Markerless Upper Body Movement Tracking During Gait in Children with HIV Encephalopathy: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Presentation of Preliminary Data
2.2. Participants
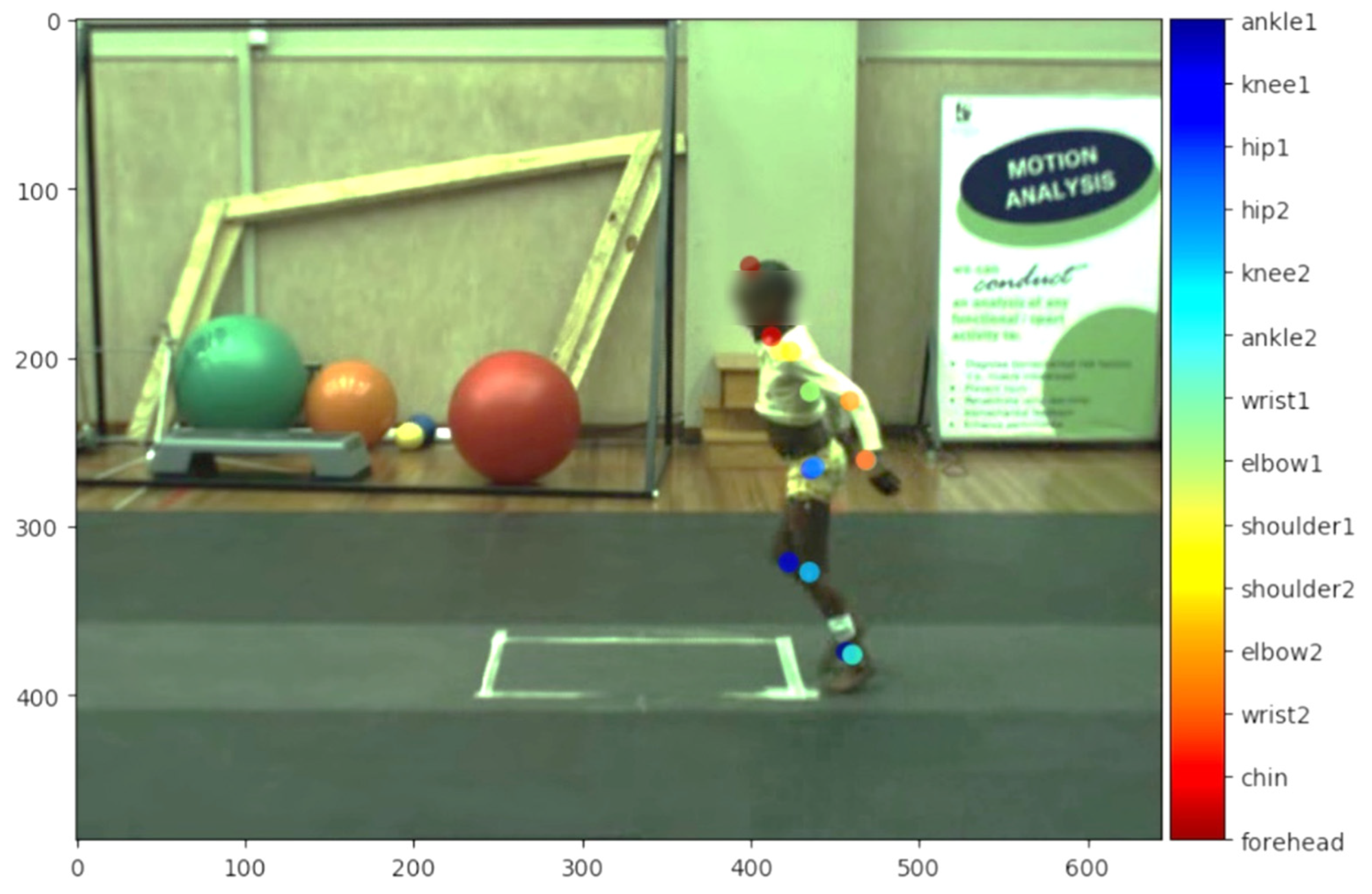
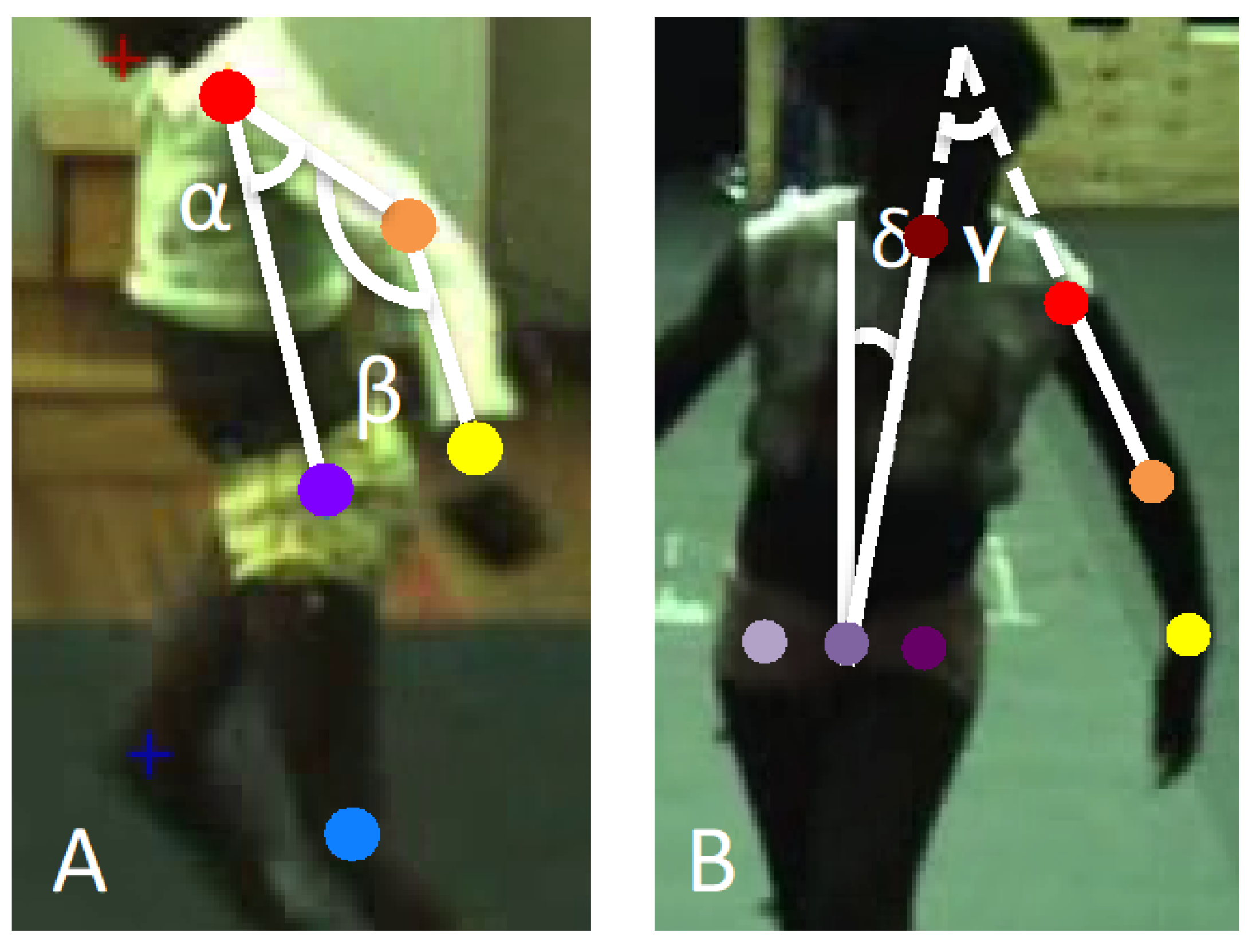
2.3. Assessment Procedures
2.4. Statistical Analysis
3. Results
3.1. Participants’ Background
3.2. Accuracy of Pre-Trained Human Network (ResNet101)
3.3. Arm Swing and Trunk Sway
4. Discussion
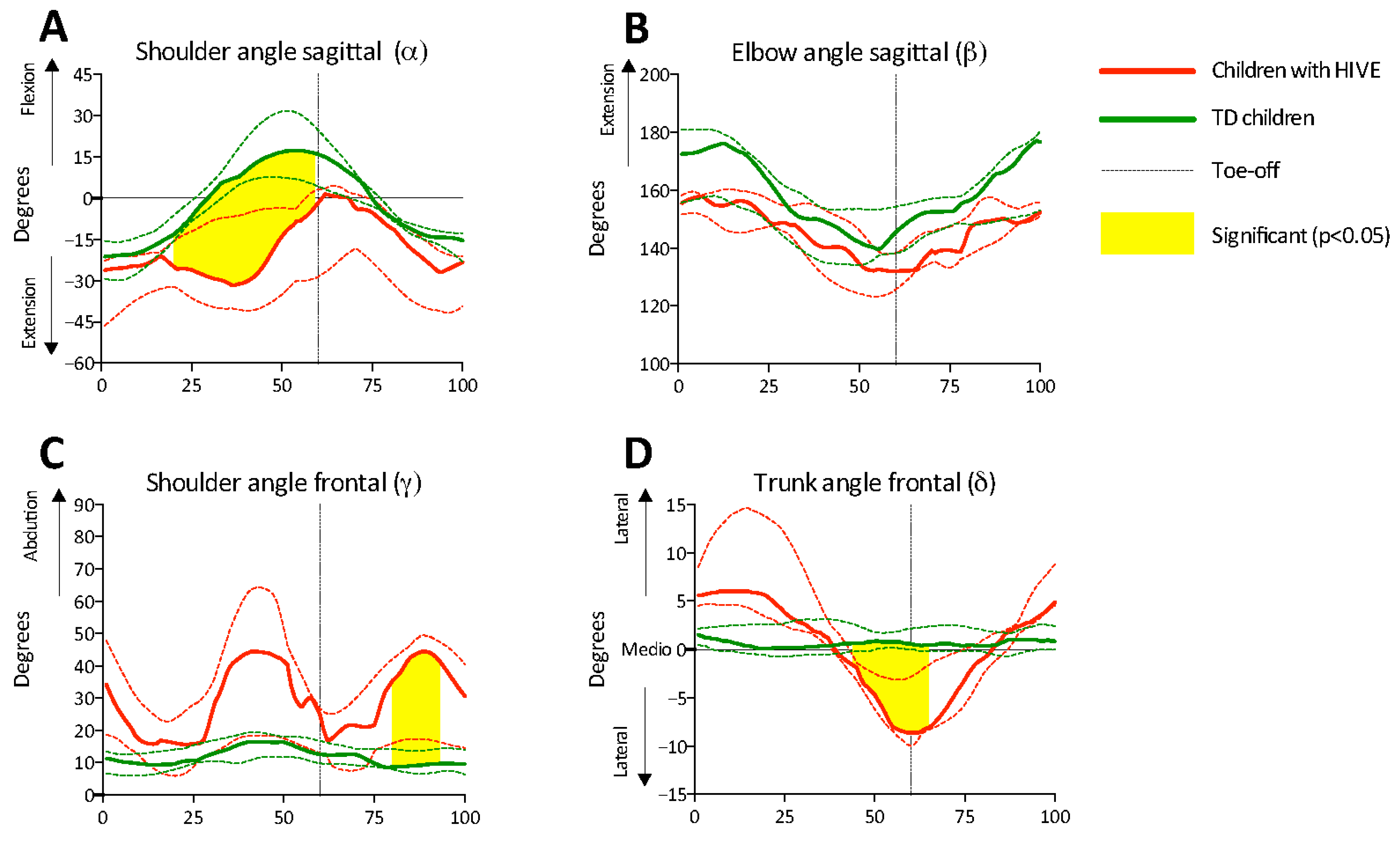
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benedetti, M.G.; Beghi, E.; De Tanti, A.; Cappozzo, A.; Basaglia, N.; Cutti, A.G.; Cereatti, A.; Stagni, R.; Verdini, F.; Manca, M.; et