Utilizing Natural Deep Eutectic Solvents (NADESs) for Sustainable Phytonutrient Recovery: Optimization and Multi-Matrix Extraction of Bioactive Compounds
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NADES Preparation
2.3. Betalain Content Determination
2.4. Carotenoid Content Determination
2.5. Anthocyanin Determination
2.6. Total Phenolic Content
2.7. Antioxidant Activity Measurements
2.8. Optimization of Water Addition to NADES
2.9. Statistical Analysis
3. Results and Discussion
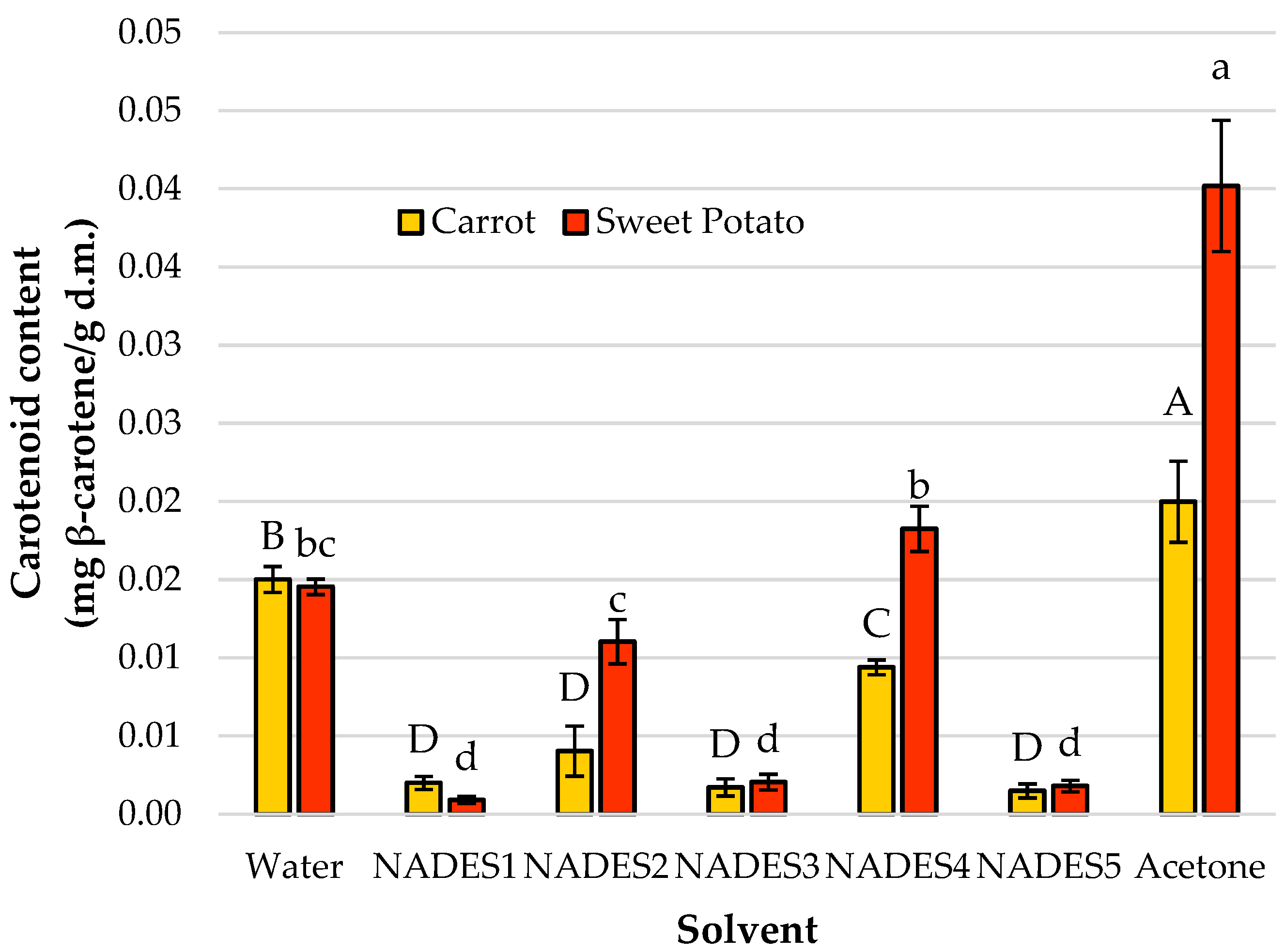
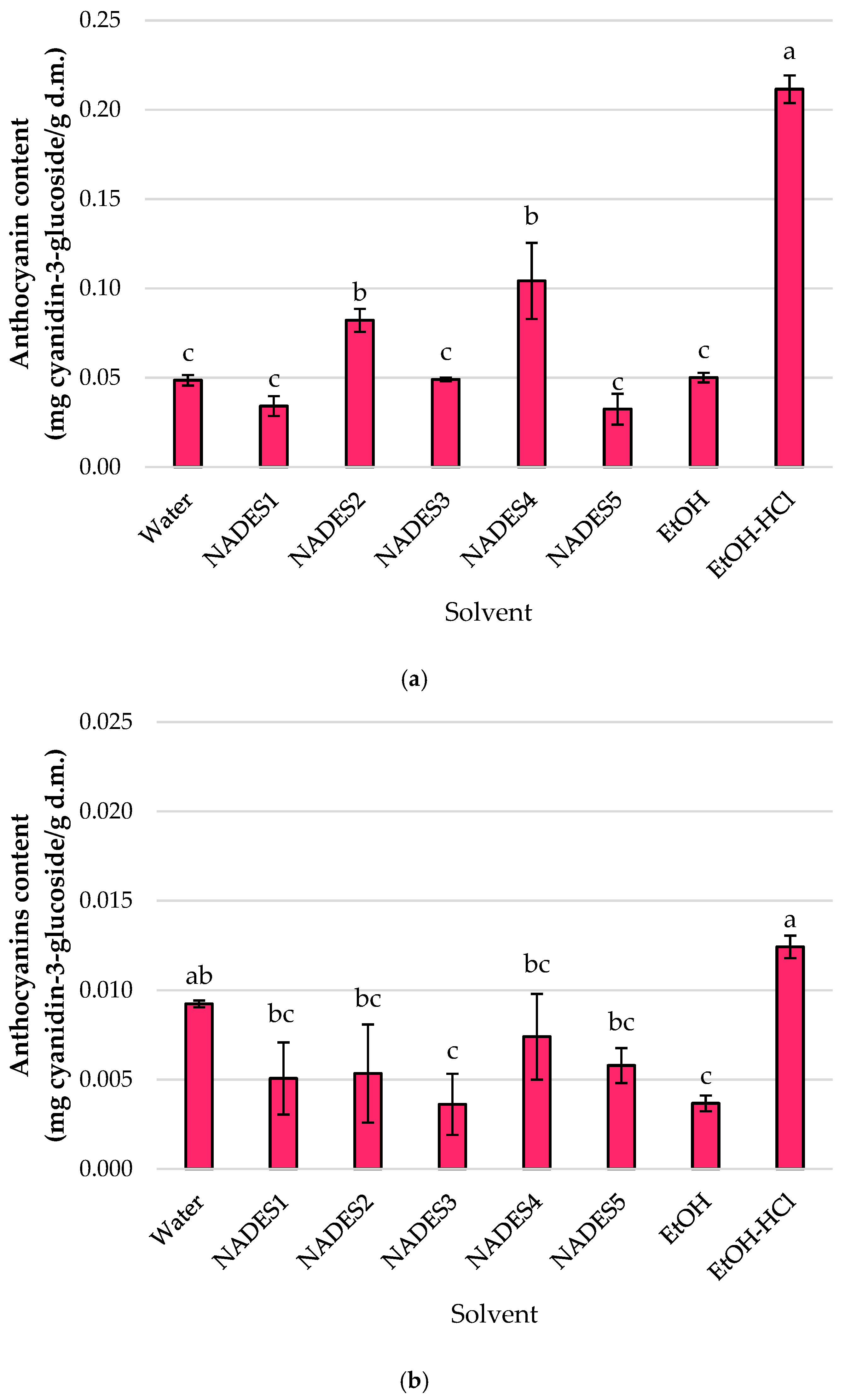
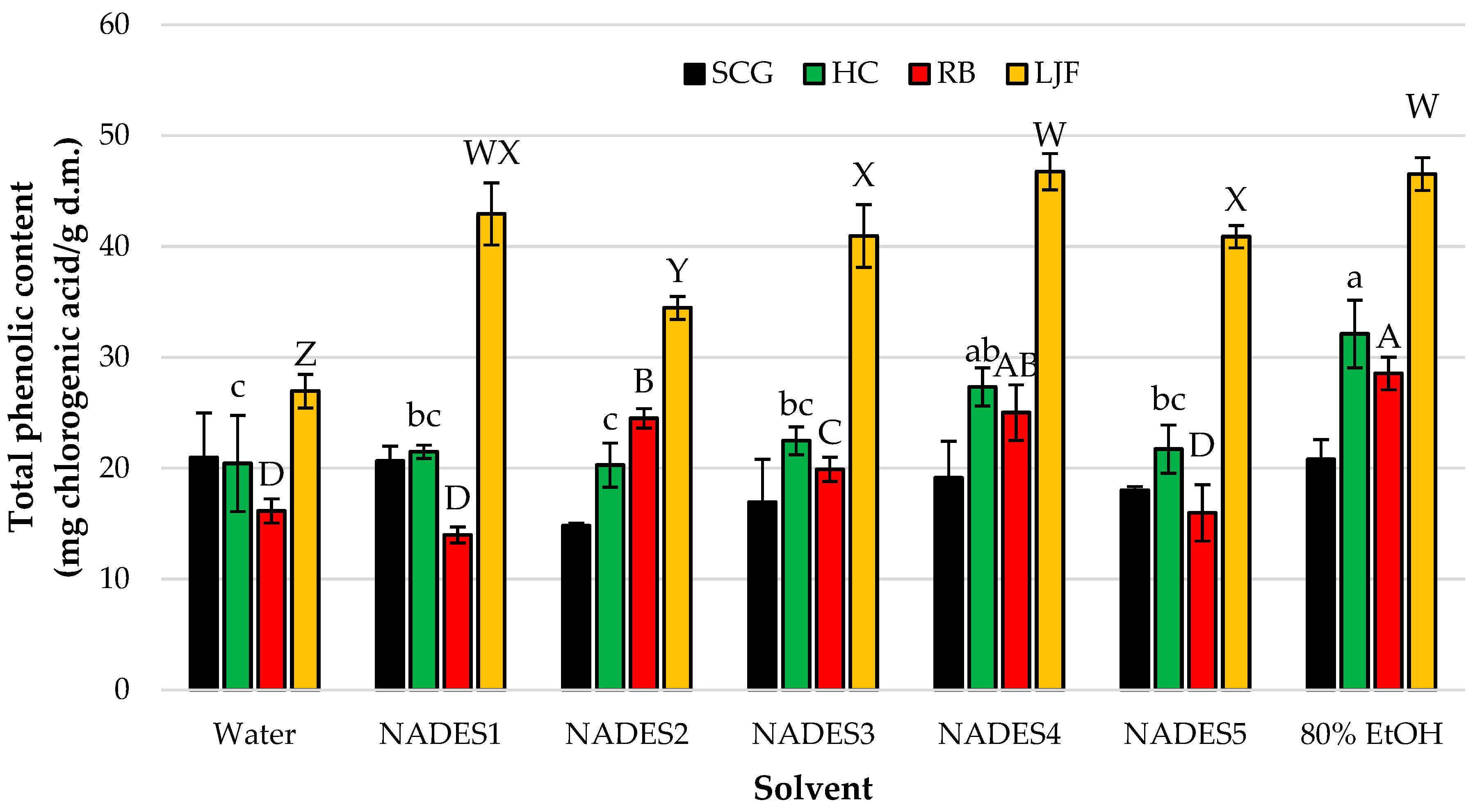
3.1. Screening of NADES for Phytochemical Recovery Across Diverse Plant Matrices
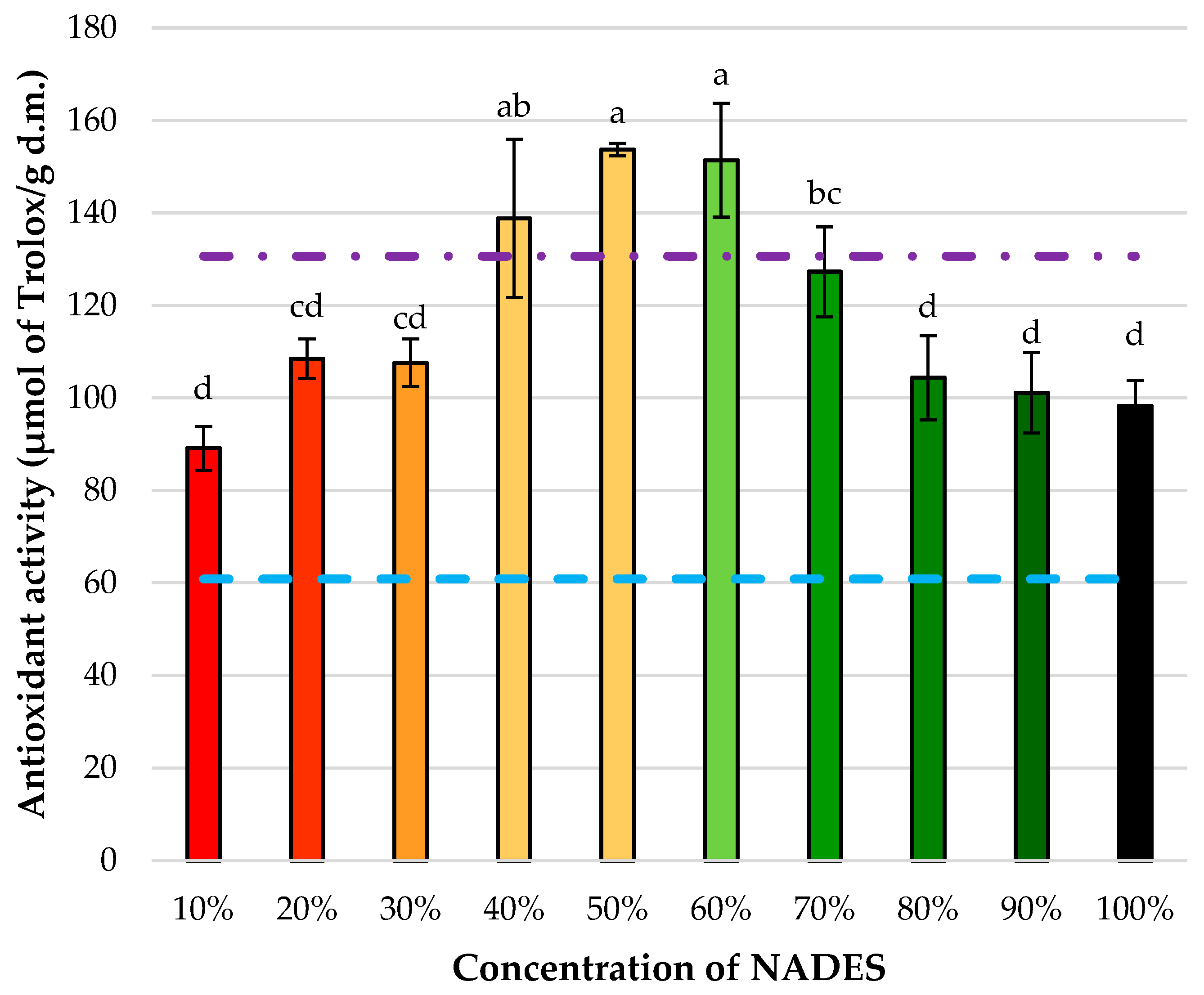
3.2. Optimization of NADES4 Aqueous Dilution for Enhanced Polyphenol Recovery from L. japonica Flowers
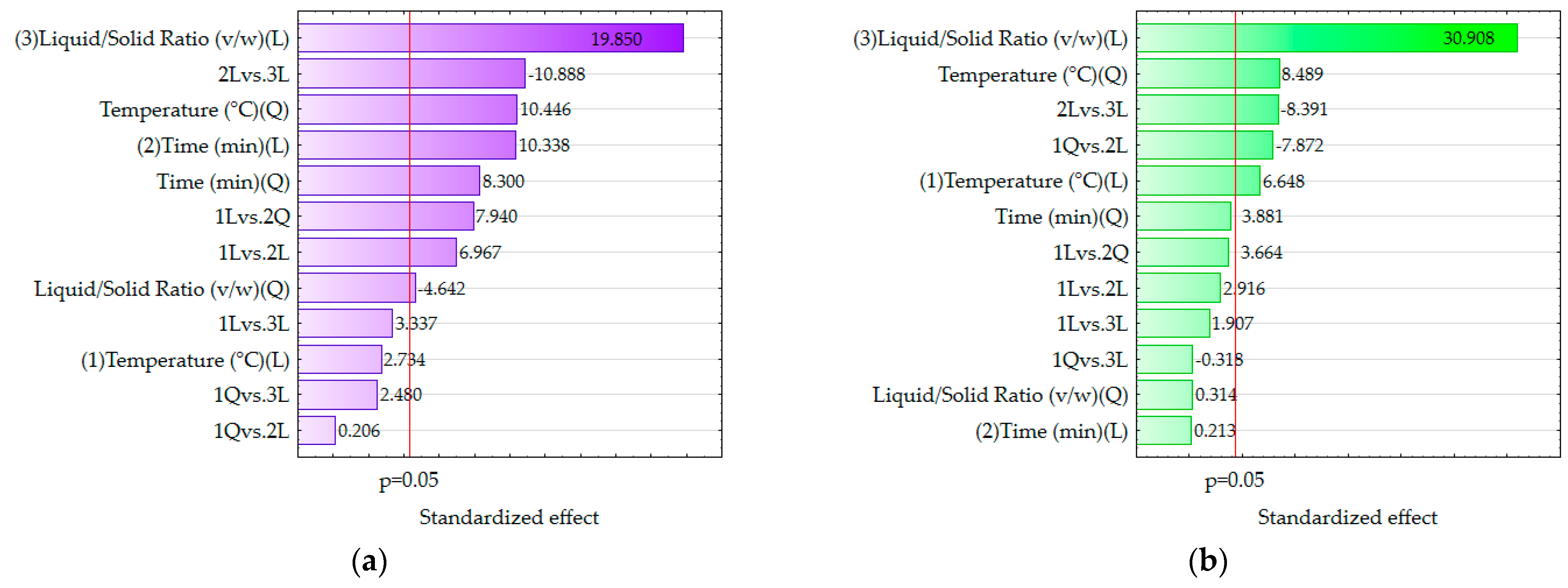
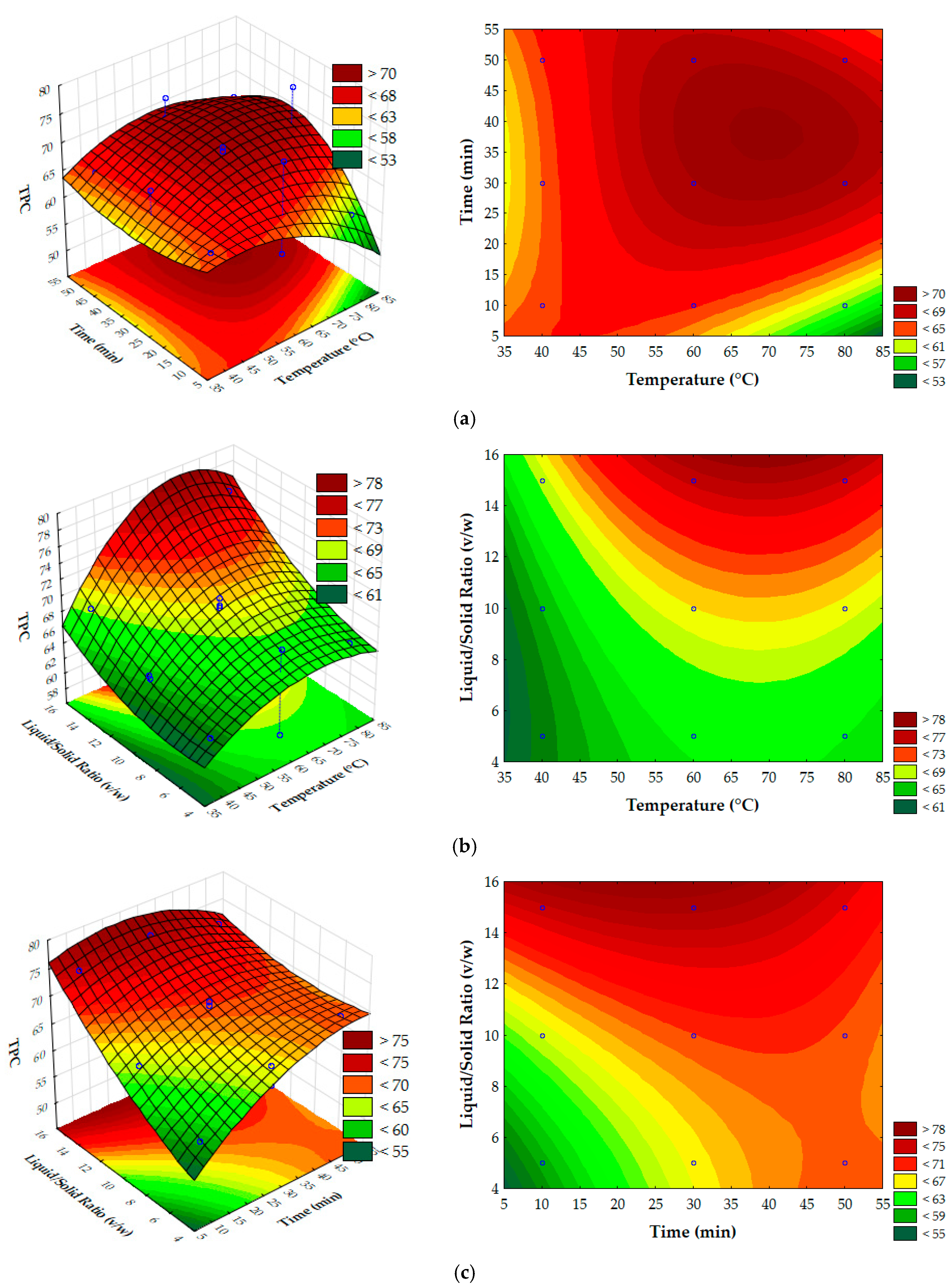
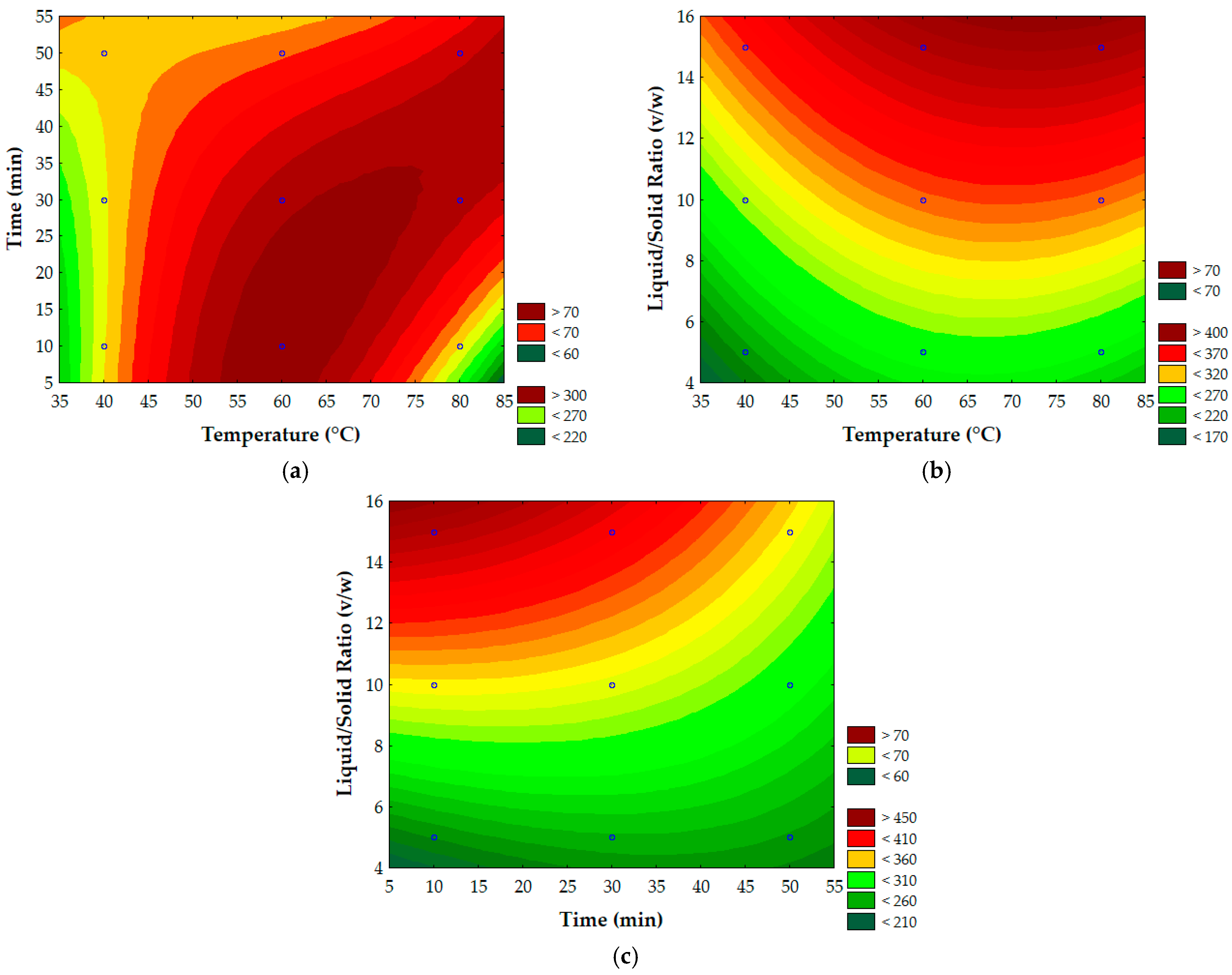
3.3. Process Parameter Optimization Using Response Surface Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Antioxidant activity |
| ANN | Artificial Neural Network |
| ANOVA | Analysis of variance |
| DES | Deep eutectic solvent |
| d.m. | Dry mass |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GAE | Gallic Acid Equivalent |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| HDES | Hydrophobic deep eutectic solvent |
| HCs | Hop Cones |
| LJF | Lonicera japonica Flowers |
