1. Introduction
Supercritical fluid injections have many applications ranging from internal combustion engines to supercritical fluid extraction [
1,
2]. It is well-known that the critical point is a singularity in thermodynamics. Fluids in supercritical conditions have distinct properties from subcritical conditions. In the supercritical state, the interface between the liquid and the gas phases no longer exists, as the liquid surface tension and the latent heat of evaporation tend towards zero. So there is no longer a distinction between liquid and gas, but it is collectively referred to as a homogeneous supercritical fluid. Its density is close to that of the liquid and its transport characteristics are close to those of the gas. When a liquid approaches the critical point, the isothermal compression coefficient, constant pressure heat and gas solubility in the liquid are sharply increased. In that condition, the phenomena such as liquid jet disintegration, atomizing and droplet evaporation (usually occurring at subcritical condition during the fuel injection and the mixture formation) no longer exist. More specifically, the injection is more like gas-gas mixing in supercritical conditions. Therefore, the mechanism of supercritical injection is very complicated and essentially different from subcritical spray. A good understanding of the destabilization mechanism of supercritical jets is demanded urgently.
In recent years, many numerical studies on shear injection and mixing at supercritical conditions have been performed [
3,
4,
5,
6,
7,
8]. However, there are limitations to current injection modeling techniques. For subcritical conditions a means for tracking the phase boundaries must be included, and since all traditional methods for modeling such processes are based on the solutions of the Navier-Stokes equations, an accurate knowledge of the constituent transport and material properties along with an accurate equation of state are required. This can be particularly difficult because of the dramatic changes in the physical properties of the fluid near the critical point. Molecular dynamics simulations have been proven to be a suitable method for studying the behavior of liquid in supercritical conditions. Molecular dynamics simulations are based purely on first principles. As a result, there is no need for material or transport properties as a function of pressure and temperature, and there is no need to track phase boundaries. In addition, the simulation is three-dimensional. All physical processes are spontaneous and the supercritical mixing occurs naturally without the need for tracking the phase boundary of the jet.
Also, as stated by Dahms and Oefelein [
9], two distinctively different interfacial conditions can be classified using the Knudsen number criterion. The Knudsen number
, where
is the molecular mean free path and
is the corresponding interface thickness calculated using linear gradient theory. Knudsen numbers greater than 0.1 characterize non-continuum molecular scales, while Knudsen numbers smaller than 0.1 correspond to continuum regimes. According to the theory of Dahms and Oefelein [
9], as the Knudsen number is greater than 0.1 in the test conditions in the present study, molecular dynamics simulation is suitable to investigate the processes in the present study, which are governed by molecular dynamics [
10].
Various studies have been conducted on supercritical or subcritical injection. Of these studies, Ludwig et al. [
11] conducted a molecular dynamics simulation and experiment of nitrogen injection under supercritical conditions. In the study, a new model of supercritical injection was proposed and proved to be suitable for studying supercritical injection. Branam [
12] described in detail the method of using molecular dynamics to simulate supercritical jets. Shin et al. [
13] investigated the phenomenon of nanojet injection in a high-pressure environment by non-equilibrium molecular dynamics. The characteristics of the jet breakup were compared from subcritical to supercritical conditions. They hypothesized that the high pressure would accelerate the jet breakup processes. A continuous phase transition became dominant and no droplet formation was observed. However, all the above studies are concerned only with the phenomenological study of supercritical jet injection. There is still a lack of relevant research on the instability of the supercritical jet shear layer using molecular dynamics.
Depending on the conditions of the initial fluid and the environment, there are typically four kinds of supercritical injection processes [
14]: I. The supercritical jet is injected into the supercritical environment; II. The subcritical jet is injected into the supercritical environment; III. The supercritical jet is injected into the subcritical environment; IV. The subcritical jet is injected into the supercritical environment and then into the subcritical environment. Injections of type II, type III and type IV are generally considered to be transcritical because they pass through the critical point during the injection process. More importantly, type II is becoming more and more important because this type of condition occurs most often in liquid rocket engines. Therefore, we focus on type I and type II in our study.
