Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from the Aerial Part of Plants in the Chenopodiaceae Family Using a Box–Behnken Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Plant Material
2.3. Experimental Design
2.4. Preparation of Extract
2.4.1. Calculation of the Extraction Yield
2.4.2. Phytochemical Analysis
Determination of Total Phenolic Content (TPC)
Determination of the Total Flavonoid Content (TFC)
2.4.3. Model Optimization of Phenolic Compounds Extraction Conditions
2.4.4. Evaluation of Antioxidant Activity
DPPH Free-Radicals Scavenging Capacity
ABTS+ Scavenging Capacity
Antioxidant Reducing Capacity of Copper (CUPRAC)
Phenanthroline Assay
Reducing Power Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Impact of Varied Ultrasound-Assisted Extraction Parameters on the Extraction Responses of Phenolic Compounds from Cornulaca monacantha Del
3.1.1. Impact of the Different Extraction Factors on the Extraction Yield Percentage
3.1.2. Impact of Different Extraction Factors on the Total Phenolic Content (TPC)
3.1.3. Impact of Different Extraction Factors on the Total Flavonoid Content (TFC)
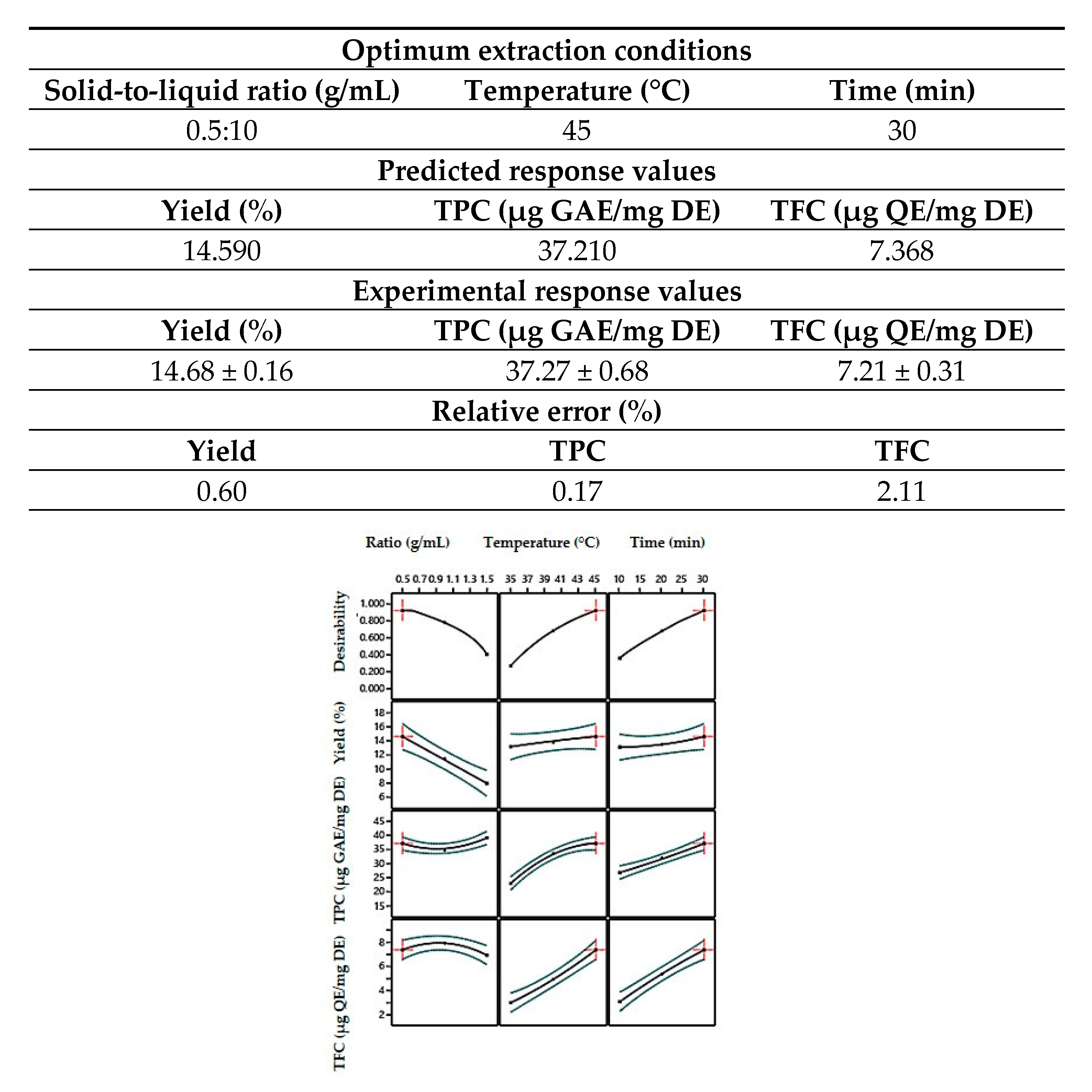
3.2. Validation of the Optimized Model for Polyphenol Extraction from Cornulaca monacantha Del. and Their Application for Anabasis articulata (Forssk.) Moq. Phenolic Compound Extraction
3.3. Extraction Yields Determination and Phytochemicals Analysis
3.4. Antioxidant Activities of Cornulaca monacantha Del. and Anabasis articulata (Forssk.) Moq. Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miara, M.D.; Teixidor-Toneu, I.; Sahnoun, T.; Bendif, H.; Ait Hammou, M. Herbal Remedies and Traditional Knowledge of the Tuareg Community in the Region of Illizi (Algerian Sahara). J. Arid Environ. 2019, 167, 65–73. [Google Scholar] [CrossRef]
- Djebili, S.; Taş, M.; Bouguerra, A.; Kucukaydin, S.; Ceylan, O.; Duru, M.E.; Barkat, M. Volatile Compound Profile and Essential Oil Composition of Three Wild Algerian Aromatic Plants with Their Antioxidant and Antibiofilm Activities. J. Food Meas. Charact. 2021, 16, 987–999. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds Characterization by LC-DAD- ESI/MSn and Bioactive Properties of Thymus Algeriensis Boiss. & Reut. and Ephedra Alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef]
- Aissaoui, M.; Rahmoun, M.N.; Latrache, H.; Barek, S.; Elassri, A.; Bensouici, C.; El Haci, I.A.; Choukchou-Braham, N. Structural Characterization, Antioxidant, and Antibiofilm Activities of Coffea canephora Green Seeds. J. Complement. Integr. Med. 2021, 18, 107–112. [Google Scholar] [CrossRef]
- Saidi, A.; Hambaba, L.; Bensaad, M.S.; Melakhessou, M.A.; Bensouici, C.; Ferhat, N.; Kahoul, M.A.; Helal, M.; Sami, R.; Alharthy, S.A.; et al. Phenolic Characterization Using cLC-DAD Analysis and Evaluation of In Vitro and In Vivo Pharmacological Activities of Ruta Tuberculata Forssk. Antioxidants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Shameem, N.; Kamili, A.N.; Parray, J.A.; Hamid, R.; Bandh, S.A. Antimicrobial and Antioxidant Activity of Methanol Extracts of Arnebia Benthamii (Wall Ex. G. Don) Johnston—A Critically Endangered Medicinal Plant of North Western Himalaya. J. Anal. Sci. Technol. 2015, 6, 36. [Google Scholar] [CrossRef]
- Abbas, M.; Ahmed, D.; Qamar, M.T.; Ihsan, S.; Noor, Z.I. Optimization of Ultrasound-Assisted, Microwave-Assisted and Soxhlet Extraction of Bioactive Compounds from Lagenaria Siceraria: A Comparative Analysis. Bioresour. Technol. Rep. 2021, 15, 100746. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Ou, H.; Gregersen, H.; Zuo, J. Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Polyphenols from Cosmos Sulphureus. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100444. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives: Plant Polyphenols for Food Preservation. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, Antimicrobial, Antioxidant Properties and Effects on Cell Migration of Phenolic Compounds of Selected Transylvanian Medicinal Plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Tandon, D.; Gupta, A.K. Comparative Assessment of Antimicrobial and Antioxidant Activity between Whole Plant and Parts of Sphaeranthus indicus Linn. (Asteraceae). Clin. Phytosci. 2020, 6, 23. [Google Scholar] [CrossRef]
