Abstract
In recent years, growing interest has emerged in the use of psychobiotics and adaptogens for regulating stress and anxiety. However, it is essential to assess their effectiveness as treatment alternatives, particularly given the limitations of conventional approaches, such as adverse effects of pharmacological therapies and the limited remission rates associated with psychotherapy alone. This systematic review primarily aims to assess how effective psychobiotics and adaptogens are in alleviating stress- and anxiety-related psychophysiological symptoms. A secondary aim is to identify specific bacterial strains and herbal compounds most consistently linked to beneficial outcomes. An extensive search was conducted in PubMed, Scopus, ScienceDirect, and Google Scholar using the terms ((adaptogens) OR (psychobiotics)) AND (stress), resulting in 23 selected articles. The findings suggest that both psychobiotics and adaptogens show promise in reducing stress- and anxiety-related symptoms. Bifidobacterium longum and Lactobacillus rhamnosus were frequently associated with improved gut–brain axis regulation, while Withania somnifera and Rhodiola rosea demonstrated physiological benefits through cortisol reduction and stress adaptation. Although results are encouraging, further research is needed to confirm long-term efficacy and clarify the specific mechanisms and compounds responsible for these effects.
1. Introduction
Stress is a multifaceted physiological and psychological response influenced by environmental, chemical, and psychological factors. Acute stress specifically involves an immediate psychophysiological response to a perceived or real threat, challenge, or significant event, which is typically short-lived and subsides once the stressor is removed [1]. It is driven by the activation of the hypothalamic–pituitary–adrenal (HPA) axis, leading to the release of stress hormones such as cortisol, epinephrine, and norepinephrine, which facilitate the ‘fight or flight’ reaction ([2]; for a review, see [3]). Furthermore, one possible response to stress, particularly in acute situations, is anxiety. Unlike acute stress, anxiety is characterized by its anticipatory nature, arising in response to perceived future threats and manifesting feelings of apprehension and worry [4]. Anxiety symptoms are key indicators of stress-related dysregulation [5].
Life events significantly contribute to stress reactions, with chronic illness, divorce, house moving, caregiving, and exam periods being recognized as major stressors [6,7,8,9,10,11]. While an efficient stress response enables effective coping, chronic overstimulation—known as allostatic load—can disrupt HPA axis function, leading to physical and mental health issues such as cardiovascular disease, post-traumatic stress disorder, and depression [12,13,14,15]. In Spain, anxiety-related mental health issues have increased by 105% from 2016 to 2021, making anxiety disorders the most prevalent mental health concern, affecting 126.9 per 1000 residents and leading to a higher consumption of psychotropic medications, particularly anxiolytics, which account for 9.1% of total medication use [16]. The widespread prescription and consumption of these medications raise concerns, as pharmacological treatments for emotional disorders are associated with several adverse side effects [17]. Moreover, a substantial proportion of individuals receiving psychotherapy alone do not achieve full remission [18,19]. Given the undeniable role of stress as a mediator in mental and physical health, exploring diverse strategies to mitigate its effects could yield substantial societal benefits.
Emerging research highlights the role of gut microbiota in regulating emotions, cognition, and behavior [20]. Microbiota are understood as the multitude of microorganisms, such as bacteria, archaea, yeasts, etc., that the human gut hosts [21,22,23]. The gut–brain axis enables bidirectional signaling via endocrine, neural, and immune pathways [24]. It is of paramount interest to know that microbiota affects the HPA axis, and consequently, the release of cortisol, involved in the stress response [25,26]. Dysbiosis, or alterations in gut microbiome composition, has been linked to emotional disorders, such as anxiety-related disorders [27]. Modulating microbiota presents potential benefits for mental health, particularly through substances like psychobiotics and adaptogens.
1.1. Background on Psychobiotics
The term “psychobiotics” was defined as “live microbes that have a positive mental health benefit” [28]. Probiotics and prebiotics have shown promising potential as psychobiotic agents [29,30] with increasing evidence on how they affect the central nervous system [31]. Evidence shows that psychobiotics exert significant benefits on stress, mood, anxiety, and cognitive processes [32,33,34,35]. Specifically, psychobiotics have been shown to reduce anxiety-like and depression-like symptoms [36] while normalizing key physiological markers such as cortisol, corticosterone, noradrenaline, brain-derived neurotrophic factor, and immune function [37,38,39,40].
Notably, certain bacterial strains, such as Bif. longum and L. helveticus, have been extensively studied for their potential role in modulating emotional behavior and stress responses [41,42,43,44]. When administered together, these strains may promote beneficial changes in psychosocial factors and psychiatric symptoms, supporting their classification as psychobiotics [45]. However, while some studies report positive outcomes, others have found limited or no effects. For instance, Romijn et al. [46] found no significant effects on depression, while Messaoudi et al. [47] reported no changes in perceived stress. In contrast, Gruenwald et al. [48] observed improvements in general well-being, and Rao et al. [49] noted improvements in anxiety. Furthermore, Messaoudi et al. [47] observed a reduction in urinary cortisol levels over time; however, this combination failed to enhance the cortisol awakening response (CAR) [50] or salivary cortisol levels [51].
1.2. Background on Adaptogens
Adaptogens are pharmacologically active compounds or extracts derived from various plant classes [52], recognized for their ability to enhance the body’s adaptation to stress and maintain metabolic homeostasis [53]. Their consumption has been linked to improvements in both mental and physical performance [54,55] and has demonstrated anti-fatigue, antidepressant, anxiolytic, stress-reducing, and healthy-aging properties [56,57,58]. Their primary mechanism of action involves regulating the HPA axis and modulating key stress mediators, including cortisol [56,59], which enhances resistance to stress by mitigating physiological changes induced by various stressors [55].
Of particular interest are two adaptogenic plants, W. somnifera (Ashwagandha) and R. rosea, both of which have been shown to influence the gut–brain axis, contributing to improved stress and mental health outcomes [60]. These effects are believed to stem from their immunomodulatory and anti-inflammatory properties, which support gut homeostasis [56]. Ashwagandha is generally considered a safe adaptogen [56] and has been shown to reduce perceived stress and cortisol levels [61], exert anxiolytic effects [62], and alleviate stress and anxiety symptoms [63], including in conditions such as anxiety and bipolar disorder [56]. However, the degree of effectiveness varies across studies, depending on factors such as dosage, population characteristics, and study design. Similarly, while R. rosea has been associated with reductions in self-reported anxiety and stress [64], and demonstrated anxiolytic properties [65] as well as cortisol modulation [66], the consistency of these effects is not uniform.
