Metabolomics Approach for Sweat Mapping the Performance of Soccer Players in Pretoria, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects’ Selection
2.2. Collection of Soccer Players’ Profiles
2.3. Sweat Collection
2.4. Metabolites Profiling
2.4.1. Reagents and Chemicals
2.4.2. Sample Preparation
2.4.3. GCxGC-TOFMS System
2.4.4. Peak Identification
2.5. Data Analysis
3. Results
3.1. Soccer Player’s Profiles
3.2. Sweat Quantification
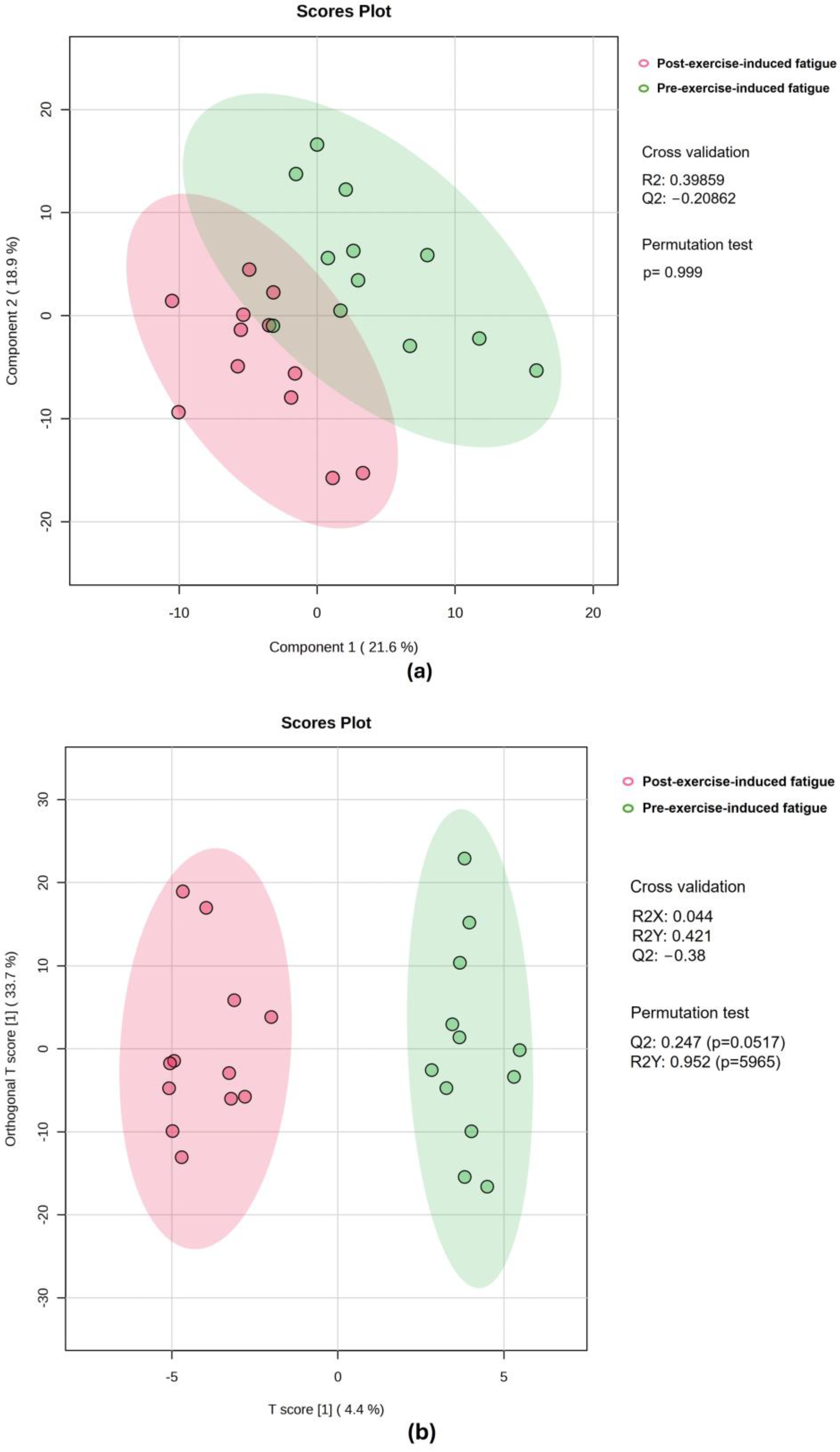
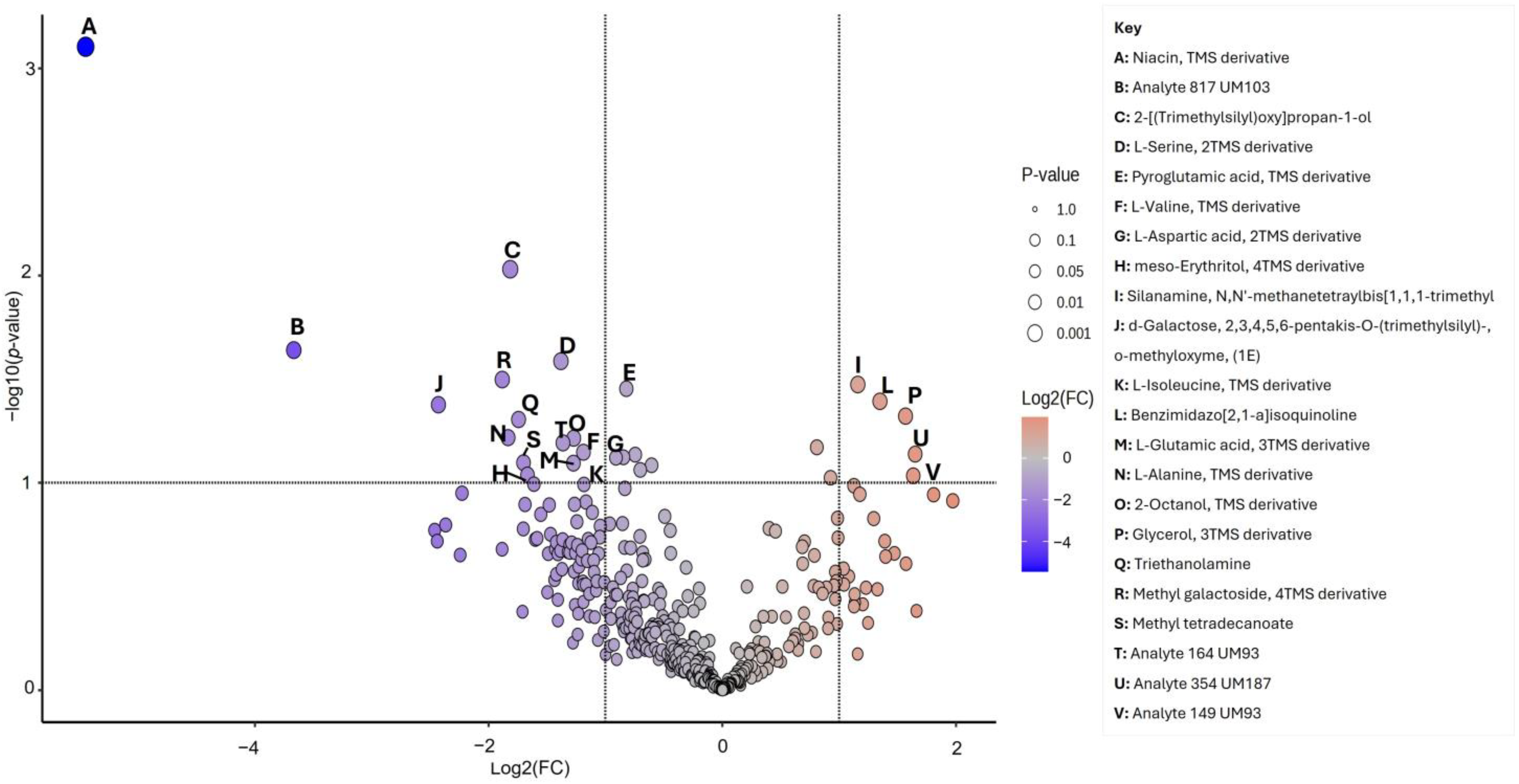
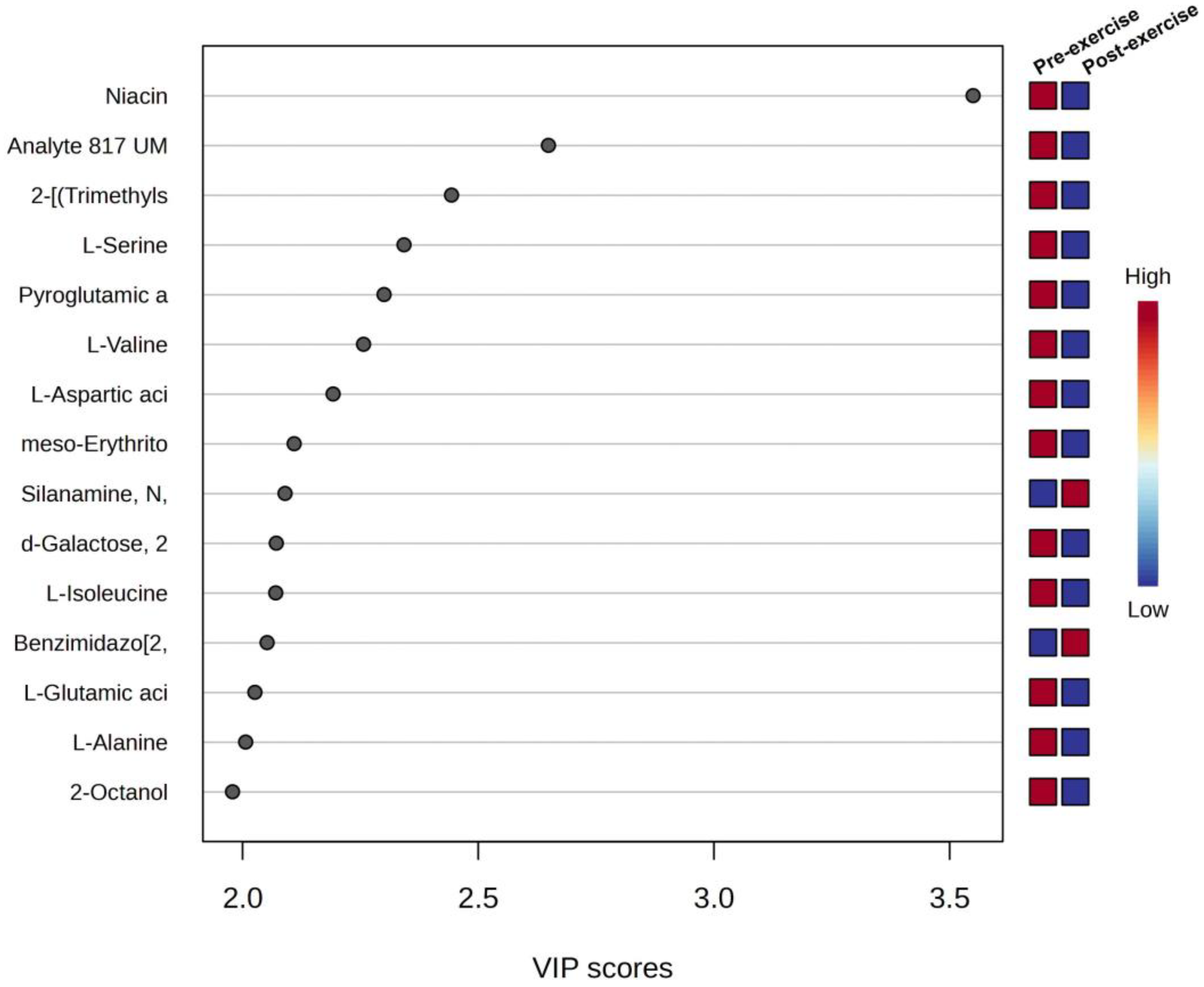
3.3. Univariate and Multivariate Data Analyses of Sweat Metabolome
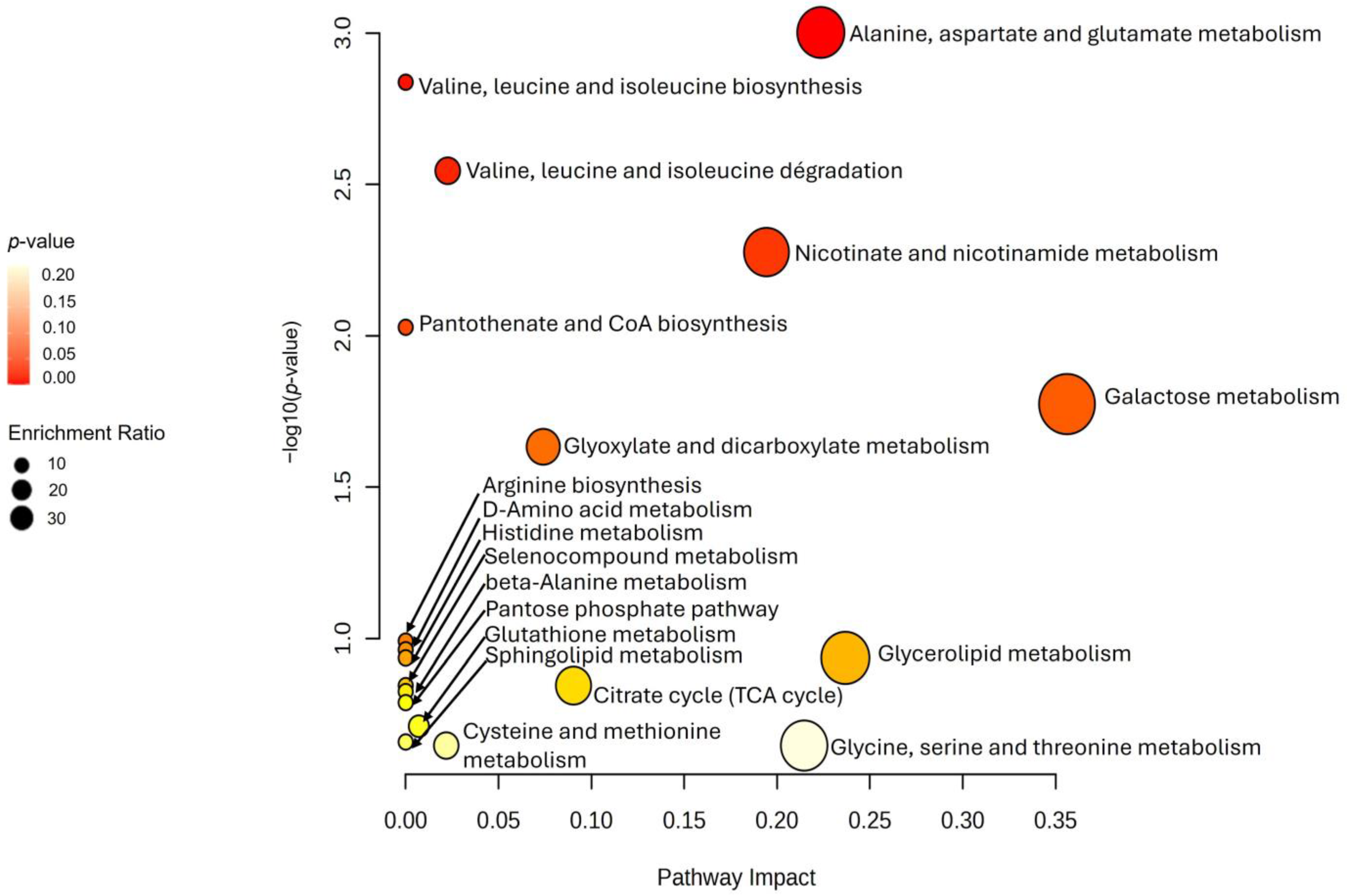
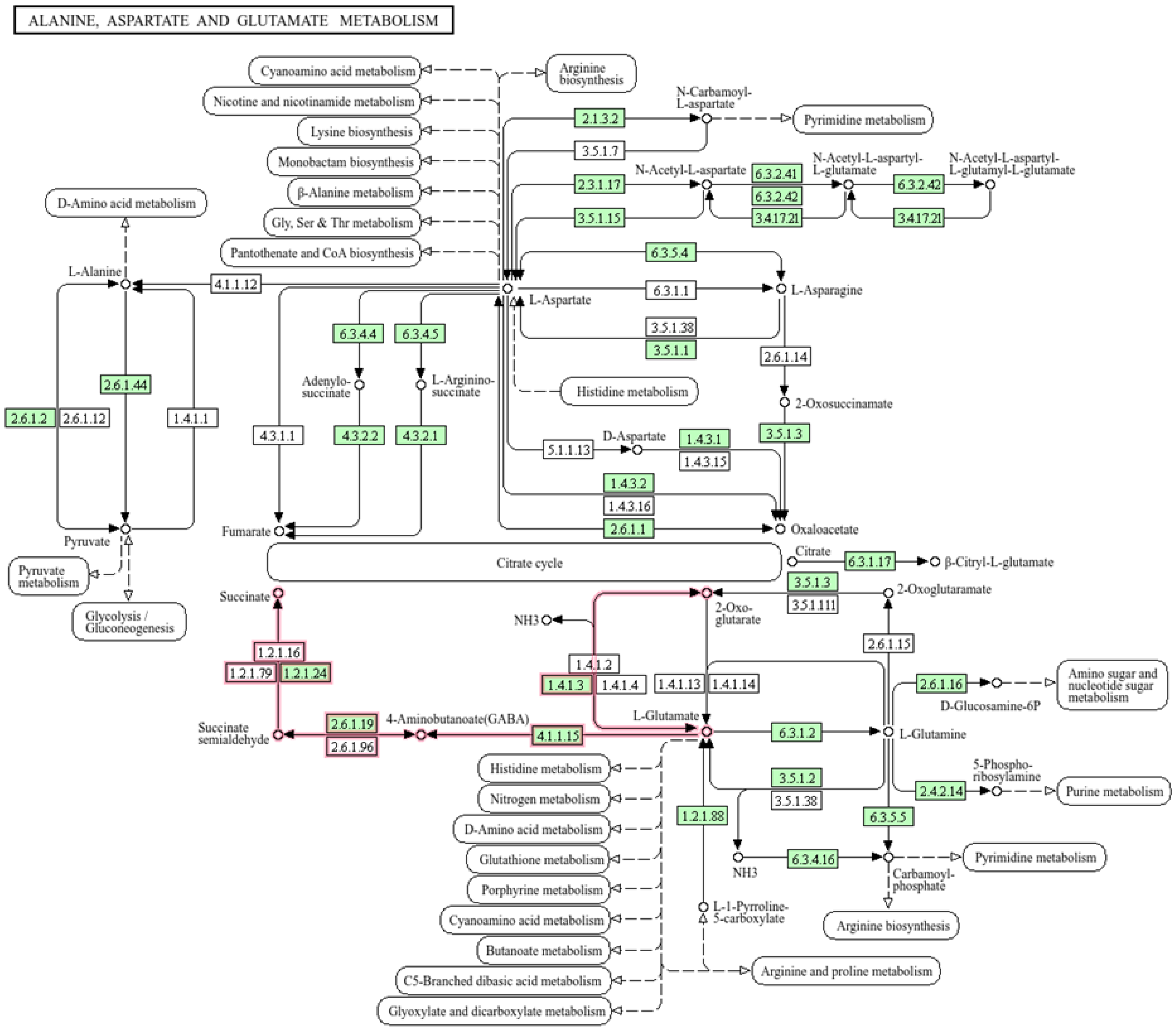
3.4. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leiper, J.B.; Carnie, A.; Maughan, R.J. Water turnover rates in sedentary and exercising middle aged men. Br. J. Sports Med. 1996, 30, 24–26. [Google Scholar] [CrossRef]
- Wendt, D.; van Loon, L.J.; Lichtenbelt, W.D. Thermoregulation during exercise in the heat: Strategies for maintaining health and performance. Sports Med. 2007, 37, 669–682. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; Calderón-Santiago, M.; Priego-Capote, F.; Luque de Castro, M.D. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography-mass spectrometry in high resolution mode. Anal. Chim. Acta 2016, 28, 115–125. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 3, 211–259. [Google Scholar] [CrossRef]
- Passos, J.; Lopes, S.I.; Clemente, F.M.; Moreira, P.M.; Rico-González, M.; Bezerra, P.; Rodrigues, L.P. Wearables and Internet of Things (IoT) Technologies for Fitness Assessment: A Systematic Review. Sensors 2021, 21, 5418. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Di Cagno, A.; De Luca, E.W.; Aldegheri, S.; Zara, V.; Ferramosca, A. Advanced Wearable Devices for Monitoring Sweat Biochemical Markers in Athletic Performance: A Comprehensive Review. Biosensors 2024, 14, 574. [Google Scholar] [CrossRef]
