ther. 2025, 37, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Torres, R.; Montes, J. Fatigue and Fatigability in Spinal Muscular Atrophy; a Proposed Taxonomy to Enhance Assessment and Treatment. Muscle Nerve 2025, 71, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Govoni, A.; Ricci, G.; Bonanno, S.; Bello, L.; Magri, F.; Meneri, M.; Torri, F.; Caponnetto, C.; Passamano, L.; Grandis, M.; et al. Six-minute walk test as outcome measure of fatigability in adults with spinal muscular atrophy treated with nusinersen. Muscle Nerve 2024, 70, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.; Tscherter, A.; Henzi, B.; Branca, M.; Carda, S.; Enzmann, C.; Fluss, J.; Jacquier, D.; Neuwirth, C.; Ripellino, P.; et al. Chronic Pain in Patients with Spinal Muscular Atrophy in Switzerland: A Query to the Spinal Muscular Atrophy Registry. J. Clin. Med. 2024, 13, 2798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kahraman, A.; Mutlu, A.; Livanelioğlu, A. General movements in spinal muscular atrophy type 1. Physiother. Theory Pract. 2024, 40, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Coratti, G.; Pera, M.C.; Montes, J.; Pasternak, A.; Scoto, M.; Baranello, G.; Messina, S.; Dunaway Young, S.; Glanzman, A.M.; Duong, T.; et al. Different trajectories in upper limb and gross motor function in spinal muscular atrophy. Muscle Nerve 2021, 64, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Oude Lansink, I.L.B.; Gorter, J.W.; van der Pol, W.L.; Bartels, B.; Beelen, A. Impact of contractures on daily functioning in adolescents with spinal muscular atrophy: A qualitative study. Disabil. Rehabil. 2024, 46, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, P.; Cielecka, J.; Harrison, E.; Zwicker, J.G. Rehabilitation practices for childhood spinal muscular atrophy. Disabil. Rehabil. 2025, 1–10, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Muni-Lofra, R.; Coratti, G.; Duong, T.; Medina-Cantillo, J.; Civitello, M.; Mayhew, A.; Finkel, R.; Mercuri, E.; Marini-Bettolo, C.; Muntoni, F. Assessing disease progression in spinal muscular atrophy, current gaps, and opportunities: A narrative review. Neuromuscul. Disord. 2025, 49, 105341. [Google Scholar] [CrossRef] [PubMed]
- Corti, S.; Sansone, V.; Bitetti, I.; Brolatti, N.; Gadaleta, G.; Patanella, A.K.; Coratti, G.; Mercuri, E.; SMAkers working group. Italian survey on evolving SMA care with disease-modifying therapies: A consensus workshop on nutrition, swallowing, respiratory and rehabilitation care. Neuromuscul. Disord. 2025, 48, 105278. [Google Scholar] [CrossRef]
- Shin, H.I. shin Strategies for Patients with Spinal Muscular Atrophy in the Era of Disease-Modifying Therapy. Ann. Rehabil. Med. 2024, 48, 229–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, D.; Zhang, T.; Li, Y.; Liu, J.; Jia, Y.; Xiao, N. Rehabilitation for spinal muscular atrophy patients in China: A national cross-sectional study. Orphanet J. Rare Dis. 2024, 19, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, F.; Islam, A.; Akter, S.; Al Zubayer, M.A.; Mahmud, M.N.; Yeasmin, H.; Mawa, Z. Multidisciplinary physical rehabilitation program of individuals with spinal muscular atrophy in an inclusive school setting. J. Pediatr. Rehabil. Med. 2024, 17, 247–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ippolito, C.; Canthiya, L.; Floreani, A.; Luckhart, K.; Hoffman, A.; McAdam, L. Twice- Weekly Outpatient Rehabilitation Intervention for Young Children with Spinal Muscular Atrophy Treated with Genetic-Based Therapies: Protocol for a Feasibility Study. JMIR Res. Protoc. 2023, 12, e46363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sweat, M.; Skalsky, A. The Critical Importance of Early and Combination Treatment for Spinal Muscular Atrophy Type. Muscle Nerve 2025, 71, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Schroth, M.K.; Deans, J.; Bharucha Goebel, D.X.; Burnette, W.B.; Darras, B.T.; Elsheikh, B.H.; Felker, M.V.; Klein, A.; Krueger, J.; Proud, C.M.; et al. Spinal Muscular Atrophy Update in Best Practices: Recommendations for Treatment Considerations. Neurol. Clin. Pract. 2025, 15, e200374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bagga, P.; Singh, S.; Ram, G.; Kapil, S.; Singh, A. Diving into progress: A review on current therapeutic advancements in spinal muscular atrophy. Front. Neurol. 