Effects of β-Cryptoxanthin on Cisplatin-Treated Human Oral Mucosa-Derived Keratinocytes and Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Cellular Growth and Morphology
2.4. Quantitative RT-PCR
2.5. ELISA
2.6. Reactive Oxygen Species (ROS) Assay
2.7. SA-β-Gal
2.8. Statistical Analysis
3. Results
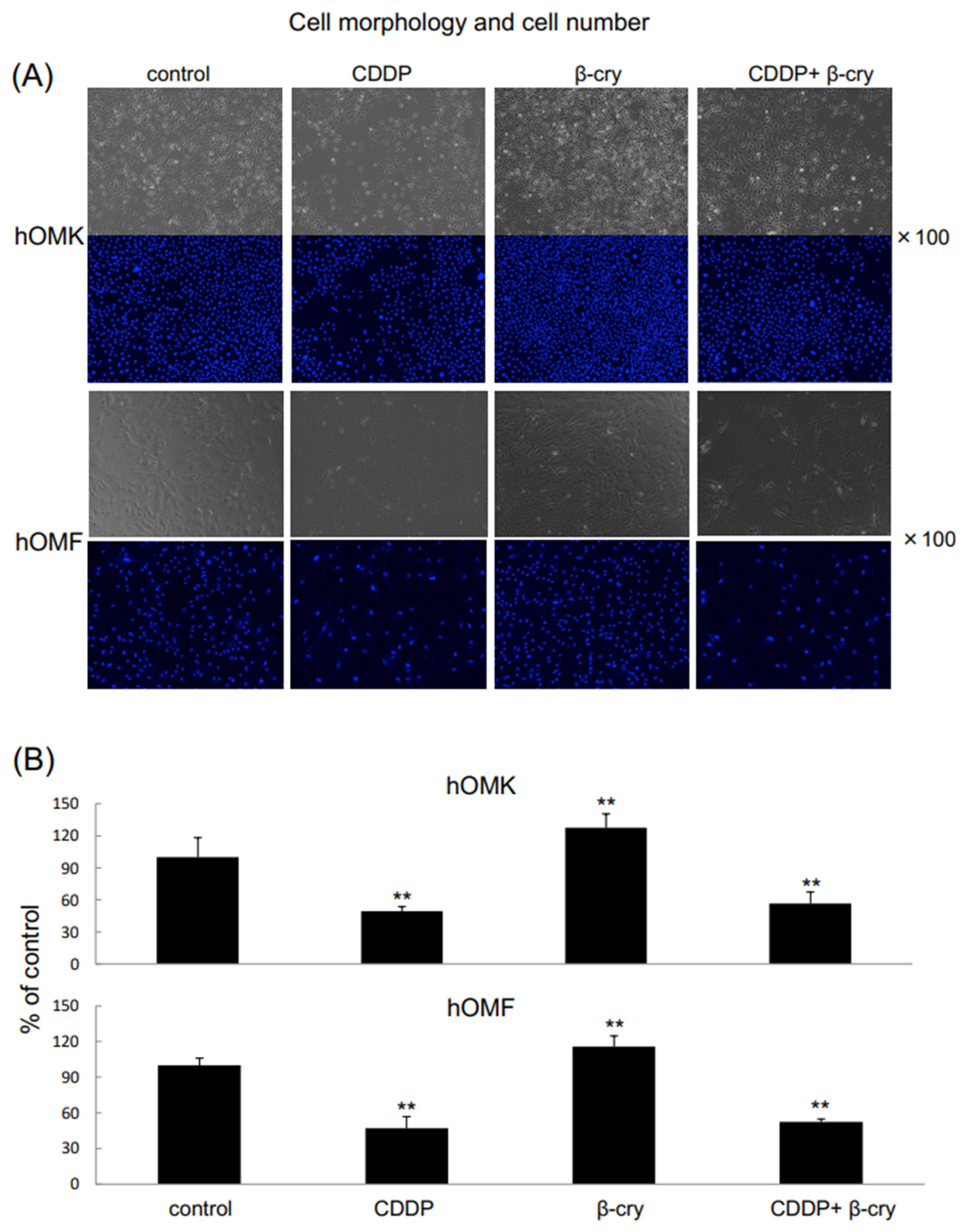
3.1. Cell Growth and Morphology of CDDP- and/or β-Cry-Treated hOMK and hOMF
3.2. Effects of β-Cry on Inflammatory Cytokine and MMP mRNA Expression Levels in CDDP-Treated hOMK and hOMF
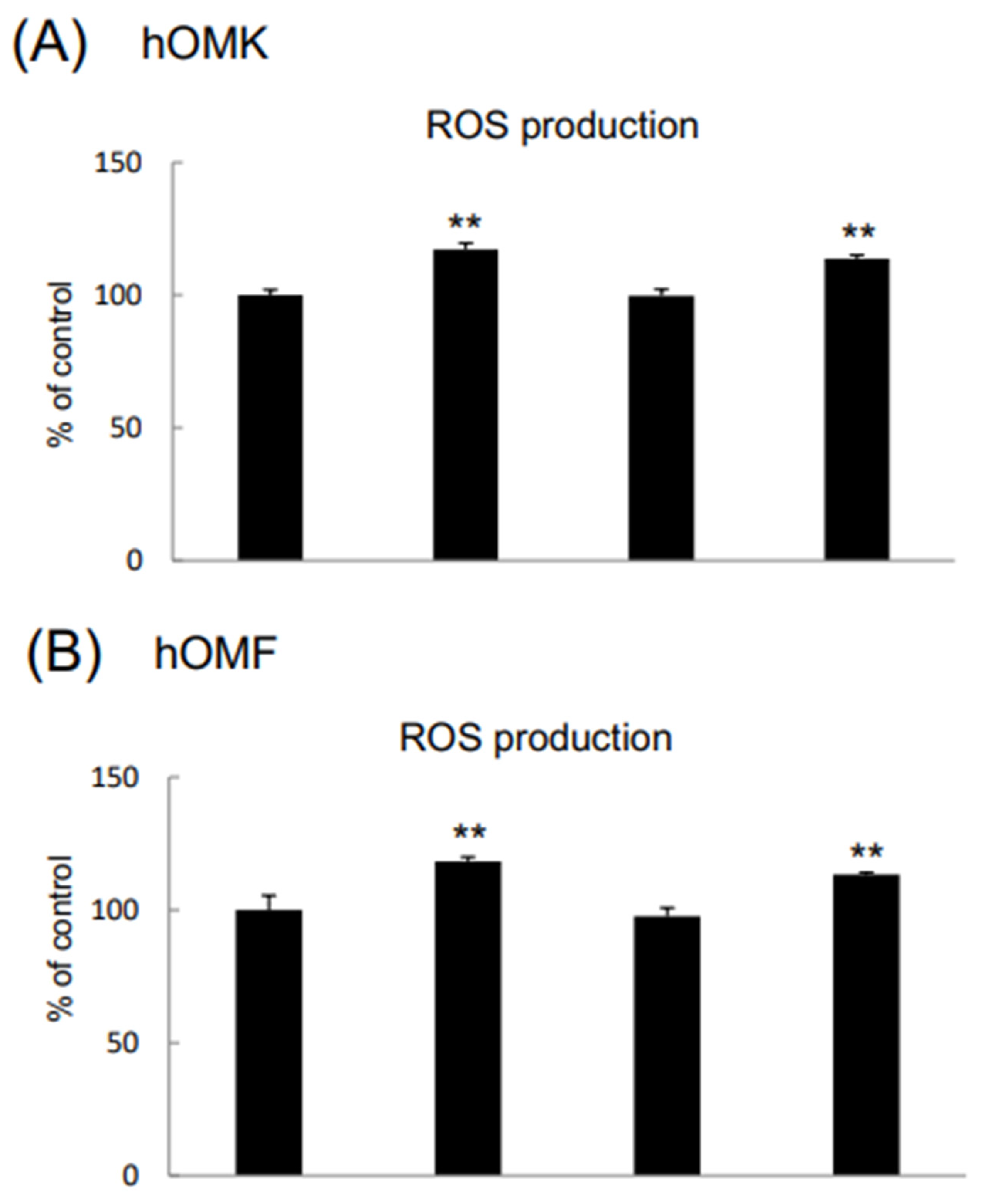
3.3. Effects of β-Cry on IL-6, IL-8, and ROS Levels in CDDP-Treated hOMK and hOMF
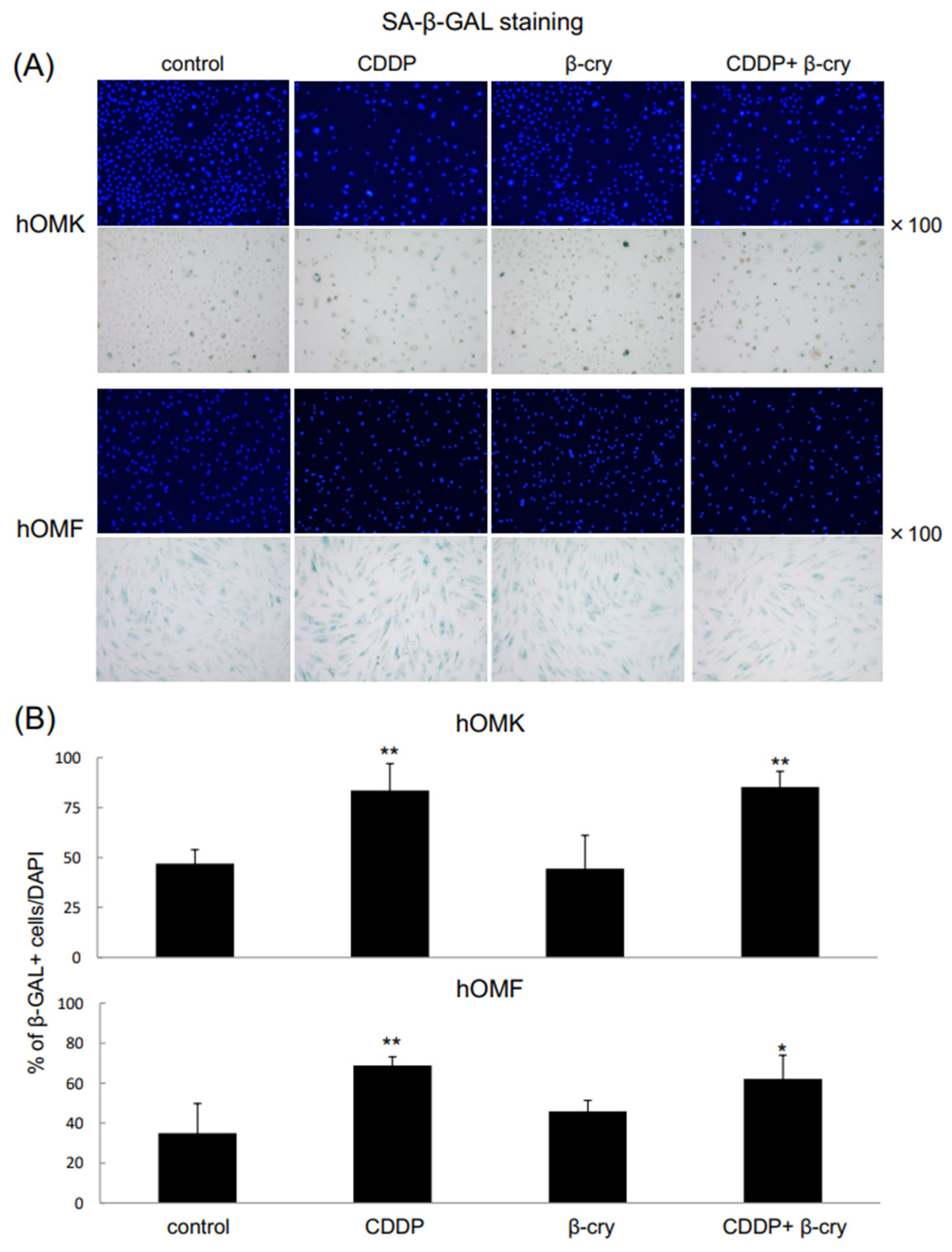
3.4. SA-β-Gal-Positive Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cheng, Y.; Li, S.; Gao, L.; Zhi, K.; Ren, W. The molecular basis and therapeutic aspects of Cisplatin resistance in oral squamous cell carcinoma. Front. Oncol. 2021, 11, 761379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Liu, K.; Huo, Z.J.; Li, X.C.; Wang, M.; Liu, P.; Pang, B.; Wang, S.-J. A cell-targeted chemotherapeutic nanomedicine strategy for oral squamous cell carcinoma therapy. J. Nanobiotechnol. 2015, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Menashe, D.S.; Jacobs, S.C. Complications of hypogastric artery cisplatin infusions. J. Surg. Oncol. 1989, 41, 160–164. [Google Scholar] [CrossRef]
- Kaneyasu, Y.; Okawa, M.K.; Okawa, T. Clinical evaluation of chemoradiotherapy for advanced cervical cancer. Gan Kagaku Ryoho. 1997, 24, 2084–2091. [Google Scholar]
- Kralovánszky, J.; Prajda, N.; Kerpel-Fronius, S.; Gál, F.; Kiss, F. Comparison of intestinal toxic effects of platinum complexes: Cisplatin (CDDP), carboplatin (CBDCA), and iproplatin (CHIP). Cancer Chemother. Pharmacol 1988, 21, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Takeda, S.; Kanekiyo, S.; Nishiyama, M.; Kitahara, M.; Ueno, T.; Yamamoto, S.; Yoshino, S.; Hazama, S.; Okayama, N.; et al. Association of tumor necrosis factor-α polymorphism with chemotherapy-induced oral mucositis in patients with esophageal cancer. Mol. Clin. Oncol. 2017, 6, 125–129. [Google Scholar] [CrossRef][Green Version]