- Luo, T.T.; Sun, Z.H.; Li, C.X.; Feng, J.L.; Xiao, Z.X.; Li, W.D. Monitor for lactate in perspiration. J. Physiol. Sci. 2021, 71, 26. [Google Scholar] [CrossRef]
- Gibson, O.R.; James, C.A.; Mee, J.A.; Willmott, A.G.B.; Turner, G.; Hayes, M.; Maxwell, N.S. Heat alleviation strategies for athletic performance: A review and practitioner guidelines. Temperature 2019, 7, 3–36. [Google Scholar] [CrossRef]
- Egan, B.; Hawley, J.A.; Zierath, J.R. SnapShot: Exercise Metabolism. Cell Metab. 2016, 24, 342–342.e1. [Google Scholar] [CrossRef]
- Pohjanen, E.; Thysell, E.; Jonsson, P.; Eklund, C.; Silfver, A.; Carlsson, I.B.; Lundgren, K.; Moritz, T.; Svensson, M.B.; Antti, H. A multivariate screening strategy for investigating metabolic effects of strenuous physical exercise in human serum. J. Proteome Res. 2007, 6, 2113–2120. [Google Scholar] [CrossRef]
- Nordsborg, N.B.; Connolly, L.; Weihe, P.; Iuliano, E.; Krustrup, P.; Saltin, B.; Mohr, M. Oxidative capacity and glycogen content increase more in arm than leg muscle in sedentary women after intense training. J. Appl. Physiol. 2015, 119, 116–123. [Google Scholar] [CrossRef]
- Nyberg, M.; Fiorenza, M.; Lund, A.; Christensen, M.; Rømer, T.; Piil, P.; Hostrup, M.; Christensen, P.M.; Holbek, S.; Ravnholt, T.; et al. Adaptations to speed endurance training in highly trained soccer players. Med. Sci. Sports Exerc. 2016, 48, 1355–1364. [Google Scholar] [CrossRef]
- Franchi, M.V.; Wilkinson, D.J.; Quinlan, J.I.; Mitchell, W.K.; Lund, J.N.; Williams, J.P.; Reeves, N.D.; Smith, K.; Atherton, P.J.; Narici, M.V. Early structural remodeling and deuterium oxide-derived protein metabolic responses to eccentric and concentric loading in human skeletal muscle. Physiol. Rep. 2015, 3, e12593. [Google Scholar] [CrossRef]
