Neurobiomechanical Characterization of Feedforward Phase of Gait Initiation in Chronic Stroke: A Linear and Non-Linear Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Participants
2.3. Instruments
2.3.1. Sample Selection and Characterization
2.3.2. Kinematic and Kinetic Data
2.4. Procedures
2.4.1. Data Processing
2.4.2. Statistical Analysis
3. Results
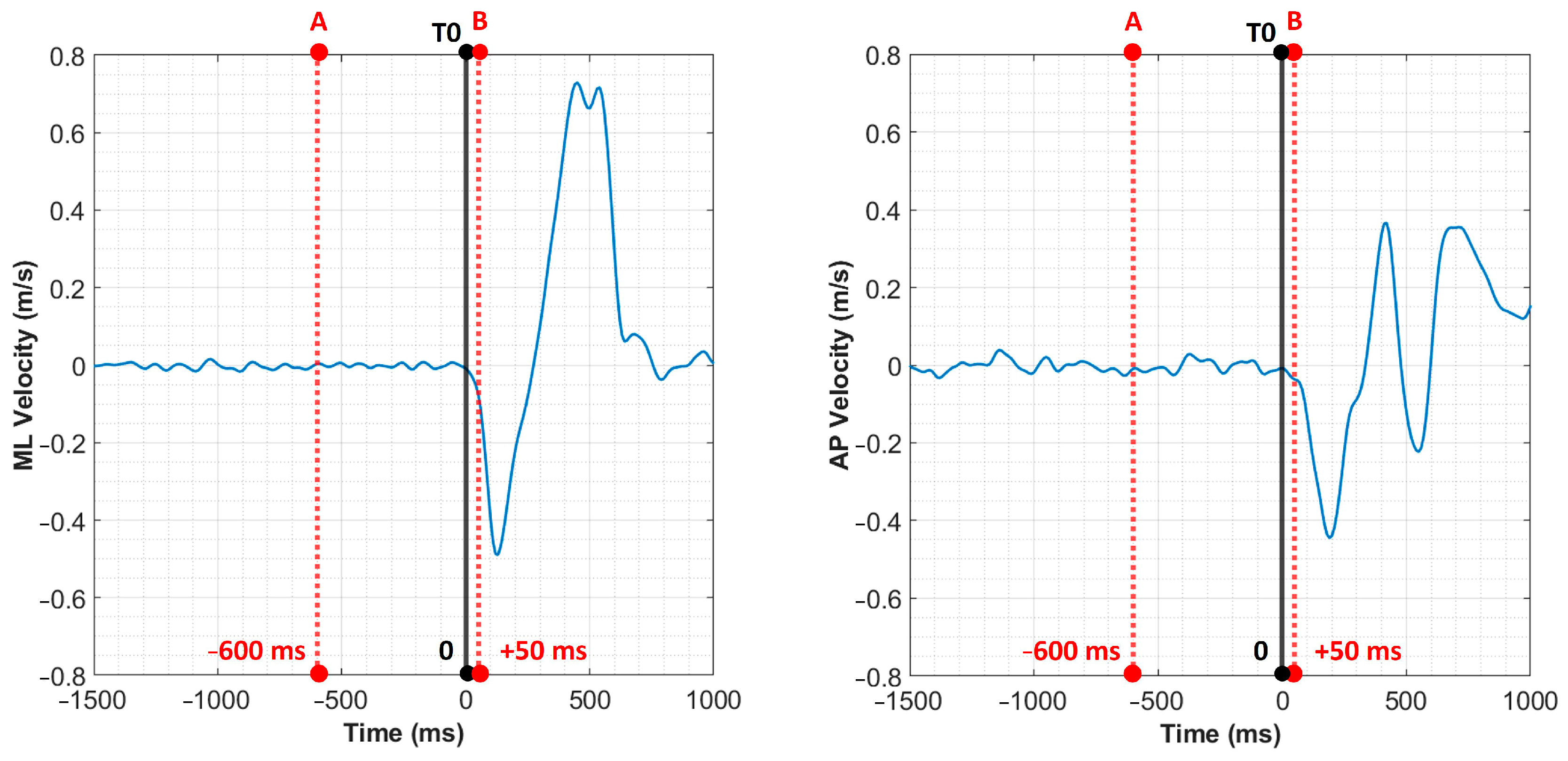
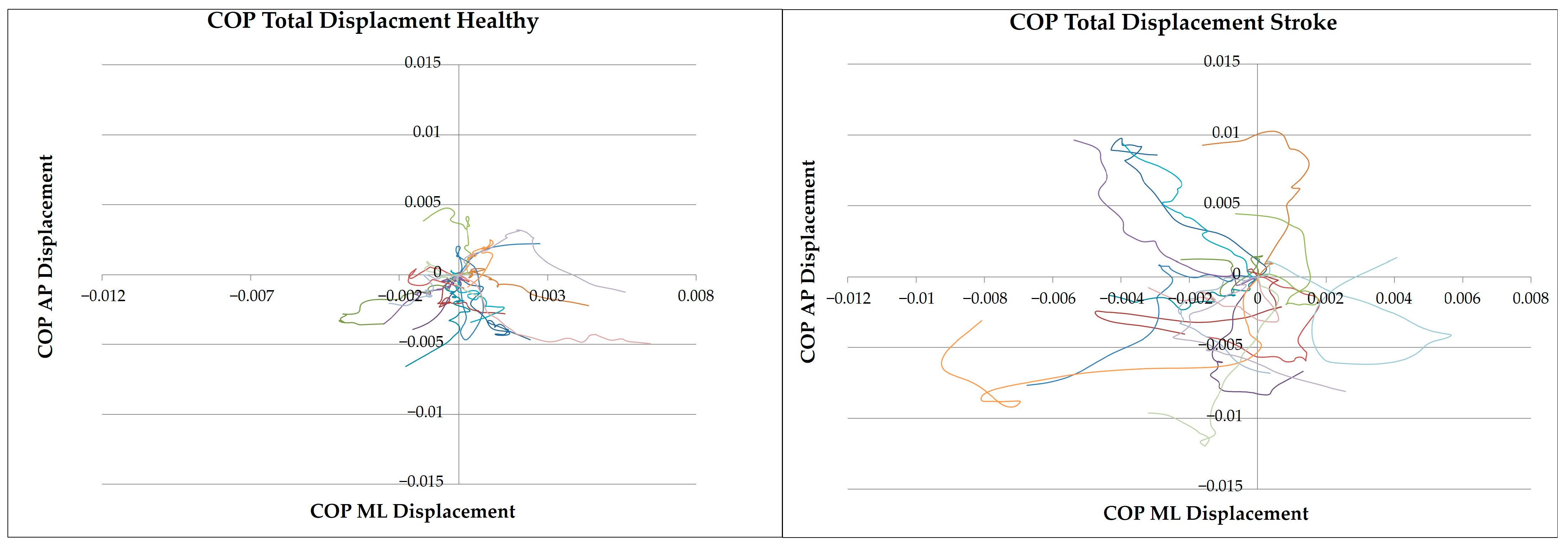
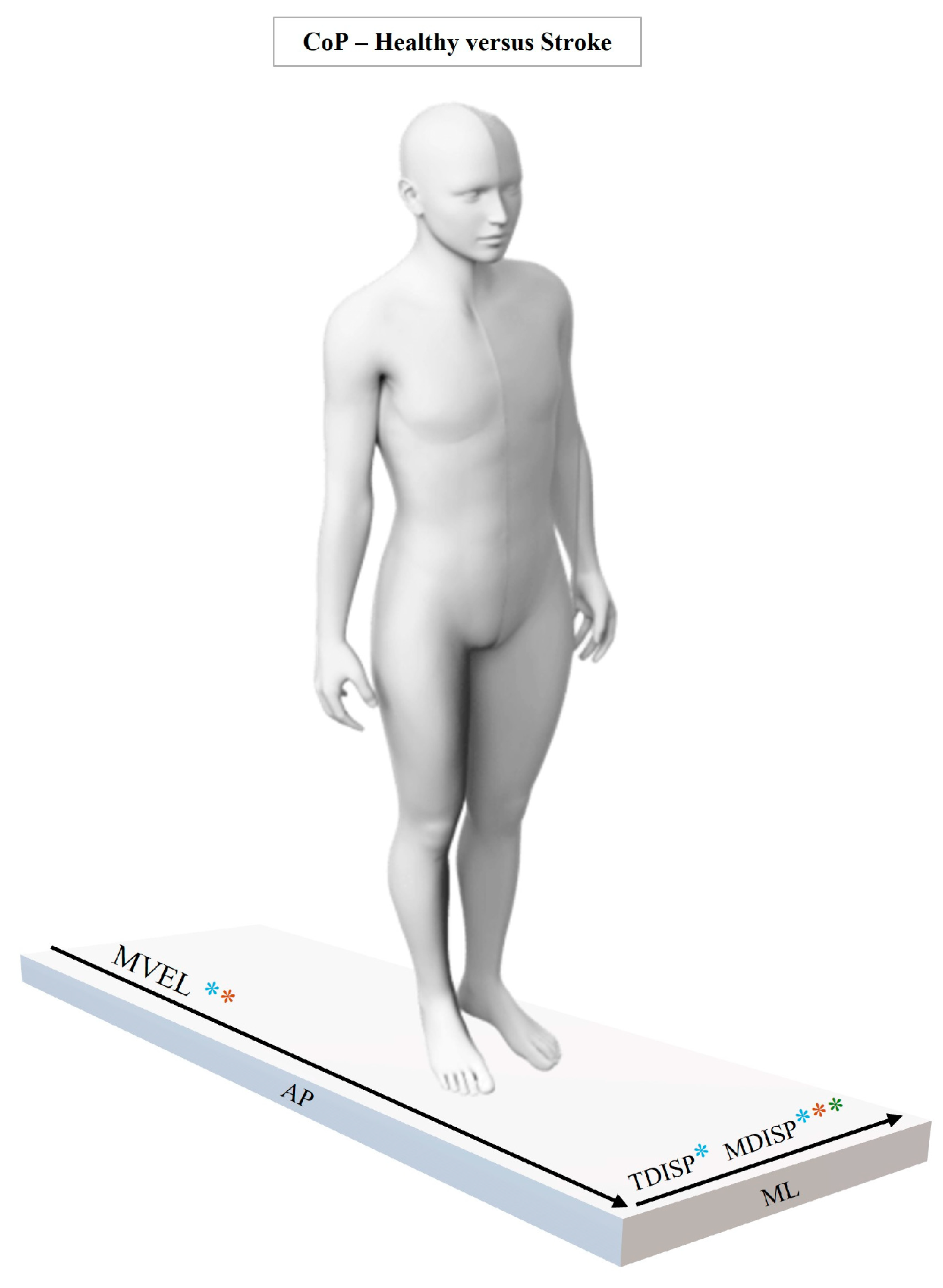
3.1. Kinetic Analysis
3.1.1. Linear Analysis
3.1.2. Non-Linear Analysis
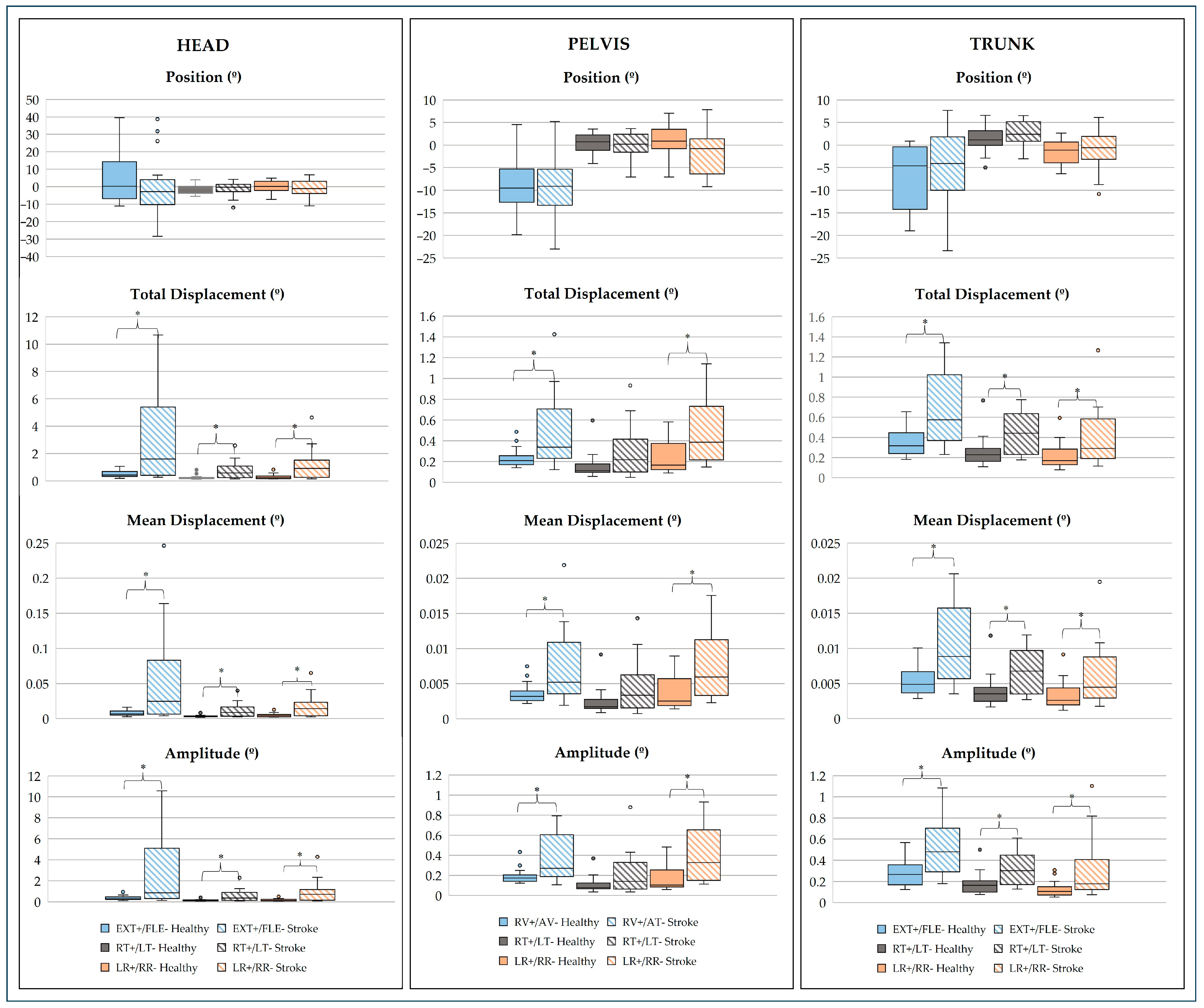
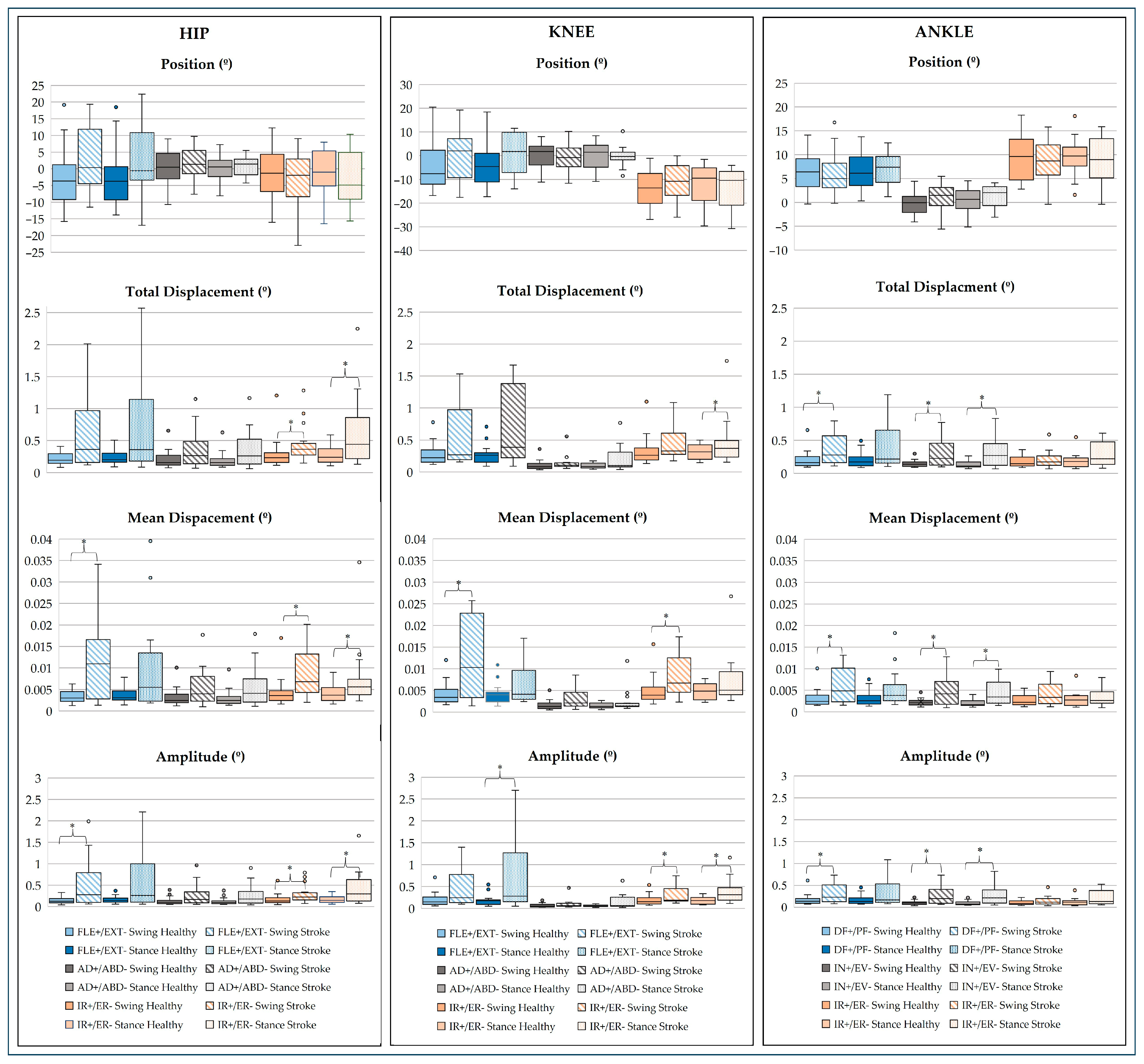
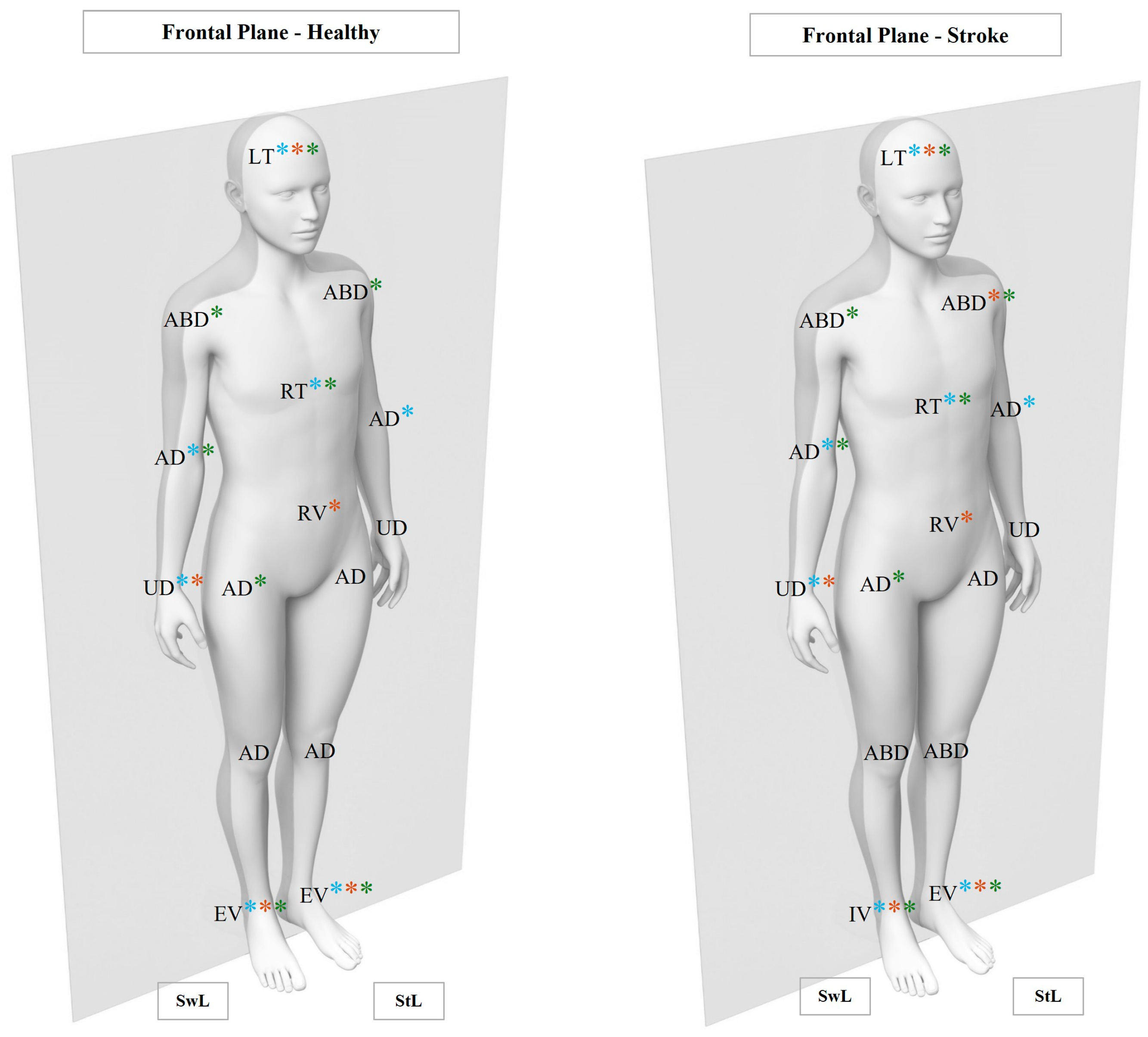
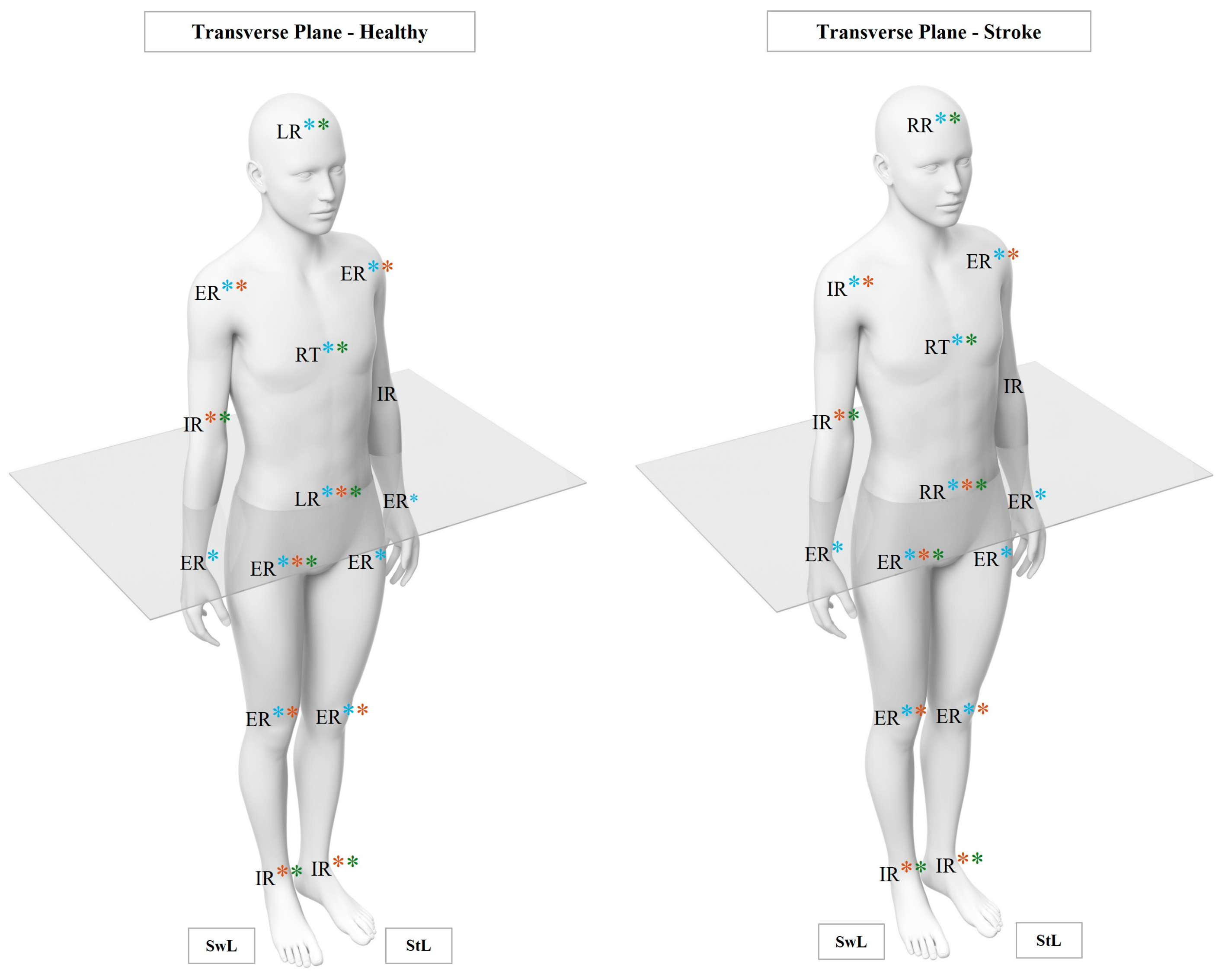
3.2. Kinematic Analysis
3.2.1. Linear Analysis
- Head, Pelvis, and Trunk Joints
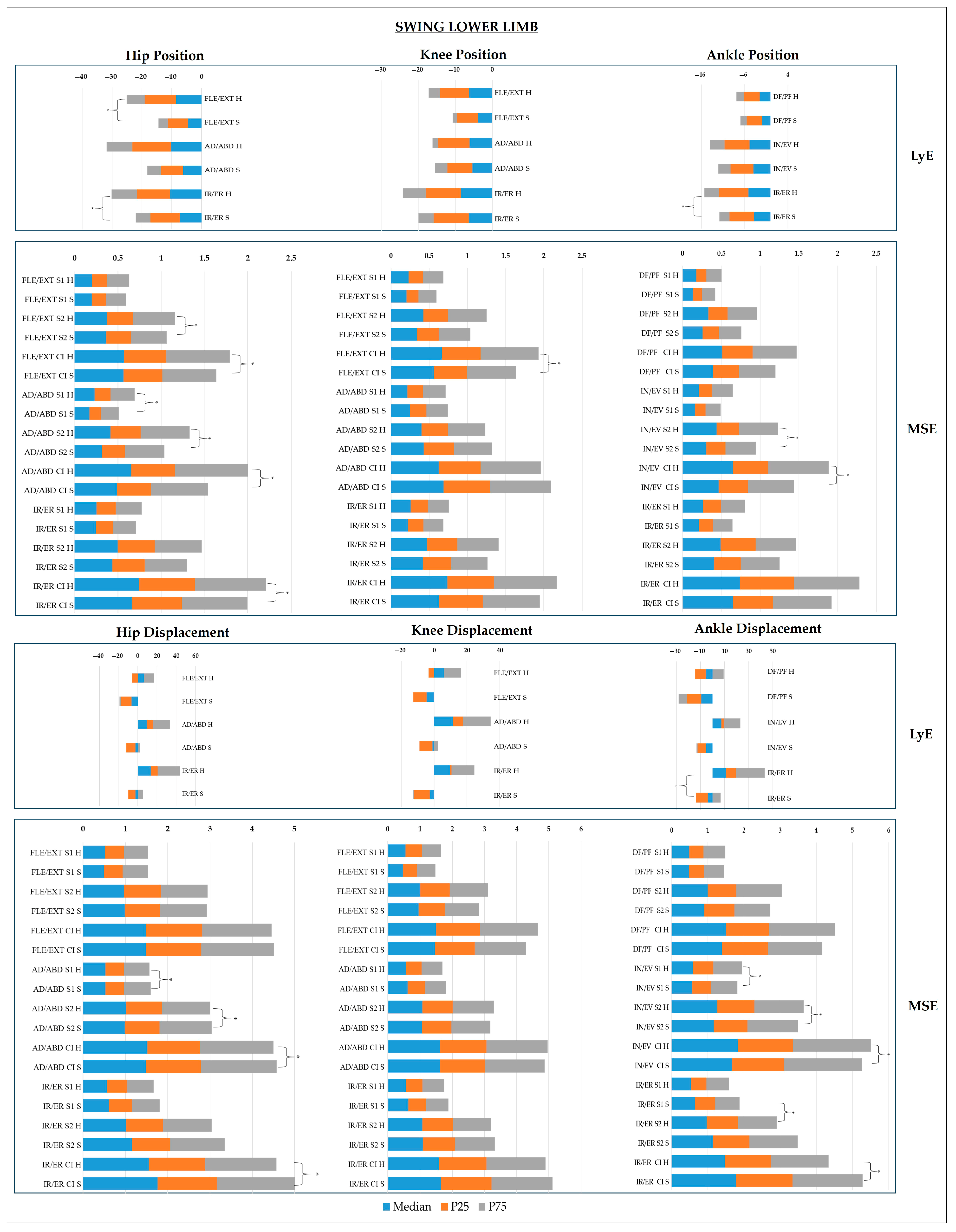
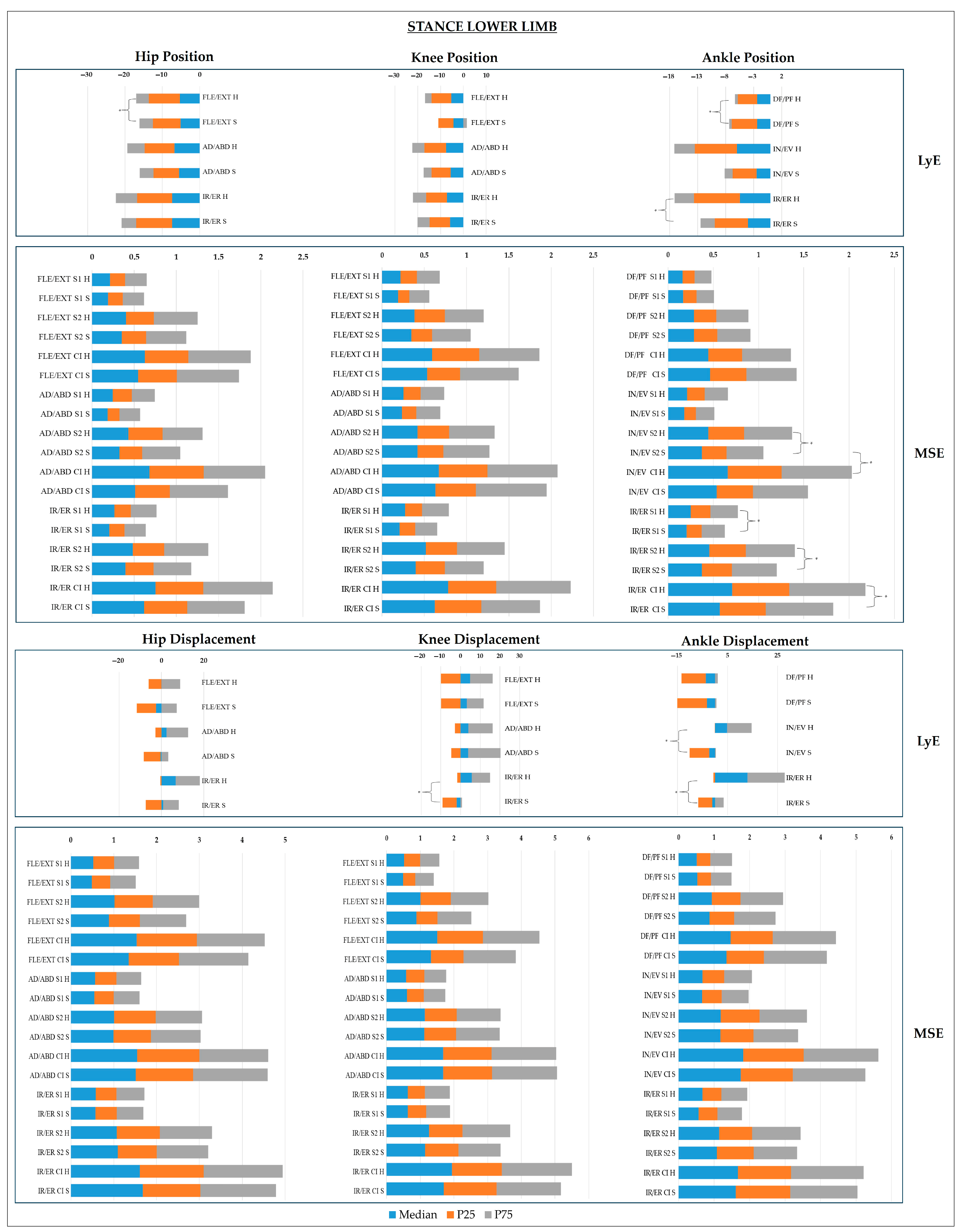
- Lower Limb Joints
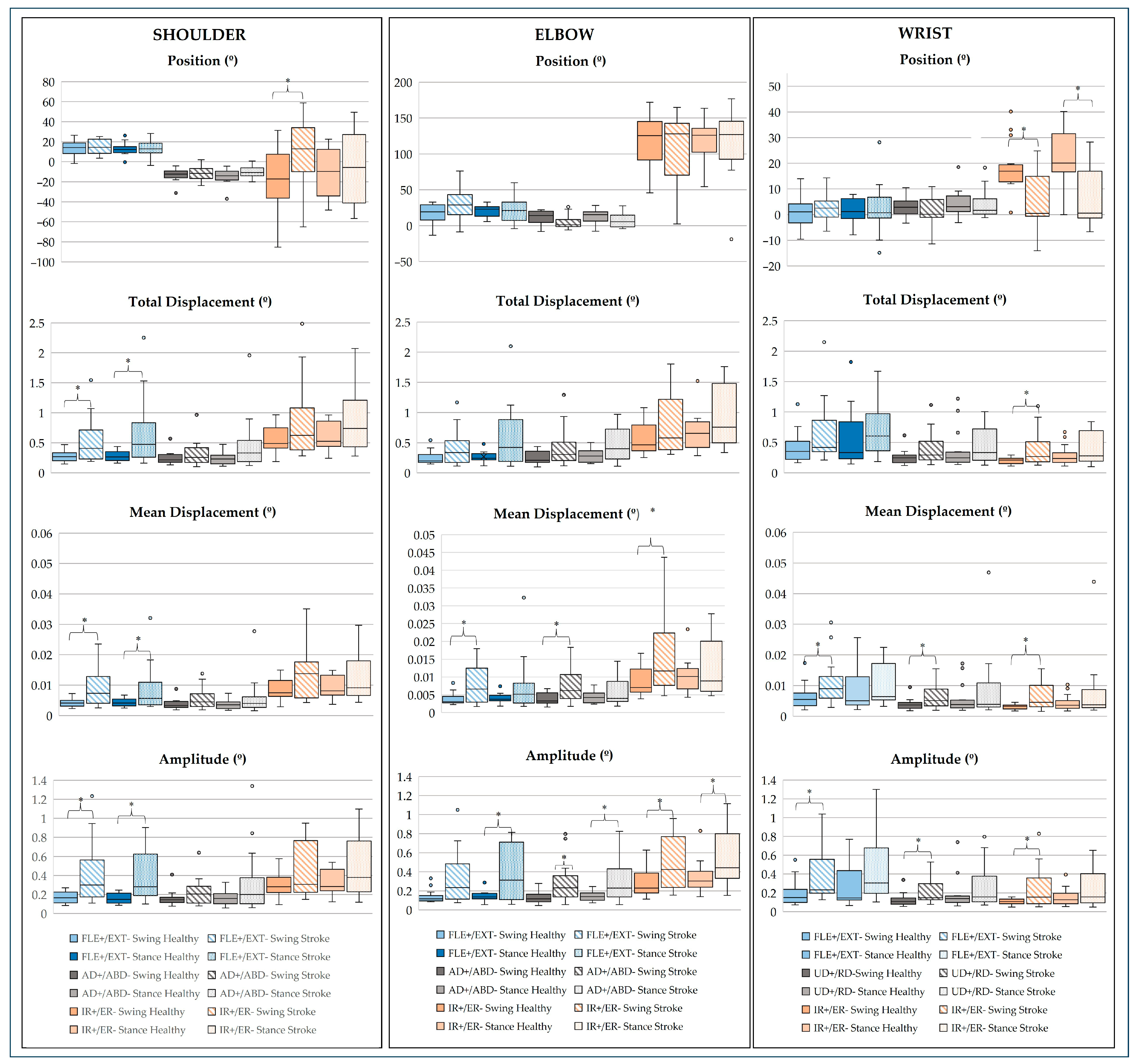
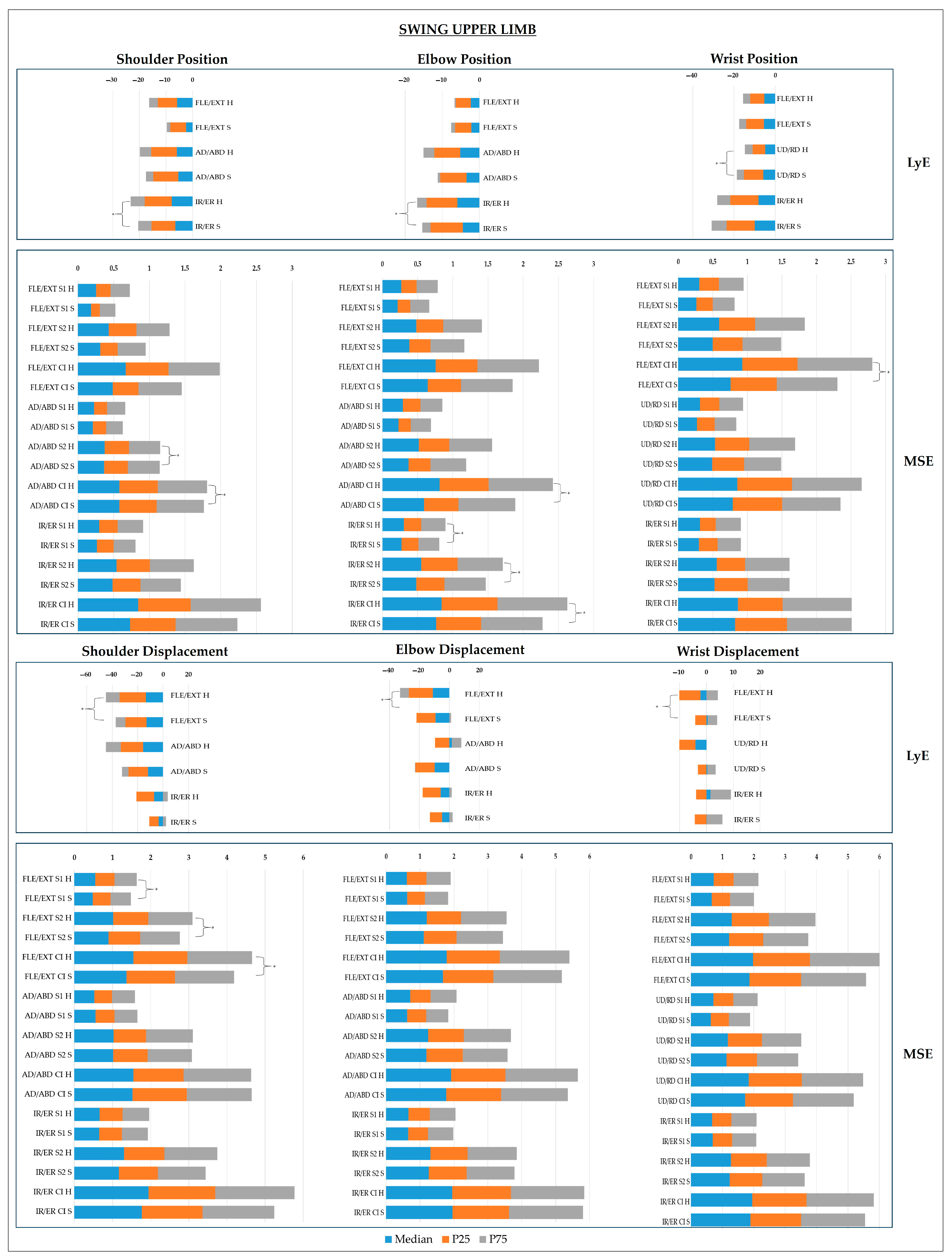
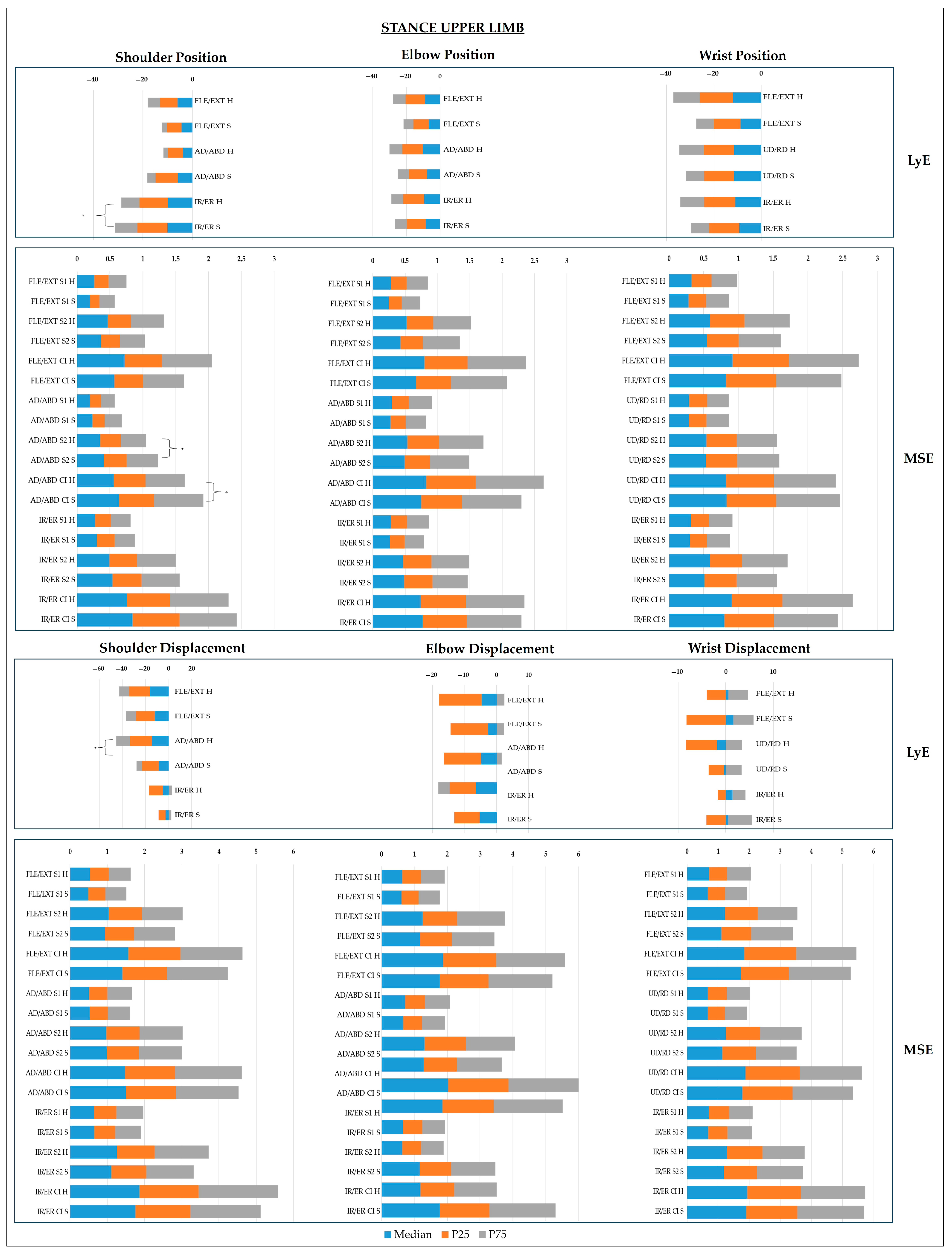
- Upper Limb Joints
3.2.2. Non-Linear Analysis
- Head, Pelvis, and Trunk Joints
- Lower Limb Joints
- Upper Limb Joints
4. Discussion
4.1. CoP Dynamics
4.2. Joint Dynamics
- Sagittal Plane
- Frontal Plane
- Transverse Plane
4.3. Multidimensional Postural Control: Neurobiomechanical Integration of Findings
4.4. Clinical Relevance
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABD | Abduction |
| AD | Adduction |
| AMP | Amplitude |
| AP | Anteroposterior |
| APAs | Anticipatory postural adjustments |
| AV | Anteversion |
| AVC | Cerebrovascular accident |
| BMI | Body mass index |
| CCL | Contralesional left |
| C FuzzyEn | Cross fuzzy entropy |
| CI | Complexity Index |
| CIFI2D | Centre for Research, Training, Innovation, and Intervention in Sport |
| CIR | Centre for Rehabilitation Research |
| CLR | Contralesional right |
| CNS | Central nervous system |
| COM | Centre of mass |
| CoP | Centre of pressure |
| CRS | Corticoreticular systems |
| CSS | Corticospinal systems |
| DISP | Displacement |
| DF | Dorsiflexion |
| EPAs | Early postural adjustments |
| ER | External rotation |
| EV | Eversion |
| EXT | Extension |
| FLE | Flexion |
| FMA-LE | Fugl-Meyer assessment of sensorimotor recovery after stroke—lower extremity |
| GI | Gait initiation |
| H2M | Health and Human Movement Unit |
| IPAQ-SF | International Physical Activity Questionnaire—short form |
| IPSN | Instituto Politécnico de Saúde do Norte |
| IR | Internal rotation |
| ITC | Item total correlation |
| IV | Inversion |
| LABIOMEP | Porto Biomechanics Laboratory |
| LE_FM | Fugl–Meyer assessment—lower extremity |
| LL | Lower limb |
| LR | Left rotation |
| LT | Left tilt |
| LyE | Largest Lyapunov exponent |
| M | Mean |
| m | Metres |
| MCA | Middle cerebral artery |
| MDISP | Mean displacement |
| ML | Mediolateral |
| MMSE | Mini mental state examination |
| MSE | Multiscale entropy |
| MVC | Maximal voluntary contraction |