| NADES | Natural deep eutectic solvent |
| RBs | Rowanberries |
| RSM | Response Surface Methodology |
| SCGs | Spent coffee grounds |
| TPC | Total phenolic content |
| VOCs | Volatile organic compounds |
References
- Cámara, M.; Sánchez-Mata, M.C.; Fernández-Ruiz, V.; Cámara, R.M.; Cebadera, E.; Domínguez, L. A Review of the Role of Micronutrients and Bioactive Compounds on Immune System Supporting to Fight against the COVID-19 Disease. Foods 2021, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Groborz, K.; Wołoszynowska, M.; Ratusz, K.; Białecka-Florjańczyk, E.; Fabiszewska, A. Enzymatic Synthesis of Lipophilic Esters of Phenolic Compounds, Evaluation of Their Antioxidant Activity and Effect on the Oxidative Stability of Selected Oils. Biomolecules 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, B.; Boselli, E. Blackcurrant Pomace as a Rich Source of Anthocyanins: Ultrasound-Assisted Extraction under Different Parameters. Appl. Sci. 2024, 14, 821. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Martinez, R.M.; Melo, C.P.B.; Pinto, I.C.; Mendes-Pierotti, S.; Vignoli, J.A.; Verri, W.A.; Casagrande, R. Betalains: A Narrative Review on Pharmacological Mechanisms Supporting the Nutraceutical Potential towards Health Benefits. Foods 2024, 13, 3909. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated Volatile Organic Compounds (Cl-VOCs) in Environment—Sources, Potential Human Health Impacts, and Current Remediation Technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate Change, Phenology, and Phenological Control of Vegetation Feedbacks to the Climate System. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Lynge, E.; Anttila, A.; Hemminki, K. Organic Solvents and Cancer. Cancer Causes Control 1997, 8, 406–419. [Google Scholar] [CrossRef]
- Aboagye, E.A.; Chea, J.D.; Yenkie, K.M. Systems Level Roadmap for Solvent Recovery and Reuse in Industries. iScience 2021, 24, 103114. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Nahar, Y.; Thickett, S.C. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Molnar, M.; Periš, I.; Komar, M. Choline Chloride Based Deep Eutectic Solvents as a Tuneable Medium for Synthesis of Coumarinyl 1,2,4-Triazoles: Effect of Solvent Type and Temperature. Eur. J. Org. Chem. 2019, 2019, 2688–2694. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; Blesa, J.; López-Malo, D.; Frígola, A.; Esteve, M.J. Choline Chloride-Based Natural Deep Eutectic Solvents for the Extraction and Stability of Phenolic Compounds, Ascorbic Acid, and Antioxidant Capacity from Citrus sinensis Peel. Lebenson. Wiss. Technol. 2023, 177, 114595. [Google Scholar] [CrossRef]
- Niemira, J.; Galus, S. Valorization of Red Beetroot (Beta vulgaris L.) Pomace Combined with Golden Linseed (Lini Semen) for the Development of Vegetable Crispbreads as Gluten-Free Snacks Rich in Bioactive Compounds. Molecules 2024, 29, 2105. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of Anthocyanins and Polymeric Color Formation during Heat Treatment of Purple Sweet Potato Extract at Different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Quality of Red Bell Pepper Subjected to Freeze-Drying Preceded by Traditional and Novel Pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Valério, A.; de Oliveira, J.V.; de Oliveira, D.; Araújo, P.H.H.d.; Riella, H.G.; Fiori, M.A. Synthesis of Geranyl Cinnamate by Lipase-Catalyzed Reaction and Its Evaluation as an Antimicrobial Agent: Lipase-Catalyzed Synthesis of Geranyl Cinnamate. J. Chem. Technol. Biotechnol. 2017, 92, 115–121. [Google Scholar] [CrossRef]
- Hernández-Aguirre, O.A.; Muro, C.; Hernández-Acosta, E.; Alvarado, Y.; Díaz-Nava, M.D.C. Extraction and Stabilization of Betalains from Beetroot (Beta vulgaris) Wastes Using Deep Eutectic Solvents. Molecules 2021, 26, 6342. [Google Scholar] [CrossRef] [PubMed]
- Kaba, B.; Zannou, O.; Ali Redha, A.; Koca, I. Enhancing Extraction of Betalains from Beetroot (Beta vulgaris L.) Using Deep Eutectic Solvents: Optimization, Bioaccessibility and Stability. Food Prod. Process. Nutr. 2024, 6, 38. [Google Scholar] [CrossRef]
- Gupta, V.; Saraswat, V.; Alagarasan, G.; Nallamilli, T.; Ramireddy, E.; K.S.M.S., R. Ultrasound-Assisted Extraction of Betalains from the Amaranthus Using Natural Deep Eutectic Solvents. Plant Foods Hum. Nutr. 2024, 80, 15. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-Carotene from Pumpkin Using Switchable Natural Deep Eutectic Solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Sportiello, L.; Tolve, R.; Galgano, F.; Giarola, M.; Musollini, S.; Favati, F. Optimizing Carotenoids NaHDES Extraction for Enhancing Spreadable Chocolate’s Nutritional Value. Food Biosci. 2024, 62, 105109. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; López-Malo, D.; Esteve, M.J.; Frígola, A.; Blesa, J. Improving Carotenoid Extraction, Stability, and Antioxidant Activity from Citrus Sinensis Peels Using Green Solvents. Eur. Food Res. Technol. 2023, 249, 2349–2361. [Google Scholar] [CrossRef]
- Foroutani, Z.; Afshar Mogaddam, M.R.; Ghasempour, Z.; Ghareaghajlou, N. Application of Deep Eutectic Solvents in the Extraction of Anthocyanins: Stability, Bioavailability, and Antioxidant Property. Trends Food Sci. Technol. 2024, 144, 104324. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ibrahim, S.A.; Koca, I. Extraction of Anthocyanins from Borage (Echium amoenum) Flowers Using Choline Chloride and a Glycerol-Based, Deep Eutectic Solvent: Optimization, Antioxidant Activity, and In Vitro Bioavailability. Molecules 2022, 27, 134. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Koca, I. Greener Extraction of Anthocyanins and Antioxidant Activity from Blackberry (Rubus spp) Using Natural Deep Eutectic Solvents. Lebenson. Wiss. Technol. 2022, 158, 113184. [Google Scholar] [CrossRef]