- Khoramipour, K.; Sandbakk, Ø.; Keshteli, A.H.; Gaeini, A.A.; Wishart, D.S.; Chamari, K. Metabolomics in exercise and sports: A systematic review. Sports Med. 2022, 52, 547–583. [Google Scholar] [CrossRef]
- Wishart, D.S.; Li, C.; Marcu, A.; Badran, H.; Pon, A.; Budinski, Z.; Patron, J.; Lipton, D.; Cao, X.; Oler, E.; et al. PathBank: A comprehensive pathway database for model organisms. Nucleic Acids Res. 2020, 48, D470–D478. [Google Scholar] [CrossRef]
- Corrie, S.R.; Coffey, J.W.; Islam, J.; Markey, K.A.; Kendall, M.A. Blood, sweat, and tears: Developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 2015, 13, 4350–4364. [Google Scholar] [CrossRef]
- Su, M.; Jin, J.; Li, Y.; Zhao, S.; Zhan, J. Research on sweat metabolomics of athlete’s fatigue induced by high intensity interval training. Front. Physiol. 2023, 14, 1269885. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med.-Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Lin, Y.S.; Weibel, J.; Landolt, H.P.; Santini, F.; Meyer, M.; Brunmair, J.; Meier-Menches, S.M.; Gerner, C.; Borgwardt, S.; Cajochen, C.; et al. Daily Caffeine Intake Induces Concentration-Dependent Medial Temporal Plasticity in Humans: A multimodal double-blind randomized controlled trial. Cereb. Cortex. 2021, 31, 3096–3106. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.; van Duijnhoven, J.P.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Kjeldahl, K.; Bro, R. Some common misunderstandings in chemometrics. J. Chemom. 2010, 24, 558–564. [Google Scholar] [CrossRef]
- Souza, S.L.; Graça, G.; Oliva, A. Characterization of sweat induced with pilocarpine, physical exercise, and collected passively by metabolomic analysis. Skin Res. Technol. 2018, 24, 187–195. [Google Scholar] [CrossRef]
