A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
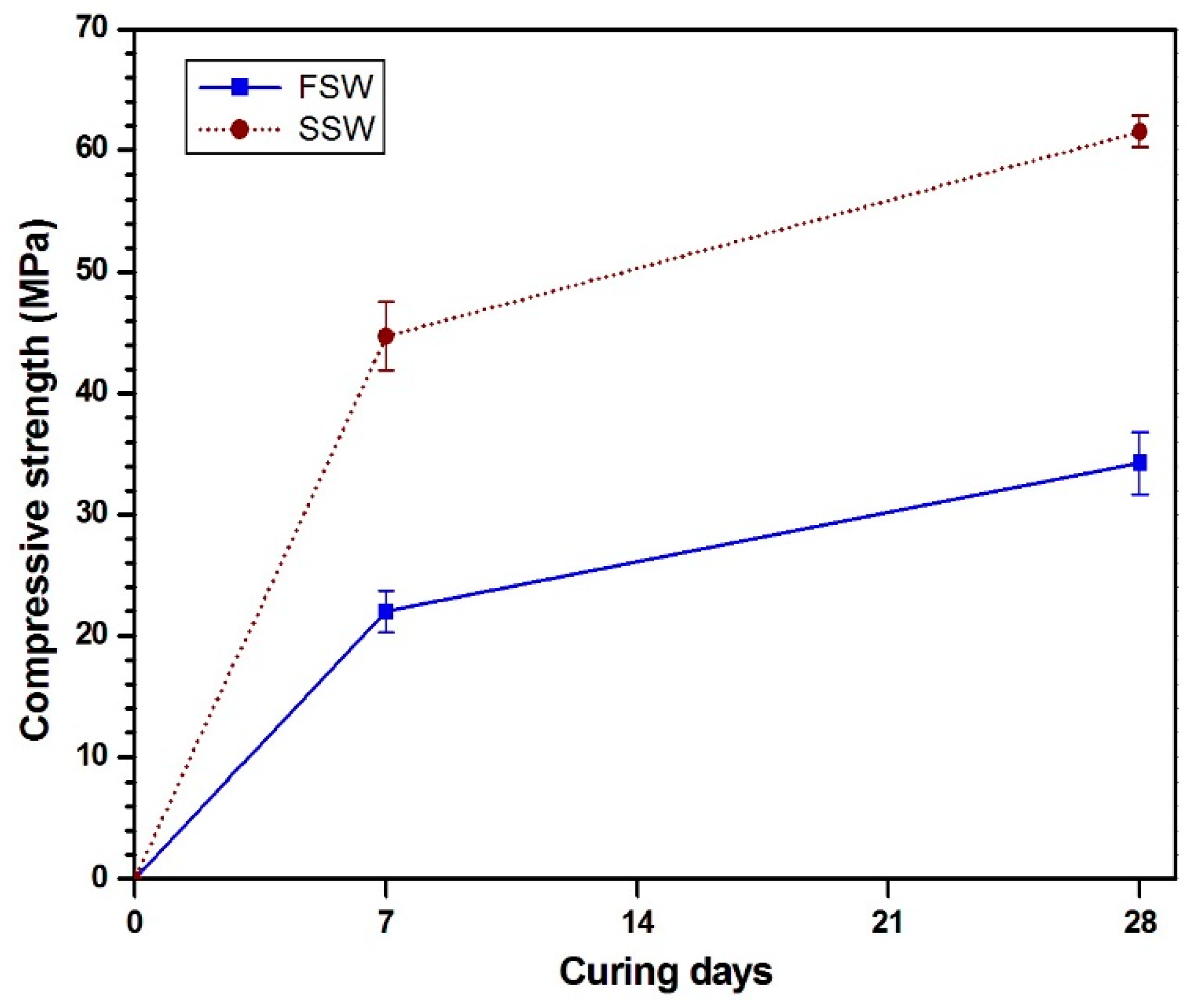
2.3.1. Compressive Strength Test
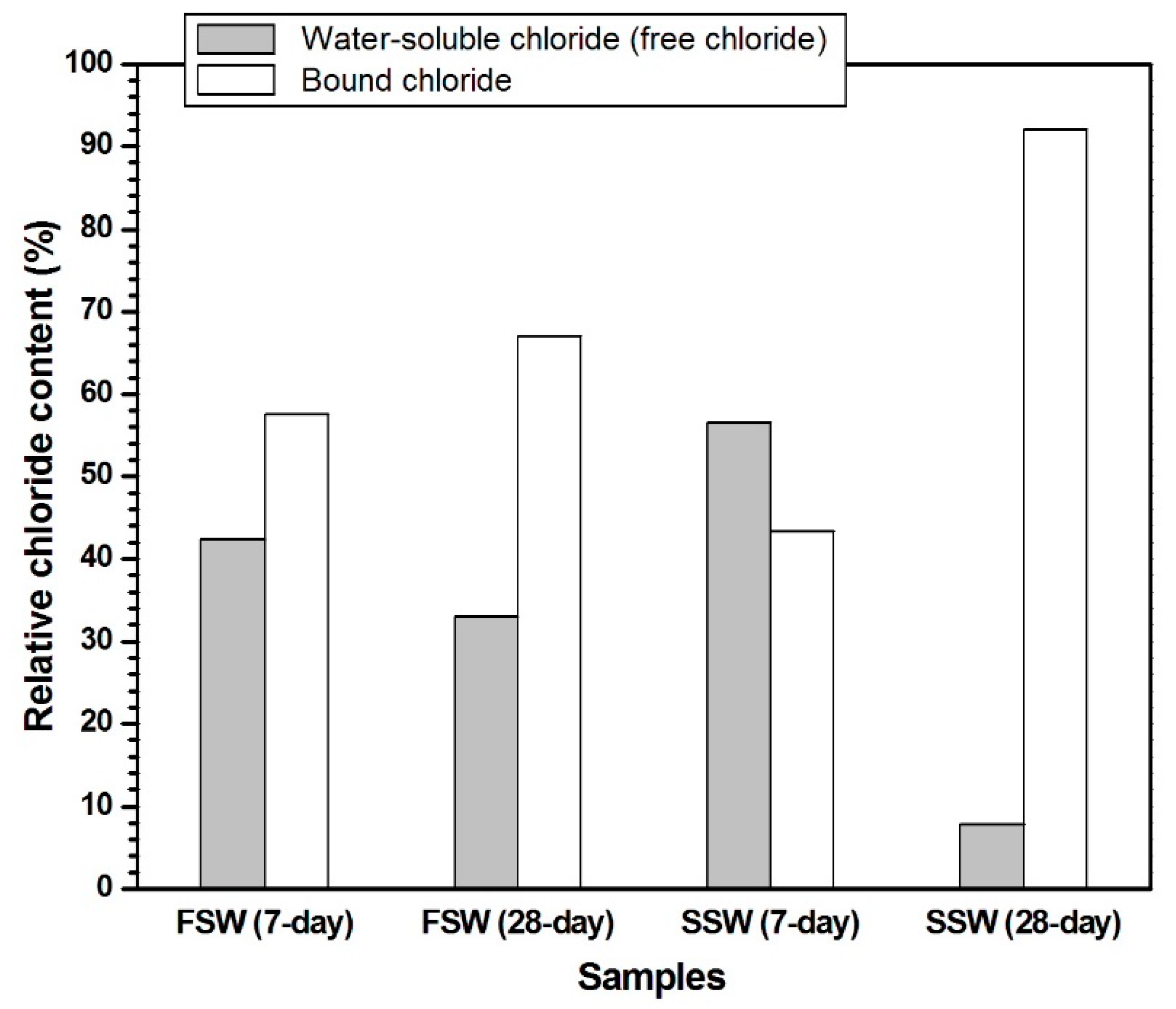
2.3.2. Measurement of Free and Bound Chloride Contents