2024, 15, 1368658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishio, H.; Niba, E.T.E.; Saito, T.; Okamoto, K.; Takeshima, Y.; Awano, H. Spinal Muscular Atrophy: The Past, Present, and Future of Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 11939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, M.C.; Laforêt, P.; van der Pol, W.L.; Pegoraro, E.; Attarian, S.; Bartels, B.; Gorni, K.; Goemans, N.; Gusset, N.; Hodgkinson, V.; et al. 254th ENMC international workshop. Formation of a European network to initiate a European data collection, along with development and sharing of treatment guidelines for adult SMA patients. Virtual meeting 28–30 January 2022. Neuromuscul. Disord. 2023, 33, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Leibrock, B.; Landfeldt, E.; Hussong, J.; Huelle, T.; Mattheus, H.; Thiele, S.; Walter, M.C.; Zemlin, M.; Moehler, E.; Dillman, U.; et al. Areas of improvement in the medical care of SMA: Evidence from a nationwide patient registry in Germany. Orphanet J. Rare Dis. 2023, 18, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krosschell, K.J.; Dunaway Young, S.; Peterson, I.; Curry, M.; Mazzella, A.; Jarecki, J.; Cruz, R. Clinical and Research Readiness for Spinal Muscular Atrophy: The Time Is Now for Knowledge Translation. Phys Ther. 2022, 102, pzac108. [Google Scholar] [CrossRef] [PubMed]
- Pitarch Castellano, I.; Cabrera-Serrano, M.; Calvo Medina, R.; Cattinari, M.G.; Espinosa García, S.; Fernández-Ramos, J.A.; García Campos, O.; Gómez-Andrés, D.; Grimalt Calatayud, M.A.; Gutiérrez Martínez, A.J.; et al. Delphi consensus on recommendations for the treatment of spinal muscular atrophy in Spain (RET-AME consensus). Neurologia 2022, 37, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Rad, N.; Cai, H.; Weiss, M.D. Management of Spinal Muscular Atrophy in the Adult Population. Muscle Nerve 2022, 65, 498–507. [Google Scholar] [CrossRef] [PubMed]
- de Lemus, M.; Cattinari, M.G.; Pascual, S.I.; Medina, J.; García, M.; Magallón, A.; Dumont, M.; Rebollo, P. Identification of the most relevant aspects of spinal muscular atrophy (SMA) with impact on the quality of life of SMA patients and their caregivers: The PROfuture project, a qualitative study. J. Patient Rep. Outcomes 2024, 8, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poitou, T.; Boyer, F.C.; Benoit, C.; Ferté, J.B.; Pineau, C.; Taiar, R.; Rapin, A. Mobility and quality of life among adults with 5q-spinal muscular atrophy: The influence of individual history. Ann. Phys. Rehabil. Med. 2022, 65, 101552. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1, Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Gnasso, R.; Corrado, B.; Iommazzo, I.; Migliore, F.; Magliulo, G.; Giardulli, B.; Ruosi, C. Assessment, pharmacological therapy and rehabilitation management of musculoskeletal pain in children with mucopolysaccharidoses: A scoping review. Orphanet J. Rare Dis. 2022, 17, 255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sommese, M.; Corrado, B. A Comprehensive Approach to Rehabilitation Interventions in Patients with Angelman Syndrome: A Systematic Review of the Literature. Neurol. Int. 2021, 13, 359–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrado, B.; Giardulli, B.; Costa, M. Evidence-Based Practice in Rehabilitation of Myasthenia Gravis. A Systematic Review of the Literature. J. Funct. Morphol. Kinesiol. 2020, 5, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrado, B.; Ciardi, G.; Lucignano, L. Supervised Physical Therapy and Polymyositis/Dermatomyositis-A Systematic Review of the Literature. Neurol. Int. 2020, 12, 77–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fonzo, M.; Sirico, F.; Corrado, B. Evidence-Based Physical Therapy for Individuals with Rett Syndrome: A Systematic Review. Brain Sci. 2020, 10, 410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrado, B.; Ciardi, G.; Iammarrone, C.S. Rehabilitation management of Pompe disease, from childhood through adulthood: A systematic review of the literature. Neurol. Int. 2019, 11, 7983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrado, B.; Ciardi, G. Hypermobile Ehlers-Danlos syndrome and rehabilitation: Taking stock of evidence based medicine: A systematic review of the literature. J. Phys. Ther. Sci. 2018, 30, 843–847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrado, B.; Ciardi, G.; Bargigli, C. Rehabilitation Management of the Charcot-Marie-Tooth Syndrome: A Systematic Review of the Literature. Medicine 2016, 95, e3278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Sayers, A. Tips and tricks in performing a systematic review. Br. J. General. Pract. 2007, 57, 759. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.R.; Baird, J.S.; Plosky, D.; Navado, J.; Weaver, B. Spinal muscular atrophy type 1: Management and outcomes. Pediatr. Pulmonol. 2002, 34, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.R.; Niranjan, V.; Weaver, B. Spinal muscular atrophy type 1: A noninvasive respiratory management approach. Chest 2000, 117, 1100–1105. [Google Scholar] [CrossRef]