- Kirkland, J.B.; Meyer-Ficca, M.L. Niacin. Adv. Food Nutr. Res. 2018, 83, 83–149. [Google Scholar] [CrossRef]
- Deen, C.P.J.; van der Veen, A.; Gomes-Neto, A.W.; Geleijnse, J.M.; Borgonjen-van den Berg, K.J.; Heiner-Fokkema, M.R.; Kema, I.P.; Bakker, S.J.L. Urinary Excretion of N1-methyl-2-pyridone-5-carboxamide and N1-methylnicotinamide in Renal Transplant Recipients and Donors. J. Clin. Med. 2020, 9, 437. [Google Scholar] [CrossRef]
- Jacob, R.A.; Swendseid, M.E.; McKee, R.W.; Fu, C.S.; Clemens, R.A. Biochemical markers for assessment of niacin status in young men: Urinary and blood levels of niacin metabolites. J. Nutr. 1989, 119, 591–598. [Google Scholar] [CrossRef]
- Prousky, J.E. Niacin for Detoxification: A Little-known Therapeutic Use. J. Orthomol. Med. 2011, 26, 85–92. [Google Scholar]
- Zhou, S.S.; Li, D.; Sun, W.P.; Guo, M.; Lun, Y.Z.; Zhou, Y.M.; Xiao, F.C.; Jing, L.X.; Sun, S.X.; Zhang, L.B.; et al. Nicotinamide overload may play a role in the development of type 2 diabetes. World J. Gastroenterol. 2009, 15, 5674–5684. [Google Scholar] [CrossRef]
- Penberthy, W.T.; Kirkland, J.B. Niacin. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 293–306. [Google Scholar]
- Li, D.; Sun, W.P.; Zhou, Y.M.; Liu, Q.G.; Zhou, S.S.; Luo, N.; Bian, F.N.; Zhao, Z.G.; Guo, M. Chronic niacin overload may be involved in the increased prevalence of obesity in US children. World J. Gastroenterol. 2010, 16, 2378–2387. [Google Scholar] [CrossRef]
- Newsholme, P.; Bender, K.; Kiely, A.; Brennan, L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007, 35, 1180–1186. [Google Scholar] [CrossRef]