- Hechaichi, F.Z.; Bendif, H.; Bensouici, C.; Alsalamah, S.A.; Zaidi, B.; Bouhenna, M.M.; Souilah, N.; Alghonaim, M.I.; Benslama, A.; Medjekal, S.; et al. Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts. Molecules 2023, 28, 2263. [Google Scholar] [CrossRef] [PubMed]
- Hellal, K.; Maulidiani, M.; Ismail, I.S.; Tan, C.P.; Abas, F. Antioxidant, α-Glucosidase, and Nitric Oxide Inhibitory Activities of Six Algerian Traditional Medicinal Plant Extracts and 1H-NMR-Based Metabolomics Study of the Active Extract. Molecules 2020, 25, 1247. [Google Scholar] [CrossRef]
- Chamali, S.; Bendaoud, H.; Bouajila, J.; Camy, S.; Saadaoui, E.; Condoret, J.-S.; Romdhane, M. Optimization of Accelerated Solvent Extraction of Bioactive Compounds from Eucalyptus Intertexta Using Response Surface Methodology and Evaluation of Its Phenolic Composition and Biological Activities. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100464. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; González-Manzano, S. Phenolic Acids and Derivatives: Description, Sources, Properties, and Applications. In Natural Secondary Metabolites; Carocho, M., Heleno, S.A., Barros, L., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 37–72. ISBN 978-3-031-18586-1. [Google Scholar]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A Green Ultrasound-Assisted Extraction Process for the Recovery of Antioxidant Polyphenols and Pigments from Onion Solid Wastes Using Box–Behnken Experimental Design and Kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Mighri, H.; Akrout, A.; Bennour, N.; Eljeni, H.; Zammouri, T.; Neffati, M. LC/MS Method Development for the Determination of the Phenolic Compounds of Tunisian Ephedra Alata Hydro-Methanolic Extract and Its Fractions and Evaluation of Their Antioxidant Activities. S. Afr. J. Bot. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Di Marco, G.; Canini, A.; Atik Bekkara, F. GC/MS Analysis, and Antioxidant and Antimicrobial Activities of Alkaloids Extracted by Polar and Apolar Solvents from the Stems of Anabasis Articulata. Med. Chem. Res. 2019, 28, 754–767. [Google Scholar] [CrossRef]
- Ben Menni, D.; Belyagoubi-Benhammou, N.; Benmahieddine, A.; Ben Menni, H.; Gismondi, A.; Monteleone, V.; Di Marco, G.; D’Agostino, A.; Canini, A.; Benamar, H.; et al. Identification of Sterols from Anabasis Articulata (Forssk.) Moq. (Chenopodiaceae) Growing in Algeria and Study of Their Potential Bioactivity. Waste Biomass Valorization 2022, 13, 3283–3295. [Google Scholar] [CrossRef]
- Gangwar, H.; Kumari, P.; Jaiswal, V. Phytochemically Rich Medicinally Important Plant Families. In Phytochemical Genomics; Swamy, M.K., Kumar, A., Eds.; Springer Nature: Singapore, 2022; pp. 35–68. ISBN 978-981-19577-8-9. [Google Scholar]
- Hussien, R.M.; Badawy, A.M.; Ahmed, S.A.E.; Eltamany, E.E. Review Article on Phytochemical Constituents and Biological Activity of Cornulaca Monacantha. Rec. Pharm. Biomed. Sci. 2023, 7, 13–18. [Google Scholar] [CrossRef]
- Mhiri, R.; Koubaa, I.; Chawech, R.; Auberon, F.; Allouche, N.; Michel, T. New Isoflavones with Antioxidant Activity Isolated from Cornulaca monacantha. Chem. Biodivers. 2020, 17, e2000758. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, G.; Kumar, A.; Siddiqi, Z.M.; Sharma, G. Exclusion of Organic Dye Using Neoteric Activated Carbon Prepared from Cornulaca monacantha. Stem: Equilibrium and Thermodynamics Studies. Mater. Sci. Forum 2016, 875, 1–15. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Abdalla, M.S.; Rizk, M.Z.; Mahdy, E.-S.M.E.; Farrag, A.-R.H.; El-Sharabasy, F.S.; Aly, H.F.; Mohamed, M.R. Alleviation of Dimethylnitrosamine-Induced Liver Injury and Fibrosis by Supplementation of Anabasis Articulata Extract in Rats. Indian J. Clin. Biochem. 2014, 29, 418–429. [Google Scholar] [CrossRef]
