Antimony Oxide-Doped 0.99Pb(Zr0.53Ti0.47)O3–0.01Bi(Y1?xSbx)O3 Piezoelectric Ceramics for Energy-Harvesting Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analysis of Pre-Synthesized PZT and Bi[Y(1−x)Sbx]O3
3.2. Comparative Study of Sb2O3 and Sb2O5 Doping on the PZT–BY System
3.3. Effects of Pre-Synthesized BYS(x) Content on the Densities of PZT–BYS(x) Ceramics
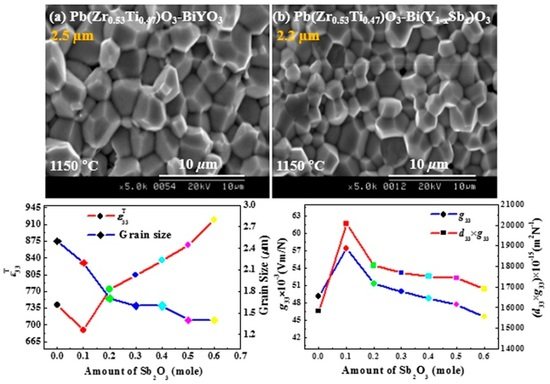
3.4. Effects of Pre-Synthesized BYS(x) on the Crystal Structure and Microstructure of PZT–BYS(x) Ceramics
3.5. The Piezoelectric and Dielectric Properties of PZT–BYS(x) Ceramics
3.6. Feasibility of Sb2O3-Doped PZT–BYS(x) Ceramics for Use in an Energy Harvester
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kang, M.G.; Jung, W.S.; Kang, C.Y.; Yoon, S.J. Recent progress on PZT based piezoelectric energy harvesting technologies. Actuators 2016, 5, 5. [Google Scholar] [CrossRef]
- Tang, G.; Yang, B.; Hou, C.; Li, G.; Liu, J.; Chen, X.; Yang, C. A piezoelectric micro generator worked at low frequency and high acceleration based on PZT and phosphor bronze bonding. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Almusallama, A.; Luob, Z.; Komolafea, A.; Yanga, K.; Robinsonc, A.; Toraha, R.; Beeby, S. Flexible piezoelectric nano-composite films for kinetic energy harvesting from textiles. Nano Energy 2017, 33, 146–156. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Sameoto, D.; Evoy, S. A review of piezoelectric polymers as functional materials for electromechanical transducers. Smart Mater. Struct. 2014, 23, 1–26. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Deng, Z.D. Energy harvesting from low frequency applications using piezoelectric materials. Appl. Phys. Rev. 2014, 1, 1–19. [Google Scholar] [CrossRef]
- Lupascu, D.C. Fatigue in Ferroelectric Ceramics and Related Issues; Springer: Berlin, Germany, 2004; pp. 43–46. [Google Scholar]
- Yuan, D.; Yang, Y.; Hu, Q.; Wang, Y. Structures and properties of Pb(Zr0.5Ti0.5)O3–Pb(Zn1/3Nb2/3)O3–Pb(Ni1/3Nb2/3)O3 ceramics for energy harvesting devices. J. Am. Ceram. Soc. 2014, 97, 3999–4004. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kang, H.W.; Kucheiko, S.I.; Kim, H.J.; Jung, H.J.; Lee, D.K.; Ahn, H.K. Piezoelectric properties of Pb[Zr0.45Ti0.5−xLux(Mn1/3Sb2/3)0.05]O3 ceramics. J. Am. Ceram. Soc. 1998, 81, 2473–2476. [Google Scholar] [CrossRef]
- Choi, C.H.; Seo, I.T.; Song, D.; Jang, M.S.; Kim, B.Y.; Nahm, S.; Sung, T.H.; Song, H.C. Relation between piezoelectric properties of ceramics and output power density of energy harvester. J. Eur. Ceram. Soc. 2013, 33, 1343–1347. [Google Scholar] [CrossRef]
- Lonkar, C.M.; Premkumar, S.; Kharat, D.K.; Kumar, H.H.; Prasad, S.; Balasubramanian, K. Effect of La on piezoelectric properties of Pb(Ni1/3Sb2/3)O3–Pb(ZrTi)O3 ferroelectric ceramics. J. Mater. Sci. Mater. Electron. 2013, 24, 1989–1993. [Google Scholar] [CrossRef]
- Xu, R.; Kim, S.G. Figures of merits of piezoelectric materials in energy harvesters. In Proceedings of the PowerMEMS, Atlanta, GA, USA, 2–5 December 2012. [Google Scholar]