al. SIAMOC position paper on gait analysis in clinical practice: General requirements, methods and appropriateness. Results of an Italian consensus conference. Gait Posture 2017, 58, 252–260. [Google Scholar] [CrossRef]
- Castelli, A.; Paolini, G.; Cereatti, A.; Della Croce, U. A 2D Markerless Gait Analysis Methodology: Validation on Healthy Subjects. Comput. Math. Methods Med. 2015, 2015, 186780. [Google Scholar] [CrossRef]
- Fernández-González, P.; Koutsou, A.; Cuesta-Gómez, A.; Carratalá-Tejada, M.; Miangolarra-Page, J.C.; Molina-Rueda, F. Reliability of Kinovea® Software and Agreement with a Three-Dimensional Motion System for Gait Analysis in Healthy Subjects. Sensors 2020, 20, 3154. [Google Scholar] [CrossRef] [PubMed]
- Damsted, C.; Nielsen, R.O.; Larsen, L.H. Reliability of video-based quantification of the knee- and hip angle at foot strike during running. Int. J. Sports Phys. Ther. 2015, 10, 147–154. [Google Scholar]
- Van Rie, A.; Harrington, P.R.; Dow, A.; Robertson, K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: A global perspective. Eur. J. Paediatr. Neurol. 2007, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. UNAIDS Data. 2019. Available online: https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data (accessed on 19 April 2025).