2.3.3. XRD and MIP Tests
3. Results and Discussion
3.1. Free and Bound Cl Contents
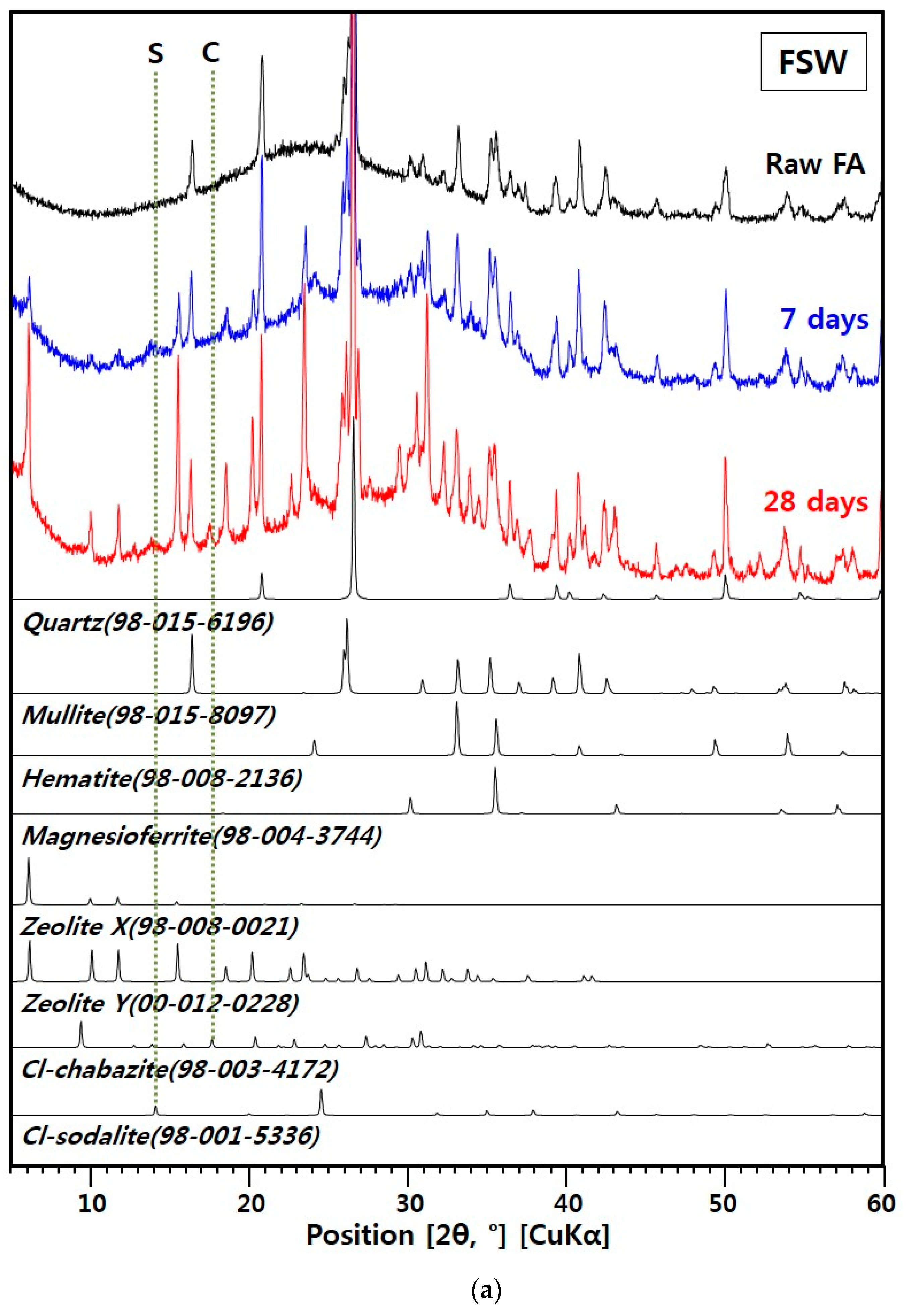
3.2. XRD Results
3.2.1. XRD for the FSW Sample
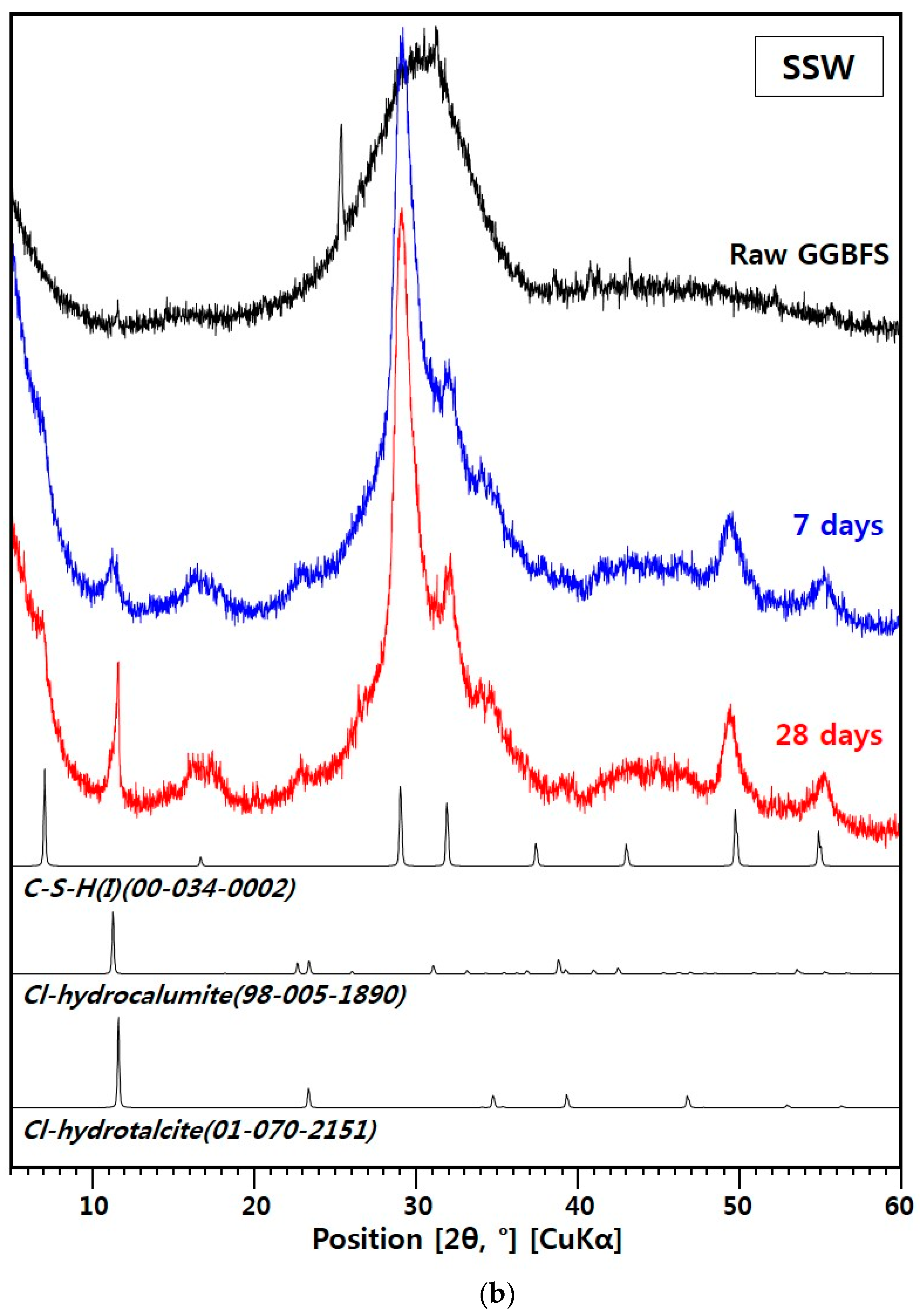