In the present study, an accurate simulation method of the supercritical spray using molecular dynamics was performed and the spatial instability of the jet mixing layer was studied. Firstly, the molecular dynamics simulation of the thermodynamic property of nitrogen was carried out and compared with the Soave-Redlich-Kwong (SRK) equation of state. Then the molecular dynamics simulation of the supercritical nitrogen jet was performed at different conditions. The working conditions of the supercritical and the subcritical nitrogen jets ejected into a supercritical environment were calculated. Through the analysis of the results, the distribution of jet velocity, density, temperature and other jet characteristics were obtained. The interface was determined and the disturbance growth rate of the transcritical shear layer under different conditions was obtained. Finally, the growth rate obtained by molecular simulation was compared to the results of the linear stability analysis.
2. Methodology
Molecular dynamics simulation is a technique for calculating the evolution of classical multibody systems. In this case, the term classical means that the nuclear motion of the constituent particles obeys the laws of classical mechanics. Therefore, the motion of each atom follows the Newtonian motion formula:
where
,
and
are the position, acceleration and mass of atom
, respectively.
is the total force exerted on the atom. In molecular dynamics and statistical mechanics, it is customary to use the Hamiltonian form of motion equations to represent molecular motion, which is completely equivalent to the Newtonian equation of motion:
where
is a Hamiltonian function. It is a function of generalized momentum
and generalized coordinates
. It can be proved that the Hamiltonian function is the sum of the kinetic energy and the potential energy, that is:
where
and
are kinetic energy and potential energy, respectively. It can be seen that the Hamiltonian function is equal to the total energy of the system. It also introduces the concept of phase space, which is
.
N is the number of atoms. The state of the system is determined by