- Tindyebwa, D.; Kayita, J.; Musoke, P.; Eley, B.; Nduati, R.; Coovadia, H.; Bobart, R.; Mbori-Ngacha, D.; Kieffer, P.M. Handbook on Paediatric AIDS in Africa, 4th ed.; African Network for the Care of Children Affected by HIV/AIDS—ANECCA Publishers: Kampala, Uganda, 2017. [Google Scholar]
- Cronin, N.J.; Avela, J.; Finni, T.; Peltonen, J. Differences in contractile behaviour between the soleus and medial gastrocnemius muscles during human walking. J. Exp. Biol. 2013, 216, 909–914. [Google Scholar] [CrossRef]
- Langerak, N.G.; du Toit, J.; Burger, M.; Cotton, M.F.; Springer, P.E.; Laughton, B. Spastic diplegia in children with HIV encephalopathy: First description of gait and physical status. Dev. Med. Child. Neurol. 2014, 56, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.N.; Donald, K.A.; Laughton, B.; Lamberts, R.P.; Langerak, N.G. HIV encephalopathy with bilateral lower limb spasticity: Upper limb motor function and level of activity and participation. Dev. Med. Child. Neurol. 2017, 59, 412–419. [Google Scholar] [CrossRef]
- Kidziński, L.; Yang, B.; Hicks, J.L.; Rajagopal, A.; Delp, S.L.; Schwartz, M.H. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat. Commun. 2020, 11, 4054. [Google Scholar] [CrossRef]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef]
- Nath, T.; Mathis, A.; Chen, A.C.; Patel, A.; Bethge, M.; Mathis, M.W. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 2019, 14, 2152–2176. [Google Scholar] [CrossRef] [PubMed]
- Meyns, P.; Desloovere, K.; Van Gestel, L.; Massaad, F.; Smits-Engelsman, B.; Duysens, J. Altered arm posture in children with cerebral palsy is related to instability during walking. Eur. J. Paediatr. Neurol. 2012, 16, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Meyns, P.; Kerkum, Y.L.; Brehm, M.A.; Becher, J.G.; Buizer, A.I.; Harlaar, J. Ankle foot orthoses in cerebral palsy: Effects of ankle stiffness on trunk kinematics, gait stability and energy cost of walking. Eur. J. Paediatr. Neurol. 2020, 26, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Meyns, P.; Duysens, J.; Desloovere, K. The arm posture in children with unilateral Cerebral Palsy is mainly related to antero-posterior gait instability. Gait Posture 2016, 49, 132–135. [Google Scholar] [CrossRef]
- Delabastita, T.; Desloovere, K.; Meyns, P. Restricted Arm Swing Affects Gait Stability and Increased Walking Speed Alters Trunk Movements in Children with Cerebral Palsy. Front. Hum. Neurosc 2016, 10, 354. [Google Scholar] [CrossRef]
- Eken, M.M.; Meyns, P.; Lamberts, R.P.; Langerak, N.G. Markerless movement tracking using a machine-learning algorithm to assess arm movements during gait in children with HIV encephalopathy. Gait Posture 2020, 81, 85–86. [Google Scholar] [CrossRef]
- Mann, T.N.; Laughton, B.; Donald, K.A.; Langerak, N.G. HIV encephalopathy with bilateral lower limb spasticity: Gross motor function and antiretroviral therapy. Dev. Med. Child. Neurol. 2017, 59, 407–411. [Google Scholar] [CrossRef]
- Caldwell, M.; Oxtoby, M.; Simonds, R.; Lindegren, M.; Rogers, M. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. CDC Morbodity Mortal. Wkly. Rep. 1994, 43, 1–10. [Google Scholar]