Taken together, existing evidence suggest that psychobiotics and adaptogens may contribute to the regulation of stress- and anxiety-related symptoms through mechanisms such as cortisol and corticosterone modulation and regulation of the HPA axis. Nevertheless, the variability in findings reflects the heterogeneity of current research designs, populations, and outcome measures. As the application of psychobiotics and adaptogens in the treatment of stress- and mood-related disorders is still emerging, further investigation is required to better understand their efficacy, underlying mechanisms, and potential therapeutic value.
The objective of this systematic review is to assess the efficacy of alternative interventions—namely psychobiotics and adaptogens—in alleviating stress-related psychophysiological symptoms. Specifically, the review aims to determine whether these compounds lead to measurable improvements in stress and anxiety outcomes. In addition to this primary goal, a secondary aim is to identify the specific bacterial strains and herbal compounds most consistently associated with beneficial effects, thereby contributing to a more precise understanding of their therapeutic potential.
2. Materials and Methods
2.1. Search Strategy
This review was conducted in accordance with the PRISMA guidelines [67]. The research question was structured using the PICO framework [68] as follows: in individuals from the general population as well as those with stress-related conditions, overweight, or specific dietary patterns (P), does the administration of psychobiotics or adaptogens (I) lead to a reduction in stress biomarkers (O) when compared to baseline stress levels before the intervention or between groups (C)? [69].
A comprehensive literature search was conducted between March and April 2024 across PubMed, ScienceDirect, Scopus, and Google Scholar using the following Boolean search strategy: ((adaptogens) OR (psychobiotics)) AND (stress). A manual search was conducted after the database searches to ensure no relevant studies were overlooked, particularly those not indexed in the major databases.
2.2. Study Selection
The initial literature search was conducted following predefined inclusion criteria. Studies were considered eligible if they were empirical research (excluding reviews, single-case studies, books, or manuals) and focused on the general population or individuals with stress-related conditions, obesity, or specific dietary patterns. Additionally, selected studies had to employ validated stress measures, such as cortisol levels or psychological assessments of stress and anxiety; in the latter case, studies on anxiety were included in the review, as several papers examined stress-related phenomena while utilizing anxiety measures. The review included research examining psychobiotics or adaptogens within the fields of psychology, medicine, or neuroscience, published between 2014 and 2024, with full-text availability in open access. Only studies published in English or Spanish were considered.
Studies that were excluded were reviews, single-case studies, books, and manuals; studies focusing exclusively on clinical populations unrelated to stress (e.g., severe psychiatric or neurodegenerative disorders) without clear relevance to stress modulation; interventions not involving psychobiotics or adaptogens as primary components; studies lacking validated stress-related outcome measures; publications in languages other than English or Spanish; and studies without full-text open-access availability.
Two independent reviewers (I.L. and M.A.S.) assessed the search results from each database separately, selecting studies based on their abstracts and full texts. Articles were included only if both reviewers reached an agreement. If any discrepancies arose, a third reviewer (N.S.-M.) was involved in resolving the disagreement and establishing a consensus.
2.3. Data Extraction
Following the initial study selection, data extraction was conducted by the first author (I.L). For each study meeting the final inclusion and exclusion criteria, the following information was systematically gathered: (1) bibliographic details, including the title, authors, and year of publication; (2) characteristics of the experimental design, such as study type, number of groups, and time points at which measurements were obtained; (3) the specific psychobiotic or adaptogen administered, including the strain used; (4) stress- and anxiety-related outcomes, evaluated through cortisol levels and validated psychological measures; and (5) the study’s key findings.
2.4. Risk of Bias Assessement
The evaluation of methodological quality and potential sources of bias in the selected studies was conducted using the revised version of the Cochrane Risk of Bias tool (RoB 2, 2008; updated in 2011 [70]). This instrument assesses five critical domains that may introduce bias in randomized controlled trials: the process of random sequence generation and allocation concealment (D1), deviations from intended interventions (D2), completeness of outcome data (D3), accuracy and reliability of outcome measurement (D4), and selective reporting of results (D5). Each domain was independently rated as presenting a ‘low risk’, ‘some concerns’, or a ‘high risk’ of bias.
3. Results
A comprehensive database search yielded a total of 1645 bibliographic records, with 284 entries retrieved from PubMed, 997 from ScienceDirect, 360 from Scopus, and 4 from Google Scholar. The flow diagram detailing the selection process for this systematic review is presented in Figure 1. Based on the predefined inclusion and exclusion criteria, 28 articles were initially selected. After identifying and removing three duplicate records, 25 articles remained.
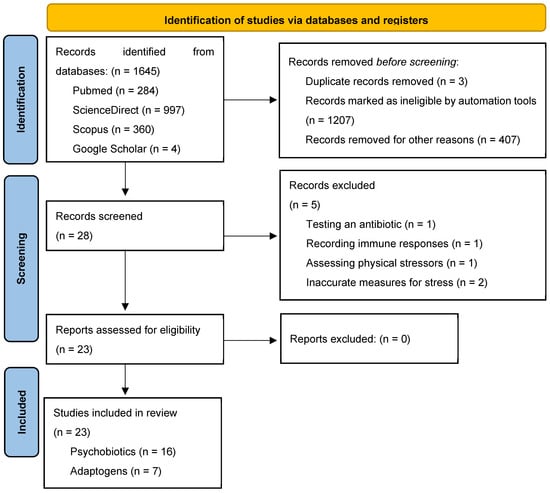
Figure 1.
The PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases and registers only.
Following abstract screening, five additional articles were excluded for the following reasons: evaluating an antibiotic rather than a psychobiotic or adaptogen (n = 1); focusing exclusively on immune responses without assessing endocrine or psychological stress markers (n = 1); investigating physical rather than psychological stressors (n = 1); and employing inadequate stress assessment measures (n = 2). Consequently, 23 articles met the final inclusion criteria and were selected for systematic review.
3.1. Effects of Treatment with Psychobiotics
Table 1 summarizes the sixteen studies that compared the effects of psychobiotics on stress measures.

Table 1.
A summary of sixteen studies investigating the effects of psychobiotics.
3.1.1. Type of Psychobiotic Treatment
Among the 16 included studies, one investigated the effects of a psychobiotic diet [72]. Regarding individual psychobiotic strains, Bif. longum [71,76], L. rhamnosus [73,74], L. gasseri [78,79], and L. plantarum [81,85] were each examined in two studies, while Bif. breve [75], L. helveticus [77], and L. paracasei [86] were evaluated in a single study each. Multistrain formulations were tested in four studies [80,82,83,84]. These included combinations such as omega-3 fish oil alongside various bacterial strains [80]; Lactoflorene® Plus, containing L. and Bif. strains along with zinc and B vitamins [82]; and SAMe combined with probiotics [83].
