Abstract
The purpose of this study was to evaluate the bactericidal effect of 270 nm UV-C light-emitting diode (LED) light delivered through a newly designed prototype device with thin optical fiber against Enterococcus faecalis (E. faecalis). The prototype device, developed to integrate UV-C light into a thin optic fiber (diameter 124 µm) connected to a UV-C LED (Luminous Device; Sunnyvale, CA, USA) via a specialized double-lens system that focuses divergent light to achieve a 65 mm working distance and a numerical aperture of 0.22. E. faecalis, was cultured at 37 °C under aerobic conditions for 24 h. The UV-C LED optical fiber was positioned 10 mm above the bacterial culture prepared in the wells of a 96-well plate. The E. faecalis cells were exposed to UV-C irradiation for 0, 10, 30, 60, 90, 120 and 180 s. Following irradiation, the OD600 values were measured after incubation at 37 °C for an additional 24 h. The data were statistically analyzed using one-way ANOVA, followed by Tukey’s honestly significant difference (HSD) test at a significance level of 0.05. UV irradiation at 270 nm significantly reduced E. faecalis growth in a time-dependent manner (p < 0.05). No significant changes were observed at 0 and 10 s, while peak reductions occurred at 120 and 180 s, with effects beginning at 30 s and increasing over time. The 270 nm UV-C wavelength was highly effective in bactericidal action against E. faecalis. The custom-designed UV-C delivery system effectively integrated the light source into a thin optical fiber, allowing for efficient UV-C light transmission and demonstrating its potential for application in narrow spaces such as root canals.
1. Introduction
The primary goals of root canal therapy are a sufficient reduction in bacteria and their by-products from the root canal system and the prevention of reinfection [1]. It is accomplished traditionally with a combination of mechanical instrumentation and irrigation with a disinfecting solution. However, the complexity of the root canal anatomy and the limited penetration depth of irrigation make it a challenge to achieve optimal results [2]. Reinfection of the root canal system causes root canal treatment to fail, and Enterococcus faecalis (E. Faecalis) is a facultative anaerobic Gram-positive bacteria that is frequently associated with recurrent root canal infections [3]. It has shown resistance to calcium hydroxide [4] and the ability to colonize 800–1000 µm into dentinal tubules, while sodium hypochlorite with the conventional method has been shown to only be effective by penetrating up to 300 µm [5]. In order to overcome these limitations, irrigant activation methods such as sonic and ultrasonic activators are sometimes used, but achieving complete sterilization of the root canal system with these methods remains difficult [6].
To address this limitation, lasers have been suggested as an adjunct method to enhance endodontic treatment. When lasers come into contact with living tissue, they can produce various effects, depending on the wavelength and the optical properties of the irradiated tissue [7]. Er/YAG and Nd/YAG lasers have demonstrated the ability to reduce microorganisms [8] and remove the smear layer [9]. Previous studies [10,11] have shown that lasers as an adjunct therapy achieve more efficient bacterial elimination than traditional chemo-mechanical preparation due to their greater penetration into deep radicular dentin. Combined treatment with MBG-Ag and Er/YAG lasers not only contributes to bacterial reduction but also improves the mechanical properties of dentin through the formation of hydroxyapatite-like structures [12]. Furthermore, Er/YAG laser-assisted antimicrobial treatment has been explored as a means to enhance biofilm disruption and improve dentinal tubule disinfection [12,13]. In addition to biomaterial-based disinfection methods, the integration of optical fiber technology in antimicrobial light-based therapy has gained attention. However, the limitations of these techniques include undesirable thermal damage and morphological changes and ablations in the dentin structure in the case of inadequate energy levels and durations [14,15,16].
A recent study demonstrated that UVC LED at wavelengths of 265 nm and 280 nm effectively kills oral microorganisms within 30–90 s, showing potential as an alternative or adjunct to sodium hypochlorite (NaOCl) with its inherent limitations. This therapeutic approach is based on the principle that UVC, a source of bactericidal light energy, penetrates small microbial cells (0.5–2.0 μm) and disrupts DNA transcription, translation, and replication by forming cyclobutyl pyrimidine dimers in the DNA structure [17]. The germicidal efficiency of UV light is strongest in the UV-C range, as the shorter wavelength possesses the ability to reach the DNA in the cytoplasm of smaller microbial cells, especially in the range of 255–280 nm [18]. Recently, advancements in light-emitting diode (LED) technology have led to the development of UV-C-range LEDs, which have been shown to be efficient in disinfection while using less power than other sources of UV energy [19]. Several studies have suggested its potential as an adjunct disinfection method for root canal treatments [17,20]. Aung et al. [21] also reported that LED-UV on denture surfaces devitalizes bacteria without producing thermal damage on surrounding periodontal tissues, which presents an advantage over high-power lasers. The potential of combining antimicrobial biomaterials with advanced light-based technologies has been recognized; however, challenges remain in effectively integrating and optimizing these approaches for practical use in root canal disinfection.
To maximize the efficacy of UV-C disinfection in confined anatomical structures, such as root canals, the integration of LED light into a thin optical fiber is crucial. The proper integration of UV-C LED into a thin optical fiber not only improves energy transmission but also enables the effective application of photo disinfection in intricate dental structures. Ensuring efficient coupling of UV LED light into these slender fibers is critical for maximizing disinfection efficacy [22,23]. Studies have demonstrated that optimizing the interface between the UV-C LED source and the optical fiber enhances light transmission efficiency, leading to more efficient outcomes in confined spaces [22,23,24]. The use of lenses, precise alignment, and coupling techniques can significantly reduce light loss and increase the intensity of UV-C LED light delivered to target areas, but the development and optimization of such devices for practical application still present many challenges to be addressed.