- Kambouche, N.; Tadjeddine, A.; Medjdoub, H.; Meddah, B.; Dicko, A.; Saidi, S.; Derdour, A. Antidiabetic Effect of Anacyclus valentinus L. Aqueous Extract in Normoglycemic and Streptozotocin Induced-Diabetic Rats. Am. J. Phytomed. Clin. Ther. 2013, 1, 424. [Google Scholar]
- Monteleone, V.; Ben Menni, D.; Belyagoubi-Benhammou, N.; Di Marco, G.; Canini, A.; Gismondi, A. Anabasis articulata (Forssk.) Moq. Food Aqueous Extract Triggers Oxidative Stress-Induced Senescence and Reduces Metastatic Power in MDA-MB-231 Cells. J. Funct. Foods 2024, 116, 106203. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet Extraction of Phenolic Compounds from Vernonia cinerea Leaves and Its Antioxidant Activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Hentabli, M.; Remini, H.; Mouni, L. Optimization of Ultrasound-Assisted Extraction of Phenolic-Saponin Content from Carthamus caeruleus L. Rhizome and Predictive Model Based on Support Vector Regression Optimized by Dragonfly Algorithm. Chemom. Intell. Lab. Syst. 2022, 222, 104493. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Wang, C.; Chang, T.; Jiang, H. Ultrasound-Homogenization-Assisted Extraction of Polyphenols from Coconut Mesocarp: Optimization Study. Ultrason. Sonochem. 2021, 78, 105739. [Google Scholar] [CrossRef]
- Tang, Z.; Lin, W.; Yang, J.; Feng, S.; Qin, Y.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T.; et al. Ultrasound-Assisted Extraction of Cordyceps Cicadae Polyphenols: Optimization, LC-MS Characterization, Antioxidant and DNA Damage Protection Activity Evaluation. Arab. J. Chem. 2022, 15, 103953. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.; Elbir Abca, T.; Kamiloğlu, A.; Kutlu, N. Ultrasound Assisted Choline Chloride-Based Deep Eutectic Solvent Extraction of Bioactive Compounds from Salsify (Tragopogon buphthalmoides (DC.) Boiss. Var. Latifolius Boiss.): A Box–Behnken Design Approach for Optimization. Microchem. J. 2024, 199, 110218. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Fonteles, T.V.; Rodrigues, S.; De Brito, E.S.; Tiwari, B.K. Ultrasound-Assisted Extraction of Anthocyanins and Phenolics from Jabuticaba (Myrciaria cauliflora) Peel: Kinetics and Mathematical Modeling. J. Food Sci. Technol. 2020, 57, 2321–2328. [Google Scholar] [CrossRef]
- Khalaf, D.; Pradal, D.; Dimitrov, K. Multi-Criteria Optimization Including Environmental Impacts of Ultrasound-Assisted Extraction of Phenolic Antioxidants from Blackcurrant Pomace by-Product. Chem. Eng. Process. Process Intensif. 2024, 204, 109935. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Dubey, A.K.; Varghese, E.; Kaur, C. Optimization and Comparison of Non-Conventional Extraction Technologies for Citrus paradisi L. Peels: A Valorization Approach. J. Food Sci. Technol. 2019, 56, 1221–1233. [Google Scholar] [CrossRef]
- Bouaoudia-Madi, N.; Boulekbache-Makhlouf, L.; Madani, K.; Silva, A.M.S.; Dairi, S.; Oukhmanou-Bensidhoum, S.; Cardoso, S.M. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Myrtus communis L. Pericarp. Antioxidants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Janarny, G.; Ranaweera, K.K.D.S.; Gunathilake, K.D.P.P. Optimization of Ethanol Based Extraction of Phenolics from Ocimum Sanctum Flowers by Response Surface Methodology. Biocatal. Agric. Biotechnol. 2022, 45, 102493. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K. Microwave and Ultrasound Assisted Extraction of Phytocompounds from Black Jamun Pulp: Kinetic and Thermodynamics Characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102913. [Google Scholar] [CrossRef]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of Ultrasound-Assisted Extraction of Antioxidant Compounds from Tunisian Zizyphus Lotus Fruits Using Response Surface Methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of Ultrasonic-Assisted Extraction of Flavonoids, Polysaccharides, and Eleutherosides from Acanthopanax Senticosus Using Response Surface Methodology in Development of Health Wine. LWT 2022, 165, 113725. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J.; Wang, Y.; Li, X.; Zhou, H.; Zhu, L. Optimization of Ultrasonic-Assisted Extraction of Wedelolactone and Antioxidant Polyphenols from Eclipta Prostrate L Using Response Surface Methodology. Sep. Purif. Technol. 2014, 138, 55–64. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J.Š.; Castro, E. Optimization of Ultrasound-Assisted Extraction of Biomass from Olive Trees Using Response Surface Methodology. Ultrason. Sonochem. 2019, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Basics in Analysis of Phenolic Antioxidants. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; pp. 437–479. ISBN 978-3-030-74767-1. [Google Scholar]
- Jovanović, M.; Mudrić, J.; Drinić, Z.; Matejić, J.; Kitić, D.; Bigović, D.; Šavikin, K. Optimization of Ultrasound-Assisted Extraction of Bitter Compounds and Polyphenols from Willow Gentian Underground Parts. Sep. Purif. Technol. 2022, 281, 119868. [Google Scholar] [CrossRef]
- Afoakwah, N.A.; Tchabo, W.; Owusu-Ansah, P. Ultrasound-Assisted Extraction (UAE) of Jerusalem Artichoke Tuber Bio-Active Ingredient Using Optimized Conditions of Box-Behnken Response Surface Methodology. Heliyon 2024, 10, e25645. [Google Scholar] [CrossRef]
- Diwathe, M.C.; Anandkumar, J.; Mazumdar, B. Optimization of Ultrasound-Assisted Extraction of Phenolics from Cordia Dichotoma Leaves Using Response Surface Methodology. Chem. Eng. Res. Des. 2024, 208, 572–587. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Chamorro, F.; Simal-Gandara, J.; Prieto, M.A.; Cassani, L. Improving Phenolic Compound Extraction from Arnica Montana Flowers through Multivariate Optimization of Heat and Ultrasound-Assisted Methods. Sustain. Chem. Pharm. 2024, 41, 101722. [Google Scholar] [CrossRef]
- Ngamkhae, N.; Monthakantirat, O.; Chulikhit, Y.; Boonyarat, C.; Maneenet, J.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Daodee, S. Optimization of Extraction Method for Kleeb Bua Daeng Formula and Comparison between Ultrasound-Assisted and Microwave-Assisted Extraction. J. Appl. Res. Med. Aromat. Plants 2022, 28, 100369. [Google Scholar] [CrossRef]
- Dimitrov, K.; Pradal, D.; Vauchel, P.; Baouche, B.; Nikov, I.; Dhulster, P. Modeling and Optimization of Extraction and Energy Consumption during Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Pomegranate Peels. Environ. Prog. Sustain. Energy 2019, 38, 13148. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Teksee, A.; Pongsetkul, J.; Sinthusamran, S.; Rawdkuen, S. Optimization of Ultrasonic Assisted Ethanolic Extraction for Natural Pigments from Butterfly Pea Flower Applied in Thai Dessert Using Box-Behnken Approach. Food Chem. X 2024, 22, 101484. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-Physical and Sono-Chemical Effects of Ultrasound: Primary Applications in Extraction and Freezing Operations and Influence on Food Components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef]
- Ozcan, H.; Damar, I. Valorization of Spinach Roots for Recovery of Phenolic Compounds by Ultrasound-Assisted Extraction: Characterization, Optimization, and Bioaccessibility. Eur. Food Res. Technol. 2023, 249, 1899–1913. [Google Scholar] [CrossRef]
- Saeed, R.; Ahmed, D.; Mushtaq, M. Ultrasound-Aided Enzyme-Assisted Efficient Extraction of Bioactive Compounds from Gymnema Sylvestre and Optimization as per Response Surface Methodology. Sustain. Chem. Pharm. 2022, 29, 100818. [Google Scholar] [CrossRef]