- Bulut, N.; Yardimci, B.N.; Ayvat, E.; Aran, O.T.; Yilmaz, Ö.; Karaduman, A. The effect of two different aerobic training modalities in a child with spinal muscular atrophy type II: A case report. J. Exerc. Rehabil. 2019, 15, 322. [Google Scholar] [CrossRef]
- Chatwin, M.; Bush, A.; Simonds, A.K. Outcome of goal-directed non-invasive ventilation and mechanical insufflation/exsufflation in spinal muscular atrophy type I. Arch. Dis. Child 2011, 96, 426–432. [Google Scholar] [CrossRef]
- Cunha, M.C.B.; Oliveira, A.S.B.; Labronici, R.H.D.D.; Gabbai, A.A. Spinal muscular atrophy type II (intermediary) and III (Kugel-berg-Welander). Evolution of 50 patients with physiotherapy and hydrotherapy in a swimming pool. Arq. Neuropsiquiatr. 1996, 54, 402–406. [Google Scholar] [CrossRef]
- Fehlings, D.L.; Kirsch, S.; McComas, A.; Campbell, K.; Chipman, M. Evaluation of therapeutic electrical stimulation to improve muscle strength and function in children with types II/III spinal muscular atrophy. Dev. Med. Child. Neurol. 2002, 44, 741–744. [Google Scholar] [CrossRef]
- Gobbo, M.; Lazzarini, S.; Vacchi, L.; Gaffurini, P.; Bissolotti, L.; Padovani, A.; Filosto, M. Exercise Combined with Electrotherapy Enhances Motor Function in an Adolescent with Spinal Muscular Atrophy Type III. Case Rep. Neurol. Med. 2019, 2019, 4839793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lemoine, T.J.; Swoboda, K.J.; Bratton, S.L.; Holubkov, R.; Mundorff, M.; Srivastava, R. Spinal muscular atrophy type 1: Are proactive respiratory interventions associated with longer survival? Pediatr. Crit. Care Med. 2012, 13, e161–e165. [Google Scholar] [CrossRef]
- Lewelt, A.; Krosschell, K.J.; Stoddard, G.J.; Weng, C.; Xue, M.; Marcus, R.L.; Gappmaier, E.; Viollet, L.; Johnson, B.A.; White, A.T.; et al. Resistance strength training exercise in children with spinal muscular atrophy. Muscle Nerve 2015, 52, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Hansen, R.S.; Preisler, N.; Thøgersen, F.; Berthelsen, M.P.; Vissing, J. Training improves oxidative capacity, but not function, in spinal muscular atrophy type III. Muscle Nerve 2015, 52, 240–244. [Google Scholar] [CrossRef]
- Markström, A.; Cohen, G.; Katz-Salamon, M. The effect of long term ventilatory support on hemodynamics in children with spinal muscle atrophy (SMA) type II. Sleep. Med. 2010, 11, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Mellies, U.; Dohna-Schwake, C.; Stehling, F.; Voit, T. Sleep disordered breathing in spinal muscular atrophy. Neuromuscul. Disord. 2004, 14, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Mirea, A.; Leanca, M.C.; Onose, G.; Sporea, C.; Padure, L.; Shelby, E.S.; Dima, V.; Daia, C. Physical Therapy and Nusinersen Impact on Spinal Muscular Atrophy Rehabilitative Outcome. Front. Biosci. 2022, 27, 179. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.; Garber, C.E.; Kramer, S.S.; Montgomery, M.J.; Dunaway, S.; Kamil-Rosenberg, S.; Carr, B.; Cruz, R.; Strauss, N.E.; Sproule, D.; et al. Single-Blind, Randomized, Controlled Clinical Trial of Exercise in Ambulatory Spinal Muscular Atrophy: Why are the Results Negative? J. Neuromuscul. Dis. 2015, 2, 463–470. [Google Scholar] [CrossRef]
- Novikov, A.; Maldova, M.; Shandybina, N.; Shalmiev, I.; Shoshina, E.; Epoyan, N.; Moshonkina, T. First Use of Non-Invasive Spinal Cord Stimulation in Motor Rehabilitation of Children with Spinal Muscular Atrophy. Life 2023, 13, 449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrone, A.; Pavone, M.; Testa, M.B.; Petreschi, F.; Bertini, E.; Cutrera, R. Noninvasive ventilation in children with spinal muscular atrophy types 1 and 2. Am. J. Phys. Med. Rehabil. 2007, 86, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Salem, Y.; Jaffee Gropack, S. Aquatic therapy for a child with type III spinal muscular atrophy: A case report. Phys. Occup. Ther. Pediatr. 2010, 30, 313–324. [Google Scholar] [CrossRef]