The aim of this study was to investigate the bactericidal effect of 270 nm UV-C light-emitting diode (LED) light delivered through a newly designed prototype device with thin optical fiber against Enterococcus faecalis (E. faecalis).
2. Materials and Methods
2.1. Characteristic of End-Firing Optical Fiber
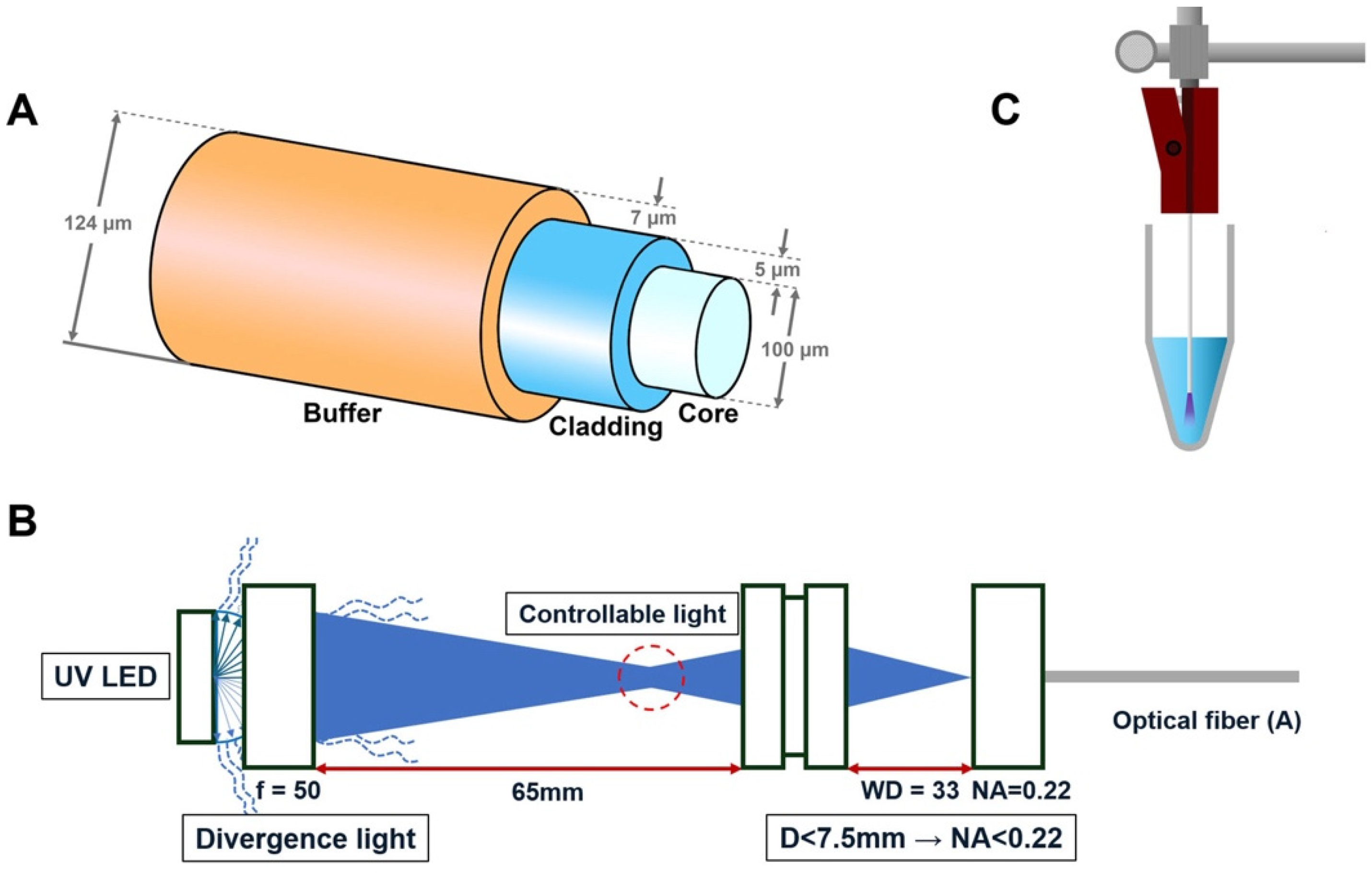
The end-firing optical fiber (UM22-100, Thorlabs, NJ, USA) utilized in this study consists of three layers: buffer, cladding, and core. The outer diameter of the fiber is 124 ± 3 µm. The buffer, composed of PI (polyimide) film, has a thickness of 7 µm; the cladding, made of doped silica, has a thickness of 5 µm; and the core, composed of high-OH silica, has a diameter of 100 µm. The fiber has a numerical aperture of 0.22 ± 0.02, which defines the range of angles over which it can accept light. The end-firing optical fiber transmits and emits light exclusively from the distal end of the fiber, maintaining its original structure without any modifications.
A laboratory-fabricated prototype device was developed to integrate and deliver UV-C light through a thin optical fiber system. The 270 nm LED light source (XST-3535; Luminus Device, Sunnyvale, CA, USA) with a power output of 100 mW/cm2 was used in this experiment. The optical fiber was connected to a UV-C LED (270 nm, XST-3535-UV-A60-CD270-00, Luminous Device, Sunnyvale, CA, USA) light source using a specialized double-lens (f = 50, WD = 33, Thorlabs, Newton, NJ, USA) optical system, as illustrated in Figure 1B. The divergent light is focused through a series of lenses to achieve a working distance of 65 mm and a numerical aperture (NA) of 0.22. Since the NA value of the lens in front of the optical fiber is smaller than the NA value of the single-mode optical fiber, using this double-lens optical system, all light passing through the first lens at a sub-horizontal angle can be condensed into the optical fiber. This controlled light path ensures precise and consistent delivery of UV light to the optical fiber.