- Deng, W.-W.; Sun, B.; Yang, H.; Hou, X.-J.; Zhang, Y.-J.; Gan, T.-X.; Cheng, X.-Y.; Yuan, A.; Dong, X.-Y.; Zhou, C.-Y.; et al. Enhanced Antioxidant Extraction from Lonicerae japonicae Flos Based on a Novel Optimization Strategy with Tailored Deep Eutectic Solvents. Separations 2024, 11, 189. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Gao, Q.; Qiao, L.; Li, H.; Yang, S.; Yan, G.; Lei, J.; Liang, B.; Kuang, A.; et al. Eco-Friendly and Efficient Extraction of Lonicera macranthoides Phenylpropanoid Based on Natural Deep Eutectic Solvents: Process Optimization, Extraction Mechanism and Biological Activity. Microchem. J. 2024, 198, 110133. [Google Scholar] [CrossRef]
- Fan, Z.; Li, J.; Zhou, J.; Chen, Y.; Cui, Z. Extraction of Chlorogenic Acid from Lonicera japonica Thunb. Using Deep Eutectic Solvents: Process Optimization and Mechanism Exploration by the Combination of Experiment and Molecular Simulation. Microchem. J. 2025, 212, 113280. [Google Scholar] [CrossRef]
- Peng, X.; Duan, M.-H.; Yao, X.-H.; Zhang, Y.-H.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Green Extraction of Five Target Phenolic Acids from Lonicerae japonicae Flos with Deep Eutectic Solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Isci, A.; Kaltschmitt, M. Recovery and Recycling of Deep Eutectic Solvents in Biomass Conversions: A Review. Biomass Convers. Biorefin. 2022, 12, 197–226. [Google Scholar] [CrossRef]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Dragičević, T.L.; Redovniković, I.R. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]



