1. Introduction
Sampling and elution are the very first steps in the preparation of biochemical assays, which are used in the clinical testing or analysis of environmental samples. Swabs are widely used to collect biological samples, such as saliva, nasal sputum, semen, blood, or urine from the human body. They are made of cotton, rayon, polyurethane foam, or polyester, and are available in a spun or flocked tipped format. Cotton based collection methods are the most widely used sample collection techniques with salivary or sputum based immunoassays because of its easy availability, low cost and high absorbance. Apart from biological samples, swabbing techniques are also used for DNA analysis of touched evidence [
1], recovery of spores from environmental surfaces [
2,
3], or the removal of dried blood stains [
4]. Depending on the field of application, the type of swab used could vary (dry and pre-moistened swabs [
1], or macrofoam swabs [
2]). Recovery of samples from these swab tips requires a stable and high efficiency elution technique, and the success rate of all the downstream processes, like lysis and DNA amplification, are governed by the number of captured and lysed cell.
The concentration of microbial cells in body fluids has a significant influence on diagnostic sensitivity of any assay. The concentration of pathogens can range from 1 × 10
4 CFU/mL in nasal sputum samples of nosocomial pneumonia patients [
5] to 1.6 × 10
9 CFU/mL in the saliva of periodontitis patients [
6]. Apart from these common body fluids, samples from blood, vaginal fluid, urinary tracts, or semen are also collected by swabs [
7,
8]. Sperm cell recovery is a critical for elution in sexual assault samples of forensic cases. Recovery efficiency of spermatozoa, as low as 10% [
8], collected from a victim’s body hinders the subsequent process of lysis and PCR amplification, thereby incorrectly characterizing the sample as negative to sperms. Higher recovery is essential for cases where trace amounts of bacterial cells are available in the body fluids.
Several methods have been used to increase the recovery of the sample constituents from the swab. Among them, chemically enhanced recovery [
8,
9,
10] and water extraction techniques involving vortexing [
2,
3] are the most common techniques for elution and recovery of sperm cells. Enzymatic dissolution of cotton fibres result in 18–23% recovery [
8], which could be increased when detergents like Sarkosyl (54.4%) and sodium dodecyl sulfate (SDS) (78.50%) were used [
9]. Vortexing can be used for the recovery of bacterial spores in environmental samples from cotton, macrofoam, polyester, and rayon swabs, with an average recovery of 6.6% and 26.7% for dry and pre-moistened swabs, respectively [
3]. Sometimes, convective flow has been associated with vortexing to enhance the recovery (41.7%) of
Bacillus anthracis spores [
2,
3]. A comparative performance by Lutz et al. [
11] for extraction of
Staphylococcus aureus from metal surfaces with abrasive techniques showed an apparent recovery efficiency of 18% for electrostatic wipes, 10% for roller sampler and 0.04% for contact plates, which are lesser than those that were obtained from ultrasonic elution processes (32.1%) [
12,
13,
14]. Another widely used process is the thermal elution technique [
13,
15,
16], which possess the added advantage of eluting and lysing the cells simultaneously. Mechanical and magnotephoretic agitation methods require higher volumes or a continuous flow of eluent and highly intricate systems for detecting the target analyte to yield a maximum of only 55% recovery.
Several swabbing devices have also been reported in the patent literature. These devices use periodic vibration or compressible forces for elution. For example, a swab analyser with a porous swabbing pen was designed by Wuske et al. [
17], which utilises 5 mL of eluent flow for the removal of immunological components taken from skin and pharyngeal cavity. Pelssers et al. [
18] provided a compressible device for the elution of the receiving end of swabs, which allows for a conditioning fluid to be flown into the elution zone from a blister along a transfer path. Piezolelectric methods [
19] allow for an actuator to strike a flexible film at low frequency ranges between 10 and 100 Hz, but also require a flow of conditioning fluid across the swab. Nason [
20] used one or more test fluids to be delivered to the tip of the swab through the shank after being squeezed out of porous filter membranes with a hydrophobic sealant. Alternating magnetic fields [
21] have also been used for extraction of analytes from samples by tagging antibodies with magnetic beads, which binds to antigens on bacteria and gets removed on the influence of the magnetic trap.