| MVEL | Mean velocity |
| NIHSS | National Institutes of Health Stroke Scale |
| PC | Postural control |
| PCA | Principal component analysis |
| PF | Plantar flexion |
| POS | Position |
| RD | Radial deviation |
| ROM | Range of motion |
| RR | Right rotation |
| RT | Right tilt |
| RV | Retroversion |
| SampEn | Sample entropy |
| S | Scale |
| SD | Standard deviation |
| SEM | Standard error of measurement |
| SMA | Supplementary motor area |
| SPSS | Statistical Package for Social Science |
| StL | Stance limb |
| SwL | Swing limb |
| TDISP | Total displacement |
| TUG | Timed Up and Go |
| UD | Ulnar deviation |
| UL | Upper limb |
References
- Gill-Body, K.M.; Hedman, L.D.; Plummer, L.; Wolf, L.; Hanke, T.; Quinn, L.; Riley, N.; Kaufman, R.; Verma, A.; Quiben, M.; et al. Movement System Diagnoses for Balance Dysfunction: Recommendations From the Academy of Neurologic Physical Therapy’s Movement System Task Force. Phys. Ther. 2021, 101, pzab153. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef]
- Sibley, K.M.; Beauchamp, M.K.; Van Ooteghem, K.; Straus, S.E.; Jaglal, S.B. Using the Systems Framework for Postural Control to Analyze the Components of Balance Evaluated in Standardized Balance Measures: A Scoping Review. Arch. Phys. Med. Rehabil. 2015, 96, 122–132. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research Into Clinical Practice; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2017. [Google Scholar]
- Vaughan-Graham, J.; Cott, C. Defining a Bobath clinical framework—A modified e-Delphi study. Physiother. Theory Pract. 2016, 32, 612–627. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- Maeda, R.S.; Gribble, P.L.; Pruszynski, J.A. Learning New Feedforward Motor Commands Based on Feedback Responses. Curr. Biol. 2020, 30, 1941–1948.e1943. [Google Scholar] [CrossRef] [PubMed]
- Farinelli, V.; Bolzoni, F.; Marchese, S.M.; Esposti, R.; Cavallari, P. A Novel Viewpoint on the Anticipatory Postural Adjustments During Gait Initiation. Front. Hum. Neurosci. 2021, 15, 709780. [Google Scholar] [CrossRef] [PubMed]
- Klous, M.; Mikulic, P.; Latash, M.L. Early postural adjustments in preparation to whole-body voluntary sway. J. Electromyogr. Kinesiol. 2012, 22, 110–116. [Google Scholar] [CrossRef]
- Latash, M.L.; Zatsiorsky, V. Biomechanics and Motor Control: Defining Central Concepts; Human Kinetics: Champaign, IL, USA, 2015; pp. 1–409. [Google Scholar]
- Kanekar, N.; Aruin, A.S. Improvement of anticipatory postural adjustments for balance control: Effect of a single training session. J. Electromyogr. Kinesiol. 2015, 25, 400–405. [Google Scholar] [CrossRef]
- Harbourne, R.T.; Deffeyes, J.E.; Kyvelidou, A.; Stergiou, N. Complexity of postural control in infants: Linear and nonlinear features revealed by principal component analysis. Nonlinear Dyn. Psychol. Life Sci. 2009, 13, 123–144. [Google Scholar]