- Holeček, M. Origin and Roles of Alanine and Glutamine in Gluconeogenesis in the Liver, Kidneys, and Small Intestine under Physiological and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 7037. [Google Scholar] [CrossRef]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021, 33, 905–922.e6. [Google Scholar] [CrossRef]
- Czumaj, A.; Szrok-Jurga, S.; Hebanowska, A.; Turyn, J.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The Pathophysiological Role of CoA. Int. J. Mol. Sci. 2020, 21, 9057. [Google Scholar] [CrossRef]
- Coelho, A.I.; Berry, G.T.; Rubio-Gozalbo, M.E. Galactose metabolism and health. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 422–427. [Google Scholar] [CrossRef]
- Lu, M.; He, W.; Xu, Z.; Lu, Y.; Crabbe, M.J.C.; De, J. The effect of high altitude on ephedrine content and metabolic variations in two species of Ephedra. Front. Plant Sci. 2023, 14, 1236145. [Google Scholar] [CrossRef]
- Hajjar, G.; Santos, M.C.B.; Bertrand-Michel, J.; Canlet, C.; Castelli, F.; Creusot, N.; Dechaumet, S.; Diémé, B.; Giacomoni, F.; Giraudeau, P.; et al. Scaling-up metabolomics: Current state and perspectives. Trends Anal. Chem. 2023, 167, 117225. [Google Scholar] [CrossRef]

| Profiles | Soccer Players (n = 22) | Range |
|---|---|---|
| Age (Years) | 21.75 ± 1.23 | 18–30 ns |
| Body Mass (Kg) | 76.26 ± 1.82 | 70.48–77.27 ns |
| Height (cm) | 157.94 ± 2.40 | 154.5–170.0 ns |
| Running speed (km/h) | 19.78 ± 0.54 | 15.4–24.12 ns |
| Average heart rate (bpm) | 179 ± 0.94 | 173–180 ns |
| Temperature (°C) | 30.46 ± 0.88 | 30.13–33.14 ns |
| Rating of Perceived Exertion (RPE) | 18.25 ± 0.96 | 17–19 ns |
| Metabolites ID | Molecular Weight | Retention Time 1D Rt(min) | Formula | VIP | FC log2 | Paired t Test p-Value | FDR Critical Value | FDR q-Value |
|---|---|---|---|---|---|---|---|---|
| Niacin, TMS derivative | 195.29 | 15.87 | C9H13NO2Si | 3.55 | 5.449 | 0.001 | 0.002 | 0.032 ⸸⸸ |