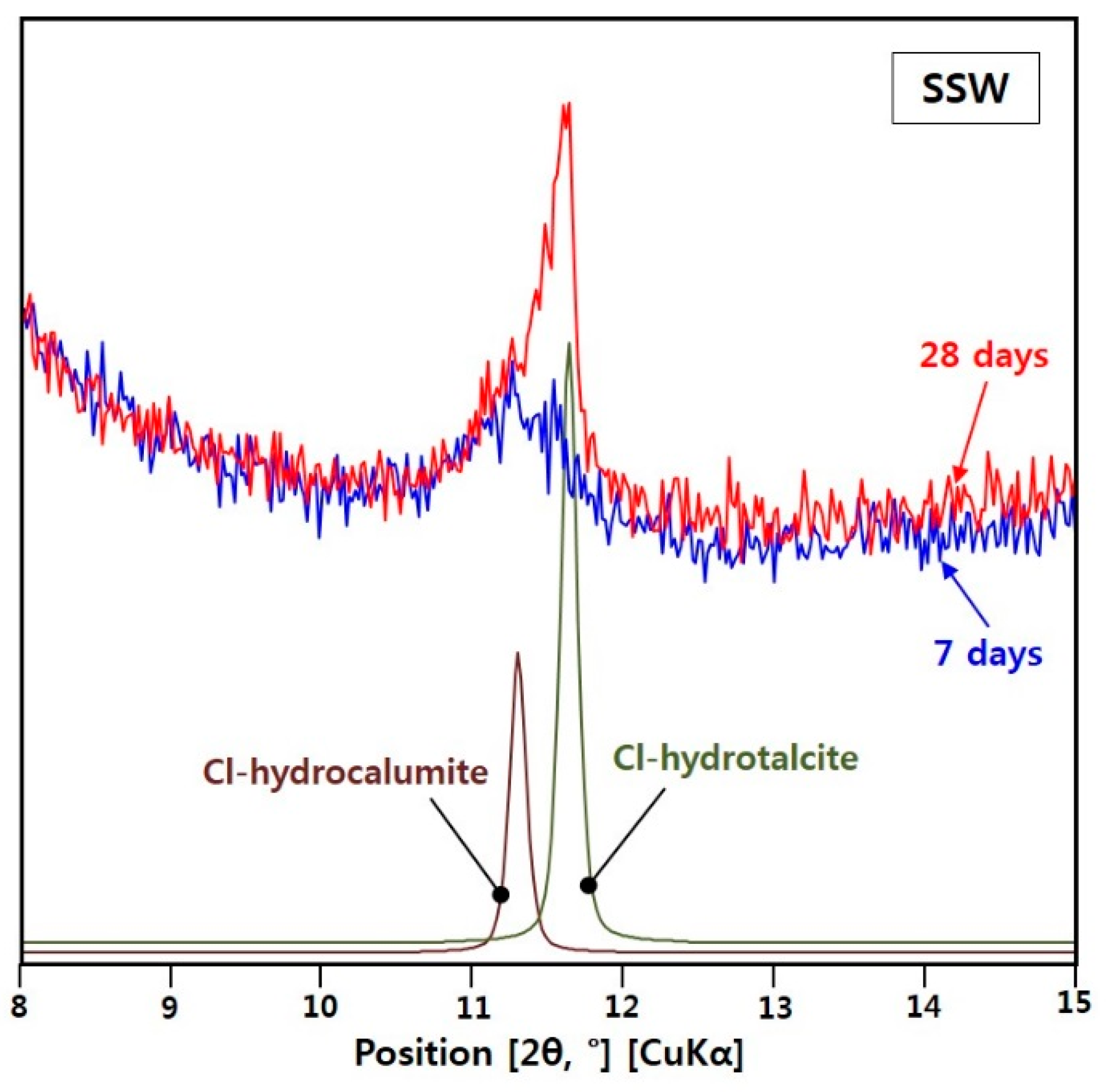
3.2.2. XRD for the SSW Sample
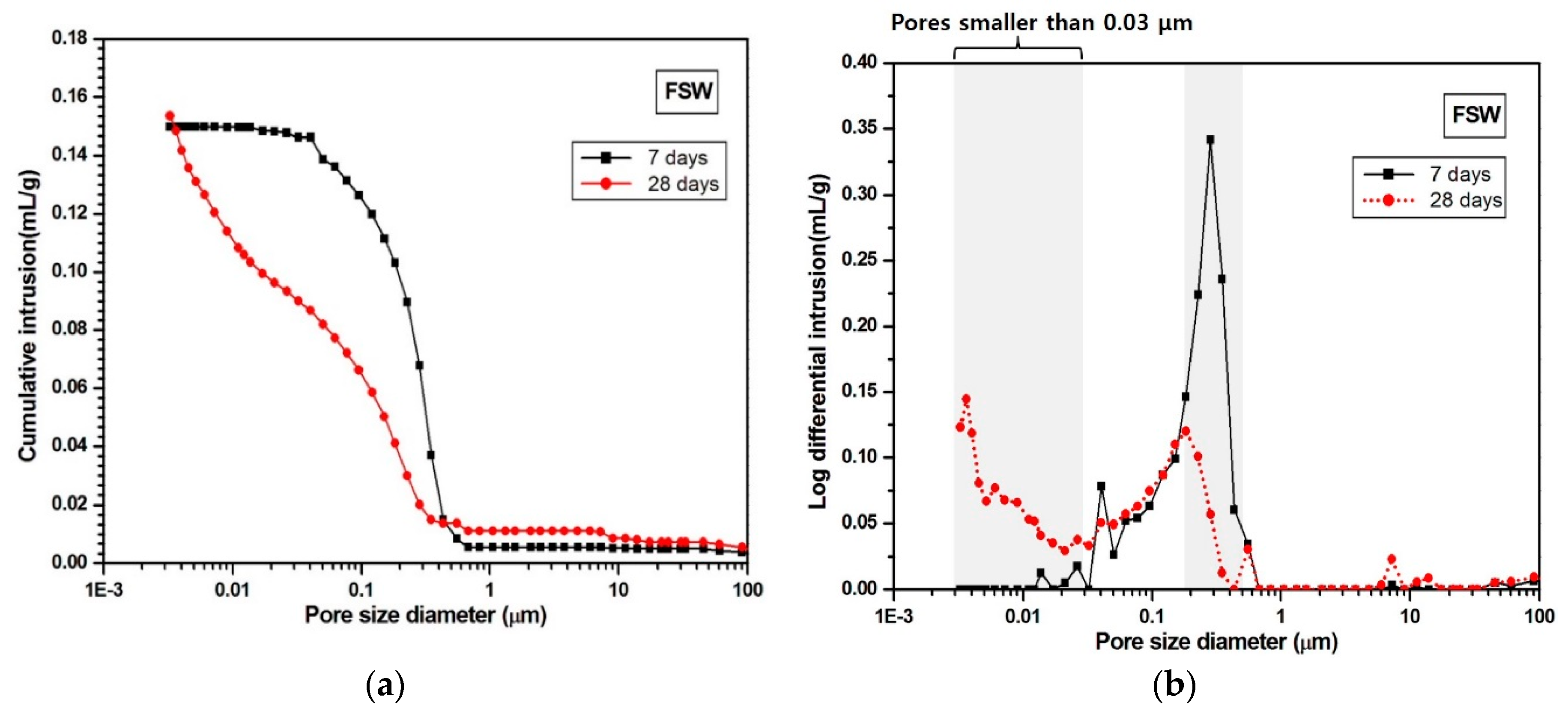
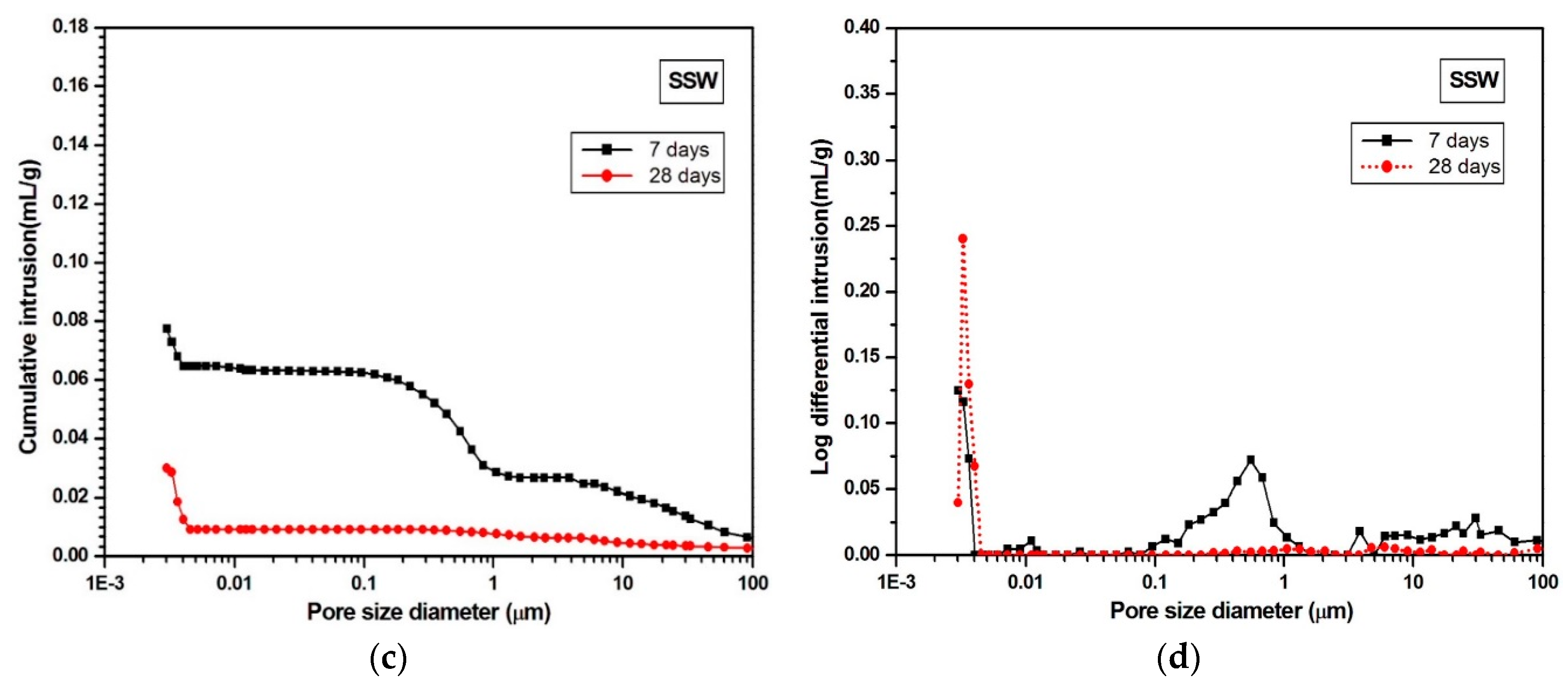
3.3. MIP
3.4. Summary and Further Discussion
4. Conclusions
- The main Cl-bearing phases were zeolites (i.e., Cl-chabazite and Cl-sodalite) in the FSW sample, while they were LDH phases (i.e., Cl-hydrotalcite and Cl-hydrocalumite) in the SSW sample.
- The bound chloride content of the SSW sample was 91% at 28 days, whereas that of the FSW sample was 69%. Therefore, at 28 days, the alkali-activated GGBFS system is more advantageous for Cl-binding than the alkali-activated fly ash system.