- Harbourne, R.T.; Stergiou, N. Movement variability and the use of nonlinear tools: Principles to guide physical therapist practice. Phys. Ther. 2009, 89, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Sumardi, N. Postural Dysfunction in Stroke Rehabilitation. Indones. J. Phys. Med. Rehabil. 2021, 9, 124–135. [Google Scholar] [CrossRef]
- Sousa, A.S.; Silva, A.; Santos, R. Reliability of Two Methods for Identifying the Postural Phase of Gait Initiation in Healthy and Poststroke Subjects. J. Appl. Biomech. 2015, 31, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.S.P.; Moreira, J.; Silva, C.; Mesquita, I.; Silva, A.; Macedo, R.; Santos, R. Postural control during turn on the light task assisted by functional electrical stimulation in post stroke subjects. Sci. Rep. 2022, 12, 6999. [Google Scholar] [CrossRef]
- Halmi, Z.; Stone, T.W.; Dinya, E.; Málly, J. Postural instability years after stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105038. [Google Scholar] [CrossRef]
- Hugues, A.; Di Marco, J.; Ribault, S.; Ardaillon, H.; Janiaud, P.; Xue, Y.; Zhu, J.; Pires, J.; Khademi, H.; Rubio, L.; et al. Limited evidence of physical therapy on balance after stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221700. [Google Scholar] [CrossRef]
- Hong, S.Y.; Moon, Y.; Choi, J.D. Effects of Cognitive Task Training on Dynamic Balance and Gait of Patients with Stroke: A Preliminary Randomized Controlled Study. Med. Sci. Monit. Basic. Res. 2020, 26, e925264. [Google Scholar] [CrossRef]
- Tasseel-Ponche, S.; Yelnik, A.P.; Bonan, I.V. Motor strategies of postural control after hemispheric stroke. Neurophysiol. Clin. 2015, 45, 327–333. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Boix-Sala, L.; Grau-Pellicer, M.; Guzmán-Bernal, J.A.; Caballero-Gómez, F.M.; Urrútia, G. The Effectiveness of Additional Core Stability Exercises in Improving Dynamic Sitting Balance, Gait and Functional Rehabilitation for Subacute Stroke Patients (CORE-Trial): Study Protocol for a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6615. [Google Scholar] [CrossRef]
- Ghamari, N.; Ghaderpanah, R.; Sadrian, S.H.; Fallah, N. Effect of a visual dual task on postural stability-A comparative study using linear and nonlinear methods. Health Sci. Rep. 2023, 6, e1437. [Google Scholar] [CrossRef]
- Djurovic, O.; Mihaljevic, O.; Radovanovic, S.; Kostic, S.; Vukicevic, M.; Brkic, B.G.; Stankovic, S.; Radulovic, D.; Vukomanovic, I.S.; Radevic, S.R. Risk Factors Related to Falling in Patients after Stroke. Iran. J. Public Health 2021, 50, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, A.; Galli, M.; Fernandes, P.; Rigoldi, C.; Carmo, A.; Barros, R. Spontaneous Improvement in PosturalControl after Stroke: A LongitudinalProspective Study. Int. J. Eng. Sci. Technol. 2015, 4, 2705–2714. [Google Scholar] [CrossRef]
- Kang, N.; Da Lee, R.; Lee, J.H.; Hwang, M.-H. Functional Balance and Postural Control Improvements in Patients with Stroke after Non-Invasive Brain Stimulation: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 101, 141–153. [Google Scholar] [CrossRef]