. The microscopic state of each system is a phase point in the 6N dimensional phase space, and the evolution of the system over time is the movement of the corresponding phase point of the system in the 6N dimensional phase space. In the Cartesian coordinate system, the form of the Hamiltonian equation of motion is:
This equation can be used to obtain the phase trajectory of the system by numerical integration through the famous Velocity-Verlet algorithm:
The molecular dynamics simulation mainly consists of three steps: the first step is to solve the total potential energy of the system. The second step is to calculate the force of each atom from the total potential energy obtained, and then use a certain integral method (for example, here we use the Velocity-Verlet algorithm) to integrate the position and velocity of each atom in each time step. The third step is to obtain the quantity we are interested in from the evolution of the phase point of the system according to the statistical mechanics principle. Theoretically, any kinetic quantity of the system, i.e., all physical properties, can be obtained from atomic trajectory information.
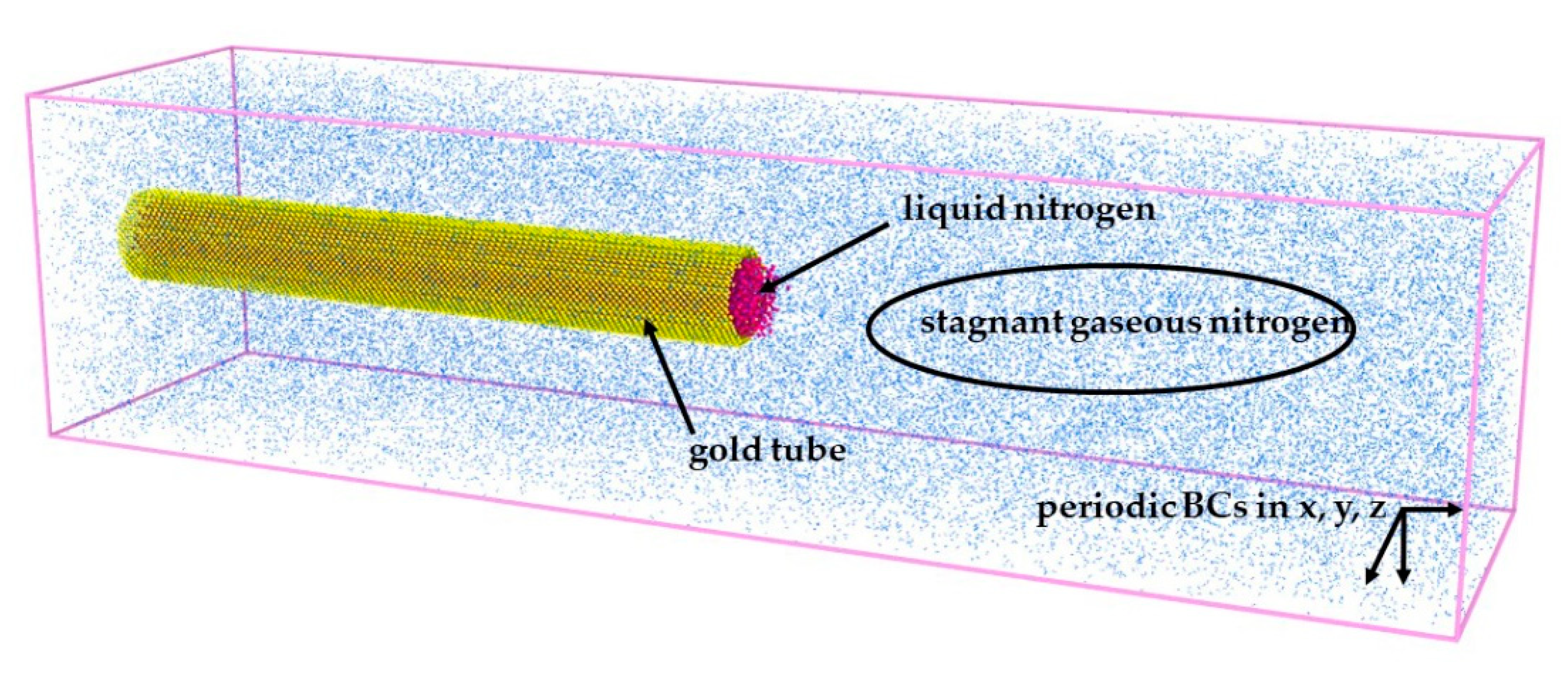
The three-dimensional (3-D) schematic diagram of the molecular dynamics (MD) model we study is given in
Figure 1. It is a closed system with the size of the simulation box being
(
,
,
). A horizontal gold tube, which is made of three layers of gold atoms on a face-centered cubic (FCC) lattice, is placed at the left center of the system. The gold tube is fixed by setting the velocities of gold atoms to zero in the whole simulation process. The lattice constant of gold is 4.08
. The length of the tube is 400
. The inside diameter is 40
and the outer diameter is 60
. The tube is full of liquid nitrogen under different thermodynamic conditions. All the space outside of the tube was filled with stagnant gaseous nitrogen as a supercritical environment. Periodic boundary conditions (BCs) were individually applied in the x, y and z directions. About 32,697 nitrogen molecules and 17,640 gold atoms were involved in the simulation. The number of atoms is different under different working conditions.