- The FSW sample contained zeolite X, zeolite Y, Cl-chabazite, and Cl-sodalite as crystalline zeolite phases. Zeolite X and Y, which do not contribute to the chemical Cl-binding, comprised a larger portion of the XRD pattern than Cl-chabazite and Cl-hydrocalumite.
- The SSW sample contained C-S-H(I), Cl-hydrocalumite, and Cl-hydrotalcite. Its Cl-binding mechanisms are similar to hydrated portland cement systems. C-S-H(I) is responsible for physical binding and Cl-LDHs contribute to chemical binding.
- Between the two Cl-LDHs in the SSW sample, the XRD peak of only Cl-hydrotalcite grew significantly from 7 to 28 days, and the bound chloride content of the SSW sample considerably increased from 42% to 91% over that time. Therefore, after 7 days, Cl-hydrotalcite is an important phase for chemical Cl-binding in the alkali-activated GGBFS system.
- The SSW sample showed both pore-size refinement and porosity reduction from 7 to 28 days. In contrast, the FSW sample only showed pore-size refinement over that time.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Pore size Diameter (μm) | Log Differential Intrusion (mL/g) | Pore size Diameter (μm) | Log Differential Intrusion (mL/g) | ||
|---|---|---|---|---|---|
| FSW 7 Days | FSW 28 Days | SSW 7 Days | SSW 28 Days | ||
| 0.00329 | 0 | 0.12304 | 0.00303 | 0.12477 | 0.03992 |
| 0.00362 | 0 | 0.14457 | 0.00329 | 0.11614 | 0.24018 |
| 0.00402 | 0 | 0.11852 | 0.00362 | 0.07304 | 0.12966 |
| 0.00452 | 0 | 0.0805 | 0.00402 | 0 | 0.06723 |
| 0.00517 | 0 | 0.06687 | 0.00452 | 0 | 0 |
| 0.00603 | 0 | 0.07686 | 0.00517 | 0 | 0 |
| 0.00724 | 0 | 0.06765 | 0.00603 | 0 | 0 |
| 0.00906 | 0 | 0.06558 | 0.00724 | 0.00464 | 0 |
| 0.01105 | 0 | 0.05297 | 0.00906 | 0.00489 | 0 |
| 0.01224 | 0 | 0.05159 | 0.01105 | 0.01095 | 0 |
| 0.01373 | 0.01259 | 0.04108 | 0.01224 | 0.00332 | 0 |
| 0.0171 | 0 | 0.03531 | 0.01373 | 0 | 0 |
| 0.02108 | 0.00507 | 0.02946 | 0.0171 | 0 | 0 |
| 0.02629 | 0.01781 | 0.03803 | 0.02108 | 0 | 0 |
| 0.03239 | 0 | 0.03331 | 0.02629 | 0.0025 | 0 |
| 0.04033 | 0.07809 | 0.05043 | 0.03241 | 0 | 0 |
| 0.05034 | 0.02662 | 0.04926 | 0.04032 | 0 | 0 |
| 0.06249 | 0.05212 | 0.05721 | 0.05035 | 0 | 0 |
| 0.0771 | 0.05404 | 0.0631 | 0.0625 | 0.00274 | 0 |
| 0.09539 | 0.06335 | 0.07466 | 0.07709 | 0 | 0 |
| 0.12075 | 0.08667 | 0.08664 | 0.09537 | 0.00672 | 0 |
| 0.15107 | 0.09919 | 0.1101 | 0.12082 | 0.01236 | 0 |
References
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, P. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Publishing: New York, NY, USA, 2006. [Google Scholar]
- Flower, D.J.M.; Sanjayan, J.G. Green house gas emissions due to concrete manufacture. Int. J. Life Cycle Assess. 2007, 12, 282. [Google Scholar] [CrossRef]
- Yoon, S.; Monteiro, P.J.M.; Macphee, D.E.; Glasser, F.P.; Imbabi, M.S.-E. Statistical evaluation of the mechanical properties of high-volume class F fly ash concretes. Constr. Build. Mater. 2014, 54, 432–442. [Google Scholar] [CrossRef]
- Mintzer, I.M. Confronting Climate Change: Risks, Implications and Responses; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Leiserowitz, A. Climate Change Risk Perception and Policy Preferences: The Role of Affect, Imagery, and Values. Clim. Chang. 2006, 77, 45–72. [Google Scholar] [CrossRef]
- Glasser, F.P.; Marchand, J.; Samson, E. Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Baoying, Y.; Jun, W.; Yuxin, G.; Jiayu, X. Studies on key technology and toughness of super sulfate cement-based compound materials. Appl. Mech. Mater. 2014, 665, 151–165. [Google Scholar]
- Fernández-Carrasco, L.; Vázquez, E. Reactions of fly ash with calcium aluminate cement and calcium sulphate. Fuel 2009, 88, 1533–1538. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2017. [Google Scholar] [CrossRef]