3.1.2. Effects of Psychobiotics on Stress Biomarkers
Salivary cortisol was assessed in eight studies [71,74,75,76,78,79,82,85], two of which employed the SECPT task [71,74]. Serum or blood cortisol was analyzed in four studies [75,81,84,85], while hair and urinary cortisol were each examined in one study [76,82,83]. Other biomarkers, including salivary α-amylase, were reported in two studies [82,85]; ACTH in one study [75]; and CgA in two studies [78,79].
3.1.3. Effects of Psychobiotics on Self-Report Questionnaires
Nine studies used the PSS to assess subjective stress levels [71,72,73,74,76,80,84,85,86], and the STAI [73,74,76,78,79] was applied in five studies to evaluate anxiety levels. Additionally, several studies employed alternative self-report measures to assess anxiety and job-related stress. The HADS was used in three studies [76,78,79], while the BAI [74], the DASS [84], and the HAM-D17 and SCL-90 anxiety subscales [81] were each employed in one study. Stress levels were also evaluated using a VAS scale in two studies [79,86], and job-related stress was measured using the JSS, also in two studies [85,86].
3.1.4. Overall Findings
Among the studies analyzed, six reported a significant reduction in both physiological and psychological stress-related measures. In a 4-week crossover study involving healthy male participants, Bif. longum 1714 significantly lowered post-SECPT salivary cortisol levels and perceived stress compared to both the placebo and baseline measurements. However, perceived stress levels increased again during the follow-up period [71]. Similarly, Bif. breve CCFM1025 significantly reduced both salivary and plasma cortisol levels in a 4-week RCT with 20 healthy participants [75]. In another study, L. gasseri CP2305 suppressed the escalation of salivary cortisol levels during an examination period in a 12-week RCT. Additionally, this psychobiotic significantly prevented an increase in the STAI-S scores in females prior to examination, although no significant differences were observed in the HADS-A [78]. However, in a separate study, the same probiotic showed no significant effects on salivary cortisol levels after a 24-week double-blind RCT, although it did reduce STAI-T anxiety scores, not STAI-S anxiety scores [79]. A multi-strain probiotic was also found to significantly decrease serum cortisol levels in a 28-day RCT involving 74 participants experiencing academic stress. The probiotic group exhibited a 43.02% reduction in serum cortisol levels, alongside a 38.62% decrease in perceived stress from baseline [84]. Furthermore, supplementation with L. plantarum PS128TM and HK-PS23 significantly reduced salivary and blood cortisol levels, self-perceived stress, anxiety, and overall job stress and job-related stress, respectively, after an 8-week intervention in a baseline-controlled study with highly stressed individuals [85,86]. Finally, the only study assessing the effects of a psychobiotic diet was conducted in a sample of 21 males and females with poor dietary habits in a 4-week RCT. The findings indicated a significant decrease in perceived stress in the diet group (−32% change from baseline) [72]. Additionally, L. rhamnosus HA-114 was administered in a RCT lasting 12 months involving 152 males and females with obesity under a weight loss program. The results showed a significant reduction in perceived stress over time in the probiotic group, although no significant group-by-time interaction effect was observed [73].
Conversely, several studies found no significant effects of psychobiotic treatment on stress biomarkers or self-reported psychological measures. One study reported that L. rhamnosus administration for 8 weeks failed to prevent the elevation of salivary cortisol output, anxiety measures, and stress measures following the SECPT in healthy male volunteers [74]. Similarly, Bif. Longum administration for 16 weeks had no effect on salivary or hair cortisol levels and in stress or anxiety in healthy male volunteers undergoing academic stress in a double-blind, cross-over RCT [76]. Furthermore, no significant effects on anxiety were observed following 4 weeks of L. helveticus supplementation in male and female participants [77] or 12-week treatment with a combination of omega-3 and various psychobiotics [80]. Additionally, in a study involving male and female patients diagnosed with major depression, 12 weeks of L. Plantarum administration did not alter cortisol concentrations nor anxiety or stress levels [81]. Likewise, double-blind, cross-over RCT in healthy males and females experiencing stress-related symptoms after 19 weeks of Lactoflorene® treatment reported no significant effects [82]. Moreover, an additional study investigated urinary cortisol levels in a double-blind, cross-over RCT but did not observe significant differences between the psychobiotic and placebo groups [83].
Additional stress-related biomarkers were also evaluated. ACTH levels in blood were assessed in one study, but no significant differences among groups were found [75]. Similarly, findings for CgA were inconsistent; while one study reported a positive effect following psychobiotic treatment [79], another found no significant differences [78].
3.1.5. Risk of Bias Results—Psychobiotics
The risk of bias analysis for the psychobiotic studies is summarized in the Supplementary Materials (Figure S1). This evaluation included a total of 15 randomized controlled trials. The study by Wu et al. [85] was excluded from this analysis, as it did not meet the inclusion criteria for randomized controlled trials.
Most studies presented a low risk of bias across all evaluated domains. The randomization process (D1) was identified as the most variable domain, with 6 out of 16 studies rated as having ‘some concerns’. In contrast, the domains related to deviations from intended interventions (D2), missing outcome data (D3), and a selection of the reported results (D5) consistently showed low risk across all studies. Minor concerns were observed in outcome measurements (D4) for a small number of studies, often due to limited reporting detail or potential issues with blinding procedures.
Overall, the majority of the studies demonstrated good methodological quality, with only a few [71,72,74,77,78,79,80,81,82,83] presenting moderate concerns in isolated domains. These findings support the robustness of the evidence base on psychobiotics while also highlighting the importance of continued methodological rigor in future trials.
3.2. Effect of Treatment with Adaptogens
Table 2 provides a summary of the seven studies that examined the effects of adaptogens on stress-related measures.

Table 2.
A summary of seven studies investigating the effects of adaptogens.
3.2.1. Type of Adaptogen Treatment
Among the seven studies included, three investigated the effects of W. somnifera (Ashwagandha root extract, [87,89,91]), and one examined R. rosea [93]. Additionally, three studies evaluated adaptogenic formulations comprising multiple plant extracts. One analyzed a combination of bioactive compounds derived from plants belonging to the Crassulaceae, Araliaceae, and Schisandraceae families (W. somnifera and Abelmoschus esculentus, [88]), another assessed a blend of W. somnifera and Abelmoschus esculentus [90], and a third tested a formulation containing Eleutherococcus sensicosus, Schizandra chinensis, R. rosea, Glycyrrhiza uralensis, Crataegus oxyacantha, and Aralia manchurica [92].