After setting up the model, the simulation was implemented under an NVE (i.e. constant atom number, constant volume and constant energy) ensemble to update all the nitrogen molecules with a time step of 0.01 ps using the classic parallel MD code [
15]. Gaussian distribution at a given initial temperature was used to determine the initial velocities of all nitrogen atoms. In practice, we followed Ludwig’s method [
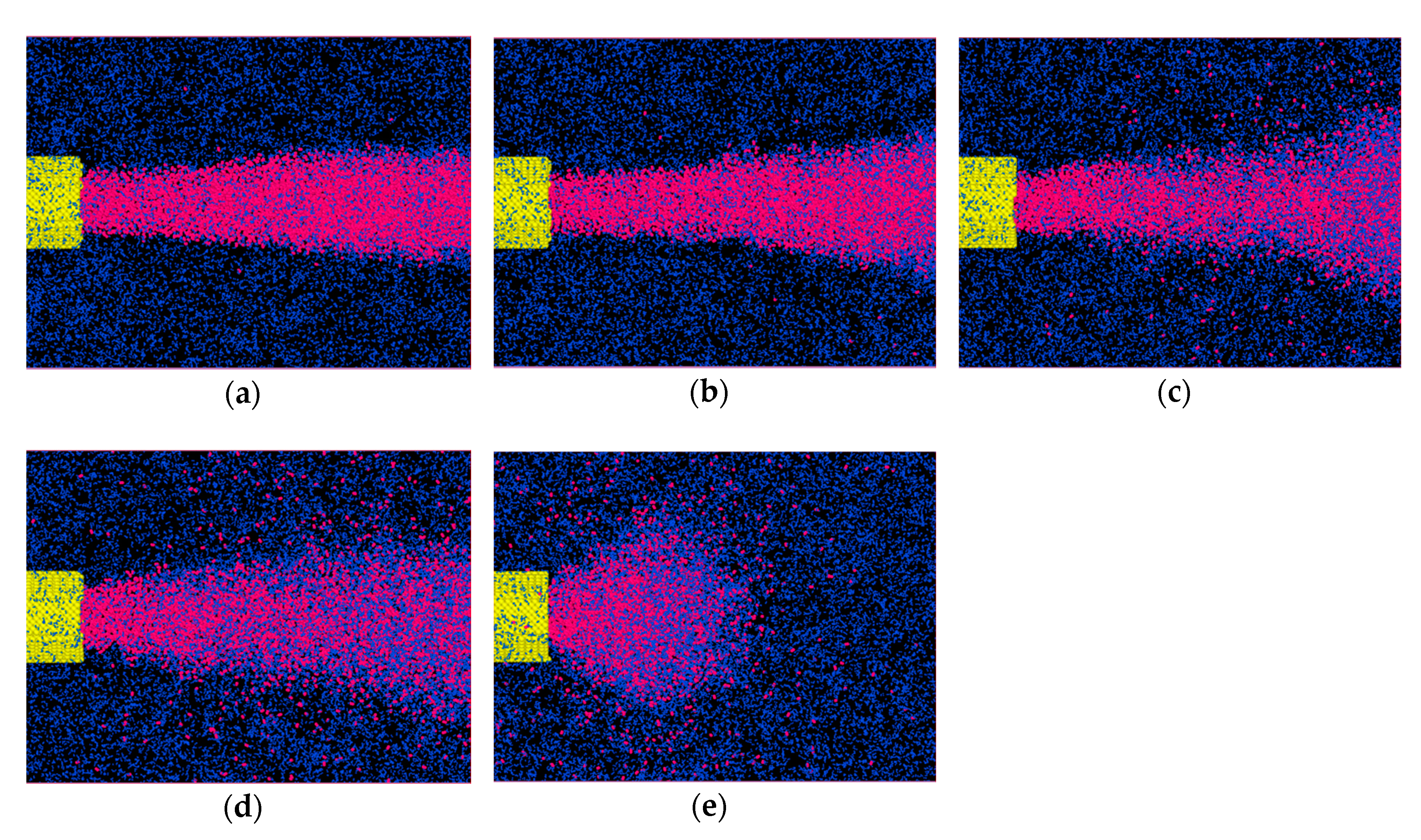
11] to divide the simulation into three stages, as illustrated in
Figure 2. The main target of step one is to form a developed pipe flow. Firstly, energy minimization was carried out for the whole system. After 10,000 steps of relaxation, the liquid and environment reached the desired temperature and pressure. Then the volume force was applied to the liquid nitrogen for a period of time. With a periodic BC, the liquid in pipe will loop back to the left boundary when it flows out of the right boundary. This enables the formation of a developed pipe flow. For the second stage, the gaseous nitrogen of the environment in a specific temperature and pressure was simulated. Finally, the system was coupled together to continue the injection simulation for 100 ps. Note that we need to be especially careful when operating the coupling process since the bond of nitrogen may cross the boundary and overlapping atoms may appear.