| Mixture | HBA–HBD | Molar Ratio (HBA–HBD) | pH (After Addition of 20% Water) |
|---|---|---|---|
| NADES1 | Choline chloride–Glucose | 2:1 | 4.80 ± 0.07 |
| NADES2 | Choline chloride–Glycerol | 1:2 | 6.67 ± 0.04 |
| NADES3 | Choline chloride–Citric acid | 2:1 | 0.83 ± 0.05 |
| NADES4 | Choline chloride–Urea | 1:2 | 9.07 ± 0.06 |
| NADES5 | Choline chloride–Sorbitol | 1:1 | 3.92 ± 0.08 |
| Factors | Name | Units | Low (−1) | Medium (0) | High (+1) |
|---|---|---|---|---|---|
| 1 | Temperature | °C | 40 | 60 | 80 |
| 2 | Time | min | 10 | 30 | 50 |
| 3 | Liquid/Solid Ratio | v/m | 5 | 10 | 15 |
| Exp. No. | Temperature (°C) | Time (min) | Liquid/Solid Ratio (v/w) | TPC * | TPC (Approximated) | AA | AA (Approximated) |
|---|---|---|---|---|---|---|---|
| 1 | 40 | 10 | 10 | 64.57 ± 6.22 | 64.57 | 278.49 ± 21.95 | 278.49 |
| 2 | 80 | 10 | 10 | 59.51 ± 5.66 | 59.51 | 280.60 ± 11.55 | 280.60 |
| 3 | 40 | 50 | 10 | 64.97 ± 6.34 | 64.97 | 284.93 ± 8.72 | 284.93 |
| 4 | 80 | 50 | 10 | 68.04 ± 5.37 | 68.04 | 326.43 ± 28.55 | 326.43 |
| 5 | 40 | 30 | 5 | 62.42 ± 4.41 | 62.42 | 204.90 ± 23.93 | 204.90 |
| 6 | 80 | 30 | 5 | 66.03 ± 1.43 | 66.03 | 248.81 ± 2.30 | 248.81 |
| 7 | 40 | 30 | 15 | 68.43 ± 3.96 | 68.43 | 348.62 ± 20.79 | 348.62 |
| 8 | 80 | 30 | 15 | 75.94 ± 2.94 | 75.94 | 418.29 ± 29.32 | 418.29 |
| 9 | 60 | 10 | 5 | 58.38 ± 2.17 | 58.38 | 240.76 ± 4.55 | 240.76 |
| 10 | 60 | 50 | 5 | 69.31 ± 5.70 | 69.31 | 248.37 ± 2.51 | 248.37 |