Similar issues of low recovery are also encountered in sample processing for immunoassays in clinical diagnostics. Rose et al. [
3] confirmed the preference of vortexing (43.6%) over sonication (17.7%) as a better technique of elution of bacterial cells from pre-moistened cotton swabs. Higher recovery efficiencies upto 55% can be obtained with vortexing, while tangential shear forces with associated flow (piezoelectric, pressurized flow) can yield a maximum of 60% recovery. Different extraction techniques by vortexing, squeezing, pummelling, maceration, rolling, and differential extraction of semen from swabs were compared by Allard et al. [
22]. This study showed higher average elution times (~5 min) with 8000–13,000 RPM and ~400 to 1000 µL of eluate. Though chemical processes can provide higher elution efficiency (~78%), they require a complex combination of buffers, enzymes, and detergent solutions, and up to 2 h of incubation period, and it is also specific to the type of cells.
In this paper, a mechanical technique of elution of cotton-tipped applicator swabs was developed that significantly improves the recovery efficiency. Here, we demonstrate the highest elution recovery procedure by inflicting physical impingement forces on the swab fibres by magnetic particles. The collection and release of polystyrene beads and E. coli bacteria are compared and characterized with physical parameters, such as rotational speed (RPM), elution time, and concentration. The ability of the device to elute micro-organisms like E. coli from artificial mucous samples has also been explored. Separate protocols for the quantification of particulates have been developed using microscopic pictures and fluorescent intensity measurements. It is expected that this method has broad applicability in sample elution of a variety of clinical and environmental samples that are collected using swabs.
Working Principle and Device Design
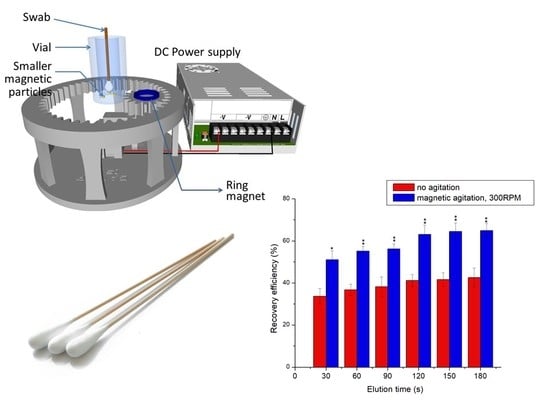
The elution device (
Figure 1a,b) consists of a rotating mechanism, ring magnet, a vial at the center with the magnetic particles, and the electrical control unit. It resembles a sun and planetary gear mechanism, with a planetary gear rotating around the teeth of an external fixed gear, the centre distance between them being 38.1 mm. The device is designed with 16 teeth on the planetary gear and a facewidth of 12 mm on both structures to prevent gear failure. The gear ratio between the mating gears is 2.9, with a module of 2.5 mm. A cylindrical vial (15 mm diameter and 45 mm height) is placed at the centre of the device, supported by clamps. Smaller magnetic particles (3.2 mm
3) are placed in confinement (gap spacing ~5 mm) inside the walls of the vial. The planetary gear has slot for holding the diametrically magnetized ring/disk magnet. The smaller magnetic particles inside the vial get attracted by the magnet, which produces simultaneous rotation around its axis and revolution around the vial axis. The rotation of the magnet along its own axis is responsible for the physical impact on the fibres. The revolutionary trajectory allows for the particles to cover the entire surface area of the swab, thereby allowing maximum exposure. By adjusting the height of the vial, the magnetic particles can be levitated from the base, which allows them to cover the entire length of the swab in the vertical direction. Smooth transfer of rotational energy has been ensured by inserting snap rings below the bearings to prevent its fallout form position. A small beam supporting the planetary gear is also connected with the motor shaft that revolves around the central axis of the device. The device is connected to a DC power supply, which provides the necessary voltage for the DC motor (
Figure 2a).