- da Silva Donadone, D.B.; Giombelli, C.; Silva, D.L.G.; Stevanato, N.; da Silva, C.; Barros, B.C.B. Ultrasound-Assisted Extraction of Phenolic Compounds and Soluble Sugars from the Stem Portion of Peach Palm. J. Food Process. Preserv. 2020, 44, e14636. [Google Scholar] [CrossRef]
- da Silva, N.C.; de Barros-Alexandrino, T.T.; Assis, O.B.G.; Martelli-Tosi, M. Extraction of Phenolic Compounds from Acerola By-Products Using Chitosan Solution, Encapsulation and Application in Extending the Shelf-Life of Guava. Food Chem. 2021, 354, 129553. [Google Scholar] [CrossRef]
- Lozano-Puentes, H.S.; Sánchez-Matiz, J.J.; Ruiz-Sanchez, E.; Costa, G.M.; Díaz-Ariza, L.A. Guadua Angustifolia Kunth Leaves as a Source for Bioactive Phenolic Compounds: Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology and Antioxidant Activities. Heliyon 2023, 9, e22445. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-Treatment and Extraction Techniques for Recovery of Added Value Compounds from Wastes throughout the Agri-Food Chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of Ultrasound-Assisted Extraction to Obtain Mycosterols from Agaricus Bisporus L. by Response Surface Methodology and Comparison with Conventional Soxhlet Extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef]
- Karunanithi, A.; Venkatachalam, S. Ultrasonic-assisted Solvent Extraction of Phenolic Compounds from Opuntia Ficus-indica Peel: Phytochemical Identification and Comparison with Soxhlet Extraction. J. Food Process Eng. 2019, 42, e13126. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response Surface Methodology and Artificial Neural Network Approach for the Optimization of Ultrasound-Assisted Extraction of Polyphenols from Garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Response Surface Optimization for Recovery of Polyphenols and Carotenoids from Leaves of Centella Asiatica Using an Ethanol-based Solvent System. Food Sci. Nutr. 2019, 7, 528–536. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous Optimization of the Ultrasound-Assisted Extraction for Phenolic Compounds Content and Antioxidant Activity of Lycium ruthenicum Murr. Fruit Using Response Surface Methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; She, X.-R.; Huang, J.-B.; Liu, M.-C.; Zhan, M.-E. Ultrasonic-Extraction of Phenolic Compounds from Phyllanthus Urinaria: Optimization Model and Antioxidant Activity. Food Sci. Technol. 2018, 38, 286–293. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess. Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Živković, J.; Janković, T.; Menković, N.; Šavikin, K. Optimization of Ultrasound-Assisted Extraction of Isogentisin, Gentiopicroside and Total Polyphenols from Gentian Root Using Response-Surface Methodology. Ind. Crops Prod. 2019, 139, 111567. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Zahnit, W.; Smara, O.; Bechki, L.; Dekmouche, M.; Bensouici, C. IN-VITRO Assessment of Anti-Cholinesterase, Anti-Lipase, Antioxidant Activities and Photoprotective Effect of Algerian Fagonia Bruguieri DC Extracts. Pharm. Chem. J. 2023, 57, 89–100. [Google Scholar] [CrossRef]
- Elhadef, K.; Smaoui, S.; Ben Hlima, H.; Ennouri, K.; Fourati, M.; Chakchouk Mtibaa, A.; Sellem, I.; Mellouli, L. Chemometric Multivariate Analyses of Phenolics and Biological Activities for Characterization and Discrimination of Tunisian Ephedra Alata. Eur. Food Res. Technol. 2020, 246, 1495–1513. [Google Scholar] [CrossRef]
- Khenifi, M.L.; Serseg, T.; Migas, P.; Krauze-Baranowska, M.; Özdemir, S.; Bensouici, C.; Alghonaim, M.I.; Al-Khafaji, K.; Alsalamah, S.A.; Boudjeniba, M.; et al. HPLC-DAD-MS Characterization, Antioxidant Activity, α-Amylase Inhibition, Molecular Docking, and ADMET of Flavonoids from Fenugreek Seeds. Molecules 2023, 28, 7798. [Google Scholar] [CrossRef]