- Topping, R.P.; Wilkinson, J.C.; Scarpinato, K.D. Mismatch repair protein deficiency compromises cisplatin-induced apoptotic signaling. J. Biol. Chem. 2009, 284, 14029–14039. [Google Scholar] [CrossRef]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug Delivery Strategies for Platinum-Based Chemotherapy. ACS Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef]
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R.; et al. Emerging evidence on the pathobiology of mucositis. Support. Care Cancer 2013, 21, 3233–3241. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; Kataoka, T.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef]
- Daugėlaitė, G.; Užkuraitytė, K.; Jagelavičienė, E.; Filipauskas, A. Prevention and treatment of chemotherapy and radiotherapy induced oral mucositis. Medicina 2019, 55, 25. [Google Scholar] [CrossRef]
- Chaveli-López, B.; Bagán-Sebastián, J.V. Treatment of oral mucositis due to chemotherapy. Clin. Exp. Dent. 2016, 8, e201–e209. [Google Scholar] [CrossRef]
- Saadeh, C.E. Chemotherapy- and radiotherapy-induced oral mucositis: Review of preventive strategies and treatment. Pharmacotherapy 2005, 25, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kobayashi, R.; Suzuki, A.; Yamada, Y.; Ishida, M.; Shakui, T.; Kitagawa, J.; Hayashi, H.; Sugiyama, T.; Takeuchi, H.; et al. Preparation and clinical evaluation of a novel lozenge containing polaprezinc, a zinc-L-carnosine, for prevention of oral mucositis in patients with hematological cancer who received high-dose chemotherapy. Med. Oncol. 2016, 33, 91. [Google Scholar] [CrossRef] [PubMed]