- Isho, T.; Usuda, S. Association of trunk control with mobility performance and accelerometry-based gait characteristics in hemiparetic patients with subacute stroke. Gait Posture 2016, 44, 89–93. [Google Scholar] [CrossRef]
- Portnoy, S.; Reif, S.; Mendelboim, T.; Rand, D. Postural control of individuals with chronic stroke compared to healthy participants: Timed-Up-and-Go, Functional Reach Test and center of pressure movement. Eur. J. Phys. Rehabil. Med. 2017, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Vonesch, A.; Duhot, C.; Lelard, T.; Léonard, G.; Błażkiewicz, M.; Mouras, H. Non-Linear Measures of Postural Control in Response to Painful and Non-Painful Visual Stimuli. Entropy 2023, 25, 1561. [Google Scholar] [CrossRef] [PubMed]
- Hua, A.; Wang, G.; Bai, J.; Hao, Z.; Liu, J.; Meng, J.; Wang, J. Nonlinear dynamics of postural control system under visual-vestibular habituation balance practice: Evidence from EEG, EMG and center of pressure signals. Front. Hum. Neurosci. 2024, 18, 1371648. [Google Scholar] [CrossRef]
- Moreno, F.J.; Caballero, C.; Barbado, D. Postural control strategies are revealed by the complexity of fractional components of COP. J. Neurophysiol. 2022, 127, 1289–1297. [Google Scholar] [CrossRef]
- Jagroop, D.; Aryan, R.; Schinkel-Ivy, A.; Mansfield, A. Reliability of novel centre of pressure measures of quiet standing balance in people with chronic stroke. Gait Posture 2023, 102, 159–163. [Google Scholar] [CrossRef]
- Lacour, M.; Bernard-Demanze, L.; Dumitrescu, M. Posture control, aging, and attention resources: Models and posture-analysis methods. Neurophysiol. Clin. 2008, 38, 411–421. [Google Scholar] [CrossRef]
- van Emmerik, R.E.A.; Ducharme, S.W.; Amado, A.C.; Hamill, J. Comparing dynamical systems concepts and techniques for biomechanical analysis. J. Sport. Health Sci. 2016, 5, 3–13. [Google Scholar] [CrossRef]
- Caballero, C.; Barbado, D.; Moreno, F. Non-linear tools and methodological concerns measuring human movement variability: An overview. Eur. J. Hum. Mov. 2014, 32, 61–81. [Google Scholar]
- Kędziorek, J.; Błażkiewicz, M. Nonlinear Measures to Evaluate Upright Postural Stability: A Systematic Review. Entropy 2020, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Pinho, F.; Pinho, L.; Silva, S.; Figueira, V.; Vilas-Boas, J.P.; Silva, A. Biomechanical Assessment Methods Used in Chronic Stroke: A Scoping Review of Non-Linear Approaches. Sensors 2024, 24, 2338. [Google Scholar] [CrossRef]
- Ghomashchi, H. Investigating the effects of visual biofeedback therapy on recovery of postural balance in stroke patients using a complexity measure. Top. Stroke Rehabil. 2016, 23, 178–183. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Khalaf, K.; Ghomashchi, H.; Taghizadeh, G.; Ebrahimi, I.; Taghavi Azar Sharabiani, P.; Mousavi, S.J.; Parnianpour, M. Effects of cognitive load on the amount and temporal structure of postural sway variability in stroke survivors. Exp. Brain Res. 2018, 236, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, R.; Talebian, S.; Hadian, M.R.; Olyaei, G.; Bagheri, H. Characteristic muscle activity patterns during gait initiation in the healthy younger and older adults. Gait Posture 2016, 43, 148–153. [Google Scholar] [CrossRef]