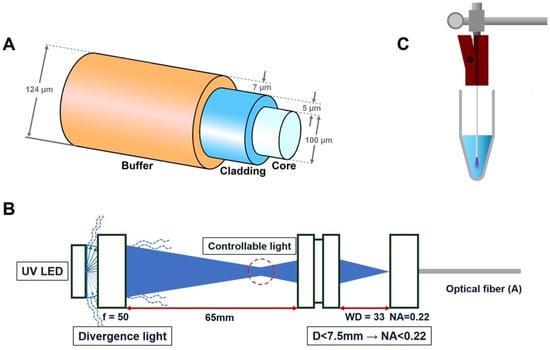
Figure 1.
Schematic illustration of the optical fiber firing apparatus. (A) Diagram of the optical fiber, which consists of a core that transmits light, a cladding with a lower refractive index that surrounds the core to confine the light, and a buffer that protects the fragile fiber. (B) Newly developed laboratory-manufactured prototype device with a double-lens optical system for UV-C LED, designed to control light divergence and ensure precise beam direction. (C) A schematic representation of the model illustrating the application of the fiber optic system.
2.2. Bacterial Culture and Growth Conditions, and UV-C LED Irradiation Through the Optical Fiber System
The E. faecalis (ATCC 29212) strain used in this study was obtained from the Ameri-can Type Culture Collection (ATCC, Manassas, VA, USA). The bacteria were cultured in Brain Heart Infusion (BHI; Becton, Dickinson and Company, Sparks, MD, USA) broth at 37 °C under aerobic conditions for 24 h. After incubation, bacterial cells were harvested via centrifugation (8000× g for 10 min) and re-suspended in sterile phosphate-buffered saline (PBS) to obtain a suspension with optical densities (ODs) of 0.4–0.5 at 600 nm, measured using a spectrophotometer. The bacterial concentration was standardized to 108 CFU/mL using the CFU counting method to ensure uniform inoculum sizes across all experimental groups.
Aliquots of 0.1 mL were used in the experiments. Bacterial suspensions (0.1 mL each) were poured into 96-well plates and irradiated with the developed laboratory-manufactured 270 nm UV-C-emitting prototype device with a double-lens optical system for different durations (10, 30, 60, 90, 120, and 180 s). The fiber tip was positioned 3 mm above the bottom of the plate for irradiation. Additionally, E. faecalis suspension (10 μL) was placed in a 96-well plate and subjected to irradiation under each condition. Following UV irradiation, the cultures were transferred to fresh broth and incubated at 37 °C under 5% CO2 for 6 h. Microbial growth over time was measured using a microplate reader at 600 nm. Incubation of the strain with fresh broth was used as a control. To ensure the reliability of the results, the experiments were repeated three times.
2.3. Statistical Analysis
All analyses were performed using R software (version 4.5.0; R Foundation for Statistical Computing, Vienna, Austria). The normality of the data was examined using the Shapiro–Wilk test. Inference statistics included the implementation of a one-way analysis of variance, followed by the post hoc Tukey’s test. The predetermined level for statistical significance was set at 0.05
3. Results
3.1. Effect of UV-C LED Irradiation on Bacterial Reduction
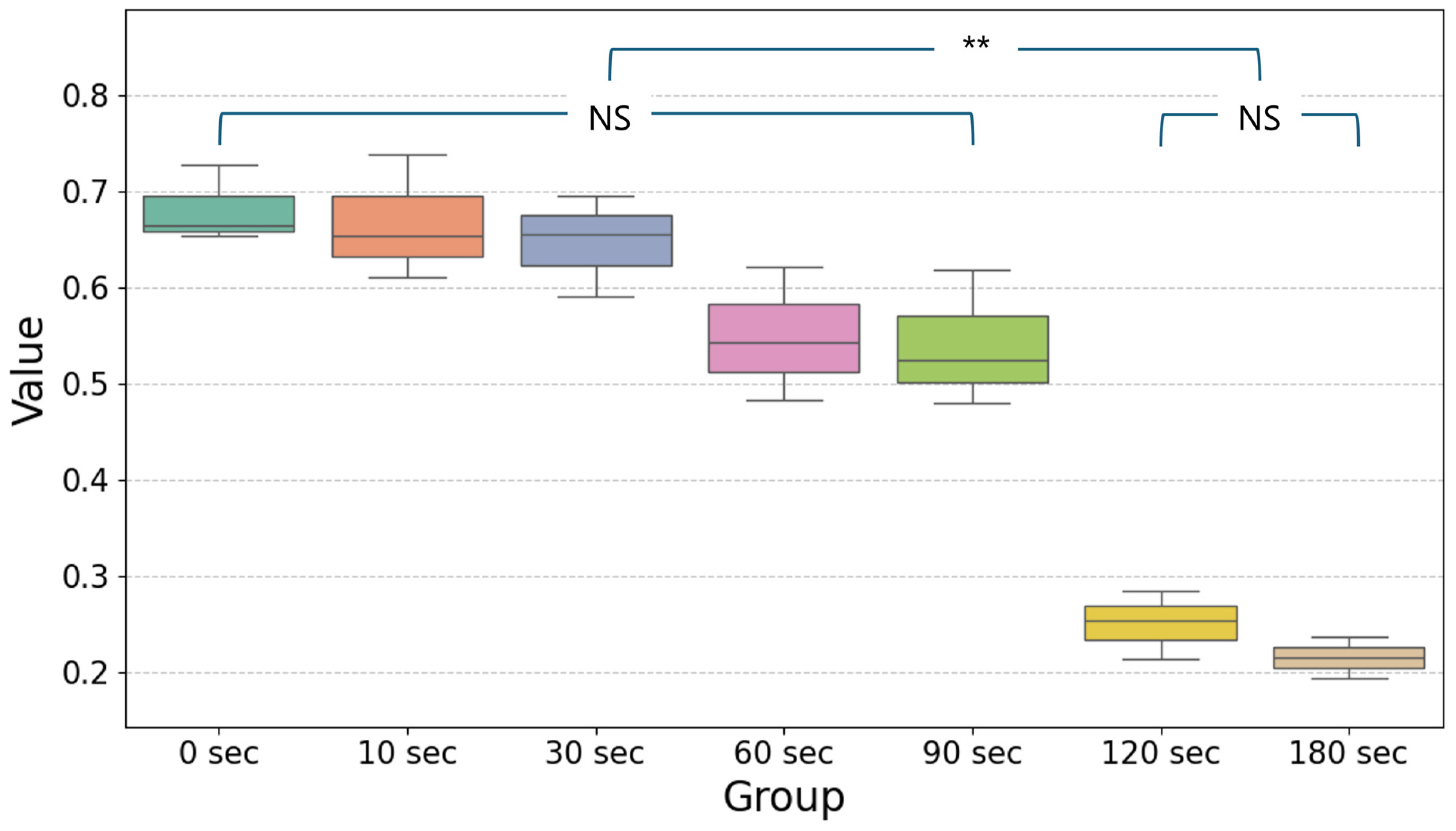