- Vry, J.; Schubert, I.J.; Semler, O.; Haug, V.; Schönau, E.; Kirschner, J. Whole-body vibration training in children with Duchenne muscular dystrophy and spinal muscular atrophy. Eur. J. Paediatr. Neurol. 2014, 18, 140–149. [Google Scholar] [CrossRef]
- Cumplido-Trasmonte, C.; Barquín-Santos, E.; Aneiros-Tarancón, F.; Plaza-Flores, A.; Espinosa-García, S.; Fernández, R.; García-Armada, E. Usability and Safety of the ATLAS 2030 Robotic Gait Device in Children with Cerebral Palsy and Spinal Muscular Atrophy. Children 2024, 11, 1500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirayama, T.; Morioka, H.; Sugisawa, T.; Shibukawa, M.; Ebina, J.; Hanashiro, S.; Nagasawa, J.; Yanagihashi, M.; Okuni, I.; Nakajima, T.; et al. A preliminary study on the effects of long-term robot suit exercise training on gait function and quality of life in patients with spinal and bulbar muscular atrophy. J. Clin. Neurosci. 2024, 128, 110778. [Google Scholar] [CrossRef] [PubMed]
- Urendes, E.; Sanchez, C.; Lerma-Lara, S.; Rojo, A.; Costa, V.; Raya, R. Design, Development, and Functional Validation of a 3D-Printed Passive Upper Limb Exoskeleton. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Watanabe, H.; Nakashiro, Y.; Iida, Y.; Nonaka, M.; Moriwaka, F.; Hamada, S. Long-term effects of the gait treatment using a wearable cyborg hybrid assistive limb in a patient with spinal and bulbar muscular atrophy: A case report with 5 years of follow-up. Front. Neurol. 2023, 14, 1143820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nistor-Cseppento, C.D.; Gherle, A.; Negrut, N.; Bungau, S.G.; Sabau, A.M.; Radu, A.F.; Bungau, A.F.; Tit, D.M.; Uivaraseanu, B.; Ghitea, T.C.; et al. The Outcomes of Robotic Rehabilitation Assisted Devices Following Spinal Cord Injury and the Prevention of Secondary Associated Complications. Medicina 2022, 58, 1447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Secciani, N.; Brogi, C.; Pagliai, M.; Buonamici, F.; Gerli, F.; Vannetti, F.; Bianchini, M.; Volpe, Y.; Ridolfi, A. Wearable Robots: An Original Mechatronic Design of a Hand Exoskeleton for Assistive and Rehabilitative Purposes. Front. Neurorobot. 2021, 15, 750385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varela-Aldás, J.; Avila-Armijos, W.; Palacios-Navarro, G. Internet of things (IoT)-based assistive system for patients with spinal muscular atrophy (SMA): A case report. Disabil. Rehabil. Assist. Technol. 2024, 19, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.; Bablok, I.; Farin-Glattacker, E.; Langer, T. Barriers and facilitating factors of care coordination for children with spinal muscular atrophy type I and II from the caregivers’ perspective: An interview study. Orphanet J. Rare Dis. 2023, 18, 136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannotta, G.; Ruggiero, M.; Pirani, G.; Oliva, M.C.; Ferrante, C.; Trabacca, A. Setting multidisciplinary intervention goals for spinal muscular atrophy patients utilizing the international classification of functioning, disability, and health: A pilot study in a small sample sizes. Acta Neurol Belg. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.J.; Menéndez, H.; Gil, L.; Martín, J.; Martín, T.; García-López, D.; Gil-Agudo, Á.; Marín, P.J. Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord 2011, 49, 554–559. [Google Scholar] [CrossRef]
- Bartels, B.; Montes, J.; Van Der Pol, W.L.; De Groot, J.F. Physical exercise training for type 3 spinal muscular atrophy. Cochrane Database Syst Rev. 2019, 2019, CD012120. [Google Scholar] [CrossRef] [PubMed]
- Sonehara, S.; Bo, R.; Nambu, Y.; Iketani, K.; Lee, T.; Shimomura, H.; Ueda, M.; Takeshima, Y.; Iijima, K.; Nozu, K.; et al. Newborn Screening for Spinal Muscular Atrophy: A 2.5-Year Experience in Hyogo Prefecture, Japan. Genes 2023, 14, 2211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruythooren, F.; Moens, P. Spinal Muscular Atrophy Scoliosis in the Era of Background Therapies-A Review of the Literature. J. Clin. Med. 2024, 13, 3467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Díaz-López, C.I.; Palomo-Carrión, R.; Romay-Barrero, H.; Pacheco-da-Costa, S.; Coello-Villalón, M.; López-Muñoz, P. Families’ experiences and perspectives on the early use of powered mobility in children with spinal muscular atrophy type I in the natural context. Disabil. Rehabil. Assist. Technol. 2025, 12, 1–9. [Google Scholar] [CrossRef]