| 11 | 60 | 10 | 15 | 74.75 ± 2.66 | 74.75 | 451.00 ± 9.46 | 451.00 |
| 12 | 60 | 50 | 15 | 72.95 ± 6.66 | 72.95 | 345.27 ± 19.99 | 345.27 |
| 13 | 60 | 30 | 10 | 69.46 ± 4.82 | 69.96 | 328.53 ± 8.59 | 336.09 |
| 14 | 60 | 30 | 10 | 69.81 ± 7.18 | 69.96 | 338.20 ± 13.93 | 336.09 |
| 15 | 60 | 30 | 10 | 70.60 ± 2.29 | 69.96 | 341.54 ± 30.97 | 336.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, A.; Özten, C.; Zieniuk, B. Utilizing Natural Deep Eutectic Solvents (NADESs) for Sustainable Phytonutrient Recovery: Optimization and Multi-Matrix Extraction of Bioactive Compounds. Appl. Sci. 2025, 15, 4843. https://doi.org/10.3390/app15094843
Makarova A, Özten C, Zieniuk B. Utilizing Natural Deep Eutectic Solvents (NADESs) for Sustainable Phytonutrient Recovery: Optimization and Multi-Matrix Extraction of Bioactive Compounds. Applied Sciences. 2025; 15(9):4843. https://doi.org/10.3390/app15094843
Chicago/Turabian StyleMakarova, Ainur, Ceylin Özten, and Bartłomiej Zieniuk. 2025. "Utilizing Natural Deep Eutectic Solvents (NADESs) for Sustainable Phytonutrient Recovery: Optimization and Multi-Matrix Extraction of Bioactive Compounds" Applied Sciences 15, no. 9: 4843. https://doi.org/10.3390/app15094843
APA StyleMakarova, A., Özten, C., & Zieniuk, B. (2025). Utilizing Natural Deep Eutectic Solvents (NADESs) for Sustainable Phytonutrient Recovery: Optimization and Multi-Matrix Extraction of Bioactive Compounds. Applied Sciences, 15(9), 4843. https://doi.org/10.3390/app15094843