3.2.2. Effects of Adaptogens on Stress Biomarkers
Six of the seven studies measured blood cortisol levels [87,88,90,91,92,93]. One study also measured ACTH levels alongside cortisol [93], and one additional study assessed salivary cortisol as a single biomarker [89].
3.2.3. Effects of Adaptogens on Self-Report Questionnaires
Perceived stress was assessed using the PSS in five studies [87,88,89,90,91], while one study used an alternative instrument to evaluate stress perception [92]. Furthermore, anxiety levels were measured in two studies using standardized questionnaires: the GAD-7 [89] and the HAM-A [91].
3.2.4. Overall Findings
Among the studies analyzed, five reported a significant reduction in both physiological and psychological stress-related measures following adaptogen treatment. In a RCT lasting 8 weeks, W. somnifera administration resulted in a significant reduction in serum cortisol levels and perceived stress in a sample of 52 males and females under chronic stress. Specifically, reductions in serum cortisol levels of 16.05% and 22.2% were observed at weeks 4 and 8, respectively. Similarly, perceived stress levels decreased by 22.1% at week 4 and 32.7% at week 8 [87]. Likewise, W. somnifera treatment significantly reduced salivary cortisol levels, self-perceived stress, and the severity of generalized anxiety disorder in a double-blind, 8-week RCT involving male and female participants with mild-to-moderate stress and anxiety levels [89]. Another RCT assessed the effects of W. somnifera in 58 highly stressed males and females over 8 weeks, where both 250 mg/day and 600 mg/day doses decreased serum cortisol levels compared to the placebo group [91]. In terms of perceived stress, reductions relative to BL were significantly higher in the treatment groups compared to the placebo in a dose-related trend.
A combination of W. somnifera and Abelmoschus esculentus contained in the proprietary composition CL18100F4 was evaluated in a 2-week RCT. The results demonstrated a significant reduction in serum cortisol levels in both 300 mg/day and 500 mg/day groups, with 27.21% and 33.01% reductions, respectively, compared to BL. Furthermore, perceived stress significantly decreased in both 300 mg/day and 500 mg/day groups, with reductions of 21.1% and 23.22%, respectively [90].
In a 60-day RCT involving 215 healthy male and female athletes, ADAPT-232S and ADAPT-S supplementation prevented the exercise-induced increase in blood cortisol levels. While cortisol levels significantly increased in the placebo group at the end of the training season, levels in the ADAPT-232S and ADAPT-S groups remained significantly lower compared to baseline. Additionally, a significant reduction in perceived stress was observed in both groups [88]. Finally, blood cortisol and ACTH levels were analyzed with no significant differences found among groups [93].
Conversely, several studies found no significant effects of adaptogen treatment on stress biomarkers or self-reported psychological measures. Specifically, Timpmann et al. [93] reported no significant differences in blood cortisol or ACTH levels following psychobiotic intervention, suggesting a lack of measurable endocrine changes. Similarly, Shara et al. [92] also observed no reduction in cortisol levels; however, their findings revealed a decrease in subjective stress perception, indicating that psychobiotics may exert beneficial effects on emotional appraisal even in the absence of detectable hormonal changes.
3.2.5. Risk of Bias Results—Adaptogens
The risk of bias assessment for the studies evaluating the effects of adaptogens is presented in the Supplementary Materials (Figure S2). All seven studies were rated as presenting a low risk of bias across all domains. Accordingly, the overall judgment for each study was also rated as low risk.
These results indicate that the evidence base on adaptogens included in this review is methodologically robust, with no significant concerns regarding internal validity across the assessed studies.
4. Discussion
The growing interest in non-pharmacological treatments for stress and related conditions has positioned psychobiotics and adaptogens as promising alternative interventions. This systematic review assesses their efficacy in alleviating stress-related psychophysiological symptoms, with particular attention to measurable improvements in stress and anxiety outcomes. It also explores which specific bacterial strains and herbal compounds are most consistently associated with beneficial effects, contributing to a more nuanced understanding of their therapeutic potential. A distinctive strength of this review lies in its dual examination of both psychobiotics and adaptogens—two emerging but often separately studied approaches—which allows for a broader perspective on microbiota- and plant-based strategies for stress regulation. This comprehensive scope contributes to a more integrated understanding of their potential benefits and limitations.
As highlighted in the review by Sharma et al. [94], psychobiotics have demonstrated beneficial effects in addressing stress and anxiety in both animal and human studies, improving psychological and neurochemical profiles. Specifically, animal studies have provided compelling evidence for the potential of psychobiotics to modulate behavior and brain function through gut–brain axis mechanisms. Thus, chronic administration of specific strains such as Bif. longum 1714 and L. rhamnosus JB-1 has been shown to reduce anxiety-like behaviors and improve stress responses in mice, including lowering corticosterone levels and enhancing performance in behavioral tests such as the elevated plus maze and forced swim test. On the other side, L. helveticus NS8 and L. plantarum have demonstrated effects on increasing neurotransmitter levels, including dopamine and serotonin in brain regions like the prefrontal cortex. In our systematic review, nine of the included studies reported positive treatment effects, showing significant improvements in stress biomarkers, anxiety-related symptoms, and self-perceived stress [71,72,73,75,78,79,84,85,86], suggesting a potential regulatory role of psychobiotics in stress response.
Regarding the specific psychobiotic strains used in studies reporting positive effects, a diverse range of formulations was observed, including multi-strain probiotics and psychobiotic-enriched diets. However, most of the studies reviewed focused on strains from Bif. and L. families, which predominantly exhibited beneficial outcomes [71,73,75,78,79,85]. This suggests that strains within these families may be particularly effective in mitigating stress and improving psychological well-being. These psychobiotics have also been consistently linked to reduced gastrointestinal discomfort and improved mental health outcomes, aligning with findings from Smith et al. [45].
The effectiveness of psychobiotics for stress reduction varies considerably across the literature [47,49,51,52] as well as in the studies analyzed in this review, with nearly half reporting no significant improvements in stress-related outcomes. For instance, Bif. Breve, L. gasseri, and L. paracasei [75,78,79,86] have been associated with reductions in cortisol levels and self-reported stress, highlighting their potential utility in stress management. Additionally, B. longum 1714 has been demonstrated to alleviate anxiety symptoms in human studies [39] and has shown to reduce salivary cortisol output and perceived stress levels [71], while another study did not show any effects [76]. Similarly, L. plantarum PS128™ significantly reduced cortisol levels, self-perceived stress, and both trait and state anxiety in individuals experiencing high stress [85]. In contrast, L. plantarum 299v did not produce any significant changes in patients diagnosed with major depression [81]. It is worth noting that although previous research has provided growing evidence of the positive effects of psychobiotics on mood-related disorders [36,95], the mechanisms of action remain unclear.