- Manzi, N.M.; Silveira, R.C.; Reis, P.E. Prophylaxis for mucositis induced by ambulatory chemotherapy: Systematic review. J. Adv. Nurs. 2016, 72, 735–746. [Google Scholar] [CrossRef]
- Lorenzo, Y.; Azqueta, A.; Luna, L.; Bonilla, F.; Domínguez, G.; Collins, A.R. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis 2009, 30, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Wang, Y.Y.; Dan, X.G.; Kumar, A.; Ye, T.Z.; Yu, Y.Y.; Yang, L.G. Anti-inflammatory potential of β-cryptoxanthin against LPS-induced inflammation in mouse Sertoli cells. Reprod. Toxicol. 2016, 60, 148–155. [Google Scholar] [CrossRef]
- Quesada-Gómez, J.M.; Santiago-Mora, R.; Durán-Prado, M.; Dorado, G.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Casado-Díaz, A. β-cryptoxanthin inhibits angiogenesis in human umbilical vein endothelial cells through retinoic acid receptor. Mol. Nutr. Food Res. 2018, 62, 201700489. [Google Scholar] [CrossRef]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef]
- Park, G.; Horie, T.; Fukasawa, K.; Ozaki, K.; Onishi, Y.; Kanayama, T.; Iezaki, T.; Kaneda, K.; Sugiura, M.; Hinoi, E. Amelioration of the development of osteoarthritis by daily intake of β-cryptoxanthin. Biol. Pharm. Bull. 2017, 40, 1116–1120. [Google Scholar] [CrossRef]
- Nishigaki, M.; Yamamoto, T.; Ichioka, H.; Honjo, K.; Yamamoto, K.; Oseko, F.; Kita, M.; Mazda, O.; Kanamura, N. Beta-cryptoxanthin regulates bone resorption related-cytokine production in human periodontal ligament cells. Arch. Oral. Biol. 2013, 58, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Yamanobe, H.; Yamamoto, K.; Kishimoto, S.; Nakai, K.; Oseko, F.; Yamamoto, T.; Mazda, O.; Kanamura, N. Anti-inflammatory effects of β-cryptoxanthin on 5-fluorouracil-induced cytokine expression in human oral mucosal keratinocytes. Molecules 2023, 28, 2935. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sato, Y.; Honjo, K.; Ichioka, H.; Oseko, F.; Sowa, Y.; Yamamoto, T.; Kanamura, N.; Kishida, T.; Mazda, O. Generation of directly converted human osteoblasts that are free of exogenous gene and xenogenic protein. J. Cell. Biochem. 