- Benouchenne, D.; Bellil, I.; Akkal, S.; Bensouici, C.; Khelifi, D. LC–MS/MS Analysis, Antioxidant and Antibacterial Activities of Algerian Fir (Abies Numidica de LANNOY Ex CARRIÈRE) Ethylacetate Fraction Extracted from Needles. J. King Saud. Univ. Sci. 2020, 32, 3321–3327. [Google Scholar] [CrossRef]
- Bendjedid, S.; Lekmine, S.; Tadjine, A.; Djelloul, R.; Bensouici, C. Analysis of Phytochemical Constituents, Antibacterial, Antioxidant, Photoprotective Activities and Cytotoxic Effect of Leaves Extracts and Fractions of Aloe Vera. Biocatal. Agric. Biotechnol. 2021, 33, 101991. [Google Scholar] [CrossRef]
- Djermane, N.; Gali, L.; Arhab, R.; Gherraf, N.; Bensouici, C.; Erenler, R.; Gok, M.; Abdessamed, A. Chemical Composition and in Vitro Evaluation of Antioxidant, Antimicrobial, and Enzyme Inhibitory Activities of Erucaria uncata and Thymeleae hirsuta. Biocatal. Agric. Biotechnol. 2020, 29, 101834. [Google Scholar] [CrossRef]
- Silva Júnior, M.E.; Araújo, M.V.R.L.; Santana, A.A.; Silva, F.L.H.; Maciel, M.I.S. Ultrasound-Assisted Extraction of Bioactive Compounds from Ciriguela (Spondias purpurea L.) Peel: Optimization and Comparison with Conventional Extraction and Microwave. Arab. J. Chem. 2021, 14, 103260. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization Extraction Conditions for Improving Phenolic Content and Antioxidant Activity in Berberis Asiatica Fruits Using Response Surface Methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Rana, M.R.; Maisha, M.R.; Sayem, A.S.M.; Rahman, M.; Ara, R. Optimization of Ultrasound-Assisted Extraction of Phenolic Content & Antioxidant Activity of Hog Plum (Spondias pinnata L. f. Kurz) Pulp by Response Surface Methodology. Heliyon 2022, 8, e11109. [Google Scholar] [CrossRef]
- Feki, F.; Klisurova, D.; Masmoudi, M.A.; Choura, S.; Denev, P.; Trendafilova, A.; Chamkha, M.; Sayadi, S. Optimization of Microwave Assisted Extraction of Simmondsins and Polyphenols from Jojoba (Simmondsia chinensis) Seed Cake Using Box-Behnken Statistical Design. Food Chem. 2021, 356, 129670. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of Polyphenol Extraction from Hypericum Perforatum (St. John’s Wort) Using Aqueous Glycerol and Response Surface Methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of Antioxidant Activity of Some Medicinal Plants Having High Content of Caffeic Acid Derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef]
- Hani, N.M.; Torkamani, A.E.; Zainul Abidin, S.; Mahmood, W.A.K.; Juliano, P. The Effects of Ultrasound Assisted Extraction on Antioxidative Activity of Polyphenolics Obtained from Momordica Charantia Fruit Using Response Surface Approach. Food Biosci. 2017, 17, 7–16. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Nivetha, C.V. Optimization of Ultrasound-Assisted Extraction of Natural Pigments from Bougainvillea Glabra Flowers. Ind. Crops Prod. 2015, 63, 182–189. [Google Scholar] [CrossRef]
- Rodrigues, S.; Fernandes, F.A.N.; de Brito, E.S.; Sousa, A.D.; Narain, N. Ultrasound Extraction of Phenolics and Anthocyanins from Jabuticaba Peel. Ind. Crops Prod. 2015, 69, 400–407. [Google Scholar] [CrossRef]
- Santos, L.G.; Martins, V.G. Optimization of the Green Extraction of Polyphenols from the Edible Flower Clitoria ternatea by High-Power Ultrasound: A Comparative Study with Conventional Extraction Techniques. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100458. [Google Scholar] [CrossRef]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound Assisted Extraction of Phenolic Compounds from Peaches and Pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, S.; Dang, S.; Han, S.; Yun, C.; Wang, W.; Wang, H. Optimized Ultrasound-Assisted Extraction of Total Polyphenols from Empetrum nigrum and Its Bioactivities. J. Chromatogr. B 2021, 1173, 122699. [Google Scholar] [CrossRef] [PubMed]