- Xie, Q.; Meng, Q.; Yu, W.; Wu, Z.; Xu, R.; Zeng, Q.; Zhou, Z.; Yang, T.; Yu, H. Design of a SMA-based soft composite structure for wearable rehabilitation gloves. Front. Neurorobot. 2023, 17, 1047493, Erratum in Front. Neurorobot. 2023, 17, 1269432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tapken, I.; Kuhn, D.; Hoffmann, N.; Detering, N.T.; Schüning, T.; Billaud, J.N.; Tugendreich, S.; Schlüter, N.; Green, J.; Krämer, A.; et al. From data to discovery: AI-guided analysis of disease-relevant molecules in spinal muscular atrophy (SMA). Hum. Mol. Genet. 2024, 33, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
| PubMed Medline Research Strategy | ||
|---|---|---|
| Search | Query | Items Found |
| #1 | (spinal muscular atrophy) AND (electrical stimulation) | 133 |
| #2 | (spinal muscular atrophy) AND (whole body vibration training) | 5 |
| #3 | (spinal muscular atrophy) AND (strength training exercise) | 36 |
| #4 | (spinal muscular atrophy) AND (aerobic training) | 72 |
| #5 | (spinal muscular atrophy) AND (hydrotherapy) | 3 |
| #6 | (spinal muscular atrophy) AND (scoliosis) | 243 |
| #7 | (spinal muscular atrophy) AND (“Respiratory Insufficiency/therapy”) | 55 |
| #8 | (spinal muscular atrophy) AND (Positive-Pressure Respiration) | 46 |
| #9 | ((spinal muscular atrophy) AND (rehabilitation)) AND (orthoses) | 15 |
| #10 | ((spinal muscular atrophy) AND (contractures)) AND (range of motion) | 10 |
| #11 | (spinal muscular atrophy) AND (Wheelchairs) | 62 |
| PEDro research strategy | ||
| #1 | “Simple research” with “spinal muscular atrophy” as keyword | 12 |
| Author and Year | Study Design | Intervention | N. of Participants | Outcomes | Main Results |
|---|---|---|---|---|---|
| Bach et al. (2002) * [43] | Retrospective Analysis | 16 patients underwent tracheostomy (group A); 33 used high-pressure PIP + PEEP nocturnal ventilation and were intubated during intercurrent respiratory infections (group B); 7 died from respiratory failure, refusing intubation and tracheostomy (group C). | 56 patients with SMA I | Survival, hospitalisation, language, and outcomes related to the need for a respirator in patients with SMA I, using non-invasive ventilation or tracheostomy. | Compared to group B, patients in group A had fewer hospitalisations until the age of 3, but more after 5 years and 15 out of 16 lost all spontaneous respiratory tolerance post-tracheostomy and could not speak. One patient from group A passed away at 16 months of age, and the others were 73.8 +/− 57 months old (the oldest being 19 years old). Two patients from group B passed away at 6 and 13 months, while the remaining 31 were 41.8 +/− 26.0 months old (and up to 8.3 years). Three of the 31 in group B required continuous PIP + PEEP with minimal tolerance for breathing on their own, and 4 could not communicate verbally. |
| Bach et al. (2000) * [44] | Retrospective Cohort Study | Non-invasive respiratory care (BiPAP, manual and mechanic assistance) versus conventional care. | 11 patients with SMA I | Successes and failures of a non-invasive respiratory management protocol vs. conventional respiratory management. | Two children survived for 37 and 66 months and were never intubated despite requiring 24-h nasal ventilation from 5 to 7 months of age. Two children underwent tracheostomy, and one child was lost to follow-up three months after successful extubation. The other six children were managed at home for 15–59 months (average 30.4) using nighttime nasal ventilation after an episode of respiratory failure. Nine children were successfully extubated 23 out of 28 times using the protocol. In contrast, the same children managed conventionally without the protocol were successfully extubated only 2 out of 20 times (p < 0.001 from a two-tailed Fisher exact test). |
| Bulut et al. (2019) [45] | Case report | Ergometric training was performed three times a week for 12 weeks. After a wash-out period of 6 weeks, hydrotherapy was applied twice a week for 12 weeks. | 1 child with SMA I | HFMS, GMFM and PedsQL 3.0. | The HFMS and GMFM scores, child’s lung function, and quality of life scores of their parents improved with both approaches. The improvements were maintained during the 1-year follow-up. |