This device provides higher impact forces on the swab fibres, desired to improve elution efficiencies. In order to introduce multiple impingements and fluid circulation, a diametrically magnetized ring magnet is used and mechanically moved around the vial in a planetary fashion. The planetary rotation periodically changes the polarity of the magnet, which causes rotation of the magnetic particles in the fluid. The rotation of the particles is responsible for creating a scrubbing action to remove materials from the swab. The revolution of the ring magnet itself causes curvilinear translation of the magnetic particles around the swab. These particles strike the swab at different locations angles to provide the necessary force to elute the entrapped beads/cells. Simultaneous rotation and revolution of the particles is desired inside the vial to cover maximum surface area of the swab head. Repeated impingements along with turbulent motions of the fluid also allow for fresh fluid to enter the fibre matrix after each impact and dissolve the samples with the elution fluid.
The most important parameters that affect the recovery efficiency are the rotational speed of the magnet around the vial, the time of elution, and the concentration of the particles/cells in the sample. The rotational magnetic field is dependent on the speed of the motor. Higher rotational speed provides more number of impingements per minute, thereby facilitating higher removal of materials from the swab fibres. Hence, RPM can be identified as the most important parameter. Elution time reflects the amount of time that the impact forces are imparted to the swab and hence the trend in recovery should also influence the elution time. Concentration of the particulates in the sample is another important criterion that shows the number of eluted particles and chances of entrapment of the organisms in the fibrous matrix. The effect of these parameters needs to be characterized in order to determine the optimal operating conditions for high recovery efficiency.
3. Experimental Methods
3.1. Experimental Process
In order to demonstrate the increase in elution from the swab due to impingement of the magnetic particles, experiments were performed in settings with and without magnetic agitation, using swabs that were dipped in artificial sputum samples containing polystyrene beads. In these experiments, dry swabs were immersed in the artificial sputum samples with 10% v/v (~109 particles/mL) polystyrene bead concentrations. Next, the swabs were air dried for 30 s and dipped into the vial containing DI water. The experiments were repeated (n = 5) for various elution times (30 s–180 s) at 30 s intervals. In another set of experiments (n = 5), swabs dipped in the same samples were dipped into the vial with DI water and were agitated using mechanical impingement caused by magnetic particles under the influence of the external magnet rotating at 300 RPM for various elution times (30 s–180 s). Recovery efficiencies were obtained by measuring the number of beads eluted into the DI water solution using techniques described in the methods section.
Rotational speed of the magnetic particles is an important parameter for elution, which determines the number of impacts to the swab fibres and therefore is related to the amount of material scrubbed per minute. In order to characterize its effect of rotational speed of the magnets on the number of eluted particles and recovery efficiency, dry swabs were immersed in artificial sputum solutions of 10% v/v (~109 polystyrene particles/mL), air dried for 30 s, immersed inside the vial with elution fluid, and exposed to various rotational speeds (0–500 RPM), for 30 s. The number of eluted particles and recovery efficiencies were both recorded for five experiments at each rotational speed. The duration of elution procedure also has a significant effect on the recovery efficiency, since it determines the number of impingements that are caused over the entire period of time. To predict the nature of elution rate with changes in time, experiments were done with 10% polystyrene in artificial sputum samples. Dry swabs were dipped in artificial sputum samples concentrated with 10% v/v (~109 particles/mL concentration) polystyrene beads, air dried for 30 s and immersed in the vial with elution fluid. The motor was set to a specific rotational speed and the number of eluted particles was noted with the change of time (30 s–120 s).