2016, 117, 2538–2545. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kishida, T.; Nakai, K.; Sato, Y.; Kotani, S.I.; Nishizawa, Y.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-β signal. Sci. Rep. 2018, 8, 8463. [Google Scholar] [CrossRef]
- Okada, H.; Oguchi, N.; Uchida, J.; Mikami, O.; Matusda, T. Study on platinum concentration in internal iliac venous blood after iliac artery cisplatin infusion for invasive bladder cancer. Hinyokika Kiyo 1999, 45, 145–148. [Google Scholar]
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003, 98, 1531–1539. [Google Scholar] [CrossRef]
- Oba, M.K.; Innocentini, L.M.A.R.; Viani, G.; Ricz, H.M.A.; de Carvalho Reis, T.; Ferrari, T.C.; de Macedo, L.D. Evaluation of the correlation between side effects to oral mucosa, salivary glands, and general health status with quality of life during intensity-modulated radiotherapy for head and neck cancer. Support. Care Cancer 2020, 29, 127–134. [Google Scholar] [CrossRef]
- Alsheyyab, F.; Al-Momani, D.; Kasht, R.; Kamal, A.; Abusalem, D.; Al-Qasem, W. Impact of severe oral mucositis in pediatric cancer patients on resource utilization and cancer treatment plans. Int. J. Clin. Pharm. 2021, 43, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, P.; Masur, K.; Shome, D.; Kaushik, N.; Nguyen, L.N.; Kaushik, N.K.; Choi, E.H. Influence of redox stress on crosstalk between fibroblasts and keratinocytes. Biology 2021, 10, 1338. [Google Scholar] [CrossRef]
- Cardoso, L.M.; Pansani, T.N.; Hebling, J.; de Souza Costa, C.A.; Basso, F.G. Chemotherapy drugs and inflammatory cytokines enhance matrix metalloproteinases expression by oral mucosa cells. Arch. Oral Biol. 2021, 127, 105159. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–5366. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. A biological approach to mucositis. J. Support Oncol. 2004, 2, 21–32. [Google Scholar] [PubMed]