- Wegian, F.M. Effect of seawater for mixing and curing on structural concrete. IES J. Part A Civ. Struct. Eng. 2010, 3, 235–243. [Google Scholar] [CrossRef]
- Kaushik, S.K.; Islam, S. Suitability of sea water for mixing structural concrete exposed to a marine environment. Cem. Concr. Compos. 1995, 17, 177–185. [Google Scholar] [CrossRef]
- Roy, D.M.; Jiang, W.; Silsbee, M.R. Chloride diffusion in ordinary, blended, and alkali-activated cement pastes and its relation to other properties. Cem. Concr. Res. 2000, 30, 1879–1884. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Arbi, K.; Nedeljković, M.; Zuo, Y.; Ye, G. A Review on the Durability of Alkali-Activated Fly Ash/Slag Systems: Advances, Issues, and Perspectives. Ind. Eng. Chem. Res. 2016, 55, 5439–5453. [Google Scholar] [CrossRef]
- Yoon, S.; Ha, J.; Chae, S.; Kilcoyne, D.; Jun, Y.; Oh, J.; Monteiro, P. Phase Changes of Monosulfoaluminate in NaCl Aqueous Solution. Materials 2016, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, K.; Orsáková, D.; Geiker, M.R. The impact of sulphate and magnesium on chloride binding in Portland cement paste. Cem. Concr. Res. 2014, 65, 30–40. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Fiore, S. Synthesis of zeolite from Italian coal fly ash: Differences in crystallization temperature using seawater instead of distilled water. Waste Manag. 2010, 30, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.H.; Kayali, O.; Troitzsch, U. Chloride binding capacity of hydrotalcite and the competition with carbonates in ground granulated blast furnace slag concrete. Mater. Struct. 2016, 49, 4609–4619. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- ASTM International. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, H.; Chen, D.; Wang, H.; Xu, H.; Zhang, R. Preparation and properties of glass–ceramics derived from blast-furnace slag by a ceramic-sintering process. Ceram. Int. 2009, 35, 3181–3184. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Riley, J.P.; Chester, R. Chemical Oceanography; Academic Press: Cambridge, MA, USA, 1976; Volume 6. [Google Scholar]
- Sathonsaowaphak, A.; Chindaprasirt, P.; Pimraksa, K. Workability and strength of lignite bottom ash geopolymer mortar. J. Hazard. Mater. 2009, 168, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Oh, J.E. Use of gypsum as a preventive measure for strength deterioration during curing in class F fly ash geopolymer system. Materials 2015, 8, 3053–3067. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Arya, C.; Buenfeld, N.R.; Newman, J.B. Factors influencing chloride-binding in concrete. Cem. Concr. Res. 1990, 20, 291–300. [Google Scholar] [CrossRef]
- Cheewaket, T.; Jaturapitakkul, C.; Chalee, W. Long term performance of chloride binding capacity in fly ash concrete in a marine environment. Constr. Build. Mater. 2010, 24, 1352–1357. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chalee, W. Effect of sodium hydroxide concentration on chloride penetration and steel corrosion of fly ash-based geopolymer concrete under marine site. Constr. Build. Mater. 2014, 63, 303–310. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Water-Soluble Chloride in Mortar and Concrete; Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 1997. [Google Scholar]
- ASTM International. Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete; Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- PANalytical. X’Pert HighScore Plus; PANalytical: Almelo, The Netherlands, 2012. [Google Scholar]
- ICDD. PDF-2 Database; International Centre for Diffraction Data: Newton Square, PA, USA, 2000. [Google Scholar]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Rousselot, I.; Taviot-Guého, C.; Leroux, F.; Léone, P.; Palvadeau, P.; Besse, J.-P. Insights on the Structural Chemistry of Hydrocalumite and Hydrotalcite-like Materials: Investigation of the Series Ca2M3+(OH)6Cl·2H2O (M3+: Al3+, Ga3+, Fe3+, and Sc3+) by X-Ray Powder Diffraction. J. Solid State Chem. 2002, 167, 137–144. [Google Scholar] [CrossRef]