Notably, studies reporting significant stress reduction effects were primarily conducted in participants experiencing high baseline stress levels or engaging in unhealthy dietary habits [40,72,73,78,79,84,85,86], like the findings in the study by Takada et al. [40], which also observed greater benefits of psychobiotic intervention in individuals with elevated stress levels. This suggests that initial gut health may play a crucial role in mediating the effectiveness of psychobiotic interventions. Participants with gut microbiota imbalances or heightened stress levels at baseline appeared to benefit the most, showing improvements in stress hormone levels, anxiety symptoms, and self-perceived stress. Conversely, studies conducted in healthy or low-stress populations often yielded null results, implying that the therapeutic potential of psychobiotics may depend on pre-existing gut dysbiosis or stress-related imbalances.
In relation to adaptogens, the vast majority of studies have demonstrated significant reductions in stress biomarkers, anxiety symptoms, and self-perceived stress [87,88,89,90,91]. In the systematic review by Todorova et al. [55], which analyzed 56 human and animal studies on adaptogens, it was concluded that natural adaptogens enhance the body’s ability to resist stress-induced changes, providing several beneficial effects. Similarly, our systematic review found homogeneous results, with most studies reporting a significant decrease in stress biomarkers, anxiety symptoms, and/or self-perceived stress following adaptogen treatment [87,88,89,90,91,92]. Regarding participant characteristics in studies showing positive effects, most included healthy individuals without any significant medical conditions [88,89,90], while a smaller number focused on participants experiencing high levels of stress or undergoing stressful situations [87,91].
Among adaptogens, W. somnifera emerges as the most frequently studied and effective one, with its consistent efficacy across varying baseline stress levels highlighting its broad applicability. These findings align with previous research demonstrating its positive effects in reducing stress and cortisol levels [61,87,96]. Therefore, it can be hypothesized that W. somnifera plays a particularly beneficial role in the treatment of stress and anxiety symptoms. While the precise mechanisms of action of adaptogens are not yet fully understood, evidence suggests that Ashwagandha root extract influences the HPA axis and key stress mediators, leading to modulations in cortisol levels [89,97]. These results are similar to those found in animal studies; in this sense, reduction in anxiety and stress indicators show the following: In domestic dogs, Ashwagandha root extract significantly reduced stress-related signs, including a decrease in urine cortisol-to-creatine ratio and improvements in fear and anxiety domains as measured by behavioral assessments [98]. Similarly, in Wistar rats, Ashwagandha demonstrated significant anxiolytic effects, improving anxiety- and depression-like behaviors and reducing stress hormones such as cortisol [99].
Regarding limitations, this review highlights several areas for improvement in future research. One of the primary challenges is the heterogeneity in psychobiotic substances and adaptogen formulations as well as the variability in participant characteristics. These factors complicate direct comparisons and limit the generalizability of findings. Additionally, the small sample sizes across studies reduce statistical power and compromise the reliability of the results, emphasizing the need for larger and more diverse cohorts.
Another key limitation is the lack of long-term follow-up assessments. While many studies report reductions in stress biomarkers and self-reported stress levels during intervention periods, it remains unclear whether these benefits are sustained after treatment discontinuation. Furthermore, methodological inconsistencies, such as variations in dosages, strains, and treatment durations, further complicate cross-study comparisons. Establishing standardized protocols using similar psychological measures (such as PSS) would enhance the reliability and reproducibility of findings in this field.
Finally, although most studies showed a low risk of bias—particularly those on adaptogens—some psychobiotic trials presented concerns, especially in randomization and outcome measurements. These limitations should be considered when interpreting results and designing future trials.
To address these limitations, future research should prioritize understanding the mechanisms by which psychobiotics and adaptogens modulate stress responses, particularly through the gut–brain axis and endocrine pathways. Identifying strain-specific effects of psychobiotics under conditions such as gut dysbiosis or high stress is crucial, as is assessing the long-term sustainability and safety of these interventions. In this regard, future studies should explicitly implement extended-duration trials with follow-up periods extending several months beyond the active intervention phase in order to assess the persistence of effects over time. These trials would provide essential insight into the long-term efficacy and clinical utility of such treatments.
Additionally, direct comparisons with traditional pharmacological treatments and psychotherapy are necessary to determine the most effective and safe options for diverse populations. Further validation of these findings requires a more rigorous methodological framework, including standardized effect size reporting, comprehensive sample descriptions, and a deeper exploration of the underlying mechanisms of action.
5. Conclusions
Psychobiotics, particularly strains from the Bif. and L. families, and adaptogens, such as W. somnifera, demonstrate significant potential for stress management, potentially through their ability to induce beneficial changes in gut microbiota composition and function. Despite certain limitations, the findings highlight the potential of these interventions as alternatives or complements to psychological and pharmacological treatments. Further research is essential to better understand their action mechanisms and optimize their clinical application through an evidence-based approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15084564/s1. Figure S1. Risk of bias assessment for included randomized controlled trials on psychobiotics and adaptogens, conducted using the Cochrane RoB 2 tool (2008; updated 2011). One study (Wu et al., 2021) was excluded from the psychobiotic analysis as it did not meet RCT criteria; Figure S2. Risk of bias assessment for the seven randomized controlled trials investigating adaptogens, conducted using the Cochrane RoB 2 tool (2008; updated 2011). All studies were rated as low risk of bias across all domains.