- Priya, S. Criterion for material selection in design of bulk piezoelectric energy harvesters. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 2610–2612. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S.; Mahmud, I.; Ur, S.C. Phase formation, microstructure and piezoelectric/dielectric properties of BiYO3 doped Pb(Zr0.53Ti0.47)O3 for piezoelectric energy harvesting devices. Ceram. Int. 2013, 39, 8581–8588. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, S.; Gu, H.; He, Y.; Kuang, A.; Sun, W. The effect of doping Sb2O3 in high d33•g33 PZT piezoelectric ceramics. Ferroelectrics 1997, 195, 101–104. [Google Scholar] [CrossRef]
- Rai, R.; Sharma, S. Structural and dielectric properties of Sb-doped PLZT ceramics. Ceram. Int. 2004, 30, 1295–1299. [Google Scholar] [CrossRef]
- Lonkar, C.M.; Kharat, D.K.; Kumar, H.H.; Prasad, S.; Balasubramanian, K. Effect of sintering time on dielectric and piezoelectric properties of Lanthanum doped Pb(Ni1/3Sb2/3)–PbZrTiO3 ferroelectric ceramics. Def. Sci. J. 2013, 63, 418–422. [Google Scholar] [CrossRef]
- Zahi, S.; Bouaziz, R.; Abdessalem, N.; Boutarfaia, A. Dielectric and piezoelectric properties of PbZrO3–PbTiO3–Pb(Ni1/3,Sb2/3)O3 ferroelectric ceramic system. Ceram. Int. 2003, 29, 35–39. [Google Scholar] [CrossRef]
- Luan, N.D.T.; Vuong, L.D.; Chanh, B.C. Microstructure, ferroelectric and piezoelectric properties of PZT-PMnSbN ceramics. Int. J. Mater. Chem. 2013, 3, 51–58. [Google Scholar]
- Choia, W.Y.; Ahn, J.H.; Lee, W.J.; Kim, H.G. Electrical properties of Sb-doped PZT films deposited by d.c. reactive sputtering using multi-targets. Mater. Lett. 1998, 37, 119–127. [Google Scholar] [CrossRef]
- Dutta, S.; Choudhary, R.N.P.; Sinha, P.K. Electrical properties of antimony doped PLZT ceramics prepared by mixed-oxide route. J. Alloys Compd. 2006, 426, 345–351. [Google Scholar] [CrossRef]
- Dutta, S.; Choudhary, R.N.P.; Sinha, P.K. Studies on structural, electrical and electromechanical properties of Sb3+-modified PLZT. Mater. Sci. Eng. B 2004, 113, 215–223. [Google Scholar] [CrossRef]
- Yoon, S.J.; Choi, J.W.; Choi, J.Y.; Dandan, W.; Li, Q.; Yang, Y. Influences of donor dopants on the properties of PZT-PMS-PZN piezoelectric ceramics sintered at low temperatures. J. Korean Phys. Soc. 2010, 57, 863–867. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Uchino, K.; Takasu, T. Property variation of piezoelectric ceramics with microstructure. Inspec 1986, 10, 29–33. [Google Scholar]
- Slouka, C.; Kainz, T.; Navickas, E.; Walch, G.; Hutter, H.; Reichmann, K.; Fleig, J. The effect of acceptor and donor doping on oxygen vacancy concentrations in lead zirconate titanate (PZT). Materials 2016, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Watanabe, N.; Takatori, K.; Hori, S. Piezoelectric and dielectric properties for doped lead zirconate titanate ceramics under strong electric field. J. Appl. Phys. 1994, 33, 5352–5355. [Google Scholar] [CrossRef]
- Takahashi, S.; Hirose, S.; Uchino, K. Stability of PZT piezoelectric ceramics under vibration level change. J. Am. Ceram. Soc. 1994, 77, 2429–2432. [Google Scholar] [CrossRef]