UV-C LED irradiation showed a time-dependent reduction in values, indicating its effectiveness in reducing bacterial populations over time. As shown in the boxplot (Figure 2), significant reductions began to appear after 60 s of UV-C LED exposure, with the most notable decreases observed at 120 and 180 s (p < 0.05). The data demonstrate that extended UV-C LED exposure results in more pronounced bactericidal effects, with minimal variability among replicates at the longer exposure times. Detailed descriptive statistics of bacterial viability measurements (OD600) at each UV-C LED exposure time are presented in Table 1. The mean values and standard deviations further confirm the progressive reduction in bacterial counts over time, emphasizing the consistent bactericidal efficacy at extended irradiation durations. The Tukey HSD post hoc test results further confirmed the statistical significance of these findings. Comparisons between groups revealed that UV-C LED irradiation for 120 and 180 s led to significantly greater reductions in bacterial populations compared to shorter exposure times, such as 0, 10, 30, 60 and 90 s (p < 0.05).
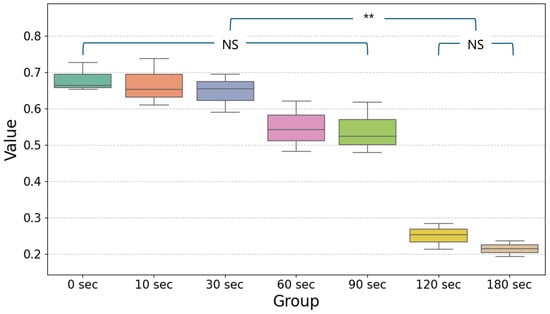
Figure 2.
Simplified boxplot showing the mean and distribution of values for different exposure times (0, 10, 30, 60, 90, 120, and 180 s) following UV-C LED irradiation. Statistical significance was determined using one-way ANOVA followed by Tukey’s HSD post hoc test (α = 0.05). Significant differences between groups are indicated by horizontal lines with ** (p < 0.05) and data without significant differences are shown as non-significant (NS). The values for 120 s and 180 s show the greatest reduction compared to the other time points, reflecting the enhanced bactericidal effect of prolonged UV-C exposure.

Table 1.
Descriptive statistics of bacterial viability (OD600) at different UV-C LED exposure times.
3.2. Time-Dependent Trends in Bacterial Viability
The decrease in bacterial counts followed a consistent trend across exposure times. At 60 and 90 s, bacterial reductions were minimal and not statistically significant when compared to the control (0 s). However, from 120 s onward, a marked decline in bacterial viability was evident. The Tukey HSD analysis indicated that significant differences existed between the 60 s group and the longer exposure groups (120 and 180 s). These results highlight the progressive bactericidal effects of UV-C LED irradiation as exposure time increases.
4. Discussion
The success of root canal therapy depends on a sufficient reduction in bacteria and their by-products from the root canal system [1]. However, achieving optimal disinfection through traditional methods, namely the combination of mechanical instrumentation and irrigation with a disinfecting solution, is difficult due to the complexity of the root canal system [25]. Previous studies have shown that laser irradiation through optical fibers can successfully disinfect root canal walls; however, these techniques are limited by the potential harm to surrounding tissues [14,15,16,26].
In this study, the effectiveness of UV-C LED light, known for its sterilizing effect, delivered through thin optical fibers and potentially applicable for root canal treatment, against E. faecalis was evaluated, specifically utilizing the UV-C band (200–280 nm) among the various ultraviolet wavelengths, including UV-C (200–280 nm), UV-B (280–320 nm), and UV-A (320–400 nm) [27].