| Analyte 817 UM103 | - | - | - | 2.65 | 3.667 | 0.023 | 0.003 | 0.105 *⸸ |
| 2-[(Trimethylsilyl)oxy]propan-1-ol | 148.28 | 29.44 | C6H16O2Si | 2.44 | 1.815 | 0.009 | 0.005 | 0.105 *⸸ |
| L-Serine, 2TMS derivative | 249.46 | 17.99 | C9H23NO3Si2 | 2.34 | 1.381 | 0.026 | 0.006 | 0.105 *⸸ |
| Pyroglutamic acid, TMS derivative | 201.30 | 25.83 | C8H15NO3Si | 2.30 | 1.323 | 0.035 | 0.008 | 0.105 *⸸ |
| L-Valine, TMS derivative | 249.45 | 10.96 | C8H19NO2Si | 2.26 | 1.188 | 0.071 | 0.009 | 0.105 *⸸ |
| L-Aspartic acid, 2TMS derivative | 277.46 | 20.03 | C10H23NO4Si2 | 2.19 | 2.002 | 0.041 | 0.011 | 0.105 *⸸ |
| meso-Erythritol, 4TMS derivative | 410.84 | 22.68 | C16H42O4Si4 | 2.11 | 1.667 | 0.092 | 0.013 | 0.105 *⸸ |
| Silanamine, N,N′-methanetetraylbis[1,1,1-trimethyl | 186.40 | 14.57 | C7H18N2Si2 | 2.09 | −1.160 | 0.034 | 0.014 | 0.105 *⸸ |
| d-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1E) | 570.10 | 33.06 | C22H55NO6Si5 | 2.07 | 0.273 | 0.050 | 0.016 | 0.105 *⸸ |
| L-Isoleucine, TMS derivative | 203.35 | 12.35 | C9H21NO2Si | 2.07 | 1.184 | 0.034 | 0.017 | 0.105 *⸸ |
| Benzimidazo[2,1-a]isoquinoline | 218.25 | 15.28 | C15H10N2 | 2.05 | −1.349 | 0.040 | 0.019 | 0.105 *⸸ |
| L-Glutamic acid, 3TMS derivative | 363.67 | 25.91 | C14H33NO4Si3 | 2.03 | 1.274 | 0.080 | 0.020 | 0.105 *⸸ |
| L-Alanine, TMS derivative | 161.27 | 5.41 | C6H15NO2Si | 2.01 | 1.833 | 0.061 | 0.022 | 0.105 *⸸ |
| 2-Octanol, TMS derivative | 202.41 | 22.66 | C11H26OSi | 1.98 | 1.272 | 0.061 | 0.023 | 0.105 *⸸ |
| Glycerol, 3TMS derivative | 308.64 | 16.06 | C12H32O3Si3 | 1.97 | −1.568 | 0.045 | 0.025 | 0.105 *⸸ |
| Analyte 42 UM197 | - | - | - | 1.96 | −1.177 | 0.055 | 0.027 | 0.105 *⸸ |
| Methyl galactoside, 4TMS derivative | 482.90 | 32.78 | C19H46O6Si4 | 1.89 | 2.430 | 0.042 | 0.028 | 0.105 *⸸ |