- Ichinose, N.; Kimura, M. Preparation and properties of lead zirconate-titanate piezoelectric ceramics using ultrafine particles. Jpn. J. Appl. Phys. 1991, 30, 2220–2223. [Google Scholar] [CrossRef]
- Martirenat, H.T.; Burfoot, J.C. Grain-size effects on properties of some ferroelectric ceramics. J. Phys. C Solid State Phys. 1974, 7, 3182–3192. [Google Scholar] [CrossRef]
- Shirane, G.; Sawaguchi, E.; Takagi, Y. Dielectric properties of lead zirconate. J. Phys. Soc. Jpn. 1951, 6, 208–209. [Google Scholar] [CrossRef]
- Okazaki, K.; Nagata, K. Mechanical behaviour of materials. J. Soc. Mater. Sci. Jpn. 1972, 4, 404. [Google Scholar]
- Okazaki, K.; Nagata, K. Effects of grain size and porosity on electrical and optical properties of PLZT ceramics. J. Am. Ceram. Soc. 1973, 56, 82–86. [Google Scholar] [CrossRef]
- Lee, K.G.S.; Shrout, T.R.; Venkataramani, S. Fabrication of fine-grain piezoelectric ceramics using reactive calcination. J. Mater. Sci. 1991, 26, 4411–4415. [Google Scholar]
- Ai, Z.; Hou, Y.; Zheng, M.; Zhu, M. Effect of grain size on the phase structure and electrical properties of PZT–PNZN quaternary systems. J. Alloys Compd. 2014, 617, 222–227. [Google Scholar] [CrossRef]
- Arlt, G. The influence of microstructure on the properties of ferroelectric ceramics. Ferroelectrics 1990, 104, 217–227. [Google Scholar] [CrossRef]
- Kang, B.S.; Choi, D.G.; Choi, S.K. Effects of grain size on pyroelectric and dielectric properties of Pb0.9La0.1TiO3 ceramic. J. Korean Phys. Soc. 1998, 32, S232–S234. [Google Scholar]
- Chamola, A.; Singh, H.; Naithani, U.C. Study of Pb(Zr0.65Ti0.35)O3(PZT(65/35) doping on structural, dielectric and conductivity properties of BaTiO3(BT) ceramics. Adv. Mater. Lett. 2011, 2, 148–152. [Google Scholar] [CrossRef]
- Miga, S.; Wojcik, K. Investigation of the diffuse phase transition in PLZT X/65/35 ceramics, x = 7–10. Ferroelectrics 1989, 100, 167–173. [Google Scholar] [CrossRef]
- Mahmud, I.; Ur, S.C.; Yoon, M.S. Effects of Fe2O3 addition on the piezoelectric and the dielectric properties of 0.99Pb(Zr0.53Ti0.47)O3–0.01Bi(Y1−xFex)O3 ceramics for energy-harvesting devices. J. Korean Phys. Soc. 2014, 65, 133–144. [Google Scholar] [CrossRef]
- Wagner, S.; Kahraman, D.; Kungl, H.; Hoffmann, M.J.; Schuh, C.; Lubitz, K.; Biesenecker, H.M.; Schmid, J.A. Effect of temperature on grain size, phase composition, and electrical properties in the relaxor-ferroelectric-system Pb(Ni1/3Nb2/3)O3–Pb(Zr,Ti)O3. J. Appl. Phys. 2005, 98, 024102. [Google Scholar] [CrossRef]
- Islam, R.A.; Priya, S. High energy density composition in the system PZT-PZNN. J. Am. Ceram. Soc. 2006, 89, 3147–3156. [Google Scholar] [CrossRef]
- Seo, I.T.; Cha, Y.J.; Kang, I.Y.; Choi, J.H.; Nahm, S.; Seung, T.H.; Paik, J.H. High energy density piezoelectric ceramics for energy harvesting devices. J. Am. Ceram. Soc. 2011, 94, 3629–3631. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, K.B.; Lee, Y.J.; Choi, J.H.; Kim, B.I.; Paik, J.H.; Nahm, S. Ferroelectric and Piezoelectric Properties of 0.72Pb(Zr0.47Ti0.53)O3–0.28Pb[(Zn0.45Ni0.55)1/3Nb2/3]O3. Jpn. J. Appl. Phys. 2012, 51, 9S2. [Google Scholar] [CrossRef]