The experimental results demonstrated that UV-C LED irradiation exerted significant bactericidal effects on E. faecalis in a time-dependent manner. As shown in the results, bacterial viability remained unchanged at 0 and 10 s but began to decline from 30 s onwards, with the most pronounced reductions observed at 120 and 180 s. The optical density (OD600) measurements revealed a progressive decline in bacterial counts over increasing exposure times, reinforcing the time-dependent nature of UV-C bactericidal activity. These findings are consistent with previous studies confirming the efficacy of UV-C in eradicating E. faecalis biofilms [28]. Although UV-C LED irradiation exhibited effective antimicrobial properties, power efficiency remains a critical factor for practical clinical application. Previous research indicates that optical fiber-mediated LED transmission can result in power losses, significantly impacting energy delivery to target sites [23,24]. In this study, a statistically significant bactericidal effect was observed at 120 s, indicating that the reduced energy delivery through the thin optical fiber is compensated by prolonged exposure time.
The prototype device utilized in this study operated at 270 nm, with a power of 100 mW, lower than conventional laser-based disinfection methods. These conventional lasers, particularly Er/YAG and Nd/YAG systems, have been widely used in endodontics due to their deep penetration and effective biofilm disruption [15,16]. However, these systems operate at significantly higher power levels (200–3000 mW), which increases the risk of excessive heat generation, potentially leading to dentinal microcracks or thermal damage to adjacent tissues [15,16]. In the present study, UV-C LED at 270 nm effectively sterilized the superficial layers of dentin by utilizing lenses, precise alignment, and coupling techniques to reduce light loss. UV-C irradiation provides an alternative antimicrobial approach by disrupting bacterial DNA replication through pyrimidine dimer formation, with peak germicidal efficiency occurring between 255 and 280 nm [17,28]. However, due to its short wavelength, its penetration depth is limited, reducing its direct impact on deeper bacterial colonies [17,28]. Unlike longer-wavelength laser systems, which can penetrate deeper but pose risks of thermal damage and structural alterations in dentinal walls, UV-C LED operates with minimal heat generation, potentially lowering the risk of collateral damage to surrounding tissues.
The integration of UV-C LED with fiber optic technology has been explored to optimize antimicrobial disinfection, particularly in confined anatomical spaces such as root canals [17,18,28]. Compared to free-space UV irradiation, fiber optic-guided UV-C delivery provides higher precision and better energy concentration within targeted regions, reducing unintended collateral damage to adjacent tissues. However, one major limitation of fiber optic-based UV-C delivery is energy attenuation as light propagates through the fiber. Optical losses due to scattering, absorption, and mode dispersion could reduce the intensity of light reaching the target bacterial biofilms, particularly in smaller-core fibers. To overcome this limitation, recent advancements have been explored, demonstrating enhanced UV-C transmission efficiency by reducing internal scattering losses [22,23]. Future designs incorporating high-reflectivity coatings and improved collimation techniques may further improve UV-C energy delivery and enhance bacterial inactivation efficiency.
In this study, a novel UV-C LED system integrated into a thin optical fiber (124 µm in diameter) was developed to effectively deliver UV-C light within confined root canal structures. Since accessing the apical third of the root canal and areas approximately 3 mm near the apex requires a fiber thickness of 100 µm or less, this study explored a similar approach using a 124 µm fiber in a newly developed prototype device. Enhancing bactericidal efficacy requires not only stronger UV-C sources but also an optimized delivery system that minimizes energy loss while ensuring safe application. The device in this study incorporated a specialized double-lens coupling system, optimizing the transmission of 270 nm UV-C light while ensuring minimal light dispersion. Proper coupling between the LED source and the optical fiber is essential to maximize energy transfer and reduce optical losses, as misalignment can significantly decrease transmission efficiency. Studies have demonstrated that lens-based coupling systems, particularly those utilizing collimating and focusing lenses, enhance beam collimation and minimize divergence, ensuring more effective light delivery through optical fibers [22,24]. Additionally, precise alignment of the LED with the fiber’s numerical aperture is critical in reducing reflection losses and improving overall energy efficiency [22]. By optimizing these factors, UV-C light can be efficiently transmitted through the thin optical fiber, improving its bactericidal potential in confined endodontic spaces.
Conventional chemo-mechanical debridement is partially effective, and UV-C LED has been proposed as an alternative or adjunct for enhancing root canal disinfection. Recent research demonstrated that 255 nm UV-C LED effectively eliminated bacterial pathogens and induced beneficial cellular responses in human embryonic palatal mesenchymal cells and gingival fibroblasts. However, its integration as an adjunct or alternative to conventional sodium hypochlorite (NaOCl) disinfection depends on the severity of endodontic infection and must be determined clinically [17]. A critical limitation in UV-C disinfection arises in surface-grown bacterial colonies or biofilms, as these structures significantly shield inner microbial cells, reducing their susceptibility to UV-induced DNA damage and diminishing the overall bactericidal efficacy [29]. Additionally, although various irrigant activation methods such as laser-activated irrigation (LAI) have shown superior biofilm removal, concerns exist regarding their impact on dentin properties. Specifically, LAI using solutions like NaOCl and EDTA can cause pronounced dentinal surface disintegration, more severe than observed with sonic or ultrasonic activation techniques [13].
Another promising approach is the use of UV-B source, where a combination of UV-C and UV-B has been shown to generate reactive oxygen species (ROS), further enhancing bacterial eradication [30]. This could improve antibacterial effectiveness while minimizing the required exposure time, reducing potential negative effects on the dentin structure. Additionally, recent studies suggest the potential benefits of combining UV-C irradiation with biomaterials such as TiO2. When TiO2 was combined with UVB irradiation, effective disinfection in complex root canals was observed, indicating potential for UV-assisted antimicrobial therapies within challenging anatomical structures [28]. Furthermore, narrowband UVB-LED irradiation has shown dual effects, directly killing oral pathogens by inducing reactive oxygen species (ROS) production from oral epithelial cells, while also supporting immune regulation and bone formation [30]. This suggests that combining UV-C disinfection with adjunctive narrowband UVB treatments could enhance comprehensive infection management in endodontics. Future research should investigate the optimal wavelength ratios and energy dosing for maximizing antimicrobial activity in endodontic applications. One of the key advantages of UV LED-based disinfection is its ability to provide targeted antimicrobial effects while minimizing tissue damage. Unlike traditional laser-based disinfection, which can cause excessive heat generation and dentinal microcracks, UV LED operates at a lower power level, reducing the risk of thermal damage [21]. Meanwhile, the disinfection of bacteria with optical fiber-mediated UV delivery, reaching deep into the dentin, remains a significant challenge in endodontics [28]. Therefore, combining UV with other biomaterials holds the potential to yield meaningful results.