| Analyte 308 UM84 | - | - | - | 1.89 | 0.298 | 0.089 | 0.030 | 0.105 *⸸ |
| Methylmalonic acid, 2TMS derivative | 262.45 | 10.53 | C10H22O4Si2 | 1.88 | −1.808 | 0.091 | 0.031 | 0.105 *⸸ |
| Heptacosane | 380.73 | 44.23 | C27H56 | 1.86 | 0.253 | 0.069 | 0.033 | 0.105 *⸸ |
| Analyte 328 UM231 | - | - | - | 1.86 | 0.693 | 0.095 | 0.034 | 0.105 *⸸ |
| Analyte 164 UM93 | - | - | - | 1.85 | 1.364 | 0.064 | 0.036 | 0.105 *⸸ |
| D-(-)-Ribofuranose, tetrakis(trimethylsilyl) ether (isomer 1) | 438.85 | 25.83 | C17H42O5Si4 | 1.84 | 0.204 | 0.073 | 0.038 | 0.105 *⸸ |
| Analyte 292 UM70 | - | - | - | 1.82 | 0.217 | 0.077 | 0.039 | 0.105 *⸸ |
| Analyte 338 UM189 | - | - | - | 1.80 | 2.499 | 0.081 | 0.041 | 0.105 *⸸ |
| Analyte 354 UM187 | - | - | - | 1.77 | 0.045 | 0.116 | 0.042 | 0.105 *⸸ |
| Analyte 412 UM74 | - | - | - | 1.76 | 1.612 | 0.120 | 0.044 | 0.105 *⸸ |
| Analyte 319 UM159 | - | - | - | 1.76 | 1.481 | 0.088 | 0.045 | 0.105 *⸸ |
| Isatin-3-oxime | 162.15 | 25.13 | C8H6N2O2 | 1.75 | −1.126 | 0.090 | 0.047 | 0.124 *⸸ |
| phenoxyethanol, TMS derivative | 210.35 | 18.13 | C11H18O2Si | 1.75 | 2.462 | 0.090 | 0.048 | 0.124 *⸸ |
| 2-Tridecanol, TMS derivative | 272.54 | 25.48 | C13H28O | 1.74 | 1.167 | 0.125 | 0.050 | 0.125 *⸸ |
| Hits a | p-Value | Holm P b | Impact Value | |
|---|---|---|---|---|
| Alanine, aspartate and glutamate metabolism | 3 | 0.001 | 0.080 | 0.224 |
| Valine, leucine and isoleucine biosynthesis | 2 | 0.001 | 0.115 | 0.000 |
| Valine, leucine and isoleucine degradation | 3 | 0.003 | 0.222 | 0.023 |
| Nicotinate and nicotinamide metabolism | 2 | 0.005 | 0.407 | 0.194 |
| Pantothenate and CoA biosynthesis | 2 | 0.009 | 0.712 | 0.000 |
| Galactose metabolism | 2 | 0.017 | 1.000 | 0.356 |
| Glyoxylate and dicarboxylate metabolism | 2 | 0.023 | 1.000 | 0.074 |