Author Contributions
Conceptualization, I.L., N.S.-M. and M.Á.S.; methodology, I.L. and M.Á.S.; formal analysis, I.L., N.S.-M. and M.Á.S.; writing—original draft preparation, I.L. and M.Á.S.; writing—review and editing, I.L., N.S.-M. and M.Á.S.; supervision, M.Á.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to Laura Miñano-Mañero for her assistance in reviewing the English language and grammar.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACTH | Adrenocorticotropic Hormone |
| AUCg | Area Under The Curve With Respect To Ground |
| AUCi | Area Under The Curve With Respect To Increase |
| B | Bacillus |
| BAI-T | Beck Anxiety Inventory Trait |
| Bif | Bifidobacterium |
| BL | Baseline |
| CAR | Cortisol Awakening Response |
| CgA | Salivary Chromogranin A |
| CG | Control Group |
| CFU | Colony Forming Unit |
| DASS | Depression, Anxiety and Stress Scale |
| EG | Experimental Group |
| GAD | Generalized Anxiety Disorder |
| HADS-A | Hospital Anxiety and Depression Scale-Anxiety Subscale |
| HAM- | Hamilton Anxiety Rating Scale |
| HAM-D17 | Hamilton Depression Rating Scale-Anxiety Subscale |
| HK | Heat-Killed |
| JSS | Job Stress Scale |
| L | Lacticaseibacillus |
| NR | Non-Responsive |
| PBO | Psychobiotic |
| PLA | Placebo |
| PRISMA | Preferred Reporting Items for Systematic Reviews and meta-Analyses |
| PSS | Perceived-Stress Scale |
| PSS-10 | Perceived-Stress Scale—10 Items |
| PSS-14 | Perceived-Stress Scale—14 Items |
| OS | Occupation Stress |
| RCT | Randomized Controlled Trial |
| SAMe | S-Adenosylmethionine |
| SAS | Stress-Associated Symptoms |
| SCL-90 | Symptom Checklist-90-Anxiety Subscale |
| SECPT | Socially Evaluated Cold-Pressor Test |
| STAI | State-Trate Anxiety Inventory |
| STAI-T | State-Trate Anxiety Inventory Trait |
| STAI-S | State-Trate Anxiety Inventory State |
| VAS | Visual Analog Scale |
| W | Withania |
| R | Rodhiola |
References
- Selye, H. A Syndrome Produced by Diverse Nocuous Agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Mcewen, B.S.; Mirsky, A.E.; Hatch, M.M. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Barlow, D.H. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic, 2nd ed.; The Guilford Press: New York, NY, USA, 2002; ISBN 1-57230-430-8. [Google Scholar]
- McEwen, B.S. Stress, Adaptation, and Disease. Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.R. Life Events, Stress and Illness. Malays. J. Med. Sci. MJMS 2008, 15, 9. [Google Scholar]
- Martin, C.M. Chronic Disease and Illness Care. Can. Fam. Physician 2007, 53, 2086. [Google Scholar]
- Lorenz, F.O.; Wickrama, K.A.S.; Conger, R.D.; Elder, G.H. The Short-Term and Decade-Long Effects of Divorce on Women’s Midlife Health. J. Health Soc. Behav. 2006, 47, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Enz, K.F.; Pillemer, D.B.; Johnson, K.M. The Relocation Bump: Memories of Middle Adulthood Are Organized around Residential Moves. J. Exp. Psychol. Gen. 2016, 145, 935–940. [Google Scholar] [CrossRef]
- Helgeson, V.S.; Jakubiak, B.; Van Vleet, M.; Zajdel, M. Communal Coping and Adjustment to Chronic Illness: Theory Update and Evidence. Personal. Soc. Psychol. Rev. 2018, 22, 170–195. [Google Scholar] [CrossRef]
- Andrews, B.; Wilding, J.M. The Relation of Depression and Anxiety to Life-Stress and Achievement in Students. Br. J. Psychol. 2004, 95, 509–521. [Google Scholar] [CrossRef]
- Dimsdale, J.E. Psychological Stress and Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 51, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B. Stress: A Risk Factor for Serious Illness. Metabolism 2002, 51, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological Stress and Disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Agorastos, A.; Chrousos, G.P. The Neuroendocrinology of Stress: The Stress-Related Continuum of Chronic Disease Development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef]
- Montalvo, M.J.F.; Latorre, A.M.; Guzmán, A.M.; Guzmán, E.A.; Sánchez, P.M.; Baraja, V.R. Informe Anual del Sistema Nacional de Salud 2023; Ministerio de Sanidad: Madrid, Spain, 2024. [Google Scholar]
- Goethe, J.W.; Woolley, S.B.; Cardoni, A.A.; Woznicki, B.A.; Piez, D.A. Selective Serotonin Reuptake Inhibitor Discontinuation: Side Effects and Other Factors That Influence Medication Adherence. J. Clin. Psychopharmacol. 2007, 27, 451–458. [Google Scholar] [CrossRef]
- Springer, K.S.; Levy, H.C.; Tolin, D.F. Remission in CBT for Adult Anxiety Disorders: A Meta-Analysis. Clin. Psychol. Rev. 2018, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rief, W.; Asmundson, G.J.G.; Bryant, R.A.; Clark, D.M.; Ehlers, A.; Holmes, E.A.; McNally, R.J.; Neufeld, C.B.; Wilhelm, S.; Jaroszewski, A.C.; et al. The Future of Psychological Treatments: The Marburg Declaration. Clin. Psychol. Rev. 2024, 110, 102417. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Gaci, N.; Borrel, G.; Tottey, W.; O’Toole, P.W.; Brugère, J.F. Archaea and the Human Gut: New Beginning of an Old Story. World J. Gastroenterol. 2014, 20, 16062–16078. [Google Scholar] [CrossRef]
- Williamson, L.L.; McKenney, E.A.; Holzknecht, Z.E.; Belliveau, C.; Rawls, J.F.; Poulton, S.; Parker, W.; Bilbo, S.D. Got Worms? Perinatal Exposure to Helminths Prevents Persistent Immune Sensitization and Cognitive Dysfunction Induced by Early-Life Infection. Brain Behav. Immun. 2016, 51, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The Human Gut Microbiota and Virome: Potential Therapeutic Implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The Microbiome: A Key Regulator of Stress and Neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The Microbiota and the Hypothalamus-Pituitary-Adrenocortical (HPA) Axis, Implications for Anxiety and Stress Disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef]
- de Weerth, C. Do Bacteria Shape Our Development? Crosstalk between Intestinal Microbiota and HPA Axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Mohanty, D. Psychobiotics: A New Approach for Treating Mental Illness? Crit. Rev. Food Sci. Nutr. 2019, 59, 1230–1236. [Google Scholar] [CrossRef]
- Bistas, K.G.; Tabet, J.P. The Benefits of Prebiotics and Probiotics on Mental Health. Cureus 2023, 15, e43217. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Logan, A.C.; Katzman, M. Major Depressive Disorder: Probiotics May Be an Adjuvant Therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef]