The effectiveness of antibacterial materials in improving root canal therapy outcomes has been widely studied. Several biomaterial-based approaches, including silver-containing mesoporous bioglass [12,31], fluoride-releasing materials [32], and chitosan-derived biomaterials [33,34], have demonstrated significant antibacterial properties and remineralization potential. Silver-based nanocomposites, in particular, have shown strong antimicrobial activity against E. faecalis, with long-term ion release contributing to sustained antibacterial effects [12,31]. Additionally, fluoride-releasing restorative materials enhance dentin remineralization while inhibiting bacterial adhesion, making them a viable adjunct to antimicrobial light-based therapies [32]. Antibacterial biomaterials have gained increasing attention as adjuncts to conventional endodontic disinfection protocols. Recent studies suggest that incorporating bioactive materials, such as silver-doped mesoporous bioactive glass (Ag-MBG) and fluoride-releasing nanocomposites, can create sustained antimicrobial environments within root canals [12]. These materials not only inhibit bacterial adhesion but also promote mineralization and dentin tubule occlusion, reducing the risk of reinfection. Future clinical trials should also explore the integration of UV-C therapy with adjunctive disinfection techniques, such as mesoporous bioglass or antimicrobial nanoparticles, to assess the potential for enhanced bacterial eradication and biofilm disruption [31,35]. This approach has potential as a future treatment modality for clinicians treating caries, periodontal diseases, peri-implant diseases, and endodontic infections and inflammation in root canals.
Chitosan-functionalized materials have also been explored for their strong antimicrobial effects against E. faecalis and ability to reinforce the dentin structure [34,36,37]. Following UV-C disinfection, the application of chitosan-based materials could further facilitate remineralization and reduce bacterial recolonization by inhibiting biofilm formation through their antibacterial properties. Furthermore, fluoride-releasing restorative materials, due to their long-term antibacterial effects, may represent a potential option for integration with UV-C LED disinfection in root canal therapy. Combining irrigants with laser-activated irrigation disinfection techniques further enhances antimicrobial efficacy by facilitating the deeper penetration of antibacterial agents into dentinal tubules [13]. This synergistic approach could improve long-term disinfection outcomes and reduce the likelihood of reinfection, which remains a critical challenge in endodontic therapy. The combination of UV-C LED disinfection with biomaterials or irrigants may represent a promising multi-modal strategy for long-term infection control. However, further studies are required to assess the durability of these materials and their interactions with UV-C irradiation. Future research should explore the long-term antimicrobial efficacy of UV-C LED disinfection in combination with antibacterial dentin materials. Investigating novel biomaterial coatings that enhance UV-C light absorption and bacterial adhesion prevention may lead to more effective and durable solutions for endodontic disinfection. Beyond root canal therapy, UV-C LED disinfection holds significant potential for broader applications in dentistry. Recent research has investigated the use of UV-C irradiation for treating caries, periodontal diseases, peri-implant diseases, and endodontic infections [17,28]. Given its broad-spectrum antimicrobial activity, UV-C technology could be explored for the chairside sterilization of dental instruments, prostheses, and orthodontic appliances.
The directional nature and power source of fiber-optic UV-C transmission play a role in energy distribution and total energy output. In this study, the optical fiber system emitted linear irradiation, requiring prolonged irradiation time to achieve bacterial elimination due to the lower energy delivered through the thin fiber. Future studies should investigate the use of thinner optical fibers with a diameter of 100 µm or less for narrower root canals, incorporating both lateral and linear irradiation capabilities, along with higher-powered UV-C LED systems, while maintaining safe operational parameters to optimize root canal disinfection. For UV-C fiber optic systems to be effectively implemented in clinical endodontic practice, several practical challenges must be addressed. First, the development of compact and ergonomic UV-C delivery devices is necessary to improve usability and integration within current endodontic workflows. Advances in miniaturized, handheld UV-C LED systems with real-time fiber optic guidance could enhance clinicians’ precision and reduce treatment time. Another critical factor is the cost-effectiveness of UV-C disinfection compared to conventional irrigation methods. While new UV-C systems may require a higher initial investment, their potential to reduce retreatment rates through additional antibacterial effects and improve long-term success could justify their clinical adoption. Additionally, studies evaluating patient-reported outcomes, such as postoperative pain reduction and healing rates, would provide valuable insights into the real-world benefits of UV-C therapy in endodontics.
In this study, the bactericidal effect of UV-C light delivered through an optical fiber was demonstrated against E. faecalis under laboratory conditions. This study is limited in that it did not investigate the effect of UV-C irradiation on other bacteria [38] or multispecies biofilms within the complex root canal system, nor did it evaluate its potential thermodynamic impact on dentin or compare the antimicrobial effects of other wavelengths of light [39].
Future studies should explore whether UV-C therapy can be adapted for preventive applications, such as a reduction in microbial biofilms in high-risk patients with recurrent dental infections. By further refining UV-C LED delivery techniques, optimizing biomaterial interactions, and expanding clinical applications, this technology has the potential to revolutionize infection control strategies in modern dentistry.