- Lelard, T.; Doutrellot, P.-L.; Temfemo, A.; Ahmaidi, S. Electromyographic Pattern during Gait Initiation Differentiates Yoga Practitioners among Physically Active Older Subjects. Front. Hum. Neurosci. 2017, 11, 300. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.L. Balance control during gait initiation: State-of-the-art and research perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef]
- Fettrow, T.; Reimann, H.; Grenet, D.; Thompson, E.; Crenshaw, J.; Higginson, J.; Jeka, J. Interdependence of balance mechanisms during bipedal locomotion. PLoS ONE 2019, 14, e0225902. [Google Scholar] [CrossRef]
- Kilby, M.C.; Molenaar, P.C.M.; Newell, K.M. Models of Postural Control: Shared Variance in Joint and COM Motions. PLoS ONE 2015, 10, e0126379. [Google Scholar] [CrossRef] [PubMed]
- Altenburger, P.; Ambike, S.S.; Haddad, J.M. Integrating Motor Variability Evaluation Into Movement System Assessment. Phys. Ther. 2023, 103, pzad075. [Google Scholar] [CrossRef] [PubMed]
- Palmieri-Smith, R.; Ingersoll, C.; Stone, M.; Krause, B. Center-of-Pressure Parameters Used in the Assessment of Postural Control. J. Sport. Rehabil. 2002, 11, 51–66. [Google Scholar] [CrossRef]
- Aghaie Ataabadi, P.; Sarvestan, J.; Alaei, F.; Yazdanbakhsh, F.; Abbasi, A. Linear and non-linear analysis of lower limb joints angle variability during running at different speeds. Acta Gymnica 2021, 51, 1–6. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Typical and atypical development of reaching and postural control in infancy. Dev. Med. Child. Neurol. 2013, 55 (Suppl. S4), 5–8. [Google Scholar] [CrossRef]
- Guerreiro, M.; Silva, A.P.; Botelho, M.; Leitão, O.; Castro-Caldas, A.; Garcia, C. Adaptação à população portuguesa da tradução do Mini Mental State Examination. Rev. Port. Neurol. 1994, 1 (Suppl. S1), 1–9. [Google Scholar]
- Morgado, J.; Rocha, C.; Maruta, C.; Guerreiro, M.; Martins, I. Novos valores Normativos do mini-mental state examination. Sinapse 2009, 9, 10–16. [Google Scholar]
- Silva, A.; Sousa, A.S.; Silva, C.; Tavares, J.M.; Santos, R.; Sousa, F. Ankle antagonist coactivation in the double-support phase of walking: Stroke vs. healthy subjects. Somat. Mot. Res. 2015, 32, 153–157. [Google Scholar] [CrossRef]
- Rowe, E.; Beauchamp, M.K.; Astephen Wilson, J. Age and sex differences in normative gait patterns. Gait Posture 2021, 88, 109–115. [Google Scholar] [CrossRef]
- Fan, M.; Lyu, J.; He, P. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ). Chin. J. Epidemiol. 2014, 35, 961–964. Available online: http://www.ipaq.ki.se (accessed on 22 April 2025).