- De Vargas, A.S.; Dal Molin, D.C.C.; Vilela, A.C.F.; Silva, F.J.D.; Pavão, B.; Veit, H. The effects of Na2O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cem. Concr. Compos. 2011, 33, 653–660. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements☆. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Oh, J.E.; Jun, Y.; Jeong, Y.; Monteiro, P.J.M. The importance of the network-modifying element content in fly ash as a simple measure to predict its strength potential for alkali-activation. Cem. Concr. Compos. 2015, 57, 44–54. [Google Scholar] [CrossRef]
- Bhatia, S. Zeolite Catalysts: Principles and Applications; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Richardson, I.G.; Brough, A.R.; Groves, G.W.; Dobson, C.M. The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (C-S-H) phase. Cem. Concr. Res. 1994, 24, 813–829. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Luping, T.; Nilsson, L.-O. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 23, 247–253. [Google Scholar] [CrossRef]
- Grover, K.; Komarneni, S.; Katsuki, H. Synthetic hydrotalcite-type and hydrocalumite-type layered double hydroxides for arsenate uptake. Appl. Clay Sci. 2010, 48, 631–637. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Kirkpatrick, R.J.; Cygan, R.T. Molecular modeling of the structure and dynamics of the interlayer and surface species of mixed-metal layered hydroxides: Chloride and water in hydrocalumite (Friedel’s salt). Am. Mineral. 2000, 85, 1046–1052. [Google Scholar] [CrossRef]
- Vieille, L.; Rousselot, I.; Leroux, F.; Besse, J.-P.; Taviot-Guého, C. Hydrocalumite and Its Polymer Derivatives. 1. Reversible Thermal Behavior of Friedel’s Salt: A Direct Observation by Means of High-Temperature in Situ Powder X-ray Diffraction. Chem. Mater. 2003, 15, 4361–4368. [Google Scholar] [CrossRef]
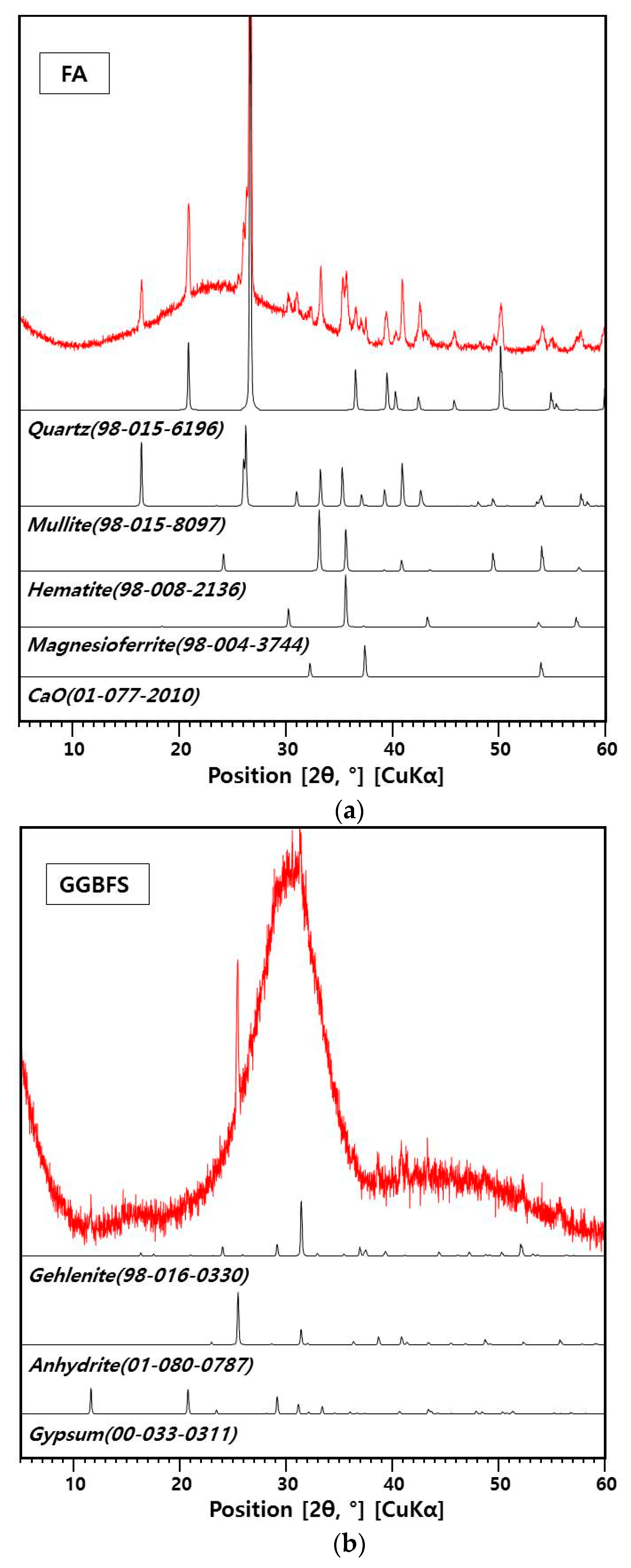
| FA | GGBFS | ||
|---|---|---|---|
| Oxide | wt % | Oxide | wt % |
| SiO2 | 51.29 | CaO | 47.42 |
| Al2O3 | 22.16 | SiO2 | 31.97 |
| Fe2O3 | 9.71 | Al2O3 | 12.51 |
| CaO | 6.44 | MgO | 3.21 |
| K2O | 1.87 | SO3 | 2.51 |
| Na2O | 1.73 | TiO2 | 0.66 |
| MgO | 1.72 | K2O | 0.53 |
| TiO2 | 1.36 | Fe2O3 | 0.41 |
| P2O5 | 1.29 | MnO | 0.34 |
| SO3 | 1.24 | Na2O | 0.21 |
| SrO | 0.37 | SrO | 0.08 |
| MoO3 | 0.34 | BaO | 0.07 |
| BaO | 0.23 | ZrO2 | 0.05 |
| MnO | 0.08 | Cl | 0.02 |
| V2O5 | 0.05 | - | - |
| ZnO | 0.02 | - | - |
| Cr2O3 | 0.02 | - | - |
| CuO | 0.02 | - | - |
| NiO | 0.02 | - | - |
| Cl | 0.01 | - | - |
| Ca2+ | K+ | Mg2+ | Na+ | Cl− | SO42− |
|---|---|---|---|---|---|
| 480 | 300 | 1200 | 10,000 | 15,000 | 2600 |
| Binder (g) (GGBFS or Fly Ash) | Activator (g) | Activator/Binder Ratio | Curing Temp (°C) | |
|---|---|---|---|---|
| 10 M NaOH in Seawater | Na2SiO3 Solution | |||
| 1600 (1240 or 1130 kg/m3) | 504 | 216 | 0.45 | 60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, Y.; Yoon, S.; Oh, J.E. A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater. Appl. Sci. 2017, 7, 971. https://doi.org/10.3390/app7100971
Jun Y, Yoon S, Oh JE. A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater. Applied Sciences. 2017; 7(10):971. https://doi.org/10.3390/app7100971
Chicago/Turabian StyleJun, Yubin, Seyoon Yoon, and Jae Eun Oh. 2017. "A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater" Applied Sciences 7, no. 10: 971. https://doi.org/10.3390/app7100971
APA StyleJun, Y., Yoon, S., & Oh, J. E. (2017). A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater. Applied Sciences, 7(10), 971. https://doi.org/10.3390/app7100971