| Doping | Sintering Temp. (°C) | Sintering Condition | Grain Size (µm) | Density (g/cc) | d33 (pC/N) | ε33T | kp (%) | g33 × 10−3 (Vm/N) | d33 × g33 (10−15 m2/N) |
|---|---|---|---|---|---|---|---|---|---|
| Sb2O5 | 1170 | air | 2.4 | 7.94 | 346 | 753 | 65.1 | 51.9 | 17,971 |
| Sb2O3 | air | 1.4 | 7.88 | 335 | 793 | 63.6 | 47.7 | 15,987 | |
| Sb2O3 | oxygen | 2.3 | 7.96 | 350 | 738 | 64.8 | 53.3 | 18,519 |
| Composition | Sintering Temp. (°C) | Grain Size (µm) | ρ (g/cc) | d33 (pC/N) | ε33T | kp (%) | Pr (µC/cm2) | EC (kV/cm) | TC (°C) | tanδ (1 kHz) 25 °C [10−3] |
|---|---|---|---|---|---|---|---|---|---|---|
| PZT–BYS(0.0) | 1150 | 2.5 | 7.91 | 324 | 742 | 63.5 | 45.6 | 11.5 | 373 | 0.0197 |
| PZT–BYS(0.1) | 1150 | 2.2 | 7.96 | 350 | 689 | 66 | 45.8 | 12.0 | 369 | 0.0213 |
| PZT–BYS(0.2) | 1150 | 1.7 | 7.95 | 352 | 774 | 65.5 | 46.3 | 12.9 | 365 | 0.0226 |
| PZT–BYS(0.3) | 1150 | 1.6 | 7.95 | 355 | 804 | 65.2 | 46.9 | 13.6 | 361 | 0.0201 |
| PZT–BYS(0.4) | 1170 | 1.6 | 7.91 | 360 | 835 | 64.9 | 47 | 13.8 | 361 | 0.0286 |
| PZT–BYS(0.5) | 1170 | 1.4 | 7.9 | 366 | 867 | 64.7 | 47.2 | 14.0 | 357 | 0.0215 |
| PZT–BYS(0.6) | 1170 | 1.4 | 7.86 | 370 | 920 | 64.5 | 47.8 | 14.1 | 354 | 0.0208 |
| Composition | Sintering Temp. (°C) | g33 × 10−3(Vm/N) | d33 × g33 (10−15 m2/N) |
|---|---|---|---|
| PZT–BYS(0.0) | 1150 | 49.1 | 15,824 |
| PZT–BYS(0.1) | 1150 | 57.4 | 20,075 |
| PZT–BYS(0.2) | 1150 | 51.3 | 18,044 |
| PZT–BYS(0.3) | 1150 | 49.9 | 17,686 |
| PZT–BYS(0.4) | 1170 | 48.7 | 17,513 |
| PZT–BYS(0.5) | 1170 | 47.7 | 17,431 |
| PZT–BYS(0.6) | 1170 | 45.6 | 16,908 |
| Composition | g33 × 10−3 (Vm/N) | d33 × g33 (10−15 m2/N) | TC (°C) | Ref. |
|---|---|---|---|---|
| 0.9Pb(Zr0.56Ti0.44)O3–0.1Pb[(Zn0.8Ni0.2)1/3Nb2/3]O3 + 2mol% MnO2 | 83 | 18,456 | ~315 | 41 |
| (0.65 + y)Pb(Zr0.47Ti0.53)–(0.35 − y)Pb[(Ni1−xZnx)1/3Nb2/3]O3 | 36 | 20,056 | … | 42 |
| 0.72Pb(Zr0.47Ti0.53)O3–0.28Pb[(Zn0.45Ni0.55)1/3Nb2/3]O3 | 35 | 13,051 | … | 43 |
| Pb0.98La0.02(NiSb)0.05[(Zr0.52Ti0.48)0.995]0.95O3 | 36.8 | 16,570 | … | 16 |
| 0.99Pb(Zr0.53Ti0.47)O3–0.01BiYO3 | 53 | 18,549 | 373 | 13 |
| 0.99Pb(Zr0.53Ti0.47)O3–0.01Bi(Y0.9Sb0.1)O3 | 57.4 | 20,075 | 369 | present |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, I.; Yoon, M.-S.; Ur, S.-C. Antimony Oxide-Doped 0.99Pb(Zr0.53Ti0.47)O3–0.01Bi(Y1?xSbx)O3 Piezoelectric Ceramics for Energy-Harvesting Applications. Appl. Sci. 2017, 7, 960. https://doi.org/10.3390/app7090960
Mahmud I, Yoon M-S, Ur S-C. Antimony Oxide-Doped 0.99Pb(Zr0.53Ti0.47)O3–0.01Bi(Y1?xSbx)O3 Piezoelectric Ceramics for Energy-Harvesting Applications. Applied Sciences. 2017; 7(9):960. https://doi.org/10.3390/app7090960
Chicago/Turabian StyleMahmud, Iqbal, Man-Soon Yoon, and Soon-Chul Ur. 2017. "Antimony Oxide-Doped 0.99Pb(Zr0.53Ti0.47)O3–0.01Bi(Y1?xSbx)O3 Piezoelectric Ceramics for Energy-Harvesting Applications" Applied Sciences 7, no. 9: 960. https://doi.org/10.3390/app7090960
APA StyleMahmud, I., Yoon, M.-S., & Ur, S.-C. (2017). Antimony Oxide-Doped 0.99Pb(Zr0.53Ti0.47)O3–0.01Bi(Y1?xSbx)O3 Piezoelectric Ceramics for Energy-Harvesting Applications. Applied Sciences, 7(9), 960. https://doi.org/10.3390/app7090960