There are two categories of force between nitrogen atoms: the bonded force between nitrogen atoms inside a nitrogen molecule and the non-bonded force between nitrogen atoms of different nitrogen molecules. The bond forces result from harmonic potential [
16]:
where
is the instantaneous bond length of a nitrogen molecular,
is the equilibrium bond length of nitrogen and
is the stretching force constant of the
triple bond. The non-bond force between nitrogen atoms of different nitrogen molecules is the so-called van der Waals force, which is described by the Lennard-Jones (L-J) 12-6 potential:
where
is the potential well depth,
is the core distance at which the potential is equal to zero,
is the distance between two atoms and
is the cut-off radius of force interaction. For N-Au pairs, the L-J parameters are determined using the Lorentz-Berthelot [
17,
18] mixing rule. It is noteworthy that the hydrophilic and hydrophobic parameters are considered for the solid-liquid interaction to avoid the liquid accumulation near the outlet of the injector. The hydrophilic and hydrophobic factors are defined as Equation (8) [
19]:
where
is the hydrophilic parameter,
is the hydrophobic parameter and
and
are actual parameters for the solid-liquid interaction. In summary, the potential parameters used for the force routine are listed in
Table 1.
When the thermodynamic conditions of the fluid are close to the critical point, the pressure and density become very sensitive to disturbances, and the ideal gas equation of state is no longer applicable. Therefore, the real gas equation of state that takes into account the true physical effects becomes especially important when studying the behavior of a fluid approaching its critical point. In order to validate the model for nitrogen, we carried out an equation of state verification of nitrogen using MD and compared the results with the modified Soave-Redich-Kwong (SRK) equation of state. Here, we provide a brief review of the SRK equation. The SRK equation of state is a commonly used semi-empirical state equation and its correctness has been verified by researchers in the past [
20,
21,
22]. Soave [
23] introduced the eccentricity factor to modify the Redich-Kwong equation of state and obtained the three-parameter SRK equation, which has the following form:
where
is the pressure,
is the universal gas constant,
is the molar volume and
,
and
are the adjustable parameters, defined as follows:
where
is the critical temperature and
is the critical pressure.
is the eccentric factor of molecules.
is the reduced temperature. For nitrogen, the critical temperature and critical pressure are 126 K and 3.4 MPa, respectively.
We followed Charpentier’s method [
24] to obtain the growth rate of the liquid spray. It is well-known that the inviscid jet model was firstly proposed by Rayleigh [
25] using linear stability theory. In his theory, the development model of the perturbation
is as follows:
where
,
,
and
are the position, time, wavenumber and phase, respectively.