Experiments were also done with a specific elution time and rotational speed to determine the effects of concentration on recovery efficiency. The concentration of the sample also influences the recovery and is a vital parameter when body fluids with varying microbial concentrations are under consideration. Since it had already been established that higher rotational speeds (400–500 RPM) and elution times (120 s) produce a better recovery of beads, experiments were performed only with those specific parameters. Dry swabs were dipped in artificial sputum solutions of different concentrations (~109–106 particles/mL), air dried for 30 s and immersed in DI water in a vial for elution. The rotational speed of the magnet was set at 400 or 500 RPM and the elution of materials from the swab was measured. Similar experiments were repeated with bacterial samples to show the respective changes in recovery efficiency with changes in rotational speed, time and concentration.
3.2. Determination of Cellular Recovery
The number of particles adhered to the swab before elution, were determined by measuring the volume of solution absorbed in the fibres, under the assumption that they were homogeneously mixed with the artificial sputum. To calculate the number of eluted particles, microscopic images (Upright Bright Field & Fluorescence Microscope, Olympus, BX53, Tokyo, Japan) of 10 µL of the post-elution solution on a microscope slide, covered by a slide cover (22 mm
2), were taken at several points on the cover. These pictures were then assessed in ImageJ software (version 3.1.0) to automatically count the number of particles at each spots of the microscope slide cover. They were then averaged over the entire area to find the number of particles in the region that were covered by the slide cover and back-calculate to obtain the actual number of eluted particles in the solution. Recovery efficiency was calculated from the formula:
3.3. Determination of Cellular Recovery for Low Concentration
With a lower concentration, direct particle counting approach was not suitable, as the random sampling at a number of different locations led to a wider distribution in the number of particles at all locations. To overcome this problem, fluorescence intensity measurements were taken with a plate reader (Tecan Infinite M1000 Pro, Tecan Trading AG, Männedorf, Switzerland) of serially diluted samples of known concentration (300 µL), and calibration curves were drawn. Separate calibration curves were drawn for E. coli and polystyrene beads to work with samples in 106–104 CFU or particles/mL concentration range. Fluorescent intensities were measured for all of the post-elution solutions and compared with the calibration curve to know the exact concentrations. Once the elution volume has been measured, the number of recovered particles/cells were calculated by using the calibrated equation.
3.4. Scanning Electron Microscopy (SEM) Measurements
For a better understanding of the release of beads from swab fibres in a working model of polystyrene beads suspended in artificial mucous, Scanning Electron Microscopy (SEM) pictures were taken. SEM images of the swab tips were taken in order to visualize the elution of the microscopic particles from the swab head. Dry swabs were immersed in a solution of artificial sputum with a bead concentration of 109 particles/mL for 30 s and air dried for 30 s. Some of these swabs were then introduced in the setup, and were eluted for 60 s in distilled water at 100 RPM. Others were preserved as is to obtain images of the pre-elution condition of the swab. Scanning Electron photomicrographs were then taken at magnifications of 1000× and 5000×.
5. Discussion
Elution by magnetic agitation has been proved to be beneficial over the control case of no agitation, as shown in
Figure 3. Two different mechanisms operate during the processes of elution by magnetic agitation. The magnetic impingement on the swab fibres causes the sample, absorbed in the swab, to be locally squeezed out at the point of impingement. This eluted sample is thoroughly mixed with the elution buffer due to the convective motion of the surrounding fluid, which is also caused by the motion of the magnets. Higher recovery efficiencies are obtained from physical impingement of particles on the swab tip, and hence is a better elution process than any technique without significant agitation. Unlike the no agitation case, where the time constant that is associated with the dissolution process is long and therefore only the sample at the surface layers of the swab get effectively eluted, under magnetic impingement, not only is the surface elution rapid, but the impingement also imparts forces to actively mix the sputum sample between the deeper layers of the swab and the surface layers that have absorbed the elution buffer from outside. This effectively helps in the dissolution of the sample that has been absorbed deep inside the swab matrix, which may not be eluted otherwise.