- Walcott, B.P.; Miller, J.C.; Kwon, C.S.; Sheth, S.A.; Hiller, M.; Cronin, C.A.; Schwamm, L.H.; Simard, J.M.; Kahle, K.T.; Kimberly, W.T.; et al. Outcomes in severe middle cerebral artery ischemic stroke. Neurocrit Care 2014, 21, 20–26. [Google Scholar] [CrossRef]
- Sousa, A.S.P.; Silva, A.; Santos, R.; Sousa, F.; Tavares, J. Interlimb coordination during the stance phase of gait in subjects with stroke. Arch. Phys. Med. Rehabil. 2013, 94, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Lannin, N.A.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, G.; Winstein, C.; van Wegen, E.E.; Wolf, S.L.; et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Campaniço, H. Validade Simultânea do Questionário Internacional de Atividade Física Através da Medição Objetiva da Atividade Física por Actigrafia Proporcional. Master's Thesis, Faculdade de Motricidade Humana, Universidade de Lisboa, Lisboa, Portugal, 2016. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; SjÖStrÖM, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.F.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.V. Adaptação e Validação Cultural e Linguística do Fugl-Meyer Assessment of Sensorimotor Recovery after Stroke. Bachelor’s Thesis, Escola Superior de Tecnologia da Saúde de Coimbra, Coimbra, Portugal, 2003. [Google Scholar]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef]
- Kwong, P.W.H.; Ng, S.S.M. Cutoff Score of the Lower-Extremity Motor Subscale of Fugl-Meyer Assessment in Chronic Stroke Survivors: A Cross-Sectional Study. Arch. Phys. Med. Rehabil. 2019, 100, 1782–1787. [Google Scholar] [CrossRef]
- Freitas, M.; Pinho, F.; Cruz-Martins, N.; Pinho, L.; Silva, S.; Figueira, V.; Vilas-Boas, J.P.; Silva, A. European Portuguese version of the Mini-BESTest: A cross-cultural adaptation and psychometric measurements in individuals with sensorimotor impairments. Disabil. Rehabil. 2024. ahead of print, 1–11. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef]
- Cappozzo, A.; Catani, F.; Della Croce, U.; Leardini, A. Position and orientation in space of bones during movement: Anatomical frame definition and determination. Clin. Biomech. 1995, 10, 171–178. [Google Scholar] [CrossRef]
- Leardini, A.; Sawacha, Z.; Paolini, G.; Ingrosso, S.; Nativo, R.; Benedetti, M.G. A new anatomically based protocol for gait analysis in children. Gait Posture 2007, 26, 560–571. [Google Scholar] [CrossRef]
- Koltermann, J.J.; Floessel, P.; Hammerschmidt, F.; Disch, A.C. The Influence of Anthropometric Variables and Filtering Frequency on Center of Pressure Data. Sensors 2023, 23, 5105. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Jaeger, S.; Schwer, J.; Seitz, A.; Hamann, I.; Werner, M.; Thorwaechter, C.; Santos, I.; Wendler, T.; Nebel, D.; et al. Accuracy measurement of different marker based motion analysis systems for biomechanical applications: A round robin study. PLoS ONE 2022, 17, e0271349. [Google Scholar] [CrossRef]
- Couto, A.G.B.; Vaz, M.A.P.; Pinho, L.; Félix, J.; Moreira, J.; Pinho, F.; Mesquita, I.A.; Mesquita Montes, A.; Crasto, C.; Sousa, A.S.P. Interlimb Coordination during Double Support Phase of Gait in People with and without Stroke. J. Mot. Behav. 2024, 56, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Gama, G.L.; Celestino, M.L.; Barela, J.A.; Barela, A.M.F. Gait initiation and partial body weight unloading for functional improvement in post-stroke individuals. Gait Posture 2019, 68, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Â.; Sousa, A.S.P.; Rocha, N.; Tavares, J. The Influence of a Cognitive Task on the Postural Phase of Gait Initiation in Parkinson’s Disease: An Electromyographic-Based Analysis. Mot. Control 2017, 21, 249–264. [Google Scholar] [CrossRef]
- Caderby, T.; Yiou, E.; Peyrot, N.; de Viviés, X.; Bonazzi, B.; Dalleau, G. Effects of Changing Body Weight Distribution on Mediolateral Stability Control during Gait Initiation. Front. Hum. Neurosci. 2017, 11, 127. [Google Scholar] [CrossRef]
- Rajachandrakumar, R.; Fraser, J.E.; Schinkel-Ivy, A.; Inness, E.L.; Biasin, L.; Brunton, K.; McIlroy, W.E.; Mansfield, A. Atypical anticipatory postural adjustments during gait initiation among individuals with sub-acute stroke. Gait Posture 2017, 52, 325–331. [Google Scholar] [CrossRef]
- Osada, Y.; Motojima, N.; Kobayashi, Y.; Yamamoto, S. Differences in mediolateral dynamic stability during gait initiation according to whether the non-paretic or paretic leg is used as the leading limb. PLoS ONE 2022, 17, e0267577. [Google Scholar] [CrossRef]
- Derrick, T.R.; van den Bogert, A.J.; Cereatti, A.; Dumas, R.; Fantozzi, S.; Leardini, A. ISB recommendations on the reporting of intersegmental forces and moments during human motion analysis. J. Biomech. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Chen, A.; Selvaraj, S.; Krishnan, V.; Asgari, S. Accuracy and Reliability of Onset Detection Algorithms in Gait Initiation for Healthy Controls and Participants With Parkinson’s Disease. J. Appl. Biomech. 2019, 35, 393–400. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.; Peng, C.-K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2005, 71, 021906. [Google Scholar] [CrossRef]
- Busa, M.A.; van Emmerik, R.E.A. Multiscale entropy: A tool for understanding the complexity of postural control. J. Sport. Health Sci. 2016, 5, 44–51. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining Lyapunov exponents from a time series. Physica D 1985, 16, 285–317. [Google Scholar] [CrossRef]
- Mademli, L.; Mavridi, D.; Bohm, S.; Patikas, D.A.; Santuz, A.; Arampatzis, A. Standing on unstable surface challenges postural control of tracking tasks and modulates neuromuscular adjustments specific to task complexity. Sci. Rep. 2021, 11, 6122. [Google Scholar] [CrossRef] [PubMed]
- Marouvo, J.; Sousa, F.; Fernandes, O.; Castro, M.A.; Paszkiel, S. Gait Kinematics Analysis of Flatfoot Adults. Appl. Sci. 2021, 11, 7077. [Google Scholar] [CrossRef]
- Broomhead, D.S.; King, G.P. Extracting qualitative dynamics from experimental data. Phys. D Nonlinear Phenom. 1986, 20, 217–236. [Google Scholar] [CrossRef]
- Matilla-García, M.; Morales, I.; Rodríguez, J.M.; Ruiz Marín, M. Selection of Embedding Dimension and Delay Time in Phase Space Reconstruction via Symbolic Dynamics. Entropy 2021, 23, 221. [Google Scholar] [CrossRef]
- Vlachos, I.; Kugiumtzis, D. State Space Reconstruction for Multivariate Time Series Prediction. arXiv 2008, arXiv:0809.2220. [Google Scholar]
- Costa, M.D.; Goldberger, A.L. Generalized Multiscale Entropy Analysis: Application to Quantifying the Complex Volatility of Human Heartbeat Time Series. Entropy 2015, 17, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Marôco, J. Análise Estatística com o SPSS Statistics: 7ª Edição; ReportNumber, Lda: Pêro Pinheiro, Portugal, 2018. [Google Scholar]
- Tomita, Y.; Mullick, A.A.; Feldman, A.G.; Levin, M.F. Altered Anticipatory Postural Adjustments During Whole-Body Reaching in Subjects With Stroke. Neurorehabilit. Neural Repair. 2024, 38, 176–186. [Google Scholar] [CrossRef]
- Lundy-Ekman, L.; Weyer, A. Neuroscience: Fundamentals for Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Delafontaine, A.; Vialleron, T.; Hussein, T.; Yiou, E.; Honeine, J.-L.; Colnaghi, S. Anticipatory Postural Adjustments During Gait Initiation in Stroke Patients. Front. Neurol. 2019, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Onuma, R.; Masuda, T.; Hoshi, F.; Matsuda, T.; Sakai, T.; Okawa, A.; Jinno, T. Measurements of the centre of pressure of individual legs reveal new characteristics of reduced anticipatory postural adjustments during gait initiation in patients with post-stroke hemiplegia. J. Rehabil. Med. 2021, 53, jrm00211. [Google Scholar] [CrossRef]
- Gandolfi, M.; Valè, N.; Dimitrova, E.; Zanolin, M.E.; Mattiuz, N.; Battistuzzi, E.; Beccari, M.; Geroin, C.; Picelli, A.; Waldner, A.; et al. Robot-Assisted Stair Climbing Training on Postural Control and Sensory Integration Processes in Chronic Post-stroke Patients: A Randomized Controlled Clinical Trial. Front. Neurosci. 2019, 13, 1143. [Google Scholar] [CrossRef]
- Moisan, G.; Chayasit, P.; Boonsinsukh, R.; Nester, C.J.; Hollands, K. Postural control during quiet standing and voluntary stepping response tasks in individuals post-stroke: A case-control study. Top. Stroke Rehabil. 2022, 29, 465–472. [Google Scholar] [CrossRef]
- Mohapatra, S.; Aruin, A.S. Static and dynamic visual cues in feed-forward postural control. Exp. Brain Res. 2013, 224, 25–34. [Google Scholar] [CrossRef]
- Nandi, T.; Fisher, B.; Hortobágyi, T.; Salem, G. Increasing mediolateral standing sway is associated with increasing corticospinal excitability, and decreasing M1 inhibition and facilitation. Gait Posture 2018, 60, 135–140. [Google Scholar] [CrossRef]
- Roerdink, M.; De Haart, M.; Daffertshofer, A.; Donker, S.F.; Geurts, A.C.; Beek, P.J. Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Exp. Brain Res. 2006, 174, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Stergiou, N.; Patterson, T.S.; Patten, C.; Richards, L.G. Speed and Rhythm Affect Temporal Structure of Variability in Reaching Poststroke: A Pilot Study. J. Mot. Behav. 2017, 49, 35–45. [Google Scholar] [CrossRef]
- Stergiou, N. Nonlinear Analysis for Human Movement Variability; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–388. [Google Scholar]
- Andreasen, S.C.; Wright, T.R.; Crenshaw, J.R.; Reisman, D.S.; Knarr, B.A. Relationships of Linear and Non-linear Measurements of Post-stroke Walking Activity and Their Relationship to Weather. Front. Sports Act. Living 2020, 2, 551542. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke: Pathophysiology and rehabilitation strategies. Neurophysiol. Clin. 2015, 45, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Plummer, P.; Altmann, L.; Feld, J.; Zukowski, L.; Najafi, B.; Giuliani, C. Attentional prioritization in dual-task walking: Effects of stroke, environment, and instructed focus. Gait Posture 2020, 79, 3–9. [Google Scholar] [CrossRef]
- Stergiou, N.; Harbourne, R.; Cavanaugh, J. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006, 30, 120–129. [Google Scholar] [CrossRef]
- Biryukova, E.; Sirotkina, I. Forward to Bernstein: Movement Complexity as a New Frontier. Front. Neurosci. 2020, 14, 1–17. [Google Scholar] [CrossRef]
- Mehdizadeh, S. The largest Lyapunov exponent of gait in young and elderly individuals: A systematic review. Gait Posture 2018, 60, 241–250. [Google Scholar] [CrossRef]
- Curuk, E.; Aruin, A.S. Perturbation-based training enhances anticipatory postural control in individuals with chronic stroke: A pilot study. Int. J. Rehabil. Res. 2022, 45, 72–78. [Google Scholar] [CrossRef]