| Arginine biosynthesis | 1 | 0.102 | 1.000 | 0.000 |
| D-Amino acid metabolism | 1 | 0.109 | 1.000 | 0.000 |
| Histidine metabolism | 1 | 0.116 | 1.000 | 0.000 |
| Glycerolipid metabolism | 1 | 0.116 | 1.000 | 0.237 |
| Selenocompound metabolism | 1 | 0.143 | 1.000 | 0.000 |
| Citrate cycle (TCA cycle) | 1 | 0.143 | 1.000 | 0.090 |
| beta-Alanine metabolism | 1 | 0.149 | 1.000 | 0.000 |
| Pentose phosphate pathway | 1 | 0.162 | 1.000 | 0.000 |
| Glutathione metabolism | 1 | 0.194 | 1.000 | 0.007 |
| Sphingolipid metabolism | 1 | 0.219 | 1.000 | 0.000 |
| Cysteine and methionine metabolism | 1 | 0.225 | 1.000 | 0.022 |
| Glycine, serine and threonine metabolism | 1 | 0.225 | 1.000 | 0.215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malefo, N.; Naidoo, C.M.; Mphephu, M.M.; Motshudi, M.C.; Mkolo, N.M. Metabolomics Approach for Sweat Mapping the Performance of Soccer Players in Pretoria, South Africa. Appl. Sci. 2025, 15, 4588. https://doi.org/10.3390/app15084588
Malefo N, Naidoo CM, Mphephu MM, Motshudi MC, Mkolo NM. Metabolomics Approach for Sweat Mapping the Performance of Soccer Players in Pretoria, South Africa. Applied Sciences. 2025; 15(8):4588. https://doi.org/10.3390/app15084588
Chicago/Turabian StyleMalefo, Nong, Clarissa Marcelle Naidoo, Mukhethwa Michael Mphephu, Mmei Cheryl Motshudi, and Nqobile Monate Mkolo. 2025. "Metabolomics Approach for Sweat Mapping the Performance of Soccer Players in Pretoria, South Africa" Applied Sciences 15, no. 8: 4588. https://doi.org/10.3390/app15084588
APA StyleMalefo, N., Naidoo, C. M., Mphephu, M. M., Motshudi, M. C., & Mkolo, N. M. (2025). Metabolomics Approach for Sweat Mapping the Performance of Soccer Players in Pretoria, South Africa. Applied Sciences, 15(8), 4588. https://doi.org/10.3390/app15084588