The recovery efficiency of polystyrene beads from the swab fibres increases with an increase in rotational speed, as shown in
Figure 5a. Multiple impingements on the swab fibres are responsible for these higher rates of elution. The magnetic particles rotate at 2 ×
i ×
Nm, where
Nm is the rotational speed of the motor and
i is the gear ratio. In one complete rotation, the magnetic particles impart two impacts. Hence, the total number of impacts per minute becomes 4 ×
i ×
Nm. As the rotational speed increases, the rate of impact on the swab fibres increases. The number of impacts at 500 RPM is 5 times higher than that offered at a low rotational speed of 100 RPM. Higher forces of impact between the particles and the swab provide better release of the samples due to efficient scrubbing action. Fresh elution fluid enters the fibre matrix after each subsequent impact and allows for the faster dissolution of artificial sputum. More particles can migrate into the elution volume, facilitating better recovery, and hence more beads are eluted from the swab tip. The acting forces on the swab were measured using a load cell, as shown in
Figure 2b. The impact forces was found to increase with the increase in the number of impacts per minute at higher rotational speeds. The forces on the swab are mostly due to impact of the rotating magnetic particles, although the whole kinetic energy is not imparted to the swabs. A part of the kinetic energy is also dissipated in the fluid, which causes convective fluid motion around the swab. The duration of elution also determines the number of impingements that are caused. When the rotational speeds are kept constant, the total number of impacts per minute remains the same over the entire time period. At lower elution times, the total number of impacts (4 ×
i ×
Nm ×
t) has a significant effect on the number of eluted beads. For 30, 60 and 90 s, the increase in total number of impacts over time results in increased average impact force on the swab fibres, thereby increasing the number of recovered particles and the efficiency. Fresh fluid entering the fibre matrix helps in dissolving the artificial sputum solutions that pushes the beads into the elution fluid. But, the dissolution reaches a steady state after a very small amount of time, and after that, the impact is the only operating force that is acting on the swab. The impact force that is surrounding the swab removes the beads from the swab matrix, but only to a certain extent. After ~90 s, most of the beads that were free to move to the eluate had already migrated. The ones trapped deeper into the fibres do not get eluted and remains inside, even at higher elution times.
The increase in recovery efficiency at a lower concentration (as shown in
Figure 6) is a surprising result and it could be due to a varied number of factors. The ability of the artificial sputum sample to dissolve into the eluent fluid could be influenced by the particle loading. High particle loading could make it more difficult to dissolve samples that are embedded in the deeper layers of the swab and therefore produce lower elution efficiency. Alternatively, the process of reabsorption of the eluent fluid after local impact into the swab and the particle loading of the surface layers due to the reabsorption can be influenced by the concentration of the particles in solution. In low concentration samples, the number of particles in the eluent solution is comparatively low. The reabsorption could preferentially absorb some of the fluid post elution and therefore retain more of the particles in the eluent fluid as compared to the high concentration case. Finally, after the elution process is complete, some of the fluid is adsorbed on the outer surface of the swab while it is being taken out, which is also directly proportional to the concentration and the number of available adsorption sites.
E.coli was chosen as a model as it is one of the common infections in many clinical situations. It is also similar in size to many other infectious pathogens. Additionally, it is easy to grow in laboratory conditions, and therefore was considered as a suitable model. The results with
E.coli showed a similar trend as the polystyrene beads indicating that the characterization performed with the beads would be useful in determining the optimal conditions for bacterial samples as well. Similar to the polystyrene beads, the bacterial cells also undergo higher rates of recovery efficiency at higher speeds, as shown in
Figure 7. The recovery efficiency was found to increase with concentration of the bacteria in the control case (no agitation). This is associated with the dissolution of the surface layer of artificial sputum dissolving into the eluent solution. In all of the other cases, the impingement of the magnetic particles on the swab creates impact forces that physically squeeze the fluid from the fibers and push the sample out, which results in higher elution efficiency.