- Lafage, R.; Duvvuri, P.; Elysee, J.; Diebo, B.; Bess, S.; Burton, D.; Daniels, A.; Gupta, M.; Hostin, R.; Kebaish, K.; et al. Quantifying the Contribution of Lower Limb Compensation to Upright Posture. Spine 2023, 48, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Cirillo, J.; Stinear, C.; Byblow, W. Neurophysiology of motor skill learning in chronic stroke. Clin. Neurophysiol. 2020, 131, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Srivastava, S.; Sutton, F.; Zhang, Y.; Badran, B.; Kautz, S. Compensatory increase in ipsilesional supplementary motor area and premotor connectivity is associated with greater gait impairments: A personalized fMRI analysis in chronic stroke. Front. Hum. Neurosci. 2024, 18, 1340374. [Google Scholar] [CrossRef]
- Takahashi, S.; Hoshino, M.; Ohyama, S.; Hori, Y.; Yabu, A.; Kobayashi, A.; Tsujio, T.; Kotake, S.; Nakamura, H. Relationship of back muscle and knee extensors with the compensatory mechanism of sagittal alignment in a community-dwelling elderly population. Sci. Rep. 2021, 11, 2179. [Google Scholar] [CrossRef]
- Chen, F.-C.; Chu, C.-H.; Pan, C.-Y.; Tsai, C.-L. Not just a light fingertip touch: A facilitation of functional integration between body sway and visual search in older adults. Gait Posture 2018, 62, 105–110. [Google Scholar] [CrossRef]
- Stamenkovic, A.; Stapley, P.J.; Robins, R.; Hollands, M.A. Do postural constraints affect eye, head, and arm coordination? J. Neurophysiol. 2018, 120, 2066–2082. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Saeys, W.; Hallemans, A.; Velghe, S.; Viskens, P.J.; Vereeck, L.; De Hertogh, W.; Truijen, S. Trunk biomechanics during hemiplegic gait after stroke: A systematic review. Gait Posture 2017, 54, 133–143. [Google Scholar] [CrossRef]
- Sharififar, S.; Vincent, H.K.; Shuster, J.; Bishop, M. Quantifying Poststroke Gait Deviations: A Meta-analysis of Observational and Cross-sectional Experimental Trials. J. Stroke Med. 2019, 2, 23–31. [Google Scholar] [CrossRef]
- Yong, M.-S.; Lee, H.; Lee, M.-Y. Correlation between head posture and proprioceptive function in the cervical region. J. Phys. Ther. Sci. 2016, 28, 857–860. [Google Scholar] [CrossRef]
- An, H.; Park, S.-J. Effects of Cervical Spine Mobilization on Respiratory Function and Cervical Angles of Stroke Patients: A Pilot Study. Healthcare 2021, 9, 377. [Google Scholar] [CrossRef]
- Darak, V.; Karthikbabu, S. Lower limb motor function and hip muscle weakness in stroke survivors and their relationship with pelvic tilt, weight-bearing asymmetry, and gait speed: A cross-sectional study. Curr. J. Neurol. 2020, 19, 1–7. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.G.; Shin, Y.J.; Choi, E.H.; Choe, Y.W. The relationship between anterior pelvic tilt and gait, balance in patient with chronic stroke. J. Phys. Ther. Sci. 2018, 30, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.; Zaltieri, M.; Bravi, M.; Morrone, M.; Caponero, M.; Schena, E.; Sterzi, S.; Massaroni, C. A Wearable System Composed of FBG-Based Soft Sensors for Trunk Compensatory Movements Detection in Post-Stroke Hemiplegic Patients. Sensors 2022, 22, 1386. [Google Scholar] [CrossRef] [PubMed]
- Rougier, P.R.; Pérennou, D. Upright standing after stroke: How loading-unloading mechanism participates to the postural stabilization. Hum. Mov. Sci. 2019, 64, 47–54. [Google Scholar] [CrossRef]
- Mengarelli, A.; Tigrini, A.; Verdini, F.; Rabini, R.; Fioretti, S. Multiscale Fuzzy Entropy Analysis of Balance: Evidences of Scale-Dependent Dynamics on Diabetic Patients With and Without Neuropathy. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1462–1471. [Google Scholar] [CrossRef]
- Watanabe, E.; Kiyono, K.; Hayano, J.; Yamamoto, Y.; Inamasu, J.; Yamamoto, M.; Ichikawa, T.; Sobue, Y.; Harada, M.; Ozaki, Y. Multiscale Entropy of the Heart Rate Variability for the Prediction of an Ischemic Stroke in Patients with Permanent Atrial Fibrillation. PLoS ONE 2015, 10, e0137144. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Mazloumi, A.; Sharifnezhad, A.; Jafari, A.; Kazemi, Z.; Keihani, A.; Mohebbi, I. Determining the interactions between postural variability structure and discomfort development using nonlinear analysis techniques during prolonged standing work. Appl. Erg. 2021, 96, 103489. [Google Scholar] [CrossRef]
- Hadamus, A.; Błażkiewicz, M.; Kowalska, A.J.; Wydra, K.T.; Grabowicz, M.; Łukowicz, M.; Białoszewski, D.; Marczyński, W. Nonlinear and Linear Measures in the Differentiation of Postural Control in Patients after Total Hip or Knee Replacement and Healthy Controls. Diagnostics 2022, 12, 1595. [Google Scholar] [CrossRef]
- Roche, N.; Bonnyaud, C.; Geiger, M.; Bussel, B.; Bensmail, D. Relationship between hip flexion and ankle dorsiflexion during swing phase in chronic stroke patients. Clin. Biomech. 2015, 30, 219–225. [Google Scholar] [CrossRef]
- Mizuta, N.; Hasui, N.; Nishi, Y.; Higa, Y.; Matsunaga, A.; Deguchi, J.; Yamamoto, Y.; Nakatani, T.; Taguchi, J.; Morioka, S. Merged swing-muscle synergies and their relation to walking characteristics in subacute post-stroke patients: An observational study. PLoS ONE 2022, 17, e0263613. [Google Scholar] [CrossRef]
- Hsiao, H.; Gray, V.; Borrelli, J.; Rogers, M. Biomechanical control of paretic lower limb during imposed weight transfer in individuals post-stroke. J. NeuroEng Rehabil. 2020, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, C.D. Chapter 1—Sensorimotor anatomy of gait, balance, and falls. In Handbook of Clinical Neurology; Day, B.L., Lord, S.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 3–26. [Google Scholar]
- Montané, E.; Cormier, C.; Scandella, M.; Cangelosi, A.; Marque, P.; Moissenet, F.; Gasq, D. ToulGaitViz: A tool for the systematic description of lower limb clearance during the swing phase of hemiparetic gait after stroke. A cohort study. Eur. J. Phys. Rehabil. Med. 2023, 59, 669–681. [Google Scholar] [CrossRef]
- Matsuda, F.; Mukaino, M.; Ohtsuka, K.; Tanikawa, H.; Tsuchiyama, K.; Teranishi, T.; Kanada, Y.; Kagaya, H.; Saitoh, E. Biomechanical factors behind toe clearance during the swing phase in hemiparetic patients. Top. Stroke Rehabil. 2017, 24, 177–182. [Google Scholar] [CrossRef]
- Gow, B.J.; Peng, C.-K.; Wayne, P.M.; Ahn, A.C. Multiscale Entropy Analysis of Center-of-Pressure Dynamics in Human Postural Control: Methodological Considerations. Entropy 2015, 17, 7926–7947. [Google Scholar] [CrossRef]
- Awad, L.N.; Lewek, M.D.; Kesar, T.M.; Franz, J.R.; Bowden, M.G. These legs were made for propulsion: Advancing the diagnosis and treatment of post-stroke propulsion deficits. J. NeuroEng Rehabil. 2020, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Celestino, M.; Van Emmerik, R.; Barela, J.; Bacca, O.; Barela, A. Effects of limited knee flexion movement in intra-limb gait coordination. J. Biomech. 2021, 128, 110712. [Google Scholar] [CrossRef]
- Kempski, K.; Awad, L.N.; Buchanan, T.S.; Higginson, J.S.; Knarr, B.A. Dynamic structure of lower limb joint angles during walking post-stroke. J. Biomech. 2018, 68, 1–5. [Google Scholar] [CrossRef]
- Mallo-López, A.; Cuesta-Gómez, A.; Fernández-Pardo, T.E.; Aguilera-Rubio, Á.; Molina-Rueda, F. Influence of Impaired Upper Extremity Motor Function on Static Balance in People with Chronic Stroke. Sensors 2024, 24, 4311. [Google Scholar] [CrossRef]
- Harbourne, R.; Kamm, K. Upper extremity function: What’s posture got to do with it? J. Hand Ther. 2015, 28, 106–112. [Google Scholar] [CrossRef]
- Phan, T.; Nguyen, H.; Vermillion, B.; Kamper, D.; Lee, S.W. Abnormal proximal-distal interactions in upper-limb of stroke survivors during object manipulation: A pilot study. Front. Hum. Neurosci. 2022, 16, 1022516. [Google Scholar] [CrossRef]
- McPherson, L.; Dewald, J. Abnormal synergies and associated reactions post-hemiparetic stroke reflect muscle activation patterns of brainstem motor pathways. Front. Neurol. 2022, 13, 934670. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Taylor, P.; Bellenger, C.; Grimshaw, P.; Crowther, R.G. The application of the Lyapunov Exponent to analyse human performance: A systematic review. J. Sports Sci. 2023, 41, 1994–2013. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, Z.; Wong, K.; Zhu, H.; Huang, Y.; Hu, X.; Zheng, Y. Pathway-specific cortico-muscular coherence in proximal-to-distal compensation during fine motor control of finger extension after stroke. J. Neural Eng. 2021, 18, 056034. [Google Scholar] [CrossRef]
- Jayasinghe, S.; Wang, R.; Gebara, R.; Biswas, S.; Ranganathan, R. Compensatory Trunk Movements in Naturalistic Reaching and Manipulation Tasks in Chronic Stroke Survivors. J. Appl. Biomech. 2021, 37, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Gray, V. The effects of stroke on weight transfer before voluntary lateral and forward steps. Front. Neurol. 2022, 13, 891439. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Truijen, S.; Schröder, J.; Maebe, Z.; Blanckaert, K.; van der Waal, C.; Vink, M.; Saeys, W. The effectiveness of trunk training on trunk control, sitting and standing balance and mobility post-stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-W.; Baune, N.; Zwir, I.; Wang, J.; Swamidass, V.; Wong, A. Measuring Activities of Daily Living in Stroke Patients with Motion Machine Learning Algorithms: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 1634. [Google Scholar] [CrossRef]
- Leteneur, S.; Simoneau, E.; Gillet, C.; Dessery, Y.; Barbier, F. Trunk’s Natural Inclination Influences Stance Limb Kinetics, but Not Body Kinematics, during Gait Initiation in Able Men. PLoS ONE 2013, 8, e55256. [Google Scholar] [CrossRef]
- De Luca, A.; Squeri, V.; Barone, L.M.; Vernetti Mansin, H.; Ricci, S.; Pisu, I.; Cassiano, C.; Capra, C.; Lentino, C.; De Michieli, L.; et al. Dynamic Stability and Trunk Control Improvements Following Robotic Balance and Core Stability Training in Chronic Stroke Survivors: A Pilot Study. Front. Neurol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Guo, Z.; Qian, Q.; Wong, K.; Zhu, H.; Huang, Y.; Hu, X.; Zheng, Y. Altered Corticomuscular Coherence (CMCoh) Pattern in the Upper Limb During Finger Movements After Stroke. Front. Neurol. 2020, 11, 410. [Google Scholar] [CrossRef]
- Michaelsen, S.M.; Dannenbaum, R.; Levin, M.F. Task-specific training with trunk restraint on arm recovery in stroke: Randomized control trial. Stroke 2006, 37, 186–192. [Google Scholar] [CrossRef]