- McKean, J.; Naug, H.; Nikbakht, E.; Amiet, B.; Colson, N. Probiotics and Subclinical Psychological Symptoms in Healthy Participants: A Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 2017, 23, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.; Li, M.; Wang, W.; Liu, Z.; Xi, C.; Huang, X.; Liu, J.; Huang, J.; Tian, D.; et al. Efficacy of Probiotics on Stress in Healthy Volunteers: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Brain Behav. 2020, 10, e01699. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Noonan, S.; Zaveri, M.; Macaninch, E.; Martyn, K. Food & Mood: A Review of Supplementary Prebiotic and Probiotic Interventions in the Treatment of Anxiety and Depression in Adults. BMJ Nutr. Prev. Health 2020, 3, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium Longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Dziedzic, A.; Maciak, K.; Bliźniewska-Kowalska, K.; Gałecka, M.; Kobierecka, W.; Saluk, J. The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota-Gut-Brain Axis: A Literature Review. Nutrients 2024, 16, 1054. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus Casei Strain Shirota Relieves Stress-Associated Symptoms by Modulating the Gut–Brain Interaction in Human and Animal Models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria Exert Strain-Specific Effects on Stress-Related Behavior and Physiology in BALB/c Mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef]
- Savignac, H.M.; Tramullas, M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria Modulate Cognitive Processes in an Anxious Mouse Strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut Feelings: A Randomised, Triple-Blind, Placebo-Controlled Trial of Probiotics for Depressive Symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Greene, M.W.; Babu, J.R.; Frugé, A.D. Psychobiotics as Treatment for Anxiety, Depression, and Related Symptoms: A Systematic Review. Nutr. Neurosci. 2021, 24, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A Double-Blind, Randomized, Placebo-Controlled Trial of Lactobacillus Helveticus and Bifidobacterium Longum for the Symptoms of Depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Gruenwald, J.; Graubaum, H.-J.; Harde, A. Effect of a Probiotic Multivitamin Compound on Stress and Exhaustion. Adv. Ther. 2002, 19, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Rode, J.; Edebol Carlman, H.M.T.; König, J.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Brummer, R.J. Multi-Strain Probiotic Mixture Affects Brain Morphology and Resting State Brain Function in Healthy Subjects: An RCT. Cells 2022, 11, 2922. [Google Scholar] [CrossRef]
- Edebol Carlman, H.M.T.; Rode, J.; König, J.; Repsilber, D.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Kiselev, A.; Pruessner, J.C.; Brummer, R.J. Probiotic Mixture Containing Lactobacillus Helveticus, Bifidobacterium Longum and Lactiplantibacillus Plantarum Affects Brain Responses to an Arithmetic Stress Task in Healthy Subjects: A Randomised Clinical Trial and Proof-of-Concept Study. Nutrients 2022, 14, 1329. [Google Scholar] [CrossRef]
- Brekhman, I.I.; Dardymov, I.V. New Substances of Plant Origin Which Increase Nonspecific Resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef]
- Wagner, H.; Nörr, H.; Winterhoff, H. Plant Adaptogens. Phytomedicine 1994, 1, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, S.; Oh, S. The Pharmacology of Actoprotectors: Practical Application for Improvement of Mental and Physical Performance. Biomol. Ther. 2012, 20, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Panossian, A. Understanding Adaptogenic Activity: Specificity of the Pharmacological Action of Adaptogens and Other Phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress-Protective Activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Evidence-Based Efficacy of Adaptogens in Fatigue, and Molecular Mechanisms Related to Their Stress-Protective Activity. Curr. Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef]
- Pawar, V.S.; Shivakumar, H. A Current Status of Adaptogens: Natural Remedy to Stress. Asian Pac. J. Trop. Dis. 2012, 2, S480–S490. [Google Scholar] [CrossRef]
- Gasmi, A.; Shanaida, M.; Oleshchuk, O.; Semenova, Y.; Mujawdiya, P.K.; Ivankiv, Y.; Pokryshko, O.; Noor, S.; Piscopo, S.; Adamiv, S.; et al. Natural Ingredients to Improve Immunity. Pharmaceuticals 2023, 16, 528. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A Prospective, Randomized Double-Blind, Placebo-Controlled Study of Safety and Efficacy of a High-Concentration Full-Spectrum Extract of Ashwagandha Root in Reducing Stress and Anxiety in Adults. Indian. J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R.; Wane, D. An Investigation into the Stress-Relieving and Pharmacological Actions of an Ashwagandha (Withania Somnifera) Extract: A Randomized, Double-Blind, Placebo-Controlled Study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J. Ashwagandha (Withania Somnifera) for the Treatment and Enhancement of Mental and Physical Conditions: A Systematic Review of Human Trials. J. Herb. Med. 2021, 28, 100434. [Google Scholar] [CrossRef]
- Cropley, M.; Banks, A.P.; Boyle, J. The Effects of Rhodiola Rosea L. Extract on Anxiety, Stress, Cognition and Other Mood Symptoms. Phytother. Res. 2015, 29, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Konstantinos, F.; Heun, R. The Effects of Rhodiola Rosea Supplementation on Depression, Anxiety and Mood–A Systematic Review. Glob. Psychiatry Arch. 2020, 3, 72–82. [Google Scholar] [CrossRef]
- Olsson, E.M.G.; Von Schéele, B.; Panossian, A.G. A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study of the Standardised Extract SHR-5 of the Roots of Rhodiola Rosea in the Treatment of Subjects with Stress-Related Fatigue. Planta Med. 2009, 75, 105–112. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R. The Well-Built Clinical Question: A Key to Evidence-Based Decisions. ACP J. Club 1995, 123, A12. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-Step Guide on How to Design, Conduct, and Successfully Publish a Systematic Review and Meta-Analysis in Medical Research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium Longum 1714 as a Translational Psychobiotic: Modulation of Stress, Electrophysiology and Neurocognition in Healthy Volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef]
- Berding, K.; Bastiaanssen, T.F.S.; Moloney, G.M.; Boscaini, S.; Strain, C.R.; Anesi, A.; Long-Smith, C.; Mattivi, F.; Stanton, C.; Clarke, G.; et al. Feed Your Microbes to Deal with Stress: A Psychobiotic Diet Impacts Microbial Stability and Perceived Stress in a Healthy Adult Population. Mol. Psychiatry 2023, 28, 601–610. [Google Scholar] [CrossRef]