- Claveau, I.; Mostefaoui, Y.; Rouabhia, M. Basement membrane protein and matrix metalloproteinase deregulation in engineered human oral mucosa following infection with Candida albicans. Matrix Biol. 2004, 23, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Laput, G.; Vyas, A.K. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco. Targets Ther. 2014, 7, 1015–1023. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Juan, C.A.; Lastra, J.M.; Plou, F.J.; Lebeña, E.P. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohtani, N.; Yamakoshi, K.; Iida, S.; Tahara, H.; Nakayama, K.; Nakayama, K.I.; Ide, T.; Saya, H.; Hara, E. Mitogenic signalling and the p16INK4a/Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006, 8, 1291–1297. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 1, 3565–3582. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Rodier, F.; Coppé, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Muñoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Jun, E.-S.; Kim, Y.J.; Kim, H.H.; Park, S.Y. Gold Nanoparticles Using Ecklonia stolonifera Protect Human Dermal Fibroblasts from UVA-Induced Senescence through Inhibiting MMP-1 and MMP-3. Mar. Drugs 2020, 19, 433. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, S.; Zu, X.; Dong, Z. CoQ10 suppression of oxidative stress and cell senescence increases bone mass in orchiectomized mice. Am. J. Transl. Res. 2020, 12, 4314–4325. [Google Scholar]
- Yang, G.; Liu, X.; Jing, X.; Wang, J.; Wang, H.; Chen, F.; Wang, W.; Shao, Y.; Cui, X. Astaxanthin suppresses oxidative stress and calcification in vertebral cartilage endplate via activating Nrf-2/HO-1 signaling pathway. Int. Immunopharmacol. 2023, 119, 110159. [Google Scholar] [CrossRef] [PubMed]