is the initial perturbation amplitude,
the spatial growth rate. The jet profile radius
obtained by the simulation can be expressed in the following form:
where
is the jet diameter. After the dimension is reduced by
and Rayleigh’s time
, with
being the density and
the surface tension, Equation (15) turns to:
where
,
,
,
and
. The standard deviation of surface fluctuations can be expressed as:
where
is the average of
at each position. It is assumed that the jet profiles are not correlated in time, and each phase obeys a uniform distribution on [0, 2π]. When
N approaches infinity, we have:
We assume that the phase variable follows a uniform distribution. For the two sides of Equation (18), we take the logarithm and then finally obtain the formula:
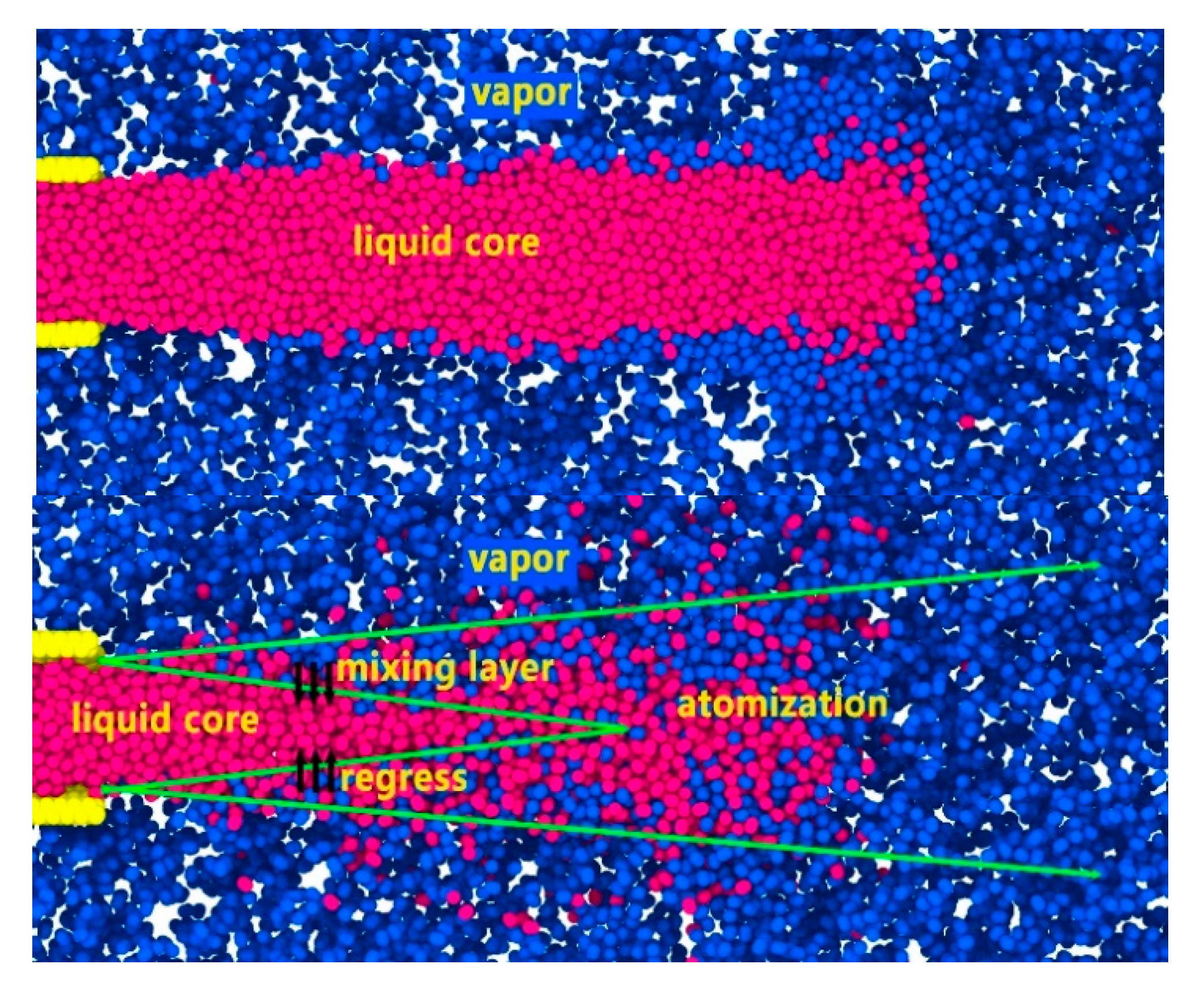
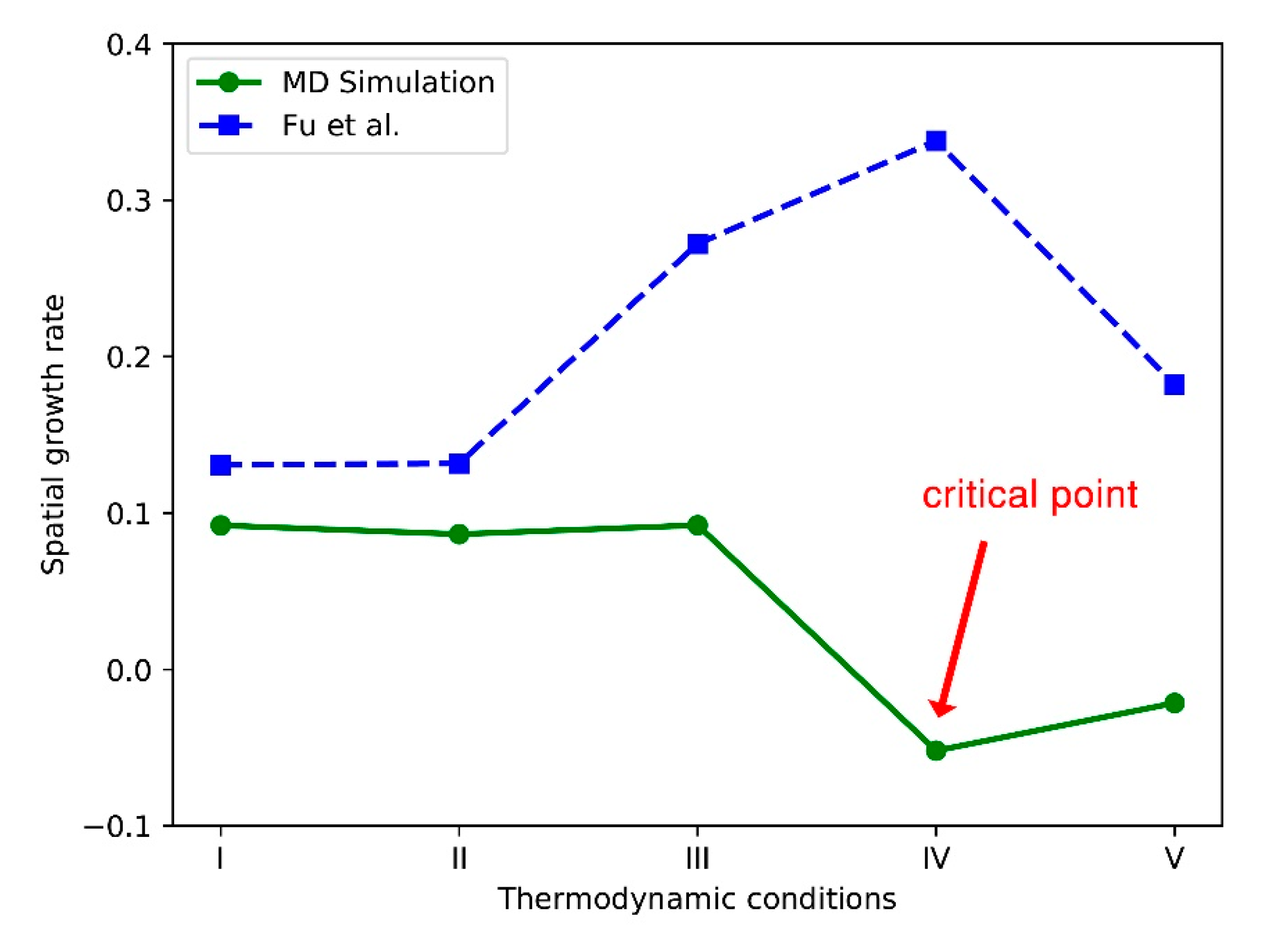
This formula associates the standard deviation of the radius at each position with the spatial growth rate . We use this formula to obtain the spatial growth rate of nitrogen jet perturbation. After we determine the gas-liquid boundary, the spatial growth rate of the supercritical jet can be obtained by fitting the data to the growth rate formula.