- Choi, B.S.Y.; Brunelle, L.; Pilon, G.; Cautela, B.G.; Tompkins, T.A.; Drapeau, V.; Marette, A.; Tremblay, A. Lacticaseibacillus Rhamnosus HA-114 Improves Eating Behaviors and Mood-Related Factors in Adults with Overweight during Weight Loss: A Randomized Controlled Trial. Nutr. Neurosci. 2023, 26, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in Translation? The Potential Psychobiotic Lactobacillus Rhamnosus (JB-1) Fails to Modulate Stress or Cognitive Performance in Healthy Male Subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, J.; Qiao, G.; Mao, X.; Zhao, J.; Wang, G.; Tian, P.; Chen, W. Bifidobacterium Breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial. Nutrients 2023, 15, 4700. [Google Scholar] [CrossRef] [PubMed]
- Moloney, G.M.; Long-Smith, C.M.; Murphy, A.; Dorland, D.; Hojabri, S.F.; Ramirez, L.O.; Marin, D.C.; Bastiaanssen, T.F.S.; Cusack, A.M.; Berding, K.; et al. Improvements in Sleep Indices during Exam Stress Due to Consumption of a Bifidobacterium Longum. Brain Behav. Immun. Health 2021, 10, 100174. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, N.; Kakiuchi, I.; Hiraku, A.; Iwabuchi, N.; Kiyosawa, K.; Igarashi, K.; Tanaka, M.; Nakamura, M.; Miyasaka, M. Heat-Killed Lactobacillus Helveticus Improves Mood States: A Randomised, Doubleblind, Placebo-Controlled Study. Benef. Microbes 2023, 14, 109–118. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily Administration of Paraprobiotic Lactobacillus Gasseri CP2305 Ameliorates Chronic Stress-Associated Symptoms in Japanese Medical Students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus Gasseri Cp2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Reigada, L.C.; Buchanan, E.M.; Hazeltine, D.B.; Shakil, H.; Polokowski, A.R. A Pilot Randomized Controlled Trial Testing Supplements of Omega-3 Fatty Acids, Probiotics, Combination or Placebo on Symptoms of Depression, Anxiety and Stress. J. Affect. Disord. Rep. 2021, 5, 100141. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Soldi, S.; Tagliacarne, S.C.; Valsecchi, C.; Perna, S.; Rondanelli, M.; Ziviani, L.; Milleri, S.; Annoni, A.; Castellazzi, A. Effect of a Multistrain Probiotic (Lactoflorene® Plus) on Inflammatory Parameters and Microbiota Composition in Subjects with Stress-Related Symptoms. Neurobiol. Stress. 2019, 10, 100138. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Esposito, C.; El-Seedi, H.R.; Khalifa, S.A.M.; Baldi, A.; Greco, A.; Santonastaso, S.; Cioffi, V.; Sperandeo, R.; et al. Efficacy of a Food Supplement Based on S-Adenosyl Methionine and Probiotic Strains in Subjects with Subthreshold Depression and Mild-to-Moderate Depression: A Monocentric, Randomized, Cross-over, Double-Blind, Placebo-Controlled Clinical Trial. Biomed. Pharmacother. 2022, 156, 113930. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, R.; Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Vinay, H.R.; Lal, A.; Thomas, G.; Stephen, S. Effect of Multi-Strain Probiotic Formulation on Students Facing Examination Stress: A Double-Blind, Placebo-Controlled Study. Probiotics Antimicrob. Proteins 2021, 13, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.I.; Wu, C.C.; Tsai, P.J.; Cheng, L.H.; Hsu, C.C.; Shan, I.K.; Chan, P.Y.; Lin, T.W.; Ko, C.J.; Chen, W.L.; et al. Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study. Front. Nutr. 2021, 8, 614105. [Google Scholar] [CrossRef]
- Wu, S.I.; Wu, C.C.; Cheng, L.H.; Noble, S.W.; Liu, C.J.; Lee, Y.H.; Lin, C.J.; Hsu, C.C.; Chen, W.L.; Tsai, P.J.; et al. Psychobiotic Supplementation of HK-PS23 Improves Anxiety in Highly Stressed Clinical Nurses: A Double-Blind Randomized Placebo-Controlled Study. Food Funct. 2022, 13, 8907–8919. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Evid. Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef]
- Hovhannisyan, A.; Nylander, M. Efficacy of Adaptogenic Supplements on Adapting to Stress: A Randomized, Controlled Trial. J. Athl. Enhanc. 2015, 4, 4. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Mundkur, L. A Standardized Ashwagandha Root Extract Alleviates Stress, Anxiety, and Improves Quality of Life in Healthy Adults by Modulating Stress Hormones: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Medicine 2023, 102, E35521. [Google Scholar] [CrossRef]
- Punukollu, R.S.; Chadalawada, A.K.; Siddabattuni, K.; Gogineni, N.T. A Blend of Withania somnifera (L.) Dunal Root and Abelmoschus esculentus (L.) Moench Fruit Extracts Relieves Constipation and Improves Bowel Function: A Proof-of-Concept Clinical Investigation. J. Ethnopharmacol. 2024, 318, 116997. [Google Scholar] [CrossRef]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-Blind, Randomized, Placebo-Controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef]
- Shara, M. Effects of an Adaptogen-Based Supplement on Stress Parameters in Healthy Volunteers. Int. J. Complement. Altern. Med. 2017, 10, 00321. [Google Scholar] [CrossRef]
- Timpmann, S.; Hackney, A.C.; Tamm, M.; Kreegipuu, K.; Unt, E.; Ööpik, V. Influence of Rhodiola Rosea on the Heat Acclimation Process in Young Healthy Men. Appl. Physiol. Nutr. Metab. 2018, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R. The Effects of Probiotics on Depressive Symptoms in Humans: A Systematic Review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef]
- Gopukumar, K.; Thanawala, S.; Somepalli, V.; Rao, T.S.S.; Thamatam, V.B.; Chauhan, S. Efficacy and Safety of Ashwagandha Root Extract on Cognitive Functions in Healthy, Stressed Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 8254344. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Panossian, A.; Hambardzumyan, M.; Hovhanissyan, A.; Wikman, G. The Adaptogens Rhodiola and Schizandra Modify the Response to Immobilization Stress in Rabbits by Suppressing the Increase of Phosphorylated Stress-Activated Protein Kinase, Nitric Oxide and Cortisol. Drug Target Insights 2007, 2, 117739280700200011. [Google Scholar] [CrossRef]
- Kaur, J.; Seshadri, S.; Golla, K.H.; Sampara, P. Efficacy and Safety of Standardized Ashwagandha (Withania Somnifera) Root Extract on Reducing Stress and Anxiety in Domestic Dogs: A Randomized Controlled Trial. J. Vet. Behav. 2022, 51, 8–15. [Google Scholar] [CrossRef]
- Dawane, J.; Seok, S.; Dhande, P.; Langade, D.; Han, H.; Kim, S.B.; Ju, J.Y. Evaluation of the Anxiolytic and Antidepressant Effects of Standardized Ashwagandha (Withania Somnifera) Root Extract in Wistar Rats. Prev. Nutr. Food Sci. 2024, 29, 414–421. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).