- Hefied, F.; B Ahmed, Z.; Yousfi, M. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activities from Pistacia atlantica Desf. Galls Using Response Surface Methodology. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100449. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Shi, N.; Zhang, Z.; Chen, Y.; Yan, M.; Li, Y. Response Surface Methodology Optimization and HPLC-ESI-QTOF-MS/MS Analysis on Ultrasonic-Assisted Extraction of Phenolic Compounds from Okra (Abelmoschus esculentus) and Their Antioxidant Activity. Food Chem. 2023, 405, 134966. [Google Scholar] [CrossRef]
- Zulkifli, S.A.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E. Optimization of Total Phenolic and Flavonoid Contents of Defatted Pitaya (Hylocereus Polyrhizus) Seed Extract and Its Antioxidant Properties. Molecules 2020, 25, 787. [Google Scholar] [CrossRef]
- Hossain, M.A.; Evan, M.S.S.; Moazzem, M.S.; Roy, M.; Zzaman, W. Response Surface Optimization for Antioxidant Extraction from Jackfruit (Artocarpus heterophyllus Lam.) Seed and Pulp. J. Sci. Res. 2020, 12, 397–409. [Google Scholar] [CrossRef]
- Boussadia, M.I.; Gueroui, Y.; Abdaoui, M.Z.; Ayad, D.; Mdjabra, A.; Boudebbouz, A.; Boumaaza, B.; Boudalia, S. Phytochemical, Antioxidant Identification, and Antibacterial Activity of a Traditional Medicinal Plant, Cornulaca monacantha Del. Vegetos 2024, 37, 1925–1937. [Google Scholar] [CrossRef]
- Ali Boutlelis, D.; Mounia, B.; Khaoula, Z.; Ahmed, G. Evaluation of Total Phenolic Contents and Antioxidant Potentials of Ten Medicinal Plants from Algerian Sahara. Int. J. Biol. Agric. Reasearch 2018, 1, 28–36. [Google Scholar]
- Al-Joufi, F.A.; Jan, M.; Zahoor, M.; Nazir, N.; Naz, S.; Talha, M.; Sadiq, A.; Nawaz, A.; Khan, F.A. Anabasis articulata (Forssk.) Moq: A Good Source of Phytochemicals with Antibacterial, Antioxidant, and Antidiabetic Potential. Molecules 2022, 27, 3526. [Google Scholar] [CrossRef]
- Benzineb, E.; Kambouche, N.; Hamiani, A.; Bellahouel, S.; Zitouni, H.; Toumi, H. Phenolics Compounds and Biological Activity of Leaves of Anabasis Articulata, an Algerian Medicinal Plant. Int. J. Pharm. Res. Allied Sci. 2019, 8, 1–5. [Google Scholar]
- Nafiu, M.O.; Salawu, M.O.; Kazeem, M.I. 21-Antioxidant Activity of African Medicinal Plants. In Medicinal Plant Research in Africa; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 787–803. ISBN 978-0-12-405927-6. [Google Scholar]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of Antioxidant Activity of Medicinal Plants Containing Polyphenol Compounds. Comparison of Two Extraction Systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept of in Foods. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–23. ISBN 978-3-030-74767-1. [Google Scholar]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.A.; Alsuwayt, B. Biological Evaluation of Cornulaca Monacantha Del. Int. J. Pharm. Phytopharm. Res. 2019, 9, 71–75. [Google Scholar]

| Factor | Code | Code Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Solid-to-liquid ratio (g/mL) | 0.5:10 | 1:10 | 1.5:10 | |
| Temperature (°C) | 35 | 40 | 45 | |
| Time (min) | 10 | 20 | 30 | |
| Run | Factor | Response Value | ||||
|---|---|---|---|---|---|---|
| Solid-to-Liquid Ratio (g/mL) | Temperature (°C) | Time (min) | Yield (%) | TPC (µg GAE/mg DE) | TFC (µg QE/mg DE) | |
| 1 | −1 (0.5:10) | −1 (35) | 0 (20) | 11.09 | 27.22 | 3.77 |
| 2 | +1 (1.5:10) | −1 (35) | 0 (20) | 7.50 | 32.96 | 4.38 |
| 3 | −1 (0.5:10) | +1 (45) | 0 (20) | 13.50 | 32.07 | 5.36 |
| 4 | +1 (1.5:10) | +1 (45) | 0 (20) | 8.14 | 29.26 | 4.26 |
| 5 | −1 (0.5:10) | 0 (40) | −1 (10) | 12.01 | 33.21 | 3.81 |
| 6 | +1 (1.5:10) | 0 (40) | −1 (10) | 8.28 | 29.25 | 2.23 |
| 7 | −1 (0.5:10) | 0 (40) | +1 (30) | 13.80 | 33.71 | 4.94 |
| 8 | +1 (1.5:10) | 0 (40) | +1 (30) | 7.95 | 39.78 | 5.13 |
| 9 | 0 (1.0:10) | −1 (35) | −1 (10) | 8.57 | 31.38 | 5.14 |
| 10 | 0 (1.0:10) | +1 (45) | −1 (10) | 10.55 | 19.89 | 2.56 |
| 11 | 0 (1.0:10) | −1 (35) | +1 (30) | 11.10 | 25.79 | 4.59 |
| 12 | 0 (1.0:10) | +1 (45) | +1 (30) | 11.47 | 34.77 | 7.94 |