- von Bültzingslöwen, I.; Brennan, M.T.; Spijkervet, F.K.; Logan, R.; Stringer, A.; Raber-Durlacher, J.E.; Keefe, D. Growth factors and cytokines in the prevention and treatment of oral and gastrointestinal mucositis. Support. Care Cancer 2006, 14, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.; Glenny, A.M.; Worthington, H.V.; Littlewood, A.; Mauleffinch, L.M.F.; Clarkson, J.E.; McCabe, M.G. Interventions for preventing oral mucositis in patients with cancer receiving treatment: Cytokines and growth factors. Cochrane Database Syst. Rev. 2017, 11, CD011990. [Google Scholar] [CrossRef]
- Gao, M.; Dang, F.; Deng, C. β-cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur. J. Pharmacol. 2019, 859, 172528. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Lambert, J.; Bush, H.; Huja, P.E.; Basu, A. Serum nutrient levels and aging effects on periodontitis. Nutrients 2018, 10, 1986. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, T.; Yamamoto, K.; Wada, N.; Oseko, F.; Mazda, O.; Kanamura, N. Effects of β-Cryptoxanthin on Cisplatin-Treated Human Oral Mucosa-Derived Keratinocytes and Fibroblasts. Appl. Sci. 2025, 15, 4803. https://doi.org/10.3390/app15094803
Yamamoto T, Yamamoto K, Wada N, Oseko F, Mazda O, Kanamura N. Effects of β-Cryptoxanthin on Cisplatin-Treated Human Oral Mucosa-Derived Keratinocytes and Fibroblasts. Applied Sciences. 2025; 15(9):4803. https://doi.org/10.3390/app15094803
Chicago/Turabian StyleYamamoto, Toshiro, Kenta Yamamoto, Naoya Wada, Fumishige Oseko, Osam Mazda, and Narisato Kanamura. 2025. "Effects of β-Cryptoxanthin on Cisplatin-Treated Human Oral Mucosa-Derived Keratinocytes and Fibroblasts" Applied Sciences 15, no. 9: 4803. https://doi.org/10.3390/app15094803
APA StyleYamamoto, T., Yamamoto, K., Wada, N., Oseko, F., Mazda, O., & Kanamura, N. (2025). Effects of β-Cryptoxanthin on Cisplatin-Treated Human Oral Mucosa-Derived Keratinocytes and Fibroblasts. Applied Sciences, 15(9), 4803. https://doi.org/10.3390/app15094803