- Bisi, M.C.; Stagni, R. Changes of human movement complexity during maturation: Quantitative assessment using multiscale entropy. Comput. Methods Biomech. Biomed. Engin 2018, 21, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Creath, R.A.; Magder, L.; Rogers, M.W.; McCombe Waller, S. Impaired posture, movement preparation, and execution during both paretic and nonparetic reaching following stroke. J. Neurophysiol. 2019, 121, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E.; Zhou, P. Post-stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Karthikbabu, S.; Chakrapani, M.; Ganeshan, S.; Rakshith, K.C.; Nafeez, S.; Prem, V. A review on assessment and treatment of the trunk in stroke: A need or luxury. Neural Regen. Res. 2012, 7, 1974–1977. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kim, Y.; Jayaraman, A.; Park, H.S. Relationship between gait quality measures and modular neuromuscular control parameters in chronic post-stroke individuals. J. NeuroEng Rehabil. 2021, 18, 58. [Google Scholar] [CrossRef]
- Dubey, L.; Karthikbabu, S.; Mohan, D. Effects of Pelvic Stability Training on Movement Control, Hip Muscles Strength, Walking Speed and Daily Activities after Stroke: A Randomized Controlled Trial. Ann. Neurosci. 2018, 25, 80–89. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kim, N.-H.; Son, S.; Cha, Y. Effects of Trunk Stabilization Exercise While Wearing a Pelvic Compression Belt on Walking and Balancing Abilities in Patients With Stroke. Am. J. Phys. Med. Rehabil. 2020, 99, 1048–1055. [Google Scholar] [CrossRef]
- Xu, P.; Yu, H.; Wang, X.; Song, R. Characterizing stroke-induced changes in the variability of lower limb kinematics using multifractal detrended fluctuation analysis. Front. Neurol. 2022, 13, 893999. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lee, C.-H.; Jiang, B.C.; Sun, T.-L. Multiscale Entropy Analysis of Postural Stability for Estimating Fall Risk via Domain Knowledge of Timed-Up-And-Go Accelerometer Data for Elderly People Living in a Community. Entropy 2019, 21, 1076. [Google Scholar] [CrossRef]
- Goh, S.; Han, K.; Ryu, J.; Kim, S.; Choi, M. Failure of Arm Movement Control in Stroke Patients, Characterized by Loss of Complexity. PLoS ONE 2015, 10, e0141996. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.; Goussev, V.; Feldman, A.G.; Levin, M.F. Arm-trunk coordination for beyond-the-reach movements in adults with stroke. Neurorehabil Neural Repair. 2014, 28, 355–366. [Google Scholar] [CrossRef]
- Okita, S.; De Lucena, D.S.; Reinkensmeyer, D. Movement Diversity and Complexity Increase as Arm Impairment Decreases After Stroke: Quality of Movement Experience as a Possible Target for Wearable Feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 2961–2970. [Google Scholar] [CrossRef]
- Ding, K.; Wang, J.; Wang, X.; Zhou, L.; Xiong, D.; Guo, L. System for Detection and Quantitative Evaluation of Compensatory Movement in Post-Stroke Patients Based on Wearable Sensor and Machine Learning Algorithm. IEEE Sens. J. 2024, 24, 22830–22842. [Google Scholar] [CrossRef]
- Lefebvre, S.; Liew, S.L. Anatomical Parameters of tDCS to Modulate the Motor System after Stroke: A Review. Front. Neurol. 2017, 8, 29. [Google Scholar] [CrossRef]
- Seo, J.P.; Koo, D.K. Degeneration of nigrostriatal pathway in patients with middle cerebral infarct: A diffusion tensor imaging study. Medicine 2023, 102, e33370. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Lim, S.H.; Kim, Y.; Kim, J.-S.; Hong, B.Y.; Yoon, M.-J.; Rim, H.; Park, G.-Y. Structural Integrity of the Cerebellar Outflow Tract Predicts Long-Term Motor Function After Middle Cerebral Artery Ischemic Stroke. Neurorehabilit. Neural Repair. 2023, 37, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public. Health 2020, 5, e551–e567. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.C. Overview of neurophysiology of movement control. Clin. Neurol. Neurosurg. 2012, 114, 432–435. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 2010, 46, 239–248. [Google Scholar]
- Jang, S.H.; Lee, S.J. Corticoreticular Tract in the Human Brain: A Mini Review. Front. Neurol. 2019, 10, 1188. [Google Scholar] [CrossRef] [PubMed]
- Serradj, N.; Carmel, J.B. Task-Specific Modulation of Corticospinal Neuron Activity During Skilled Motor Learning. Nat. Commun. 2023, 14, 1234. [Google Scholar] [CrossRef] [PubMed]
- Rosch, K.S.; Mostofsky, S. Chapter 19—Development of the frontal lobe. In Handbook of Clinical Neurology; D’Esposito, M., Grafman, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 163, pp. 351–367. [Google Scholar]
- Asan, A.S.; McIntosh, J.R.; Carmel, J.B. Targeting Sensory and Motor Integration for Recovery of Movement After CNS Injury. Front. Neurosci. 2022, 15, 791824. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Lien, H.Y.; Liu, W.Y.; Guo, S.L.; Lin, Y.H.; Yang, T.F. Early and anticipatory postural adjustments in healthy subjects under stable and unstable sitting conditions. J. Electromyogr. Kinesiol. 2018, 43, 21–27. [Google Scholar] [CrossRef]
- Levin, M.F. Principles of Motor Recovery After Neurological Injury Based on a Motor Control Theory. Adv. Exp. Med. Biol. 2016, 957, 121–140. [Google Scholar] [CrossRef]
- Foysal, K.M.R.; Baker, S.N. A hierarchy of corticospinal plasticity in human hand and forearm muscles. J. Physiol. 2019, 597, 2729–2739. [Google Scholar] [CrossRef]
- Le Mouel, C.; Brette, R. Mobility as the Purpose of Postural Control. Front. Comput. Neurosci. 2017, 11, 67. [Google Scholar] [CrossRef]
- Kamijo, A.; Furihata, C.; Kimura, Y.; Furuhata, I.; Ohtani, T.; Miyajima, T. Postural control exercise without using the upper limbs improves activities of daily living in patients with stroke. Front. Rehabil. Sci. 2023, 4, 1124515. [Google Scholar] [CrossRef]
- Hugues, A.; Marco, J.; Janiaud, P.; Bonan, I.; Gueyffier, F.; Rode, G. Efficiency of physical rehabilitation on postural imbalance after stroke: Systematic review and meta-analysis. Ann. Rehabil. Med. 2016, 59, e67–e79. [Google Scholar] [CrossRef]
- Latash, M.L. The bliss (not the problem) of motor abundance (not redundancy). Exp. Brain Res. 2012, 217, 1–5. [Google Scholar] [CrossRef]
- Hristovski, R.; Balagué, N.; Daskalovski, B.; Zivkovic, V.; Velickovska, L.; Naumovski, M. Linear and nonlinear complex systems approach to sports. Explanatory differences and applications. Res. Phys. Educ. Sport. Health 2012, 1, 25–32. [Google Scholar]



| Stroke Group (n = 17) | Healthy Group (n = 16) | |
|---|---|---|
| Age (years) | 56.76 (12.219) | 56.00 (12.247) |
| Weight (Kg) | 78.32 (11.802) | 79.48 (11.264) |
| Body Mass (m) | 1.71 (0.084) | 1.72 (0.085) |
| BMI M (kg/m2) | 26.60 (3.229) | 26.90 (2.673) |
| Post-stroke time (years); M(SD) | 5.14 (4.125) | NA |
| Stroke Location; (n; %) | ||
| Undetermined | 8 (47.01) | NA |
| Lenticulocapsular | 6 (35.29) | NA |
| Corona-radiata | 1 (5.9) | NA |
| Frontotemporal | 2 (11.8) | NA |
| Risk Factors; (n; %) | ||
| None | 6 (35.3) | NA |
| Diabetes | 3 (17.6) | NA |
| Hypertension | 2 (11.8) | NA |
| Clinical Obesity | 4 (23.5) | NA |
| Smoking | 4 (23.5) | NA |
| Alcohol Consumption | 2 (11.8) | NA |
| Hyperlipidemia | 3 (17.6) | NA |
| Ability to walk independently at stroke onset | 8 (47.06) | NA |
| Thrombolysis/reperfusion therapy | 7 (41.18) | NA |
| Mini-BESTest M(SD) | 15. 71 (2.932) | NA |
| TUG; M(SD) | 14.90 (3.635) | NA |
| FMA-LE; M(SD) | 18.29 (2.616) | NA |
| MED (P25; P75) | p Value # | |||
|---|---|---|---|---|
| Healthy | Stroke | |||
| ML-CoP | POS (m) | −0.1985 (−0.2057; −0.1916) | −0.2075 (−0.2211; −0.1865) | 0.331 |
| MDISP (m) | 0.0026 (0.0023; 0.0030) | 0.0039 (0.0029; 0.0057) | 0.004 | |
| AMP (m) | 0.0001 (0.0001; 0.0001) | 0.0001 (0.0000; 0.0001) | 0.368 | |
| MVEL (m/s) | 0.0001 (−0.0024; 0.0023) | 0.0002 (−0.0018; 0.0027) | 1.000 | |
| AP-CoP | POS (m) | 0.2268 (0.1847; 0.2824) | 0.2037 (0.1527; 0.2546) | 0.171 |
| MDISP (m) | 0.0047 (0.0040; 0.0062) | 0.0049 (0.0039; 0.0070) | 0.666 | |
| AMP (m) | 0.0001 (0.0001; 0.0001) | 0.0001 (0.0001; 0.0001) | 0.449 | |
| MVEL (m/s) | −0.004 (−0.0072; −0.0015) | −0.001 (−0.003; 0.000) | 0.021 | |
| MED (P25; P75) | p Value # | |||
|---|---|---|---|---|
| Healthy | Stroke | |||
| ML-CoP POS | LyE | −7.515 (−9.336; −3.164) | −2.881 (−4.699; −2.057) | 0.012 |
| MSE S1 | 0.337 (0.314; 0.404) | 0.281 (0.230; 0.308) | 0.002 | |
| MSE S2 | 0.666 (0.609; 0.845) | 0.562 (0.402; 0.666) | 0.016 | |
| CI | 1.001 (0.920; 1.276) | 0.843 (0.618; 0.966) | 0.004 | |
| AP-CoP POS | LyE | −4.817 (−8.132; −2.378) | −5.537 (−8.247; −3.972) | 0.494 |
| MSE S1 | 0.294 (0.234; 0.344) | 0.279 (0.199; 0.319) | 0.368 | |
| MSE S2 | 0.612 (0.442; 0.655) | 0.538 (0.381; 0.639) | 0.449 | |
| CI | 0.921 (0.676; 1.001) | 0.817 (0.576; 0.961) | 0.428 | |
| ML-CoP DISP | LyE | 23.984 (21.299; 25.079) | 21.147 (14.126; 26.359) | 0.280 |
| MSE S1 | 1.046 (0.946; 1.114) | 0.883 (0.376; 1.077) | 0.052 | |
| MSE S2 | 1.590 (1.462; 1.710) | 1.444 (0.643; 1.730) | 0.098 | |
| CI | 2.595 (2.356; 2.703) | 2.266 (1.022; 2.708) | 0.098 | |
| AP-CoP DISP | LyE | 17.589 (13.544; 22.936) | 10.254 (7.230; 19.680) | 0.140 |
| MSE S1 | 1.019 (0.898; 1.278) | 1.027 (0.397; 1.204) | 0.564 | |
| MSE S2 | 1.498 (1.333; 1.659) | 1.502 (0.640; 1.612) | 0.428 | |
| CI | 2.470 (2.181; 2.743) | 2.434 (1.055; 2.858) | 0.564 | |
| ML-CoP MVEL | LyE | 32.384 (31.123; 33.495) | 30.329 (21.765; 31.937) | 0.031 |
| MSE S1 | 0.738 (0.680; 0.788) | 0.729 (0.645; 0.812) | 0.692 | |
| MSE S2 | 1.526 (1.332; 1.615) | 1.354 (1.187; 1.539) | 0.090 | |
| CI | 2.214 (1.943; 2.365) | 1.991 (1.879; 2.278) | 0.183 | |
| AP-CoP MVEL | LyE | 28.690 (24.158; 30.212) | 22.618 (18.712; 24.454) | 0.002 |
| MSE S1 | 0.751 (0.637; 0.912) | 0.830 (0.733; 0.925) | 0.349 | |
| MSE S2 | 1.426 (1.220; 1.568) | 1.549 (1.413; 1.659) | 0.090 | |
| CI | 2.191 (1.848; 2.423) | 2.340 (2.149; 2.497) | 0.130 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.; Fonseca, P.; Alves, L.; Pinho, L.; Silva, S.; Figueira, V.; Félix, J.; Pinho, F.; Vilas-Boas, J.P.; Silva, A. Neurobiomechanical Characterization of Feedforward Phase of Gait Initiation in Chronic Stroke: A Linear and Non-Linear Approach. Appl. Sci. 2025, 15, 4762. https://doi.org/10.3390/app15094762
Freitas M, Fonseca P, Alves L, Pinho L, Silva S, Figueira V, Félix J, Pinho F, Vilas-Boas JP, Silva A. Neurobiomechanical Characterization of Feedforward Phase of Gait Initiation in Chronic Stroke: A Linear and Non-Linear Approach. Applied Sciences. 2025; 15(9):4762. https://doi.org/10.3390/app15094762
Chicago/Turabian StyleFreitas, Marta, Pedro Fonseca, Leonel Alves, Liliana Pinho, Sandra Silva, Vânia Figueira, José Félix, Francisco Pinho, João Paulo Vilas-Boas, and Augusta Silva. 2025. "Neurobiomechanical Characterization of Feedforward Phase of Gait Initiation in Chronic Stroke: A Linear and Non-Linear Approach" Applied Sciences 15, no. 9: 4762. https://doi.org/10.3390/app15094762
APA StyleFreitas, M., Fonseca, P., Alves, L., Pinho, L., Silva, S., Figueira, V., Félix, J., Pinho, F., Vilas-Boas, J. P., & Silva, A. (2025). Neurobiomechanical Characterization of Feedforward Phase of Gait Initiation in Chronic Stroke: A Linear and Non-Linear Approach. Applied Sciences, 15(9), 4762. https://doi.org/10.3390/app15094762