| 13 | 0 (1.0:10) | 0 (40) | 0 (20) | 9.46 | 30.77 | 5.21 |
| 14 | 0 (1.0:10) | 0 (40) | 0 (20) | 10.01 | 31.58 | 4.94 |
| 15 | 0 (1.0:10) | 0 (40) | 0 (20) | 10.99 | 30.26 | 4.77 |
| Yield (%) | TPC (µg GAE/mg DE) | TFC (µg QE/mg DE) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Regression Coefficient | F-Value | p-Value | Estimated Regression Coefficient | F-Value | p-Value | Estimated Regression Coefficient | F-Value | p-Value | |
| Intercept () | 10.15 | 16.19 | 0.0034 | 30.87 | 50.88 | 0.0002 | 4.97 | 38.86 | 0.0004 |
| −2.32 | 118.75 | 0.0001 | 0.6300 | 5.25 | 0.0705 | −0.2350 | 6.48 | 0.0516 | |
| 0.6750 | 10.08 | 0.0247 | −0.1700 | 0.3824 | 0.5634 | 0.2800 | 9.20 | 0.0290 | |
| 0.6138 | 8.34 | 0.0343 | 2.54 | 85.36 | 0.0002 | 1.11 | 143.89 | <0.0001 | |
| −0.4425 | 2.17 | 0.2010 | −2.14 | 30.23 | 0.0027 | −0.4275 | 10.72 | 0.0221 | |
| −0.5300 | 3.11 | 0.1382 | 2.51 | 41.60 | 0.0013 | 0.4425 | 11.49 | 0.0195 | |
| −0.4025 | 1.79 | 0.2382 | 5.12 | 173.26 | <0.0001 | 1.48 | 128.92 | <0.0001 | |
| −0.0042 | 0.0002 | 0.9899 | 2.77 | 46.81 | 0.0010 | −0.7804 | 32.98 | 0.0022 | |
| −0.0917 | 0.0858 | 0.7813 | −3.26 | 64.95 | 0.0005 | 0.2496 | 3.37 | 0.1257 | |
| 0.3608 | 1.33 | 0.3009 | 0.3487 | 0.7427 | 0.4282 | −0.1654 | 1.48 | 0.2778 | |
| Lack of Fit | 0.3362 | 0.8059 | 1.61 | 0.4057 | 1.64 | 0.4002 | |||
| 0.9668 | 0.9892 | 0.9859 | |||||||
| 0.9071 | 0.9698 | 0.9605 | |||||||
| C.V.% | 5.84 | 2.53 | 5.67 | ||||||
| Optimum Extraction Conditions | Predicted Response Values | Experimental Response Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Solid-to-Liquid Ratio (g/mL) | Temperature (°C) | Time (min) | Yield (%) | TPC (µg GAE/mg DE) | TFC (µg QE/mg DE) | Yield (%) | TPC (µg GAE/mg DE) | TFC (µg QE/mg DE) |
| 0.5:10 | 45 | 30 | 14.590 | 37.210 | 7.368 | 14.68 ± 0.16 | 37.27 ± 0.68 | 7.21 ± 0.31 |
| Sample | Yield (%) | TPC (µg GAE/mg DE) | TFC (µg QE/mg DE) |
|---|---|---|---|
| C. monacantha Del. | 14.68 ± 0.16 a | 37.27 ± 0.68 b | 7.21 ± 0.31 a |
| A. articulata (Forssk.) Moq. | 13.56 ± 0.15 b | 58.38 ± 0.65 a | 6.44 ± 0.21 b |
| Sample | Radical Scavenging Activity IC50 (µg/mL) | Reducing Power Activity A0.5 (µg/mL) | |||
|---|---|---|---|---|---|
| DPPH Assay | ABTS+ Assay | CUPRAC Assay | Phenanthroline Assay | Reducing Power Assay | |
| C. monacantha Del. | >200 | >200 | >200 | 168.09 ± 0.74 a | >200 |
| A. articulata (Forssk.) Moq. | >200 | 107.31 ± 0.26 a | 150.55 ± 0.70 a | 108.86 ± 0.75 b | >200 |
| BHA | 5.50 ± 0.10 b | 2.92 ± 0.01 c | 5.72 ± 0.17 b | 3.73 ± 0.09 c | 5.17 ± 0.12 b |
| BHT | 32.69 ± 0.18 a | 4.62 ± 0.07 b | 6.55 ± 0.18 b | 4.75 ± 0.10 c | 9.99 ± 0.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemmadi, S.; Adoui, F.; Dumas, E.; Karoune, S.; Santerre, C.; Gharsallaoui, A. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from the Aerial Part of Plants in the Chenopodiaceae Family Using a Box–Behnken Design. Appl. Sci. 2025, 15, 4688. https://doi.org/10.3390/app15094688
Lemmadi S, Adoui F, Dumas E, Karoune S, Santerre C, Gharsallaoui A. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from the Aerial Part of Plants in the Chenopodiaceae Family Using a Box–Behnken Design. Applied Sciences. 2025; 15(9):4688. https://doi.org/10.3390/app15094688
Chicago/Turabian StyleLemmadi, Sara, Faïza Adoui, Emilie Dumas, Samira Karoune, Cyrille Santerre, and Adem Gharsallaoui. 2025. "Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from the Aerial Part of Plants in the Chenopodiaceae Family Using a Box–Behnken Design" Applied Sciences 15, no. 9: 4688. https://doi.org/10.3390/app15094688
APA StyleLemmadi, S., Adoui, F., Dumas, E., Karoune, S., Santerre, C., & Gharsallaoui, A. (2025). Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from the Aerial Part of Plants in the Chenopodiaceae Family Using a Box–Behnken Design. Applied Sciences, 15(9), 4688. https://doi.org/10.3390/app15094688