5. Conclusions
Based on the findings of this in vitro study, the following conclusions can be drawn:
- The 270 nm UV-C LED light, delivered through a thin optical fiber, exhibited time-dependent bactericidal effects against E. faecalis.
- The development of a specialized optical system allowed for precise light transmission, enhancing the disinfection potential of UV-C irradiation.
- UV-C LED-based disinfection represents a promising alternative to conventional endodontic irrigation methods and may contribute to improving the success rates of root canal treatments.
Author Contributions
M.-J.J. and Y.-S.C. contributed equally to this work. M.-J.J. and Y.-S.C., and D.-G.S. participated in the conception and design of the study. M.-J.J. and Y.-S.C. performed all experimental procedures and contributed to the acquisition of data. M.-J.J., Y.-S.C. and D.-G.S. substantially contributed to the interpretation of data and analysis. M.-J.J., Y.-S.C. and D.-G.S. were involved in drafting the manuscript and revising it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grant no. 07-2020-0008 from the SNUDH Research Fund and by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology [RS-2023-NR077207].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Weissheimer, T.; Pinto, K.P.; da Silva, E.; Hashizume, L.N.; da Rosa, R.A.; Só, M.V.R. Disinfectant effectiveness of chlorhexidine gel compared to sodium hypochlorite: A systematic review with meta-analysis. Restor. Dent. Endod. 2023, 48, e37. [Google Scholar] [CrossRef] [PubMed]
- Berutti, E.; Marini, R.; Angeretti, A. Penetration ability of different irrigants into dentinal tubules. J. Endod. 1997, 23, 725–727. [Google Scholar] [CrossRef]
- Sundqvist, G.; Figdor, D.; Persson, S.; Sjögren, U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 86–93. [Google Scholar] [CrossRef]
- Evans, M.; Davies, J.K.; Sundqvist, G.; Figdor, D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int. Endod. J. 2002, 35, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Orstavik, D.; Haapasalo, M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod. Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.; Aminsobhani, M.; Sohrabi, K.; Chiniforush, N.; Ghafari, S.; Shamshiri, A.R.; Noroozi, N. Penetration Depth of Sodium Hypochlorite in Dentinal Tubules after Conventional Irrigation, Passive Ultrasonic Agitation and Nd:YAG Laser Activated Irrigation. J. Lasers Med. Sci. 2016, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Alfredo, E.; Marchesan, M.A.; Sousa-Neto, M.D.; Brugnera-Júnior, A.; Silva-Sousa, Y.T. Temperature variation at the external root surface during 980-nm diode laser irradiation in the root canal. J. Dent. 2008, 36, 529–534. [Google Scholar] [CrossRef]
- Meire, M.A.; Coenye, T.; Nelis, H.J.; De Moor, R.J. Evaluation of Nd:YAG and Er:YAG irradiation, antibacterial photodynamic therapy and sodium hypochlorite treatment on Enterococcus faecalis biofilms. Int. Endod. J. 2012, 45, 482–491. [Google Scholar] [CrossRef]
- Goya, C.; Yamazaki, R.; Tomita, Y.; Kimura, Y.; Matsumoto, K. Effects of pulsed Nd:YAG laser irradiation on smear layer at the apical stop and apical leakage after obturation. Int. Endod. J. 2000, 33, 266–271. [Google Scholar] [CrossRef]
- de Souza, E.B.; Cai, S.; Simionato, M.R.; Lage-Marques, J.L. High-power diode laser in the disinfection in depth of the root canal dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, e68–e72. [Google Scholar] [CrossRef]
- Asnaashari, M.; Sadeghian, A.; Hazrati, P. The Effect of High-Power Lasers on Root Canal Disinfection: A Systematic Review. J. Lasers Med. Sci. 2022, 13, e66. [Google Scholar] [CrossRef]
- Kung, J.C.; Wang, W.H.; Chiang, Y.C.; Yang-Wang, Y.T.; Wang, Y.C.; Chen, W.C.; Shih, C.J. The Antibacterial and Remineralization Effect of Silver-Containing Mesoporous Bioactive Glass Sealing and Er-YAG Laser on Dentinal Tubules Treated in a Streptococcus mutans Cultivated Environment. Pharmaceuticals 2021, 14, 1124. [Google Scholar] [CrossRef] [PubMed]
- Meire, M.; De Moor, R.J.G. Principle and antimicrobial efficacy of laser-activated irrigation: A narrative review. Int. Endod. J. 2024, 57, 841–860. [Google Scholar] [CrossRef]
- Roper, M.J.; White, J.M.; Goodis, H.E.; Gekelman, D. Two-dimensional changes and surface characteristics from an erbium laser used for root canal preparation. Lasers Surg. Med. 2010, 42, 379–383. [Google Scholar] [CrossRef]
- DiVito, E.E.; Colonna, M.P.; Olivi, G. The photoacoustic efficacy of an Er: YAG laser with radial and stripped tips on root canal dentin walls: An SEM evaluation. J. Laser Dent. 2011, 19, 156–161. [Google Scholar]
- Kaitsas, V.; Signore, A.; Fonzi, L.; Benedicenti, S.; Barone, M. Effects of Nd: YAG laser irradiation on the root canal wall dentin of human teeth: A SEM study. Bull. Group. Int. Rech. Sci. Stomatol. Odontol. 2001, 43, 87–92. [Google Scholar]
- Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Dataset of endodontic microorganisms killed at 265 nm wavelength by an ultraviolet C light emitting diode in root canals of extracted, instrumented teeth. Data Brief. 2022, 40, 107750. [Google Scholar] [CrossRef] [PubMed]
- Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Using ultraviolet (UV) light emitting diodes (LED) to create sterile root canals and to treat endodontic infections. Curr. Opin. Biomed. Eng. 2022, 23, 100397. [Google Scholar] [CrossRef]
- Wengraitis, S.; McCubbin, P.; Wade, M.M.; Biggs, T.D.; Hall, S.; Williams, L.I.; Zulich, A.W. Pulsed UV-C disinfection of Escherichia coli with light-emitting diodes, emitted at various repetition rates and duty cycles. Photochem. Photobiol. 2013, 89, 127–131. [Google Scholar] [CrossRef]
- Metzger, Z.; Better, H.; Abramovitz, I. Immediate root canal disinfection with ultraviolet light: An ex vivo feasibility study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 425–433. [Google Scholar] [CrossRef]
- Aung, N.; Aoki, A.; Takeuchi, Y.; Hiratsuka, K.; Katagiri, S.; Kong, S.; Shujaa Addin, A.; Meinzer, W.; Sumi, Y.; Izumi, Y. The Effects of Ultraviolet Light-Emitting Diodes with Different Wavelengths on Periodontopathic Bacteria In Vitro. Photobiomodul Photomed. Laser Surg. 2019, 37, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Q.; Yang, F.; Hong, W.; Bai, Y.; Zhou, Z. Coupling efficiency improvement of light source with a convex lens for space charge managements. Optik 2021, 248, 167999. [Google Scholar] [CrossRef]
- Ruan, Y.; Li, H.; Jia, J.; Gu, Y.; Zhang, Z.; Shen, D.; Chen, X.; Li, Q.; Hong, W.; Cui, X.; et al. High efficiency deep ultraviolet micro-LED and optical fiber coupling for low power charge management applications. Opt. Laser Technol. 2025, 181, 111902. [Google Scholar] [CrossRef]
- Park, E.-H.; Kim, M.-J.; Kwon, Y.-S. Microlens for efficient coupling between LED and optical fiber. IEEE Photonics Technol. Lett. 1999, 11, 439–441. [Google Scholar] [CrossRef]
- Chrepa, V.; Kotsakis, G.A.; Pagonis, T.C.; Hargreaves, K.M. The effect of photodynamic therapy in root canal disinfection: A systematic review. J. Endod. 2014, 40, 891–898. [Google Scholar] [CrossRef]
- Walsh, L.J. The current status of laser applications in dentistry. Aust. Dent. J. 2003, 48, 146–155; quiz 198. [Google Scholar] [CrossRef] [PubMed]
- Montero Magalló, P.; Roger Laparra, I.; Milara, J.; Cortijo, J. Damaging effects of UVA, blue light, and infrared radiation: In vitro assessment on a reconstructed full-thickness human skin. Front. Med. 2023, 10, 1267409. [Google Scholar] [CrossRef]
- Haraguchi, A.; Yoshida, S.; Takeshita, M.; Sumi, Y.; Mitarai, H.; Yuda, A.; Wada, H.; Nishimura, F.; Maeda, H.; Wada, N. Effects of ultraviolet irradiation equipment on endodontic disease–related bacteria. Lasers Dent. Sci. 2022, 6, 31–40. [Google Scholar] [CrossRef]
- Mohsin, M.S.; Avdic, M.; Fitzpatrick, K.; Lanzarini-Lopes, M. UV-C side-emitting optical fiber-based disinfection: A promising approach for infection control in tight channels. Microbiol. Spectr. 2024, 12, e0004024. [Google Scholar] [CrossRef]
- Takada, A.; Matsushita, K.; Horioka, S.; Furuichi, Y.; Sumi, Y. Bactericidal effects of 310 nm ultraviolet light-emitting diode irradiation on oral bacteria. BMC Oral Health 2017, 17, 96. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Wang, Y.C.; Kung, J.C.; Shih, C.J. Antibacterial silver-containing mesoporous bioglass as a dentin remineralization agent in a microorganism-challenged environment. J. Dent. 2021, 106, 103563. [Google Scholar] [CrossRef]
- Sungurtekin-Ekci, E.; Ozdemir-Ozenen, D.; Duman, S.; Acuner, I.C.; Sandalli, N. Antibacterial surface properties of various fluoride-releasing restorative materials in vitro. J. Appl. Biomater. Funct. Mater. 2015, 13, e169–e173. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, D.H. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor. Dent. Endod. 2013, 38, 36–42. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fan, W.; Fan, B. Synergistic effects of silver ions and metformin against enterococcus faecalis under high-glucose conditions in vitro. BMC Microbiol. 2021, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.S.; Nitisusanta, L.I.; Iqbal, K.; Daood, U.; Beng, L.T.; Neo, J. Chitosan/Riboflavin-modified demineralized dentin as a potential substrate for bonding. J. Mech. Behav. Biomed. 2013, 17, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef]
- Martu, M.A.; Luchian, I.; Mares, M.; Solomon, S.; Ciurcanu, O.; Danila, V.; Rezus, E.; Foia, L. The Effectiveness of Laser Applications and Photodynamic Therapy on Relevant Periodontal Pathogens (Aggregatibacter actinomycetemcomitans) Associated with Immunomodulating Anti-rheumatic Drugs. Bioengineering 2023, 10, 61. [Google Scholar] [CrossRef]
- Panhoca, V.H.; Corrêa, T.Q.; Bagnato, V.S. New possibilities on the application of violet light in dentistry combining aesthetics and microbiological control: Report of two clinical cases. J. Dent. Health Oral Res. 2022, 3, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).