- Palisano, R.J.; Hanna, S.E.; Rosenbaum, P.L.; Russell, D.J.; Walter, S.D.; Wood, E.P.; Raina, P.S.; Galuppi, B.E. Validation of a Model of Gross Motor Function for Children with Cerebral Palsy. Phys. Ther. 2000, 80, 974–985. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Romkes, J.; Peeters, W.; Oosterom, A.M.; Molenaar, S.; Bakels, I.; Brunner, R. Evaluating upper body movements during gait in healthy children and children with diplegic cerebral palsy. J. Pediatr. Orthop. B 2007, 16, 175–180. [Google Scholar] [CrossRef] [PubMed]
- De Vlieger, D.; Defour, A.; Bar-On, L.; Cambier, D.; Swinnen, E.; Van der Looven, R.; Van Bladel, A. Speed-dependent changes in the arm swing during independent walking in individuals after stroke. PLoS ONE 2025, 20, e0315332. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Saeys, W.; Hallemans, A.; Velghe, S.; Viskens, P.J.; Vereeck, L.; De Hertogh, W.; Truijen, S. Trunk biomechanics during hemiplegic gait after stroke: A systematic review. Gait Posture 2017, 54, 133–143. [Google Scholar] [CrossRef]
- Chaparro-Rico, B.D.M.; Cafolla, D.; Tortola, P.; Galardi, G. Assessing Stiffness, Joint Torque, and ROM for Paretic and Non-Paretic Lower Limbs during the Subacute Phase of Stroke Using Lokomat Tools. Appl. Sci. 2020, 10, 6168. [Google Scholar] [CrossRef]
- Wolfsegger, T.; Pichler, R.; Assar, H.; Topakian, R. Quantitative trunk sway analysis under challenging gait conditions in early and untreated Parkinson’s disease. Neurol. Sci. 2022, 43, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Butler, E.E.; Rose, J. Neurologic Correlates of Gait Abnormalities in Cerebral Palsy: Implications for Treatment. Front. Hum. Neurosci. 2017, 11, 103. [Google Scholar] [CrossRef]
- Wang, K.K.; Munger, M.E.; Chen, B.P.J.; Novacheck, T.F. Selective dorsal rhizotomy in ambulant children with cerebral palsy. J. Child. Orthop. 2018, 12, 413–427. [Google Scholar] [CrossRef]
- Wagenaar, R.C.; Emmerik, R.E.A. Resonant frequencies of arms and legs identify different walking patterns. J. Biomech. 2000, 33, 853–861. [Google Scholar] [CrossRef]
- Wagenaar, R.C.; Emmerik, R.E.A. Dynamics of pathological gait. Hum. Mov. Sci. 1994, 13, 441–471. [Google Scholar] [CrossRef]
- Van Bladel, A.; De Ridder, R.; Palmans, T.; Van Der Looven, R.; Verheyden, G.; Meyns, P.; Cambier, D. Defining characteristics of independent walking persons after stroke presenting with different arm swing coordination patterns. Hum. Mov. Sci. 2024, 93, 103174. [Google Scholar] [CrossRef]
- Puig-Diví, A.; Escalona-Marfil, C.; Padullés-Riu, J.M.; Busquets, A.; Padullés-Chando, X.; Marcos-Ruiz, D. Validity and reliability of the Kinovea program in obtaining angles and distances using coordinates in 4 perspectives. PLoS ONE 2019, 14, e0216448. [Google Scholar] [CrossRef]
- Iijima, S.; Shiomi, M.; Hara, T. Verification of Reliability and Validity of Trunk Forward Tilt Angle Measurement During Gait Using 2-Dimensional Motion Analysis. J. Chiropr. Med. 2023, 22, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ugbolue, U.C.; Papi, E.; Kaliarntas, K.T.; Kerr, A.; Earl, L.; Pomeroy, V.M.; Rowe, P.J. The evaluation of an inexpensive, 2D, video based gait assessment system for clinical use. Gait Posture 2013, 38, 483–489. [Google Scholar] [CrossRef]