| Chatwin et al. (2011) * [46] | Case series | Sleep study; non-invasive positive pressure ventilation (NIPPV) for ventilatory support and physiotherapy; use of mechanical insufflation/exsufflation (MI-E). | 13 patients with SMA I | Oxygen saturation (SpO2) and transcutaneous carbon dioxide (TcCO2). | NIPPV and MI-E were used for successful extubations guided by the protocol (No. = 9) but not for those not guided by the protocol (No. = 3). NIPPV was essential for home discharge in ventilator-dependent patients (n = 7) and used for palliating respiratory symptoms (n = 4). The chest wall shape improved with NIPPV. The parents of the deceased children (n = 5) were positive about the use of these techniques. |
| Cunha et al. (1996) [47] | Multi-case study | Individual physiotherapy once a week and hydrotherapy and therapeutic swimming (Halliwick method) for 30 min for children and 45 min for adults, twice a week for 2 years. | 50 patients (30 with SMA II and 20 with SMA III) | Deformities in joints, development of scoliosis, Manual Muscular Test, Barthel Ladder, and motorial activities. | The degrees of deformity in the hips, knees, and feet increased in all patients; scoliosis was more pronounced in type II patients compared to type III. Muscle strength stabilised or improved in patients with SMA type III. A total of 93% of type II patients and 100% of type III patients showed improvement in activities of daily living (Barthel Ladder); type II patients improved their motor activities except for ambulation. Type III patients improved all motor activities, including ambulation. |
| Fehlings et al. (2002) [48] | RCT | Therapeutic electrical stimulation (TES) at low intensity was applied for 6 to 12 months on the deltoid and biceps muscles in the treatment arm. The control arm received placebo stimulation. | 13 patients with SMA II and III | Myometry, manual muscle testing, maximum evoked muscle response amplitudes (M waves), and the PEDI. | There was no statistically significant difference between the treatment arm and the control arm at baseline, 6 and 12 months in quantitative myometry, manual muscle testing, M-wave amplitude (p = 0.12), and PEDI (p = 0.11). |
| Gobbo et al. (2019) [49] | Case report | During phase I (weeks 1–8) was provided a home-based program for quadriceps strengthening through neuromuscular electrical stimulation (NMES). During phase II (weeks 9–18), at-home NMES was combined with functional electrical stimulation (FES) assisting volitional cycling for a broader, systemic conditioning. | 1 patient with SMA type III | Quadriceps circumference and strength (MIVC), Tinetti scale, Hammersmith scale (HFSME), Heart rate (HR); oxygen consumption (VO2); and metabolic equivalents of task (METs). | By the end of Phase I, quadriceps isometric strength showed a significant increase, rising from 1.7 to 2.2 kg on the right side and from 0.8 to 2.0 kg on the left. Thigh circumference expanded by 7 mm and 3 mm on the right leg and by 5 mm and 3 mm on the left. At the conclusion of Phase II, thigh circumference further increased compared to baseline, reaching 15 mm and 9 mm on the right leg and 12 mm and 6 mm on the left. Maximum voluntary isometric contraction (MVIC) of the right quadriceps rose by 70.6%, while the left quadriceps nearly tripled in strength, increasing from 0.8 to 2.3 kg. Motor function assessments indicated a 7-point improvement on the HFMSE scale. The Tinetti score increased by 4 points for both balance and gait. Energy expenditure during FES-assisted cycling progressively increased from 2.3 METs to a final value of 3.1 standard METs and from 2.6 to 3.4 measured METs. Cycling power also improved, with average power rising from 7 to 9.8 watts and maximum power increasing from 14.4 to 16.8 watts between the first and final sessions. |
| Lemoine et al. (2012) * [50] | Retrospective Cohort Study | Proactive respiratory care with BiPAP during sleep and cough assistance twice a day in the first group; respiratory support care through suction, with or without supplemental oxygen in the second group. | 44 children with SMA I | The primary outcome was time of death comparing the children who received proactive respiratory care versus supportive care. | Children treated with early proactive respiratory care had statistically longer survival compared to support therapy (log rank 0.047); however, the hazard ratio for adjusted survival concerning confounding variables was not statistically different (2.44 [95% confidence interval 0.84–7.1]). Children in the proactive group were more likely to be hospitalised for respiratory failure (83% vs. 46%), and the time from diagnosis to the first hospitalisation for respiratory failure was reduced (median 118 vs. 979 days). |