- Fatone, S.; Stine, R. Capturing quality clinical videos for two-dimensional motion analysis. J. Prosthet. Orthot. 2015, 27, 27–32. [Google Scholar] [CrossRef]
- Wade, L.; Needham, L.; McGuigan, P.; Bilzon, J. Applications and limitations of current markerless motion capture methods for clinical gait biomechanics. PeerJ 2022, 10, e12995. [Google Scholar] [CrossRef]
- Needham, L.; Evans, M.; Cosker, D.P.; Wade, L.; McGuigan, P.M.; Bilzon, J.L.; Colyer, S.L. The accuracy of several pose estimation methods for 3D joint centre localisation. Sci. Rep. 2021, 11, 20673. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaart, M.; Jacobs, N.; Hallemans, A.; Meyns, P. Validity of Deep Learning-Based Motion Capture Using DeepLabCut to Assess Proprioception in Children. Appl. Sci. 2025, 15, 3428. [Google Scholar] [CrossRef]
- Sipari, D.; Chaparro-Rico, B.D.M.; Cafolla, D. SANE (Easy Gait Analysis System): Towards an AI-Assisted Automatic Gait-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10032. [Google Scholar] [CrossRef]
| No. | Age (Years) | BMI (kg/m2) | BMI Category | Videos Included (n) | |
|---|---|---|---|---|---|
| Sagittal | Frontal | ||||
| HIVE | |||||
| 1 | 8.3 | 17.9 | normal | 3 | 3 |
| 2 | 10.8 | 21.7 | overweight | 3 | 3 |
| 3 | 10.5 | 15.9 | normal | 3 | 3 |
| 4 | 9.4 | 18.0 | normal | 3 | 2 |
| 5 | 12.8 | 22.5 | overweight | 3 | 3 |
| TD | |||||
| 1 | 6.8 | 13.9 | normal | 3 | 3 |
| 2 | 7.9 | 13.6 | normal | 3 | 3 |
| 3 | 10.8 | 17.8 | normal | 3 | 2 |
| 4 | 10.8 | 15.0 | normal | 3 | 3 |
| 5 | 8.2 | 19.5 | overweight | 3 | 3 |
| HIVE | TD | ||||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p Value | |
| Sagittal plane | |||||
| Shoulder flexion/extension (°) | 31.0 | [24.4–51.5] | 37.2 | [25.2–61.8] | 0.602 |
| Elbow flexion/extension (°) | 25.4 | [20.1–38.7] | 30.7 | [24.8–36.1] | 0.602 |
| Frontal plane | |||||
| Shoulder abduction/adduction (°) | 41.5 | [11.1–49.2] | 6.5 | [4.5–10.4] | 0.028 * |
| Trunk sway (°) | 14.7 | [8.5–24.7] | 1.9 | [1.6–2.9] | 0.009 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eken, M.M.; Meyns, P.; Lamberts, R.P.; Langerak, N.G. Markerless Upper Body Movement Tracking During Gait in Children with HIV Encephalopathy: A Pilot Study. Appl. Sci. 2025, 15, 4546. https://doi.org/10.3390/app15084546
Eken MM, Meyns P, Lamberts RP, Langerak NG. Markerless Upper Body Movement Tracking During Gait in Children with HIV Encephalopathy: A Pilot Study. Applied Sciences. 2025; 15(8):4546. https://doi.org/10.3390/app15084546
Chicago/Turabian StyleEken, Maaike M., Pieter Meyns, Robert P. Lamberts, and Nelleke G. Langerak. 2025. "Markerless Upper Body Movement Tracking During Gait in Children with HIV Encephalopathy: A Pilot Study" Applied Sciences 15, no. 8: 4546. https://doi.org/10.3390/app15084546
APA StyleEken, M. M., Meyns, P., Lamberts, R. P., & Langerak, N. G. (2025). Markerless Upper Body Movement Tracking During Gait in Children with HIV Encephalopathy: A Pilot Study. Applied Sciences, 15(8), 4546. https://doi.org/10.3390/app15084546