| Lewelt et al. (2015) [51] | Prospective cohort study | Supervised resistance exercise program performed at home, 3 times a week for 12 weeks. | 9 patients with SMA II and SMA III | Feasibility, safety, QMA, HHD, MMT, and MHFMS-Extend. | The average amount of weight lifted by the participants as a group increased significantly (p < 0.001) by 0.27 (0.05) kg; the perceived exertion level remained unchanged (p = 0.76). Pain was perceived as absent 99.5% of the time on the FACES scale. For strength, there was a significant change in the total composite MMT score (p = 0.01), a non-significant increase in QMA, and no change in HHD. MHFMS-Extend scores for motor function significantly improved (p = 0.04). |
| Madsen et al. (2015) [52] | Non-randomised control trial | A 12-week training program with a cycle ergometer, consisting of 42 sessions of 30 min at 65–70% of VO2max, performed 2 to 4 times a week. | 6 patients with SMA III and 9 healthy people of the same age and gender | VO2max, ADL, changes in Wmax, isometric leg muscle strength (hand-held-dynamometer), body composition, and performance in functional tests (6MWT; 6SST, TUG; 5STST). | The training improved VO2max in patients with SMA III by 27 ± 3% (p < 0.001). There was no change or increase in fatigue in all subjects. The maximum workload capacity (Wmax) remained unchanged in half of the patients and increased in the other half. There were no significant changes in muscle strength, 6MWT, 6SST, TUG, and 5STST in any patient. |
| Markström et al. (2010) * [53] | Cohort Study | Nightly BiPAP use for more than 12 months (median 32 months, min. 14, max. 72 months) and comparison with parameters in the absence of support. | 10 patients with SMA II | Oxygen saturation (SpO2), transcutaneous partial pressure of carbon dioxide (TcPaCO2) and oxygen (TcPaO2), phase angle between chest and abdominal movements, and electrocardiogram (HR, PTT, PTT range). | The HR and PTT parameters between breathing without support and with optimal Bi-PAP were comparable (p = 0.85 and 0.79, respectively), as were the blood gases (SaO2, TcO2, TcCO2 p = 0.79, 0.88, 0.79, respectively). Respiratory efficiency improved when Bi-PAP was optimal (reduction in phase angle from 42 to 22). Suboptimal Bi-PAP due to mask air leakage was associated with significant increases in breath-to-breath variability in HR, PTT, and phase angle. |
| Mellies et al. (2004) * [54] | Case–ontrol Study | Non-invasive ventilation use for 7–12 h at night in SMA patients with sleep disturbances; Supportive treatment in SMA patients without sleep disturbances. | 12 patients with SMA I and II | Inspiratory vital capacity (IVC), PIP, PEEP, complete polysomnography with transcutaneous carbon dioxide partial pressure (PtcCO2), respiratory disturbance index (RDI), and a visual analogue scale. | The non-invasive ventilation during sleep eliminated disordered breathing, normalised sleep architecture, and improved symptoms (p < 0.05 for all). |
| Mirea et al. (2022) [55] | Retrospective observational study | Correction of posture, reduced stiffness, increased range of motion and muscle strengthening at least 5 times per week. | 55 patients with SMA I, II, III | CHOP INTEND was performed in SMA type I patients, while HFMSE in type II and III patients. | Motor skill improvements were statistically significantly (p < 0.001) higher in the study group, being almost four times better (12.66%), effect size, in comparison to the control group (3.18%). |
| Montes et al. (2015) [56] | RCT | A muscle strengthening program (3 times per week for 30 min/day), combined with a home-based aerobic exercise program using a cycle ergometer (5 times per week for 30 min/day), for 19 months in the experimental group and 12 months in the control group. | 14 patients with SMA IIIa and IIIb | 6MWT, HFMSE, TUG, FVC, PedsQL Generic Quality of Life Inventory, Multidimensional Fatigue Scale and Fatigue Severity Scale. | At baseline, the two groups were similar in all clinical variables. There were no significant changes in the experimental group after 6 months in the primary outcome measure (6MWT walking distance), or in measures of strength or motor function. VO2 max improved by 4.9% in all participants at 6 months (p = 0.036) (n = 10). |
| Novikov et al. (2023) [57] | Case series | Spinal cord stimulation was applied alongside physical therapy. The therapy included both passive and active stretching of the upper and lower joints, positioning techniques, weight movements, exercises to prevent scoliosis, stepping and kicking actions, as well as breathing exercises. Stimulation targeted one or two spinal cord regions, either above the cervical or lumbar enlargements, or above the cauda equina. | 5 patients with SMA II or III | RULM, HFMSE, FVC and goniometry. | Testing of HFMSF of participants’ sitters showed an increase of 0, 2 and 1 points, respectively. The length of sitting independently of one participant increased from 20 s to 3 min; another participant learned to move from the couch to the wheelchair, from the wheelchair to the floor and back to the couch, and managed to pull himself to a standing position while holding on to the bars of the Swedish Wall. FVC increased by 7%, 1% and 3% of predicted values based on height and age in three participants. |
| Petrone et al. (2007)* [58] | Case series | Baseline sleep study (polysomnography) and use of BiPAP for 20 min, 2/3 times a day. | 9 patients with SMA I and II | Assessment of the sleep apnea/hypopnea index (AHI), mean oxygen haemoglobin saturation (SpO2), oxygen desaturation index, transcutaneous carbon dioxide tension (tcpCO2), and the mean phase angle during sleep as a measure of thoracoabdominal coordination. | Comparing the baseline sleep studies with those conducted after non-invasive ventilation, a significant improvement was observed in the oxygen desaturation index (p 0.010), mean tcpCO2 (p 0.001), and phase angle (p 0.001). For five patients, the improvement in thoracoabdominal phase angle became significant when using high-pressure biphasic airway pressure (PAP) at two levels. |
| Salem et al. (2010) [59] | Case report | Hydrotherapy for 45 min per session, 2 times a week for 14 weeks. | 1 patient with SMA III | GMFM, PDMS-2 and GAITRite system. | There was an improvement in muscle strength of the lower limbs, pelvic movements, hip flexion, knee flexion, and ankle flexion during the swing phase. The GMFM improved by 11%. The gross motor quotient for PDMS-2 improved from 66 to 74. Additionally, spatial and temporal gait measures (walking speed, stride length, etc.) showed improvement. |
| Vry et al. (2014) [60] | Multi-case study | 8 weeks of whole-body vibration training (15–18 Hz) at home using an alternating side-to-side platform (3 sets x 3 min, twice a day, 5 days a week). | 22 patients (8 SMA and 14 with DMD) | Serum creatine kinase levels, function tests, muscle strength and angular degree of dorsiflexion of the ankles. | In patients with SMA, laboratory parameters (CK, PCR, electrolytes) were unchanged. Secondary outcomes on training effectiveness (muscle strength, ankle dorsiflexion, temporal functional testing) showed mild, but not significant, improvements, except for the 6-min walk test (6MWT) (p < 0.01). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammarano, S.; Chirico, V.A.; Giardulli, B.; Mazzuoccolo, G.; Ruosi, C.; Corrado, B. Physical and Respiratory Rehabilitation in Spinal Muscular Atrophy: A Critical Narrative Review. Appl. Sci. 2025, 15, 4398. https://doi.org/10.3390/app15084398
Cammarano S, Chirico VA, Giardulli B, Mazzuoccolo G, Ruosi C, Corrado B. Physical and Respiratory Rehabilitation in Spinal Muscular Atrophy: A Critical Narrative Review. Applied Sciences. 2025; 15(8):4398. https://doi.org/10.3390/app15084398
Chicago/Turabian StyleCammarano, Serena, Vincenzo Alessio Chirico, Benedetto Giardulli, Giovanna Mazzuoccolo, Carlo Ruosi, and Bruno Corrado. 2025. "Physical and Respiratory Rehabilitation in Spinal Muscular Atrophy: A Critical Narrative Review" Applied Sciences 15, no. 8: 4398. https://doi.org/10.3390/app15084398
APA StyleCammarano, S., Chirico, V. A., Giardulli, B., Mazzuoccolo, G., Ruosi, C., & Corrado, B. (2025). Physical and Respiratory Rehabilitation in Spinal Muscular Atrophy: A Critical Narrative Review. Applied Sciences, 15(8), 4398. https://doi.org/10.3390/app15084398







